Abstract
1 A study of the mechanism of release of [3H]-noradrenaline ([3H]-NA) by nicotine from isolated vas deferens of the rat was made using incubation media of different ionic composition.
2 Nicotine (20 μg/ml)-induced release of [3H]-NA was significantly potentiated in K+-free Krebs solution as compared to that in normal Krebs-Ringer solution.
3 Nicotine-induced release of [3H]-NA was significantly reduced in Na+-deficient Krebs solution (containing only 11 mM Na+) and was abolished in Na+-free Krebs solution.
4 In totally depolarized tissues, nicotine failed to cause an outflow of [3H]-NA but Ca2+ (5 mM) did so.
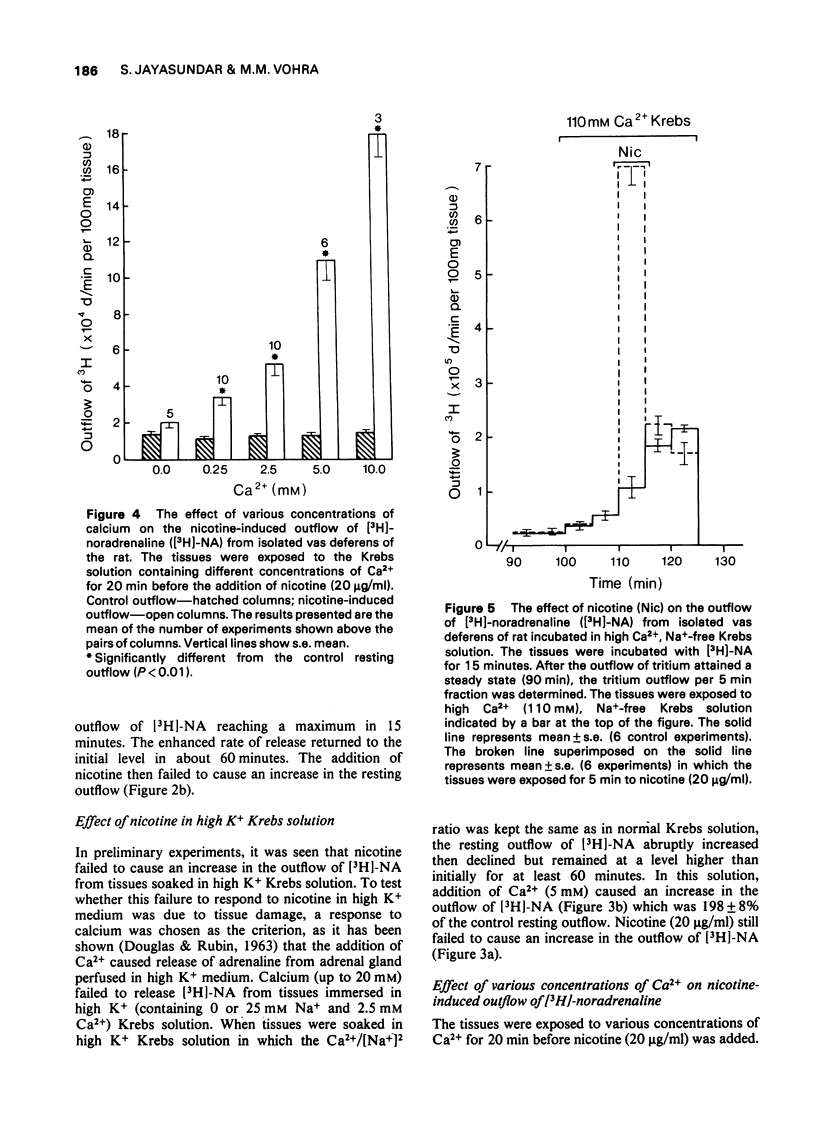
5 Nicotine required the presence of Ca2+ in the incubation medium to cause release of [3H]-NA from adrenergic nerve terminals, the magnitude of release being dependent upon the concentration of Ca2+.
6 Nicotine-induced release of [3H]-NA was demonstrated in high Ca2+, Na+-free Krebs solution in which all Na+ had been replaced with Ca2+.
7 It is concluded that nicotine increases the membrane permeability to both Na+ and Ca2+. It is also suggested that the increase in permeability to Ca2+ alone is not sufficient but a local depolarizing action of nicotine is necessary to cause release of noradrenaline from adrenergic nerve endings.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armett C. J., Ritchie J. M. The ionic requirements for the action of acetylcholine on mammalian non-myelinated fibres. J Physiol. 1963 Jan;165(1):141–159. doi: 10.1113/jphysiol.1963.sp007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Su C. Uptake of nicotine by the sympathetic nerve terminals in the blood vessel. J Pharmacol Exp Ther. 1972 Sep;182(3):419–426. [PubMed] [Google Scholar]
- Bogdanski D. F., Brodie B. B. The effects of inorganic ions on the storage and uptake of H3-norepinephrine by rat heart slices. J Pharmacol Exp Ther. 1969 Feb;165(2):181–189. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Local activity at a depolarized nerve-muscle junction. J Physiol. 1955 May 27;128(2):396–411. doi: 10.1113/jphysiol.1955.sp005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRY C. B. The synpathomimetic effect of acetylcholine on the spleen of the cat. J Physiol. 1963 Jul;167:487–504. doi: 10.1113/jphysiol.1963.sp007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF FREQUENCY OF STIMULATION AND CALCIUM CONCENTRATION ON CA45 EXCHANGE AND CONTRACTILITY ON THE ISOLATED GUINEA-PIG AURICLE. J Pharmacol Exp Ther. 1964 Jan;143:120–130. [PubMed] [Google Scholar]
- Gillis C. N., Paton D. M. Cation dependence of sympathetic transmitter retention by slices of rat ventricle. Br J Pharmacol Chemother. 1967 Mar;29(3):309–318. doi: 10.1111/j.1476-5381.1967.tb01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H., NICHOLLS J. G. Contractures and permeability changes produced by acetylcholine in depolarized denervated muscle. J Physiol. 1961 Nov;159:111–127. doi: 10.1113/jphysiol.1961.sp006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall T. C., Brasted M. The mechanism of action of nicotine on adrenergic neurons in the perfused guinea-pig heart. J Pharmacol Exp Ther. 1972 Sep;182(3):409–418. [PubMed] [Google Scholar]



