Abstract
Temperate zone woody plants cold acclimate in response to both short daylength (SD) and low temperature (LT). We were able to show that these two environmental cues induce cold acclimation independently by comparing the wild type (WT) and the transgenic hybrid aspen (Populus tremula × Populus tremuloides Michx.) line 22 overexpressing the oat (Avena sativa) PHYTOCHROME A gene. Line 22 was not able to detect the SD and, consequently, did not stop growing in SD conditions. This resulted in an impaired freezing tolerance development under SD. In contrast, exposure to LT resulted in cold acclimation of line 22 to a degree comparable with the WT. In contrast to the WT, line 22 could not dehydrate the overwintering tissues or induce the production of dehydrins (DHN) under SD conditions. Furthermore, abscisic acid (ABA) content of the buds of line 22 were the same under SD and long daylength, whereas prolonged SD exposure decreased the ABA level in the WT. LT exposure resulted in a rapid accumulation of DHN in both the WT and line 22. Similarly, ABA content increased transiently in both the WT and line 22. Our results indicate that phytochrome A is involved in photoperiodic regulation of ABA and DHN levels, but at LT they are regulated by a different mechanism. Although SD and LT induce cold acclimation independently, ABA and DHN may play important roles in both modes of acclimation.
Cold acclimation capacity is much higher in temperate zone woody plants compared with herbaceous species. Herbaceous plants survive normally under an insulating snow cover and a moderate low temperature (LT) tolerance is sufficient for survival. However, trees have to be able to face extremes of temperature and light conditions, and because of their long generation time and age, a high capacity for cold acclimation is paramount for their survival. The extreme freezing tolerance of woody plants is achieved by sequential stages of cold acclimation of which the first is initiated by short daylength (SD) and second and third by LT and freezing temperatures, respectively (Weiser, 1970). Although recent breakthroughs have increased our knowledge of the molecular basis of frost hardiness in herbaceous species, which acclimate primarily in response to LT (Thomashow, 1999), very little is known about cold acclimation of woody plants.
Phytochromes are the photoreceptors responsible for photoperiod detection in plants. Photosignal perception by the receptor activates signaling pathways leading to changes in gene expression that underlie the physiological and developmental responses to light. In Arabidopsis, five different phytochrome genes have been found, named phytochrome A (phyA) to phyE (Quail, 1991). The involvement of phytochrome in photoperiodic induction of cold acclimation in trees has been known for a long time (Williams et al., 1972; McKenzie et al., 1974). More recent molecular studies have strengthened and underlined the central role of phyA in daylength sensing of woody plants. Overexpression of oat (Avena sativa) phyA gene (PHYA) in hybrid aspen (Populus tremula × Populus tremuloides Michx.) significantly changes the critical daylength and prevents cold acclimation (Olsen et al., 1997). On the other hand, reduction of PHYA level in transgenic hybrid aspen leads to increased sensitivity to daylength (Eriksson, 2000).
It is acknowledged that the ability of plants to cold acclimate is a quantitative trait involving the action of many genes with small additive effects (Thomashow, 1999). One of the most important factors in cold acclimation is the development of tolerance to cellular dehydration induced by extracellular freezing. Accumulation of compatible solutes, sugars, and certain proteins is thought to protect cell structures during dehydration by binding water molecules (see Ingram and Bartels, 1996). One of the most extensively studied classes of such protective proteins is dehydrins (DHN), which are induced in vegetative organs by stresses leading to water deficit or in seeds during late stages of embryogenesis (Ingram and Bartels, 1996; Campbell and Close, 1997). DHN gene expression is also, in most cases, responsive to the phytohormone abscisic acid (ABA; see Campbell and Close, 1997). The function of DHNs is not fully understood, but it seems that they are providing a cohesive water layer to a number of macromolecules, preventing their coagulation under extreme dehydration (Campbell and Close, 1997) or rescuing hydrolytic enzyme function under dry conditions (Rinne et al., 1999). The structure of DHN and their subcellular localization has led to the proposition that DHN stabilize membranes under dehydrative conditions (Danyluk et al., 1998; Ismail et al., 1999). Wisniewski et al. (1999) recently showed that a peach DHN has also cryoprotective and antifreeze activity.
The purpose of the present study was to investigate factors involved in cold acclimation of woody plants. Because temperate zone woody plants can acclimate in response to both SD and LT, we used transgenic approach to dissect the LT- and SD-induced acclimation. We employed transgenic hybrid aspen overexpressing oat PHYA gene, which is not responding to SD by growth cessation, leading to impaired freezing tolerance development under SD (Olsen et al., 1997). By using this model, we were able to show that LT induces development of freezing tolerance independently of the photoperiodic acclimation. Our results demonstrate also that ABA and DHN are regulated in a different way under SD and at LT and suggest their involvement in both types of acclimation.
RESULTS
PhyA Overexpression Leads to Altered Growth Habit under Long Daylength (LD) and SD Conditions
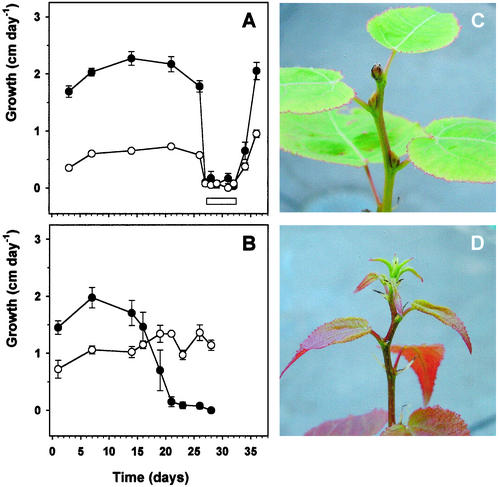
In a previous study, Olsen et al. (1997) introduced transgenic lines of hybrid aspen overexpressing oat PHYA gene. In contrast to the wild type (WT), the transgenic line 22 overexpressing of oat PHYA gene did not respond to SD by growth cessation or apical bud set. Another transgenic line (8) had very low expression level of oat PHYA gene, and it responded to SD in a similar way as the WT (Olsen et al., 1997). We wanted to study whether the WT and line 22 had the same growth characteristics at LT under LD as under SD. Under LD conditions, growth of the WT was faster compared with PHYA overexpressing line 22 (Fig. 1A), although the percentage height increment was about the same in both lines (data not shown). LT (4°C) prevented growth of the WT and line 22 immediately, and growth was restored to normal 1 d after the plants were transferred back to 18°C (Fig. 1A). Under SD conditions, growth of the WT stopped after 3 weeks (Fig. 1B) and terminal bud was formed on all WT plants after 4 weeks (Fig. 1C). Line 22 continued growth in SD even more vigorously than in LD (Fig. 1B), suggesting that growth cessation in SD was not due to depletion of e.g. photosynthetic energy but was a developmentally controlled phenomenon. Line 22 did not form terminal bud under SD (Fig. 1D), neither did LT induce terminal bud formation even after 5 weeks of exposure to 0.5°C in any of the lines (data not shown). Taken together, these results indicate that hybrid aspen perceives SD and LT signal in a distinct manner. Both environmental cues lead to growth cessation, but in SD, it is due to a slow developmental shift from vegetative growth to dormancy, whereas LT prevents the elongation growth of the plants more directly without an induction of dormancy.
Figure 1.
Growth characteristics of the hybrid aspen WT and line 22 overexpressing oat PHYA gene. A, Growth of the WT (●) and line 22 (○) under LD (16 h, 18°C) and LT (16 h, 4°C). The duration of the LT treatment is marked with a white bar. B, Growth of the WT (●) and line 22 (○) under SD (10 h, 18°C). C, Terminal bud of the WT and D, apex of line 22 after 3 weeks of SD treatment (10 h, 18°C). Values are means of three to five trees. The vertical bars represent ±se.
PhyA Overexpression and LT Prevent Dehydration of the Overwintering Tissues
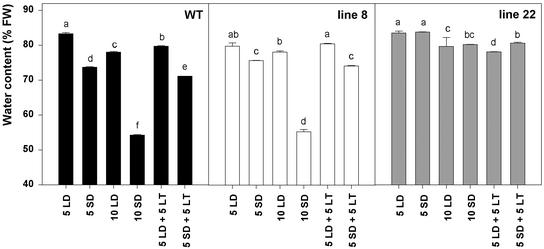
Dormancy development is characterized by disappearance of the cellular free water (Faust et al., 1991). It has been shown that phytochrome mediates this dehydration, which might be connected to the observed increase in freezing tolerance during SD (McKenzie et al., 1974). We wanted to study whether tissue dehydration is connected only to SD-induced acclimation or is it also induced by LT. Therefore, we measured the water content of the buds and the internodes after 5 and 10 weeks exposure to LD and SD at 18°C and after subsequent 5 weeks exposure to 0.5°C in the corresponding daylength. Water content of the internodes is shown in Figure 2, water content of the buds followed similar pattern (data not shown). Transfer of the plants to SD at 18°C resulted in significant reduction in water content of the internodes in the WT and line 8; after 10 weeks, water content had decreased by 30% (w/w) in both lines (Fig. 2). Line 22 did not respond to SD, and water content in this line remained at the same level as in LD throughout the study period. LT did not lead to decrease in water content under LD conditions, and if plants were transferred to LT after 5 weeks SD exposure, further dehydration was prevented (Fig. 2). Taken together, these results show that decrease of water content of overwintering tissues is mediated by phyA. This dehydration is specific to SD-induced acclimation, and it is an active process favored by high temperature.
Figure 2.
Water content of the internode segments of the WT, the line 8, and the line 22 hybrid aspen overexpressing oat PHYA gene. Plants were grown 5 weeks under LD (16 h, 18°C) or SD (10 h, 18°C) conditions, after which they were left at the same temperature for 5 weeks or they were transferred to LT (0.5°C) for 5 weeks in respective daylength. Numbers refer to duration of the treatments in weeks. The vertical bars represent ±se. Significant differences of at least 0.05 confidence level between different treatments within each line are marked with different letters.
Effect of phyA Overexpression to Freezing Tolerance of the Stem and the Leaves
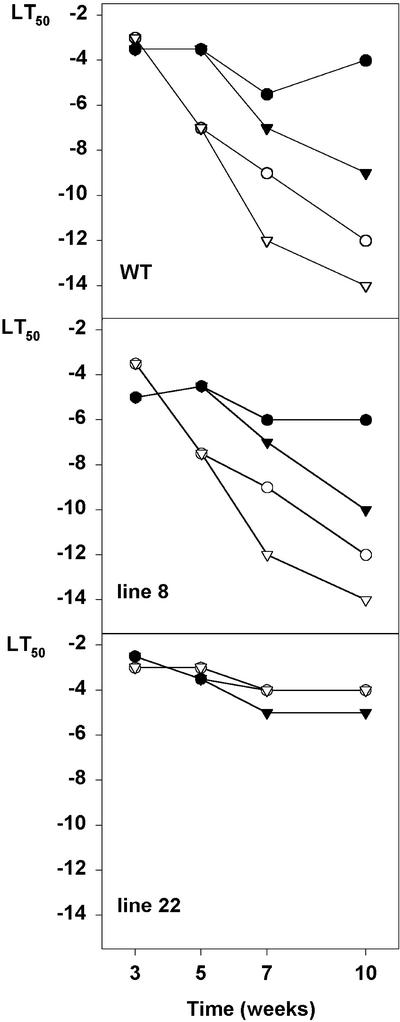
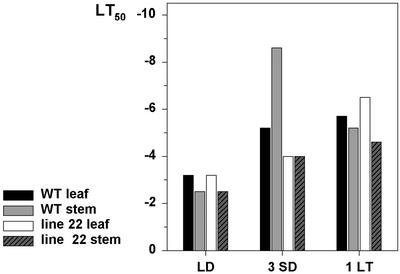
It was shown by Olsen et al. (1997) that overproduction of oat PHYA effectively prevented cold acclimation of hybrid aspen stem when the plants were acclimated in SD followed by LT. This was explained by the inability of the transgenic lines to stop growing, which has been shown to be a prerequisite for photoperiodic cold acclimation of woody plants (Fuchigami et al., 1971; Junttila and Kaurin, 1990). However, if woody plants are exposed directly to LT under LD conditions, they are able to acclimate without dormancy development (Christersson, 1978; Li et al., 2002). We wanted to study whether LT in LD conditions could induce acclimation in transgenic hybrid aspen and whether there were tissue specific differences in the acclimation capacity between these two acclimation strategies. In accordance with previous results, line 22 was severely impaired in photoperiodic induction of freezing tolerance. SD did not induce development of freezing tolerance of either leaf or stem tissue (Figs. 3 and 4). In contrast, exposure to SD resulted in significant increase in the freezing tolerance of the WT and line 8 stems (Figs. 3 and 4) and some increase in the WT leaves (Fig. 4). LT (0.5°C) alone under LD increased freezing tolerance of the stem of all the lines to some extent (Fig. 3), but a little higher temperature (4°C) was more effective in freezing tolerance induction (Fig. 4). Especially leaves of line 22 acclimated effectively under LT treatment (Fig. 4). The most effective treatment inducing freezing tolerance of the stem of the WT and line 8 was SD followed by LT, but under these conditions line 22 did not develop any freezing tolerance (Fig. 3). Results show that SD and LT can induce cold acclimation independently in hybrid aspen. SD-induced acclimation is mediated by phyA and works mainly in overwintering tissues, but LT-induced acclimation is also seen in leaves, and it does not require phyA.
Figure 3.
The effect of daylength and temperature to freezing tolerance of the stem of the WT, the line 8, and the line 22 hybrid aspen overexpressing oat PHYA gene. Plants were grown 5 weeks under LD (●; 16 h, 18°C) or SD (○; 10 h, 18°C) conditions, after which they were left to the same temperature for 5 weeks or transferred to 0.5°C for 5 weeks in LD (▾; 16 h, 0.5°C), or SD conditions (▿; 10 h, 0.5°C). LT50 shows the temperature in which one-half of the stems were killed.
Figure 4.
The effect of daylength and temperature to freezing tolerance of the leaf and stem of the WT and the line 22 hybrid aspen overexpressing oat PHYA gene. Plants were grown under LD conditions (16 h, 18°C) after which they were transferred to SD (10 h, 18°C) conditions for 3 weeks or to LT under LD (16 h, 4°C) conditions for 1 week, after which the freezing tolerance was measured. LT50 shows the temperature of 50% leakage of electrolytes.
Differential Regulation of ABA and DHN by SD and LT
ABA is thought to play a major role in dehydration stress tolerance (Chandler and Robertson, 1994). To elucidate the role of ABA in freezing tolerance development, we studied the regulation of its levels during acclimation under SD and at LT. At 18°C, ABA levels were significantly higher under LD than under SD conditions in the buds of the WT and line 8 (Table I). ABA content of line 22 buds was about the same under SD and LD and significantly lower than in line 8 or in the WT (Table I). After 7 weeks under SD conditions at 18°C, ABA levels were about the same in all lines, but from week 7 to 10, the levels decreased in line 8 and the WT and increased in line 22. Exposure to 0.5°C for 2 weeks gave a significant increase in ABA levels in all the lines under SD; under LD conditions levels increased in the WT and line 22 and decreased little in line 8 (Table I). After 3 additional weeks at 0.5°C, the levels were significantly reduced both under LD and SD in all three lines (Table I). These results suggest that photoperiod and temperature regulate ABA level differentially, and although photoperiodic regulation of line 22 was disturbed, this line responded to LT in a similar way as the WT.
Table I.
The effect of daylength and temperature to ABA content of the buds of the WT, the line 8, and the line 22 hybrid aspen overexpressing oat PHYA
| Line | Daylength | 7 Weeks at 18°C | 10 Weeks at 18°C | 5 Weeks at 18°C and 2 Weeks at 0.5°C | 5 Weeks at 18°C and 5 Weeks at 0.5°C |
|---|---|---|---|---|---|
| μg g−1 dry wt | |||||
| WT | LD | 14.08 | 18.29 | 26.76 | 6.18 |
| Line 8 | LD | 24.29 | 18.59 | 20.94 | 4.0 |
| Line 22 | LD | 5.15 | 9.37 | 11.54 | 2.48 |
| WT | SD | 4.18 | 1.48 | 11.58 | 2.53 |
| Line 8 | SD | 4.97 | 1.57 | 11.35 | 2.95 |
| Line 22 | SD | 4.48 | 7.3 | 10.58 | 0.54 |
Plants were grown for 5 weeks at 18°C under LD (16 h) or SD (10 h) conditions, after which they were left to the same temperature or transferred to 0.5°C. Samples were collected after 2 and 5 weeks of transfer. Values are means of two replicate samples, each consisting of five to 18 buds. ANOVA: Line (L), P = 0.0001; Daylength (D), P ≤ 0.0001; Temperature (T), P ≤ 0.0001; L × D, P ≤ 0.0001; L × T, P = 0.038; D × T, P = 0.025; L × D × T, P = 0.025.
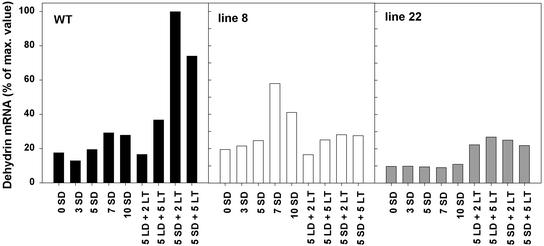
Plants increase their ability to tolerate environmental stresses by changes in gene expression (Thomashow, 1999). DHN have been studied extensively in cold acclimation research, because they are believed to contribute to protection of cellular organelles during freeze-induced cellular dehydration (Campbell and Close, 1997). To elucidate the role of DHN in SD- and LT-induced acclimation, we studied changes in DHN gene expression in hybrid aspen. As a model gene, we used DHN DSP16 of Craterostigma plantagineum Hochst. (Piatkowski et al., 1990), which was used as a probe in northern blots. The expression pattern of homologous DHN genes in buds, stem, and apex of hybrid aspen overexpressing oat PHYA was characterized under LD and SD conditions at 18°C and 0.5°C. DHN expression patterns in different tissues were similar, thus, only results from the apex are shown. The DHN transcripts were induced by a 3-week exposure to SD at 18°C in the WT and in line 8, whereas in line 22, the amount of the DHN transcripts was the same as in LD control even after 10 weeks under SD conditions (Fig. 5). In contrast, LT increased the level of DHN transcript in both transgenic lines and in the WT under LD and SD conditions (Fig. 5).
Figure 5.
Northern-blot analysis of expression of DHN gene homologous to DSP16 of C. plantagineum (Piatkowski et al., 1990) in the apex of the WT, the line 8, and the line 22 hybrid aspen overexpressing oat PHYA gene. Plants were grown under LD (16 h, 18°C) conditions, after which they were exposed to LT (16 h, 0.5°C), SD (10 h, 18°C), or SD followed by LT (10 h, 0.5°C) treatment. Numbers refer to duration of the treatment in weeks. Histogram shows normalized values of DSP16 (after standardization to ribosomal signal intensities) presented as a percentage of the highest value.
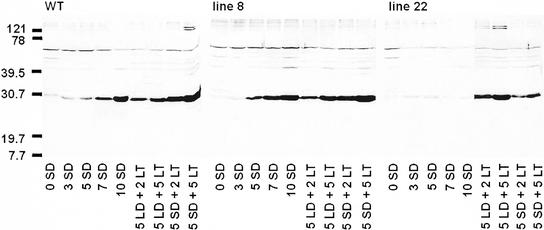
In the western-blot analysis, we used a polyclonal antibody that was raised against a synthetic polypeptide corresponding to the product of the same DSP16 gene (Piatkowski et al., 1990). Western-blot results of the buds, stem, and apex were similar, so only representative results from bud tissue are shown. The antibody recognized a number of constitutively expressed proteins in buds. Furthermore, expression of a 30-kD protein correlated with expression of gene homologous to DSP16 (Fig. 6). Similar to the DHN transcript, expression of the 30-kD DHN-related protein (DRP) was up-regulated gradually during the 10 weeks study period under SD conditions in the WT and in line 8, but its level remained at the LD control level in line 22 (Fig. 6). In contrast, LT increased the level of the 30-kD DRP in all the lines and also in line 22, both under LD and SD conditions. Induction by LT was much more rapid than SD induction; after 2 weeks at 0.5°C, the amount of 30-kD DRP was the same as after 10 weeks SD treatment in the WT and line 8. SD and LT had also additive effect to DHN protein accumulation in the WT and line 8 (Fig. 6). Taken together, these results show that both SD- and LT-induced acclimation is accompanied with DHN gene expression. Differential accumulation of DHN in line 22 by SD or LT shows that these environmental cues induce dehdydrin gene expression independently.
Figure 6.
Western-blot analysis of expression of DHN-like proteins in the buds of the WT, the line 8, and the line 22 hybrid aspen overexpressing oat PHYA. Plants were grown under LD (16 h, 18°C) conditions, after which they were exposed to LT (16 h, 0.5°C), SD (10 h, 18°C), or SD followed by LT (10 h, 0.5°C) treatment. Numbers refer to duration of the treatment in weeks. Molecular masses of the protein standards are indicated on the left. The polyclonal antibody was raised against a synthetic polypeptide from the pcC 6-19 clone of C. plantagineum (Schneider et al., 1993), corresponding to gene DSP16 (Piatkowski et al., 1990).
DISCUSSION
Our results demonstrate that two different environmental cues, SD and LT, induce cold acclimation of hybrid aspen independently. We were able to dissect these two pathways by using transgenic hybrid aspen line 22, overexpressing oat PHYA gene. This line did not respond to SD by cold acclimation, whereas the WT and the transgenic line 8, in which oat PHYA expression was negligible (Olsen et al., 1997), cold acclimated within 3 weeks under SD at 18°C (Figs. 3 and 4). However, at LT (4°C) under LD, especially leaves of line 22 were able to acclimate in a degree comparable with the WT (Fig. 4). Our results support the idea that perception of SD signal in cold acclimation of woody plants is mediated through phyA (Williams et al., 1972; McKenzie et al., 1974; Olsen et al., 1997) and suggest that LT-induced acclimation is not dependent on this mechanism.
Although SD- and LT-induced acclimation shared some common components, the mechanism behind these acclimation strategies was different. The most prominent difference was the involvement of dormancy in SD-induced acclimation and the opposite effect of LT to this dormancy development. One of the key factors in dormancy was the decrease in water content of the overwintering tissues. Dehydration has been shown to be an integral part of bud dormancy development (see Rohde et al., 2000). On the other hand, SD-induced dehydration has been shown to correlate with increased freezing tolerance (McKenzie et al., 1974; Junttila and Kaurin, 1990). We suggest that although dehydration is connected to dormancy development, it is one of the key factors that increase freezing tolerance under SD conditions and is prerequisite for maximum hardiness during subsequent LT treatment. In line 22, there was no decrease in water content of the buds or internodes nor did freezing tolerance of the stem increase under SD conditions or by the following LT treatment (Fig. 3). In contrast, decrease of water content of the buds and internodes in the WT and line 8 correlated with increased freezing tolerance under SD (Figs. 2 and 3). Dehydration may directly increase freezing tolerance through osmotic adjustment (Levitt, 1980; Sakai and Larcher, 1987), or it may induce adaptive responses that indirectly enhance freezing tolerance (Guy et al., 1992; Xiong et al., 1999). Decrease of water content under SD conditions may partly be a consequence of accumulation of dry matter, e.g. proteins and carbohydrates (Levitt, 1980). Sauter et al. (1996), for example, showed that starch accumulation under SD conditions and its conversion to sugars at LT are needed for proper acclimation in poplar. In conclusion, growth cessation, mediated by phyA leads to decrease in water content and accumulation of reserves, which are then used during the subsequent LT to achieve maximum freezing tolerance. Inability of line 22 to dehydrate and accumulate reserves in SD explains partly its inability to acclimate under SD and during subsequent LT conditions.
DHN are thought to have protective functions in plants cells against dehydration-induced stresses (Campbell and Close, 1997). We studied the role of DSP16-like DHN in SD- and LT-induced cold acclimation of the hybrid aspen. The inability of line 22 to accumulate DSP16-like DHN under SD (Figs. 5 and 6) indicates that phyA is involved in photoperiodic regulation of this gene. Slow accumulation of DSP16-like DHN during SD in the WT and line 8 suggests that this regulation is indirect, resulting from a slow dehydration of the cytoplasm. However, LT (0.5°C) induced strong and rapid accumulation of DHN transcript and protein in all three lines under LD and SD conditions (Figs. 5 and 6), revealing that LT induces a distinct pathway, which is independent of action of phyA. Earlier studies have shown that DHNs accumulate in woody plants in response to SD (Welling et al., 1997; Rinne et al., 1998) and LT (Levi et al., 1999; Richard et al., 2000). We were able to show that these environmental cues induce DHN gene expression independently. This is in accordance with Artlip et al. (1997), who suggested that LT and SD induce distinct signal transduction pathways in DHN gene expression in peach.
Accumulation of DHNs has been shown to follow seasonal changes in numerous woody species, which has raised the question of whether they are connected to dormancy or freezing tolerance (Wisniewski et al., 1996; Rinne et al., 1998; Sauter et al., 1999). We propose here that the DSP16-like DHN has a role both in dormancy and freezing tolerance development in hybrid aspen. Under SD conditions DSP16-like DHN accumulation correlated with development of dormancy (Fig. 1), dehydration of the buds and stem (Fig. 2), and increase in freezing tolerance (Fig. 3). In LD conditions, LT-induced DSP16-like DHN accumulation correlated solely with freezing tolerance, because LT did not induce dormancy development (Fig. 1). Therefore, both of these environmental cues induce expression of a DSP16-like DHN, which then can provide protection against dehydration both during dormancy development and during freezing stress. Interestingly, stems of line 22 were not able to acclimate when exposed to LT under SD conditions, although they were able to accumulate low levels of DSP16-like DHN under these conditions. This demonstrates the complexity of the cold acclimation of hybrid aspen and suggests that in woody plants the second stage of acclimation requires both SD and LT cues.
ABA is thought to be one of the key regulators in the cold acclimation response (Heino et al., 1990; Lång et al., 1994; Tamminen et al., 2001). A transient increase in ABA content preceding acclimation in those species that are able to acclimate is one of the early events in acclimation process (Chen et al., 1983; Lång et al., 1994). In birch (Betula pubescens Ehrh.), transient increase in ABA content is observed during the 1st week of SD treatment (Welling et al., 1997; Rinne et al., 1998). We could show that in woody plants, ABA content is controlled by different mechanisms during SD- or LT-induced acclimation. Photoperiod clearly influenced ABA content of the buds in the WT and line 8, but in line 22, the ABA level was approximately the same in both daylengths (Table I). However, LT induced transient increase in ABA content in all lines (Table I). Higher or similar levels of ABA at LT in line 22 compared with the WT and line 8 demonstrated that line 22 was not deficient in ABA synthesis but, rather, was unable to respond to the photoperiodic signal. These results suggest that phyA is involved in the photoperiodic regulation of ABA level, but at LT, the ABA level is regulated by a different mechanism.
Our results demonstrate that trees perceive the SD and LT signal separately and that both environmental cues can trigger cold acclimation independently. Results show that phyA-mediated apical bud formation under SD is a “main switch” that turns metabolism from vegetative growth to dormancy and to induction of freezing tolerance. Dehydration of the overwintering tissues and accumulation of proteins, especially DHN during SD are essential factors during photoperiodic acclimation. Without these changes, woody plants are not able to reach the maximum freezing tolerance in the subsequent LT. In contrast, LT-induced development of freezing tolerance and regulation of DHN and ABA levels were not dependent of phyA at LD, suggesting the presence of a distinct but interacting cold acclimation pathways.
MATERIALS AND METHODS
Plant Material
Two transgenic lines of hybrid aspen (Populus tremula × Populus tremuloides Michx.) expressing the oat (Avena sativa) PHYA gene, together with the WT were used in the experiments. Line 22 shows a strong ectopic expression of oat PHYA, which leads to reduced internode length in the LD conditions compared with the WT. Line 22 has also reduced sensitivity to SD, appearing as a continuous growth in SD conditions, whereas the WT stops growing and forms terminal bud. Expression of oat PHYA in line 8 is negligible, and its growth responses in different photoperiods are similar to WT (Olsen et al., 1997).
Plant Growth Conditions and Experimental Design
Plants were propagated by in vitro shoot culture and grown thereafter in controlled environmental chambers at 18°C (±0.5°C) under a photoperiod of 16 h for about 6 weeks before use in the experiments. Photon flux density of the light period was 150 to 200 μmol m−2 s−1 at 400 to 700 nm (TL 65W/83 fluorescent tubes, Philips, Eindhoven, The Netherlands). Humidity in the growth chambers was adjusted to give 0.5-kPa water-vapor pressure deficit. Plants were watered daily with a complete nutrient solution (Junttila, 1976). Plants were grown first for 5 weeks at 18°C (±0.5°C) in short (10 h, SD) or long (16 h, LD) photoperiod. Then one-half of the plants in corresponding photoperiods were transferred to LT (0.5°C) for 5 weeks. The rest of the plants were kept at 18°C. An additional short-term experiment was made with the WT and line 22 plants to test the freezing tolerance of the stem and the leaves. Micropropagated plants were grown in the greenhouse under LD (16 h day, 18°C) for 5 weeks before the experiments. Part of the plants were given a 3-week SD treatment in the greenhouse by reducing the daylength to 10 h with curtains; temperature was adjusted to 18°C. For LT treatment, part of the plants were moved to walk-in growth chamber. After a week of adaptation, temperature was reduced to 4°C, and the daylength was kept at 16 h. Cessation of apical growth and formation of terminal bud were monitored throughout the study period.
Sample Collection and Determination of Water Content
At each time point different types of samples were collected: (a) buds from nodes 3 to 12, (b) respective internodes along the bud and (c) apex (terminal bud with 1–2 cm of the stem beneath it). Two parallel sets of samples were collected from each genotype and treatment, and each parallel consisted of two plants. Two such samples of buds (3–5) and internodes (2–3) were taken for determination of water content. The rest of the samples were divided in two, frozen in liquid nitrogen and stored at −80°C before use for ABA and gene expression analyses. Water content of the buds and internodes was measured after 5 and 10 weeks of experiment. The dry weight of the samples was measured after drying for 24 h at 80°C. Relative water content was calculated from formula [(fresh weight − dry weight)/fresh weight] × 100%.
ABA Content of the Buds
Bud samples for ABA analyzes were collected after 7 and 10 weeks of experiment. At that time, trees were grown at 18°C under a 16- or 10-h photoperiod for 7 and 10 weeks or at 0.5°C for 2 and 5 weeks in corresponding daylength. Samples were weighted (fresh weight), frozen in liquid N2, and freeze-dried to get the dry weight. The samples were extracted for 1 h at 4°C in 0.5 mL of 50 mm sodium phosphate buffer, pH 7.0, with 0.02% (w/v) Na-diethyldithiocarbamate as an antioxidant and [2H4]ABA as an internal standard. After centrifugation, pH of the supernatant was adjusted to 2.7, and ABA was bound to Amberlite XAD-7 by shaking for 30 min at 4°C. Buffer was removed, and Amberlite XAD-7 was washed twice with 2 mL of 1% (v/v) acetic acid. The resin was dried in a Speed Vac, and ABA was eluted from Amberlite XAD-7 twice with 2 mL of dichloromethane by shaking for 30 min at 4°C. Dichloromethane was evaporated in a Speed-Vac and samples were methylated and analyzed with gas chromatography-mass spectrometry using selected ion monitoring.
Freezing Tolerance of the Stem and Leaves
Freezing tests were done with stem segments including buds; each test temperature consisted two parallels with five stem segments. Stems were freeze-tested under controlled conditions using a freezing rate of 3°C h−1 down to −17°C and further at 10°C h−1. The samples were removed at intervals of 4°C. After thawing overnight at 6°C, the samples were incubated at 18°C between moist paper towels, and injury was evaluated visually. Freezing tolerance of the leaves and corresponding stems was measured in controlled freezing bath, and injury was determined with electrolyte leakage method as described in Lång et al. (1989). Leaf sample consisted of a 1.5-cm-diameter leaf-disc cut from both sides of the middle vein from two uppermost full-grown leaves. Freezing tolerance of the stem was measured from a 10-cm part of the stem located 3 cm below the apical bud. Samples were wrapped in Miracloth and placed in test tubes in a controlled freezing bath. After initiation of ice formation at −1.5°C, bath temperature was decreased by 2°C h−1. Samples were taken out at 2°C intervals and allowed to thaw in ice at 4°C overnight. Conductivity (R0) was measured after shaking (200 rpm) the samples in 40 mL of deionized water for 2 h at room temperature. To measure the total conductivity (R1), samples were killed by boiling them for 30 min, and after chilling them to room temperature, they were re-extracted by shaking for 2 h at 200 rpm in the original solution. Ion leakage was calculated as R0/R1 × 100%. Plants showing leakage of 50% (LT50) or more of the total solutes were considered dead.
RNA Isolation and Hybridization Analysis
Total RNA from the buds, stem, and apex was purified as described by Verwoerd et al. (1989). Samples were frozen in liquid N2, homogenized to a fine powder in a mortar with a pestle, and extracted in 500 μL of hot (80°C) phenol buffer (1:1 phenol:[100 mm LiCl, 100 mm Tris, pH 8.0, 10 mm EDTA, and 1% (w/v) SDS]) by vortexing for 60 s. Samples were extracted twice with 250 μL of 1:24 chloroform:isoamylalcohol, and RNA was precipitated with 2 m LiCl at 4°C. The resulting pellet was dissolved in water and precipitated with ethanol. RNA concentration was measured with spectrophotometer, and 10 μg of total RNA was denatured and separated on a formaldehyde-agarose gel (Sambrook et al., 1989). After capillary transfer onto a positively charged nylon membrane (Roche Molecular Biochemicals, Mannheim, Germany), RNA was immobilized to the membrane by baking.
An 800-bp PstI-fragment of the DHN cDNA clone pcC6–19 from Craterostigma plantagineum Hochst., corresponding to the gene DSP16 (Piatkowski et al., 1990), was used as a probe in northern blotting. The fragment contained sequences corresponding to the N-terminal consensus sequence DEYGNP, Ser repeat, and the two copies of putative amphipathic α-helix forming domain KIKELPGH (Piatkowski et al., 1990). The probe was labeled with [α-32P]dCTP using the labeling kit Ready-To-Go (Amersham Pharmacia Biotech, Piscataway, NJ) and purified on a ProbeQuant G-50 Micro Columns (Amersham Pharmacia Biotech). Membranes were hybridized overnight at 55°C in a modified phosphate buffer (Church and Gilbert, 1984) containing 7% (w/v) SDS and 500 mm sodium phosphate buffer, pH 7.2. Washing of the membranes after hybridization was performed at 55°C according to Church and Gilbert (1984). Signals on filter were quantified by BAS 1500 scanner (Fuji Photo Film, Tokyo), and ribosomal 18S gene was used as heterologous probe to estimate differences in sample loading.
Protein Analysis
Bud, bark, or apex samples were ground in Eppendorf tubes with extraction buffer (50 mm Tris, base, 0.1% [w/v] SDS, and 200 mm DTT) and centrifuged at 10,000g twice for 10 min. Protein content was measured with Microassay Procedure for Microtiter Plates (Bio-Rad, Hercules, CA) according to manufacturers instructions. Fifteen micrograms of protein was diluted into Towbin loading buffer and separated with SDS-PAGE as described earlier (Welling et al., 1997). Two parallel gels were run. One was stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 in 40% (v/v) methanol and 10% (v/v) acetic acid for ensuring equal loading per lane. For immunoblotting, proteins from parallel unstained gels were electroblotted onto 0.45-μm nitrocellulose membranes (Micron Separations, Westborough, MA) as described earlier (Welling et al., 1997). The membrane was probed overnight in 1:1,000 dilution with polyclonal antibody, which was raised against a synthetic polypeptide from the clone pcC6–19 of C. plantagineum (Schneider et al., 1993) corresponding to gene DSP16 (Piatkowski et al., 1990).
Statistical Analyses
The influence of daylength, line, and temperature on ABA content was analyzed with two-way analysis of variance (ANOVA). One-way ANOVA was used to test the significant difference in water content between the different treatments within the lines.
ACKNOWLEDGMENTS
We thank Dr. Dorothea Bartels (Max-Planc-Institut, Köln, Germany) for her gift of the clone pcC6-19 and the corresponding antibody. We thank Dr. Pekka Heino and Dr. Markku Aalto (University of Helsinki) for helpful remarks on the manuscript.
Footnotes
This work was supported by the Academy of Finland (project nos. 44252, 44262, 44883, and 49952; Finnish Center of Excellence Program 2000–2005), by the Biocentrum Helsinki, by the National Technology Agency, by the Helsinki University Foundation, and by The Emil Aaltonen Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003814.
LITERATURE CITED
- Artlip TS, Callahan AM, Basset CL, Wisniewski ME. Seasonal expression of a dehydrin gene in sibling deciduous and evergreen genotypes of peach (Prunus persica [L.] Batch.) Plant Mol Biol. 1997;33:61–70. doi: 10.1023/a:1005787909506. [DOI] [PubMed] [Google Scholar]
- Campbell SA, Close TJ. Dehydrins: genes, proteins, and associations with phenotypic traits. New Phytol. 1997;137:61–74. [Google Scholar]
- Chandler PM, Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:113–141. [Google Scholar]
- Chen HH, Li PH, Brenner ML. Involvement of abscisic acid in potato cold acclimation. Plant Physiol. 1983;71:362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christersson L. The influence of photoperiod and temperature on the development of frost hardiness in seedlings of Pinus silvestris and Picea abies. Physiol Plant. 1978;44:288–294. [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N, Sarhan F. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell. 1998;10:623–638. doi: 10.1105/tpc.10.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson ME. The role of phytochrome A and gibberellins in growth under long and short day conditions studies in hybrid aspen. PhD thesis. Umeå: Swedish University of Agricultural Sciences; 2000. [Google Scholar]
- Faust M, Liu D, Millard MM, Stutte GW. Bound versus free water in dormant apple buds: a theory for endodormancy. HortScience. 1991;26:887–890. [Google Scholar]
- Fuchigami LH, Weiser CJ, Evert DR. Induction of cold acclimation in Cornus stolonifera Michx. Plant Physiol. 1971;47:98–103. doi: 10.1104/pp.47.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C, Haskell D, Neven L, Klein P, Smelser C. Hydration-state-responsive proteins link cold and drought stress in spinach. Planta. 1992;188:265–270. doi: 10.1007/BF00216823. [DOI] [PubMed] [Google Scholar]
- Heino P, Sandman G, Lång V, Nordin K, Palva ET. Abscisic acid deficiency prevents development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1990;79:801–806. doi: 10.1007/BF00224248. [DOI] [PubMed] [Google Scholar]
- Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Ismail AM, Hall AE, Close TJ. Purification and partial characterization of a dehydrin involved in chilling tolerance during seedling emergence of cowpea. Plant Physiol. 1999;120:237–244. doi: 10.1104/pp.120.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila O. Apical growth cessation and shoot tip abscission in Salix. Physiol Plant. 1976;38:278–286. [Google Scholar]
- Junttila O, Kaurin Å. Environmental control of cold acclimation in Salix pentandra. Scand J For Res. 1990;5:195–204. [Google Scholar]
- Lång V, Heino P, Palva ET. Low temperature acclimation and treatment with exogenous abscisic acid induce common polypeptides in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1989;77:729–734. doi: 10.1007/BF00261251. [DOI] [PubMed] [Google Scholar]
- Lång V, Mäntylä E, Welin B, Sundberg B, Palva ET. Alterations in water status, endogenous abscisic acid content, and expression of rab18 gene during the development of freezing tolerance in Arabidopsis thaliana. Plant Physiol. 1994;104:1341–1349. doi: 10.1104/pp.104.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A, Panta GR, Parmentier CM, Muthalif MM, Arora R, Shanker S, Rowland LJ. Complementary DNA cloning, sequencing and expression on an unusual dehydrin from blueberry floral buds. Physiol Plant. 1999;107:98–109. [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stresses: Chilling, Freezing and High Temperature Stresses. Vol. 1. New York: Academic Press; 1980. [Google Scholar]
- Li C, Puhakainen T, Welling A, Viherä-Aarnio A, Ernstsen A, Junttila O, Heino P, Palva ET (2002) Cold acclimation in silver birch (Betula pendula Roth.): development of freezing tolerance in different tissues and climatic ecotypes. Physiol Plant (in press)
- McKenzie JS, Weiser CJ, Burke MJ. Effects of red and far red light on the initiation of cold acclimation in Cornus stolonifera Michx. Plant Physiol. 1974;53:783–789. doi: 10.1104/pp.53.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JE, Junttila O, Nilsen J, Eriksson ME, Martinussen I, Olsson O, Sandberg G, Moritz T. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 1997;12:1339–1350. [Google Scholar]
- Piatkowski D, Schneider K, Salamini F, Bartels D. Characterization of five abscisic acid-responsive cDNA clones isolated from the desiccation-tolerant plant Craterostigma plantagineum and their relationship to other water-stress genes. Plant Physiol. 1990;94:1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail PH. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Richard S, Morency M-J, Drevet C, Jouanin L, Séguin A. Isolation and characterization of a dehydrin gene from white spruce induced upon wounding, drought and cold stresses. Plant Mol Biol. 2000;43:1–10. doi: 10.1023/a:1006453811911. [DOI] [PubMed] [Google Scholar]
- Rinne P, Welling A, Kaikuranta P. Onset of freezing tolerance in birch (Betula pubescens Ehrh.) involves LEA proteins and osmoregulation and is impaired in an ABA-deficient genotype. Plant Cell Environ. 1998;21:601–611. [Google Scholar]
- Rinne PLH, Kaikuranta PLM, van der Plas LHW, van der Schoot C. Dehydrins in cold-acclimated apices of birch (Betula pubescens Ehrh.): production, localization and potential role in rescuing enzyme function during dehydration. Planta. 1999;209:377–388. doi: 10.1007/s004250050740. [DOI] [PubMed] [Google Scholar]
- Rohde A, Howe GT, Olsen JE, Moritz T, Van Montagu M, Junttila O, Boerjan W. Molecular aspects of bud dormancy in trees. In: Jain SM, Minocha SC, editors. Molecular Biology of Woody Plants. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publisher; 2000. pp. 89–134. [Google Scholar]
- Sakai A, Larcher W. Frost survival of plants: responses and adaptation to freezing stress. Berlin: Springer-Verlag; 1987. [Google Scholar]
- Sambrook J, Maniatis T, Fritsch EF. Molecular Cloning. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sauter JJ, Westphal S, Wisniewski M. Immunological identification of dehydrin-related proteins in the wood of five species of Populus and in Salix caprea L. J Plant Physiol. 1999;154:781–788. [Google Scholar]
- Sauter JJ, Wisniewski M, Witt W. Interrelationships between ultrastructure, sugar levels, and frost hardiness of ray parenchyma cells during frost acclimation and deacclimation in poplar (Populus × canadensis Moench <robusta>) wood. J Plant Physiol. 1996;149:451–461. [Google Scholar]
- Schneider K, Wells B, Schmelzer E, Salamini F, Bartels D. Desiccation leads to the rapid accumulation of both cytosolic and chloroplastic proteins in the resurrection plant Craterostigma plantagineum Hochst. Planta. 1993;189:120–131. [Google Scholar]
- Tamminen I, Mäkelä P, Heino P, Palva ET. Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J. 2001;25:1–8. doi: 10.1046/j.1365-313x.2001.00927.x. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser CJ. Cold resistance and injury in woody plants. Science. 1970;169:1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]
- Welling A, Kaikuranta P, Rinne P. Photoperiodic induction of dormancy and freezing tolerance in Betula pubescens: involvement of ABA and dehydrins. Physiol Plant. 1997;100:119–125. [Google Scholar]
- Williams BJ, Pellett NE, Klein RM. Phytochrome control of growth cessation and initiation of cold acclimation in selected woody plants. Plant Physiol. 1972;50:262–265. doi: 10.1104/pp.50.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M, Close TJ, Artlip T, Arora R. Seasonal patterns of dehydrins and 70-kDa heat-shock proteins in bark tissues of eight species of woody plants. Physiol Plant. 1996;96:496–505. [Google Scholar]
- Wisniewski M, Webb R, Balsamo R, Close TJ, Yu X-M, Griffith M. Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica) Physiol Plant. 1999;105:600–608. [Google Scholar]
- Xiong L, Ishitani M, Zhu J-K. Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol. 1999;119:205–211. doi: 10.1104/pp.119.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]