Abstract
Sucrose synthase (SS) is a known phosphoserine (SerP)-containing enzyme in a variety of plant “sink” organs, including legume root nodules, where it is phosphorylated primarily at Ser-11. Using immunofluorescence confocal microscopy, we documented that part of the total SS (nodulin-100) pool in mature soybean (Glycine max) nodules is apparently associated with the plasma membrane in situ, and we report that this association is very “tight,” as evidenced by a variety of chemical and enzymatic pretreatments of the isolated microsomal fraction. To investigate the in situ and in planta phosphorylation state of the membrane (m) and soluble (s) forms of nodule SS, three complementary approaches were used. First, excised nodules were radiolabeled in situ with [32P]Pi for subsequent analysis of phosphorylated m- and s-SS; second, immunopurified s- and m-SS were used as substrate in “on-bead” assays of phosphorylation by nodule Ca2+-dependent protein kinase; and third, SS-Ser-11(P) phosphopeptide-specific antibodies were developed and used. The collective results provide convincing evidence that microsomal nodulin-100 is phosphorylated in mature nodules, and that it is hypophosphorylated relative to s-SS (on an equivalent SS protein basis) in attached, unstressed nodules. Moreover, the immunological data and related phosphopeptide mapping analyses indicate that a homologous N-terminal seryl-phosphorylation domain and site reside in microsomal nodulin-100. We also observed that mild, short-term inorganic nitrogen and salt stresses have a significant negative impact on the content and N-terminal phosphorylation state of nodule m- and s-SS, with the former being the more sensitive of the two SS forms.
Suc synthase (SS; EC 2.4.1.13) is a homotetrameric enzyme that catalyzes the reversible, UDP-dependent cleavage of Suc into UDP-Glc and Fru in a variety of nonphotosynthetic “sink” organs such as tubers, developing leaves, fruits, and seeds, and root nodules. The resulting UDP-Glc and Fru are used in support of various metabolic pathways in plants, including plastid starch biosynthesis, cytoplasmic glycolysis, and the synthesis of cellulose and callose at the plasma membrane (Arrese-Igor et al., 1999; Winter and Huber, 2000; Haigler et al., 2001). In addition to its exquisite transcriptional regulation (Winter and Huber, 2000 and refs. therein), SS is posttranslationally modified by seryl-phosphorylation in various maize (Zea mays) organs (Huber et al., 1996; Lindblom et al., 1997; Winter et al., 1997; Subbaiah and Sachs, 2001), soybean (Glycine max) nodules (Zhang and Chollet, 1997), tomato (Lycopersicon esculentum) fruits (Anguenot et al., 1999), and elongating cotton (Gossypium hirsutum) fibers (Haigler et al., 2001). Huber et al. (1996) specifically reported that the cytosolic SS2 isoform in the elongation zone of developing maize leaves was phosphorylated at a plant-invariant (Curatti et al., 2000) Ser residue near the N terminus (Ser-15), and Zhang et al. (1999) provided evidence that soluble (s) SS in soybean root nodules (nodulin-100) is phosphorylated in planta at a homologous target site (Ser-11). In related studies, a number of plant Ca2+-dependent protein kinases (CDPKs) capable of phosphorylating authentic and recombinant SS in vitro have been partially purified and characterized (Huber et al., 1996; Zhang and Chollet, 1997; Nakai et al., 1998; Zhang et al., 1999; Loog et al., 2000; Asano et al., 2002). To date, little is known with certainty about the physiological function(s) of this phosphorylation event in legume nodules or in any other plant “sink” organ. Nodulin-100's Suc-cleavage activity and kinetic properties are not altered markedly by in vitro phosphorylation of Ser-11 by nodule CDPK or its N-terminal truncation or mutagenesis of the target site to an acidic (S11D) or neutral (S11A and S11C) residue (Zhang et al., 1999). Phosphorylation of maize s-SS does activate the cleavage reaction, but the effect is small and may be physiologically insignificant (Huber et al., 1996; Winter et al., 1997).
In addition to the aforementioned phosphorylation-related studies, there is ongoing work in several laboratories on mechanisms that control the intracellular partitioning of SS. Several reports have appeared that document that some of the SS protein is associated with the plasma membrane (m-SS) (Amor et al., 1995; Carlson and Chourey, 1996; Winter et al., 1997; Zhang et al., 1999; Barratt et al., 2001; Haigler et al., 2001; Konishi et al., 2001; Subbaiah and Sachs, 2001) and actin cytoskeleton (Winter et al., 1998) in various “sink” organs. In fact, Winter and coworkers (1997, 2000) and Subbaiah and Sachs (2001) have proposed that reversible phosphorylation of SS may be at least part of the mechanism controlling the intracellular distribution of the maize enzyme between the cytoplasmic and membrane compartments. Winter et al. (1997) based this view on at least three lines of experimental evidence: (a) in vitro dephosphorylation of maize s-SS caused it to associate with the microsomal membrane fraction; (b) in vitro phosphorylation of membrane proteins by mammalian protein kinase A (PKA) resulted in the release of m-SS from the membrane; and (c) in situ phosphorylation studies with [32P]Pi suggested that the membrane-associated enzyme contained significantly less 32P than the soluble isoform on an equivalent SS protein basis. However, it should be noted that in the case of PKA, the primary site(s) modified by this heterologous Ser/Thr kinase is clearly distinct from the conserved, N-terminal Ser targeted preferentially by plant CDPK (Zhang et al., 1999). Thus, the intriguing findings from the in vitro phosphorylation of m-SS by PKA are not directly applicable to the effect of phosphorylation on SS partitioning in planta. Likewise, Haigler et al. (2001) recently reported no major difference in the level of phosphorylation of s-SS versus m-SS (standardized to the amount of SS protein) isolated from cotton fibers radiolabeled in situ with 32P-orthophosphate. Thus, the exact role of this phosphorylation event(s) is still not established.
The results presented here summarize our detailed study of the in vivo and in vitro phosphorylation of m-SS and s-SS in soybean root nodules. We have exploited our existing nodulin-100 antiserum (Zhang et al., 1999), in conjunction with phosphorylation state- and site-specific (Ser-11) antibodies directed against the conserved N-terminal domain, to assess possible changes in SS distribution and phosphorylation status of this primary target site as a function of mild, short-term salt and inorganic N stress. This is of interest because such abiotic stresses are known to result in a dramatic, relatively selective down-regulation of SS gene expression and the subsequent turnover of the s-SS protein in soybean nodules (Gordon et al., 1997, 2002; Arrese-Igor et al., 1999). The results from these various immunoblotting experiments were complemented by analyses of the phosphorylation of m- and s-SS (standardized to the amount of SS protein) isolated from excised soybean nodules radiolabeled in situ with [32P]Pi and the relative ability of the purified enzyme forms to serve as phosphorylation substrates for nodule CDPK in vitro.
RESULTS AND DISCUSSION
Immunolocalization of Nodulin-100
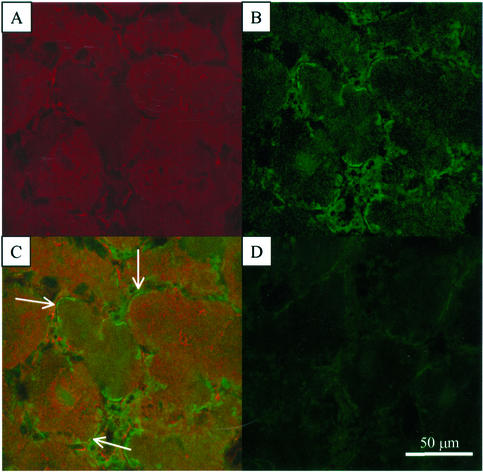
Evidence has accumulated over the past several years to indicate that a portion of SS, a traditionally viewed “cytoplasmic” enzyme in plants, is associated with the plasma membrane in soybean nodules (Zhang et al., 1999), developing cotton fibers (Amor et al., 1995; Haigler et al., 2001; Konishi et al., 2001), pea-seed embryos (Barratt et al., 2001), and various maize organs (Carlson and Chourey, 1996; Winter et al., 1997; Subbaiah and Sachs, 2001) using, in part, subcellular fractionation and immunoblot techniques. By exploiting immunolabeling with affinity-purified antibodies against soybean nodule SS and leghemoglobin and fluorescent imaging by confocal microscopy, we examined whether nodulin-100 is associated with the plasma membrane in legume nodules in situ (Fig. 1). Only a weak background signal was detected with the Cy2- and Cy5-conjugated secondary antibodies alone (Fig. 1D). Detection of leghemoglobin served as a critical marker protein because it is known that this O2-binding hemoprotein is located specifically in the cytoplasm of infected cells (Gordon, 1991). Figure 1A represents the Cy5-based fluorescent signal obtained with leghemoglobin antibodies, whereas Figure 1B (Cy2 signal with SS antibodies) and Figure 1C (Fig. 1, merged images from A and B) document that nodulin-100 is apparently colocalized to the cytoplasm and plasma membrane in mature soybean root nodules (Fig. 1C, arrows).
Figure 1.
Immunolocalization of SS in 5-week-old soybean root nodules. Nodule cross sections were pre-incubated with affinity-purified SS or primary leghemoglobin antibodies followed by incubation with secondary antibodies coupled to Cy2 or Cy5, respectively. Individual images for the Cy5 (A) and Cy2 (B) fluorochromes were collected separately and merged (C). D, Tissue autofluorescence in the absence of primary antibodies. Arrows in C point to apparent areas of SS membrane association.
In Vitro Release of Nodulin-100 from Microsomal Membranes
To assess how “tight” the documented association of SS is with nodule membranes (Fig. 1C and Zhang et al., 1999), a thoroughly washed microsomal fraction was pretreated with various reagents and enzymes. After this preincubation, the suspensions were refractionated by ultracentrifugation. Immunoblot analysis after SDS-PAGE of the resulting supernatant (S105) and pellet (P105) fractions indicated that SS was completely dissociated from the original membrane preparation only after pretreatment with a strong detergent such as 2% (w/v) SDS, 1% (v/v) Triton X-100, or 1% (v/v) Tween 20 (data not shown). In contrast, preincubation with a number of individual chaotropic, chelating, or anionic reagents, including NaBr, NaI, NaSCN (each at 2 m), EDTA (25 mm), EGTA (5 mm), and NaCl (0.5 m), had no significant effect. Likewise, in vitro phosphorylation of the microsomal fraction by a nodule-soluble CDPK in the presence of Ca2+ and ATP-Mg, or dephosphorylation by λ phosphatase, a dual-specificity protein phosphatase, was ineffective (data not shown). It is clear that SS is very tightly associated with the microsomal membrane fraction isolated from soybean nodules. This is in contrast to the results reported for maize SS in which in vitro phosphorylation/dephosphorylation caused the redistribution of SS between the isolated microsomal and cytosolic fractions (Winter et al., 1997), and for the cotton fiber enzyme where at least a substantial portion of the m-SS could be released from the P100 fraction by treatment with 10 mm EGTA (Haigler et al., 2001).
In Situ Phosphorylation of SS in Detached Soybean Nodules
Although recent evidence has documented that soluble nodulin-100 is subject to seryl-phosphorylation at residue 11 in intact soybean nodules (Zhang and Chollet, 1997; Zhang et al., 1999), it is not known whether the s-SS and m-SS enzyme forms or s-SS alone are subject to this posttranslational modification. To examine whether soybean nodule m-SS can be phosphorylated to any extent in situ, excised nodules from mature, illuminated plants were radiolabeled with [32P]Pi and the two enzyme forms were extracted and purified by immunoprecipitation and SDS-PAGE. As indicated in Figure 2A, the approximately 92-kD SS polypeptide was 32P labeled in the cytosolic and microsomal membrane fractions. Semiquantitative image analysis suggested that m-SS was possibly hyperphosphorylated relative to s-SS on an equivalent, Coomassie-stained SS protein basis. However, this apparent difference could be explained by a variety of factors, including, among others, a differential phosphorylation response of the two SS forms to the known stress of nodule excision (Sung et al., 1991; Gordon et al., 1997) and subsequent 3-h incubation, differing intracellular [32P]ATP availability or specific radioactivity for these two forms of nodulin-100, and/or different in vivo phosphorylation states of the respective target Ser residue(s) immediately prior to nodule detachment and in situ radiolabeling. Nevertheless, this 32P experiment with excised but stressed nodules, together with the related findings with elongating cotton fibers (Haigler et al., 2001), clearly indicates that the m and s forms of SS can be phosphorylated in situ from [32P]Pi.
Figure 2.
In situ phosphorylation of SS in excised soybean nodules. Detached nodules from 5-week-old plants were radiolabeled with [32P]Pi for 3 h. The immunoprecipitated, soluble (s), and solubilized microsomal (m) 32P-labeled SS proteins were resolved by SDS-PAGE and were visualized by phosphor imaging (A) and staining with Coomassie Blue (B).
Characterization of SS Phosphopeptide- Specific Antibodies
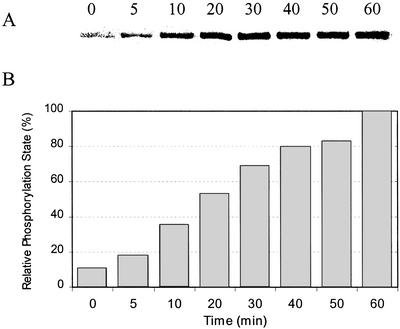
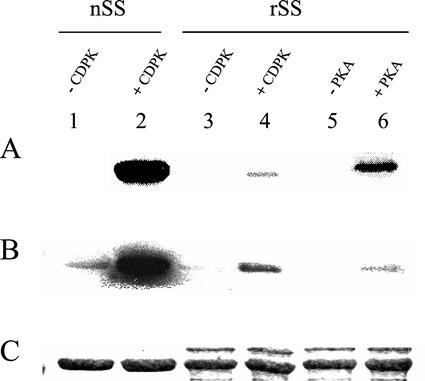
To more critically and unambiguously evaluate the relative phosphorylation states of s- and m-SS in planta (see above and Fig. 2), polyclonal antibodies directed against the phosphorylated form of nodulin-100's N-terminal domain harboring the primary target residue [Ser-11(P)] were produced and affinity purified. Similar immunological strategies for assessing the phosphorylation states of specific target residues in various phosphoproteins have been used widely in mammalian systems (e.g. Czernik et al., 1991), but only recently and sparingly with plants (Sugden et al., 1999; Chastain et al., 2000, 2002; Ueno et al., 2000). The phospho-SS antibodies were developed against a synthetic phosphopeptide corresponding to the conserved, N-terminal phosphorylation domain (residues 2–22) of the soybean nodule SS polypeptide and were proved to be highly phosphorylation state and site specific. This is evident from the immunoblot shown in Figure 3 in which purified nodule s-SS was phosphorylated in vitro by nodule CDPK for up to 60 min. The marked increase in phosphorylation state of nodulin-100 over the course of the reaction is clearly evident. The complementary immunoblot shown in Figure 4 documents that these affinity-purified antibodies are also very sensitive in comparison with phosphorimager analysis of this 32P-labeling experiment (Fig. 4, A and B, lanes 2 and 4), and are immunospecific for the phosphorylated form of nodulin-100 in that they do not crossreact with soybean recombinant SS, which is completely nonphosphorylated (Fig. 4, B and C, lanes 3 and 5). Perhaps most notable is the finding that these antibodies are highly specific to the N-terminal, Ser-11(P) domain in that phosphorylation of recombinant SS by PKA yields a relatively strong signal in our routine 32P assay, but not in the corresponding immunoblot assay (Fig. 4, A versus B, lane 6). This is consistent with our previous results showing that nodule CDPK and mammalian PKA target distinct primary phosphorylation sites in nodulin-100 (Zhang et al., 1999). Hence, given the documented specificity of these phosphopeptide antibodies directed against the conserved N-terminal phosphorylation domain of nodulin-100, a far more exacting and facile immunological assay of the in planta or in vitro phosphorylation state of legume SS is now available compared with previous 32P-based phosphorylation assays (e.g. Zhang and Chollet, 1997; Nakai et al., 1998; Zhang et al., 1999), including those with excised but stressed nodules (see above and Fig. 2).
Figure 3.
Analysis of phosphorylation state specificity of affinity-purified SS-Ser-11(P) antibodies. Fast-protein liquid chromatography (FPLC)-purified nodule s-SS was phosphorylated in vitro by CDPK in the presence of Ca2+ and ATP-Mg for 5 to 60 min at 30°C. SS was separated by SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane, and probed with SS-Ser-11(P) phosphopeptide antibodies (A). The relative enhanced chemiluminescent (ECL) signal intensities were quantified by image analysis (B). The zero-time control sample represents nodule s-SS, a known Ser-11(P)-containing phosphoprotein (Zhang and Chollet, 1997; Zhang et al., 1999), prior to in vitro phosphorylation. Quantitation was based on the results of two independent experiments.
Figure 4.
Analysis of the phosphorylation state and site specificity of the affinity-purified SS-Ser-11(P) phosphopeptide antibodies. FPLC-purified soybean nodule (lanes 1 and 2) and recombinant (lanes 3–6) SS proteins (nSS and rSS, respectively) were incubated in vitro for 30 min with [γ-32P]ATP-Mg and 0.25 mm Ca2+, in the absence of protein kinase (lanes 1, 3, and 5) or presence of nodule CDPK (lanes 2 and 4) and bovine PKA (lane 6). Radiolabeled SS was separated by SDS-PAGE on duplicate gels. One gel was analyzed by phosphorimaging (A) and Coomassie staining (C). Proteins from the second gel were transferred to a PVDF membrane and were probed with SS-Ser-11(P) phosphopeptide antibodies (B). It should be noted that for some unknown reason (e.g. protein misfolding), the fully active rSS is a much less efficient substrate for CDPK than authentic nSS (see lanes 2 and 4 in A and B, and Fig. 2 in Zhang et al., 1999).
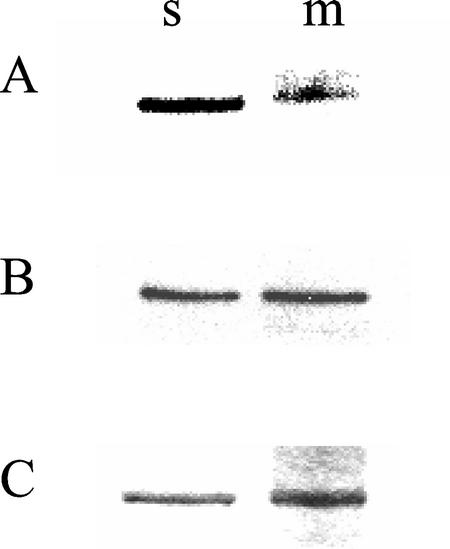
This highly effective immunological approach was used to critically evaluate the relative in vivo phosphorylation states of the cytosolic and solubilized microsomal SS proteins in attached (unstressed) 5-week-old soybean nodules. As shown in Figure 5A, both forms of immunoprecipitated nodulin-100 were phosphorylated at their N-terminal domains in planta, consistent with the far less exacting in situ [32P]Pi-radiolabeling studies with excised nodules summarized in Figure 2. However, semiquantitative image analysis indicated that the relative phosphorylation state of m-SS's N-terminal domain was only approximately 30% of that of s-SS on an equivalent SS polypeptide basis (Fig. 5, A and B), at least in mature nodules. This apparent discrepancy between the data summarized in Figures 2 and 5 could simply be due to the nature of the experimental nodule samples, i.e. detached (stressed) versus attached (unstressed), respectively.
Figure 5.
Comparison of in planta phosphorylation states of cytosolic and microsomal SS proteins in attached, unstressed 5-week-old soybean nodules. The immunoprecipitated s- and solubilized m-SS proteins were resolved by SDS-PAGE on triplicate gels. Proteins from two gels were transferred to PVDF membranes and probed with SS-Ser-11(P) phosphopeptide antibodies (A) and SS antibodies (B). The third gel was stained with Coomassie Blue (C). Semiquantitative image analysis of A and B showed that the relative Ser-11(P) content (A) per SS (B) was equal to 1.07 and 0.30 for s-SS and m-SS, respectively. Quantitation was based on the results of three independent experiments.
“On-Bead” Phosphorylation of s-SS and m-SS by Nodule CDPK
Independent confirmation of the relatively hypophosphorylated state of m-SS compared with s-SS in attached soybean nodules was provided by on-bead phosphorylation experiments. The cytosolic and solubilized microsomal nodulin-100s from 5-week-old nodules were purified by immunoprecipitation and were phosphorylated while attached to beaded protein-A by soybean nodule CDPK in the presence of Ca2+ and [γ-32P]ATP-Mg. Semiquantitative image analysis of the related SS-radiolabeling and polypeptide data (Fig. 6A) revealed that m-SS incorporated approximately three times more 32P than the soluble enzyme form on an equivalent, Coomassie-stained SS protein basis. These comparative results suggest that m-SS has significantly more target sites available for on-bead phosphorylation by CDPK than does cytosolic nodulin-100, consistent with the former's relatively hypophosphorylated state revealed by immunodecoration with the SS-Ser-11(P)-specific antibodies (see above and Fig. 5).
Figure 6.
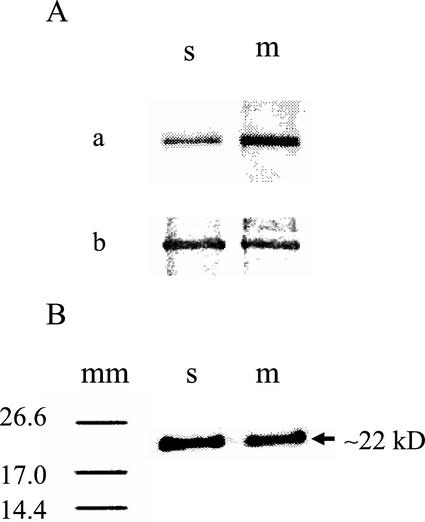
On-bead CDPK phosphorylation and subsequent 32P-phosphopeptide mapping of s- and m-SS from 5-week-old soybean nodules. Immunocomplexes of the SS proteins from the s and solubilized m fractions were incubated while attached to beaded protein A in the presence of [γ-32P]ATP-Mg, 0.25 mm Ca2+, and nodule CDPK for 60 min at 30°C. A, Radiolabeled SS was separated by SDS-PAGE and detected by phosphorimaging (a) and Coomassie staining (b). Semiquantitative image analysis of a and b showed that the relative 32P incorporation (a) per SS (b) was equal to approximately 1:3 for s-SS:m-SS, respectively. Quantitation was based on the results of three independent experiments. B, In a converse manner, the radiolabeled s- and m-SS polypeptides were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and digested in situ by CNBr. The peptide fragments were resolved by Tris-Tricine SDS-PAGE, and the phosphopeptides were detected by phosphorimaging. Molecular mass markers (mm) are shown on the left (values in kilodaltons). No 32P peptides were observed below the 14.4-kD marker in either case (data not shown).
It is well established that the primary target site for CDPK in legume s-SS is the conserved Ser residue at position 11 (Figs. 3–5; Nakai et al., 1998; Zhang et al., 1999). Likewise, the immunological data presented herein indicate that a homologous seryl-phosphorylation domain and site reside in microsomal nodulin-100 (Fig. 5). This was confirmed directly and independently by comparative phosphopeptide mapping studies with m-SS and s-SS following on-bead phosphorylation by nodule CDPK and [γ-32P]ATP-Mg. In both cases, an approximately 22-kD cyanogen bromide (CNBr) fragment was the only major phosphopeptide detected (Fig. 6B). This 32P-labeled peptide was previously assigned to the CNBr fragment spanning from Ala-2 to Met-193 in soluble nodulin-100 and nodule recombinant SS, thus harboring the N-terminal phosphorylation domain (Zhang et al., 1999).
Effect of Short-Term, Mild Salt, and Inorganic-N Stress on the Content and Relative Phosphorylation States of the Nodule s- and m-SS Proteins
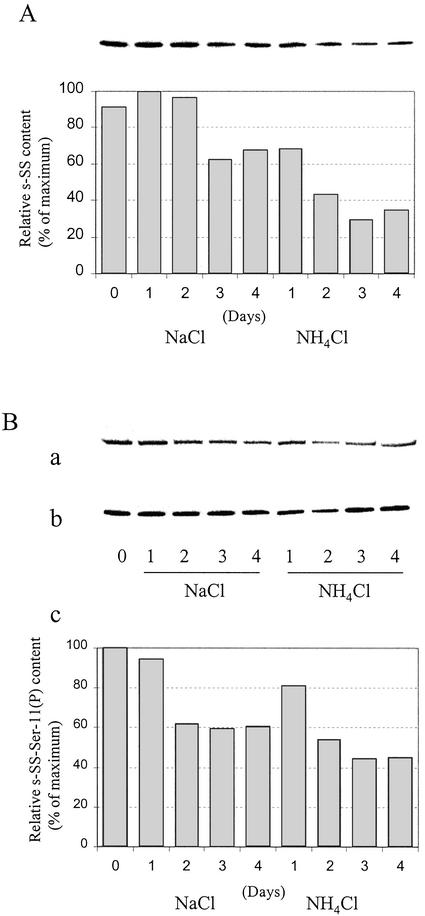
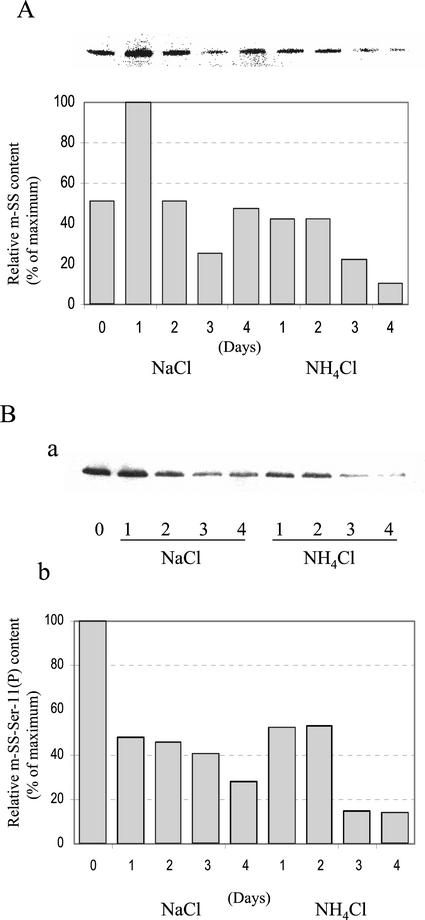
It is well established that the levels of SS transcript, protein, and activity are exquisitely sensitive to a variety of abiotic stresses in planta (Winter and Huber, 2000). For example, SS is strikingly up-regulated in anoxic maize roots (Ricard et al., 1998; Zeng et al., 1999), and recent evidence suggests that an interplay between changes in SS phosphorylation state and intracellular localization is an early event in this specific stress response (Subbaiah and Sachs, 2001). In contrast, the s-SS transcript, polypeptide, and activity levels decline significantly and rather selectively in legume root nodules when the parent plants are subjected to mild drought, salinity, and inorganic N stress (Gordon et al., 1997, 2002; Arrese-Igor et al., 1999 and refs. therein). We thus investigated possible changes in content and in planta phosphorylation state of cytosolic and microsomal nodulin-100 during such short-term abiotic stresses. In brief, 4-week-old nodulated soybean plants were irrigated daily with 15 mm NH4Cl or NaCl for up to 4 d. Figure 7 represents the data for s-SS, and Figure 8 summarizes those for m-SS. Both stress treatments caused a marked decrease in SS content by d 3, but N stress typically had a greater negative impact on the cytosolic and microsomal membrane enzyme forms (Figs. 7A and 8A). For example, only approximately 35% of the maximum s-SS and approximately 10% of the maximum m-SS levels remained in the nodules after a 4-d treatment with NH4Cl. In contrast, control nodules from 4- and 5-week-old untreated plants differed by less than 30% in their respective amounts of the two enzyme forms (data not shown). Likewise, inorganic N stress caused similarly dramatic decreases in the in vivo phosphorylation state of cytosolic and microsomal nodulin-100 on an equivalent SS basis, with m-SS being the more sensitive of the two (see histograms in Figs. 7B and 8B). In contrast, the relative phosphorylation state of soluble nodulin-100 marginally increased by approximately 10% in untreated control plants between weeks 4 and 5 (data not shown). Thus, although the in vivo phosphorylation state of the primary, N-terminal target domain and content of both nodule enzyme forms respond negatively to abiotic stresses, m-SS is relatively more sensitive, especially to inorganic N stress.
Figure 7.
Effect of mild salt and inorganic N stress on the content and relative phosphorylation state of cytosolic nodulin-100. Four-week-old soybean plants were irrigated daily for up to 4 d with N-free nutrient solution containing 15 mm NaCl or 15 mm NH4Cl. Lane 0 represents nodules from control plants irrigated with N-free nutrient solution alone for 4 weeks. Lanes 1 through 4 represent the number of treatment days. Proteins in the high-speed soluble (S105) fraction were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with SS antibodies (A; 0.1 μg of total soluble protein loaded), SS-Ser-11(P) phosphopeptide antibodies (B, a), and SS antibodies (B, b; the amount of total soluble protein loaded was adjusted to equalize the amount of s-SS loaded in a and b). The histogram in B, c, represents the relative s-SS-Ser-11(P) content per s-SS (B, a and b) as a percentage of the maximum value observed. Quantitation was based on the results of two independent experiments.
Figure 8.
Effect of mild salt and inorganic N stress on the content and relative phosphorylation state of microsomal nodulin-100. Four-week-old soybean plants were irrigated daily for up to 4 d with N-free nutrient solution containing 15 mm NaCl or NH4Cl. Lane 0 represents nodules from control plants irrigated with N-free nutrient solution alone for 4 weeks. Lanes 1 through 4 represent the number of treatment days. Proteins in the thoroughly washed microsomal (P105) fraction were resolved by SDS-PAGE, transferred to PVDF membranes, and probed with SS antibodies (A) and SS-Ser-11(P) phosphopeptide antibodies (B, a). The histogram in B, b, represents the relative m-SS-Ser-11(P) content per m-SS (B, a, and A) as a percentage of the maximum value observed. Quantitation was based on results of two independent experiments.
Concluding Remarks
There are several novel and important conclusions that can be drawn from these complementary in vivo and in vitro studies of the cytosolic and microsomal forms of soybean nodulin-100. First, part of the total SS protein pool is apparently associated with the nodule plasma membrane in situ (also see Zhang et al., 1999), and this association appears to be exceedingly “tight.” SS was completely dissociated from the original microsomal membrane fraction only after pretreatment with a strong detergent such as SDS or Triton X-100. Chaotropic, chelating, or anionic reagents, as well as in vitro phosphorylation/dephosphorylation, had no marked effect on the release of m-SS. Second, the m- and s-SS proteins are phosphorylated in attached or excised mature nodules in situ. In the physiologically more relevant case of attached (unstressed) nodules, the microsomal SS protein is hypophosphorylated relative to the cytosolic enzyme form. This important conclusion is supported by two complementary lines of evidence: the relative extents of on-bead phosphorylatability of the immunoprecipitated m- and s-SS forms (Fig. 6A); and most notably, the immunodetection and semiquantitation of the seryl-phosphorylated N-terminal domain in s-SS and m-SS with highly specific polyclonal antibodies directed against the phospho form of nodulin-100's primary phosphorylation motif harboring the target Ser-11(P) residue (Fig. 5), which were produced, affinity purified, and characterized as a critical part of this study. Taken together, these related findings with mature soybean nodules do not entirely support the earlier proposal made for maize SS (Winter et al., 1997; Winter and Huber, 2000 [see the speculative model in Fig. 2]; Subbaiah and Sachs, 2001) that reversible phosphorylation of this target protein may be a simple “on-off switch” controlling its well-documented intracellular partitioning between the cytoplasm and plasma membrane. Rather, it may be that the phosphorylation of m-SS weakens but does not completely abolish the interaction of this enzyme form with the membrane. Likewise, these differing observations may simply be explained by the vastly different physiology of the “sink” organs examined, i.e. soybean root nodules versus maize roots and developing leaves. Third, the immunoblotting data related to immunodetection of the N-terminal phosphorylation domain in s- and m-SS and the complementary phosphopeptide mapping studies establish that a homologous seryl-phosphorylation motif and site reside in microsomal and cytosolic nodulin-100. The phosphorylation state of this primary, N-terminal target domain and content of both nodule enzyme forms are clearly dynamic, responding negatively to short-term abiotic stresses such as salt and inorganic N, with m-SS being relatively more sensitive, especially to the latter. We conjecture that changes in phosphorylation state may “flag” nodulin-100 for degradation and/or that its dephospho form is a better target for intracellular proteases. Although highly speculative and in need of more extensive investigation, this hypothesis clearly has precedence in other eukaryotic protein phosphorylation systems, including plant nitrate reductase (e.g. Kaiser and Huber, 1997; Lange et al., 2000; Dumaz et al., 2001).
MATERIALS AND METHODS
Materials
Commonly used reagents were purchased at the highest level of purity available, most often from Sigma (St. Louis). Additional supplies and providers were as follows: phenyl-Sepharose CL-4B, prepacked Mono Q HR 5/5 column, [γ-32P]ATP (3 Ci mmol−1), and protein-A Sepharose beads from were purchased from Amersham Pharmacia Biotech (Piscataway, NJ); [32P]Pi (8,780 Ci mmol−1) was purchased from PerkinElmer Life Sciences (Boston); λ protein phosphatase was purchased from New England Biolabs (Beverly, MA); the catalytic subunit of bovine heart PKA and beef liver UDP-Glc dehydrogenase was purchased from Boehringer Mannheim (Indianapolis); and phenyl boronate agarose-60 was purchased from Amicon (Beverly, MA), or as noted below.
Plant Material and Bacterial Cultures
Nodulated, mature soybean (Glycine max cv Dunbar) plants were grown in a local greenhouse for 5 weeks and were irrigated daily with an N-free nutrient solution (Zhang et al., 1995). For the short-term inorganic N and salt-stress experiments, 4-week-old plants were irrigated with the same nutrient solution containing 15 mm NH4Cl or 15 mm NaCl, respectively, for up to 4 d prior to nodule harvest. Nodules were frozen immediately after harvest in liquid nitrogen and were stored at −70°C until use.
Escherichia coli cells transformed with an expression plasmid harboring an untagged nodulin-100 cDNA construct (pXUNSS; Zhang et al., 1999) were used for production of soybean recombinant SS. The cells were grown in terrific broth medium (Sambrook et al., 1989) at 37°C, and the expression of the target protein was induced by adding 0.1 mm isopropyl β-d-thiogalactoside to the 0.5-L culture. After 12 h of induction at 25°C, the cells were harvested by centrifugation, washed once with buffer containing 100 mm MOPS, pH 7.5, 5 mm MgCl2, and 2 mm EDTA, frozen in liquid nitrogen, and stored at −20°C.
FPLC Purification of Nodule s-SS and Recombinant Nodulin-100
s-SS was purified from 5-week-old soybean root nodules according to a published FPLC-based procedure (Zhang et al., 1999) except that 50 nm microcystin-Leu-Arg (MC-LR; Sigma, St. Louis) and 5 mm NaF were included in the extraction buffer to inhibit protein phosphatase 1 and 2A, and general phosphatase activity. Untagged recombinant SS was purified from freshly cultured E. coli cells exactly as described previously (Zhang et al., 1999).
In Vitro Release of Nodulin-100 from Isolated Membranes
Microsomal membranes were purified from 5-week-old soybean nodules and were stored according to a published procedure (Zhang et al., 1999). To evaluate how “tight” the physical association of SS is with these membranes (see Fig. 1 in Zhang et al., 1999), the thoroughly washed 105,000g microsomal fraction (P105) was preincubated with various individual reagents (2% [w/v] SDS, 1% [v/v] Triton X-100, 1% [v/v] Tween 20, 2 m NaBr, 2 m NaI, 2 m NaSCN, 0.5 m NaCl, 25 mm EDTA, or 5 mm EGTA) for 20 min at pH 7.5 and 30°C. In a similar manner, the membranes were pretreated enzymatically with λ phosphatase or nodule-soluble CDPK/Ca2+/ATP-Mg for 20 min at 30°C according to the supplier's instructions or the in vitro, CDPK phosphorylation protocol outlined below, respectively, followed by refractionation by ultracentrifugation for 1 h at 105,000g. Proteins in the resulting pellet and supernatant fractions were resolved by SDS-PAGE, transferred onto PVDF membrane, and probed with nodulin-100 specific antibodies (Zhang et al., 1999) by an ECL method (see below).
Immunopurification of Soybean Nodule Microsomal and Cytosolic SS
Microsomal membranes were purified from root nodules as outlined above except that 50 nm MC-LR and 5 mm NaF were included in all buffers. The thoroughly washed P105 microsomal fraction was solubilized with 1% (v/v) Triton X-100 in membrane wash buffer (50 mm HEPES-KOH, pH 7.5, 5 mm MgCl2, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 10 μg mL−1 chymostatin, 2 μg mL−1 leupeptin, and 1 μg mL−1 pepstatin A) for 30 min at 30°C, and was subsequently fractionated by ammonium sulfate precipitation (30%–45% saturation fraction). The final precipitate was redissolved in membrane wash buffer alone and was used for immunoprecipitation of m-SS. The corresponding S105 supernatant fraction from the initial ultracentrifugation was preincubated with Triton X-100 and fractionated by ammonium sulfate precipitation in the same manner. The resulting membrane-solubilized and cytosolic SS samples were preincubated with 20 μL of anti-nodulin-100 antibodies (Zhang et al., 1999) for 3 h at 4°C with gentle rotation. Five milligrams of protein-A Sepharose beads was added, and the samples were incubated for another 3 h (or overnight) at 4°C with gentle rotation. The beaded immunocomplexes were washed sequentially, three times each, with phosphate-buffered saline (PBS) containing 0.02% (w/v) SDS and 0.5% (v/v) Triton X-100, PBS alone, and SS storage buffer (25 mm HEPES-KOH, pH 7.5, 25 mm Suc, 5 mm MgCl2, 50% [v/v] glycerol, and 5 μg mL−1 leupeptin). The thoroughly washed beads were stored at −20°C or were treated immediately with SDS sample buffer (Laemmli, 1970), boiled for 5 min, and centrifuged briefly. The approximately 92-kD SS monomer in the resulting supernatant fraction was resolved by 10% (w/v) SDS-PAGE (Laemmli, 1970) and was subjected to immunoblot analysis (see below).
SS Assay and Protein Determination
SS activity in the cleavage direction (Suc + UDP → UDP-Glc + Fru) was assayed spectrophotometrically at 340 nm and 30°C (Morell and Copeland, 1985; Zhang and Chollet, 1997). In brief, the amount of UDP-Glc produced was measured continuously by enzymatic coupling to the reduction of NAD in the presence of excess UDP-Glc dehydrogenase. The standard 1-mL assay contained 20 mm HEPES-KOH, pH 7.5, 200 mm Suc, 1.5 mm UDP, 1.5 mm NAD, 5 mm MgCl2, the appropriate amount of SS, and excess beef liver UDP-Glc dehydrogenase.
The concentration of soluble and microsomal membrane proteins was determined by the Bradford and DC protein assay reagents supplied by Bio-Rad (Hercules, CA), respectively, using bovine serum albumin (BSA) as standard. Membrane fractions were solubilized with 1% (w/v) 3-[(3-cholamido-propyl) dimethylammonio]-l-propanesulfonate prior to protein quantitation.
In Situ 32P Labeling of Detached Soybean Nodules
Nodules (5 g) were excised from 5-week-old illuminated plants and were immediately incubated in 7 mL of radiolabeling solution (2 mCi of [32P]Pi, 0.25 mm KCl, 0.5 mm MgSO4, 0.2 mm CaCl2, and 10 mg L−1 NaFe-EDTA) for 3 h at room temperature. After radiolabeling, the intact nodules were thoroughly washed with distilled water, and the soluble and membrane-solubilized forms of SS were extracted and immunopurified as described above.
Phosphorylation of SS
The procedures for in vitro phosphorylation of FPLC-purified soybean SS (authentic nodulin-100 and the recombinant enzyme) by bovine PKA or a partially purified preparation of nodule-soluble CDPK and [γ-32P]ATP-Mg were described previously (see Zhang and Chollet, 1997 for details of CDPK isolation and these in vitro phosphorylation assays). Phosphorylation by the catalytic subunit of bovine PKA was carried out according to the supplier's instructions.
In the case of the on-bead phosphorylation assays, immunocomplexes of the SS proteins prepared from the cytosolic (S105) and solubilized microsomal (P105) fractions (see above) were 32P labeled while attached to beaded protein A. The procedures were similar to those for in vitro phosphorylation of the FPLC-purified, soluble SS proteins. In brief, in a 40-μL reaction mixture, immunocomplexes attached to approximately 5 mg of protein A beads were incubated with the appropriate amount of nodule CDPK, 50 mm HEPES-KOH, pH 7.5, 5 mm MgCl2, 0.1 μm MC-LR, and 5 μCi of [γ-32P]ATP (3 Ci mmol−1) for up to 60 min at 30°C in the presence of 0.25 mm Ca2+. The suspensions were briefly vortexed every 10 min during incubation. The beaded samples were then centrifuged for 1 min, and the supernatant fluid was discarded. Twenty microliters of SDS sample buffer was added, the pelleted beads were boiled for 5 min, briefly recentrifuged, and the supernatant fractions analyzed by SDS-PAGE and phosphorimaging.
CNBr Digestion and Phosphopeptide Analysis
The beaded immunocomplexes of the nodule SS proteins from the cytosolic and solubilized microsomal fractions were 32P labeled on-bead by nodule CDPK as described above for 60 min at 30°C, fractionated by SDS-PAGE (Laemmli, 1970), and transferred electrophoretically to a nitrocellulose membrane. The radiolabeled, approximately 92-kD SS polypeptide was excised and digested in situ with CNBr (Pierce, Rockford, IL) in 70% (v/v) formic acid for 3 h at room temperature (Luo et al., 1991). Following digestion, the samples were centrifuged and the supernatant fluid was taken to dryness in a CentriVap (Labconco, Kansas City, MO). The dried CNBr-peptide fragments were dissolved in Tricine sample buffer (Bio-Rad) and were resolved on 16.5% (w/v) Tris-Tricine Ready Gels (Bio-Rad). Triose-P isomerase (26, 625), myoglobin (16, 950), α-lactalbumin (14, 437), aprotinin (6, 500), oxidized insulin β chain (3, 496), and bacitracin (1, 423) were used as Mr standards. The radiolabeled, SS phosphopeptides were detected by phosphorimaging.
Production and Affinity Purification of Nodule SS-Ser-11(P)-Specific Antibodies
Antiserum against the conserved, N-terminal phosphorylation domain of cytosolic, phospho-SS was generated using a synthetic phosphopeptide corresponding to residues 2 through 22 ([C]-ATDRLTRVHS(p)LRERLDETLTA) in soybean nodulin-100 (see Zhang et al., 1999). The parent, 21-mer SS peptide was synthesized as a C-terminal amide, and a Cys residue was introduced at the extreme N terminus. This base peptide was synthesized in two forms, with or without a phosphate group at the target Ser residue. Automated synthesis was performed on a peptide synthesizer (Pioneer; PerkinElmer Biosystems, Foster City, CA), using 9-fluorenylmethoxycarbonyl-protected amino acids, PAL-PEG-PS resin (Perkin-Elmer Instruments, Norwalk, CT), and the manufacturer's recommended protocols, by the Protein Sequencing Core Facility (University of Nebraska, Lincoln).
Antibodies were raised in rabbits (University of Georgia Antibody Core Facility, Athens) against the synthetic SS phosphopeptide coupled to Keyhole limpet hemocyanin using the free sulfhydryl-specific linker m-maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce; see Chastain et al., 2000 for details). The immunoglobulin (Ig) G fraction from the collected serum was partially purified by repeated (three times) ammonium sulfate fractionation (0%–50% saturation), and was dialyzed into Tris-buffered saline (TBS). Phospho-SS-specific antibodies were purified by sequential affinity-chromatography on immobilized SS-dephosphopeptide-agarose and SS-phosphopeptide-agarose using SulfoLink coupling gel from Pierce (Chastain et al., 2000) and the manufacturer's recommended coupling protocol. The dephosphopeptide-agarose beads were preequilibrated with TBS. Approximately 3 mL of partially purified IgG from 10 to 12 mL of serum was added and mixed with the beaded dephosphopeptide matrix by gentle inversion at 4°C overnight in TBS. The beads were then packed into an approximately 1.5-mL column and washed with 5 mL of TBS. The unbound protein fraction was then added to the phosphopeptide-agarose beads preequilibrated with TBS containing 1 m NaCl. The beads were mixed by gentle inversion at 4°C overnight, a approximately 1.5-mL column was packed and thoroughly washed with 10 mL of TBS + NaCl buffer, and phosphopeptide-enriched IgG was eluted with 3 mL of Gentle Elution buffer (Pierce) into 50 μL of 1 m Tris, pH 8.8. IgG-containing fractions were combined and the dephosphopeptide-agarose column procedure was repeated once again. The final flow-through fraction, containing highly specific SS-phosphopeptide antibodies, was stored at −20°C.
Western Blotting
Following SDS-PAGE (Laemmli, 1970), immunoblotting was carried out using the SS-Ser-11(P) phosphorylation-state antibodies described above, anti-soybean nodule SS (Zhang et al., 1999), and phosphoenolpyruvate carboxylase (Zhang et al., 1995) polyclonal antibodies raised in rabbits or anti-soybean nodule leghemoglobin antibodies raised in goats (Barata et al., 2000), and the ECL reagents and general protocol from Amersham Pharmacia Biotech. The antibodies against phosphoenolpyruvate carboxylase and leghemoglobin, two nodule cytoplasmic marker proteins, were used to confirm the lack of contamination of the microsomal fractions by cytosolic proteins (e.g. see Fig. 1 in Zhang et al., 1999).
Immunoblotting was performed using standard methods of blotting and hybridization. In brief, the PVDF membranes were first blocked with 10% (w/v) low-fat milk in buffer (PBS or TBS in the case of phospho-SS immunoblots) at room temperature for 1 h, rinsed three times with TBS, and probed with the antibody of interest in TBS overnight at 4°C. After three 15-min washes, the membranes were incubated with a horseradish peroxidase-labeled anti-IgG for 1 h at room temperature, followed by three 15-min washes with TBS, and ECL detection. Semiquantitative analysis of the original SS and SS-Ser-11(P) ECL image data was performed using Gel-Doc software (Bio-Rad).
Localization of Nodulin-100 by Immunofluorescence Confocal Microscopy
Frozen sections of fresh nodules from 5-week-old soybean plants were cut (8–10 μm in thickness), collected onto poly-l-Lys-coated slides (Sigma), and stored at −20°C before use. Slides containing nodule sections were fixed/extracted in cold methanol at −20°C for 2 min, followed by two washes in PBS. After blocking for 45 min with 3% (w/v) BSA in PBS containing 0.05% (v/v) Tween 20 (PBST), samples were preincubated with goat anti-soybean nodule leghemoglobin (Barata et al., 2000) and affinity-purified rabbit anti-nodulin-100 (Zhang et al., 1999) antibodies for 2 h at room temperature. The samples were washed three times in PBST (15 min each), followed by a 1-h incubation in PBST containing 1% (w/v) BSA with Cy5-conjugated anti-goat and Cy2-conjugated anti-rabbit antibodies (1:100 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA). Slides were then washed three times in PBS (15 min each), mounted, and examined using a confocal laser-scanning microscope (MRC1024ES; Bio-Rad). Confocal images from the Cy2- (SS) and Cy5- (leghemoglobin) labeled signals were collected simultaneously using a dual-line excitation/emission mode (Ex/Em = 488/520 nm for Cy2 and 640/680 nm for Cy5) and the LaserSharp imaging program (Bio-Rad).
Distribution of Materials
Upon written request, all novel materials and reagents described in this publication will be made available in limited quantities and in a timely manner for noncommercial research purposes.
ACKNOWLEDGMENT
We thank Shirley Condon for her continued excellent technical assistance.
Footnotes
This work was supported in part by the U.S. National Science Foundation (grant no. MCB–9727236 to R.C.). This is no. 13,595 in the University of Nebraska Agricultural Research Division journal series.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002360.
LITERATURE CITED
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguenot R, Yelle S, Nguyen-Quoc B. Purification of tomato sucrose synthase phosphorylated isoforms by Fe(III)-immobilized metal affinity chromatography. Arch Biochem Biophys. 1999;365:163–169. doi: 10.1006/abbi.1999.1146. [DOI] [PubMed] [Google Scholar]
- Arrese-Igor C, Gonzalez EM, Gordon AJ, Minchin FR, Galvez L, Royuela M, Cabrerizo PM, Aparicio-Tejo PM. Sucrose synthase and nodule nitrogen fixation under drought and other environmental stresses. Symbiosis. 1999;27:189–212. [Google Scholar]
- Asano T, Kunieda N, Omura Y, Ibe H, Kawasaki T, Takano M, Sato M, Furuhashi H, Mujin T, Takaiwa F et al. Rice SPK, a calmodulin-like domain protein kinase, is required for storage product accumulation during seed development: Phosphorylation of sucrose synthase is a possible factor. Plant Cell. 2002;14:619–628. doi: 10.1105/tpc.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barata RM, Chaparro A, Chabregas SM, González R, Labate CA, Azevedo RA, Sarath G, Lea PJ, Silva-Filho MC. Targeting of the soybean leghemoglobin to tobacco chloroplasts: effects on aerobic metabolism in transgenic plants. Plant Sci. 2000;155:193–202. doi: 10.1016/s0168-9452(00)00219-3. [DOI] [PubMed] [Google Scholar]
- Barratt DHP, Barber L, Kruger NJ, Smith AM, Wang TL, Martin C. Multiple, distinct isoforms of sucrose synthase in pea. Plant Physiol. 2001;127:655–664. [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS. Evidence for plasma membrane-associated forms of sucrose synthase in maize. Mol Gen Genet. 1996;252:303–310. doi: 10.1007/BF02173776. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Botschner M, Harrington GE, Thompson BJ, Mills SE, Sarath G, Chollet R. Further analysis of maize C4 pyruvate, orthophosphate dikinase phosphorylation by its bifunctional regulatory protein using selective substitution of the regulatory Thr-456 and catalytic His-458 residues. Arch Biochem Biophys. 2000;375:165–170. doi: 10.1006/abbi.1999.1651. [DOI] [PubMed] [Google Scholar]
- Chastain CJ, Fries JP, Vogel JA, Randklev CL, Vossen AP, Dittmer SK, Watkins EE, Fiedler LJ, Wacker SA, Meinhover KC et al. Pyruvate, orthophosphate dikinase in leaves and chloroplasts of C3 plants undergoes light-/dark-induced reversible phosphorylation. Plant Physiol. 2002;128:1368–1378. doi: 10.1104/pp.010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatti L, Porchia AC, Herrera-Estrella L, Salerno GL. A prokaryotic sucrose synthase gene (susA) isolated from a filamentous nitrogen-fixing cyanobacterium encodes a protein similar to those of plants. Planta. 2000;211:729–735. doi: 10.1007/s004250000343. [DOI] [PubMed] [Google Scholar]
- Czernik AJ, Girault JA, Nairn AC, Chen J, Snyder G, Kebabian J, Greengard P. Production of phosphorylation state-specific antibodies. Methods Enzymol. 1991;201:264–283. doi: 10.1016/0076-6879(91)01025-w. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Milne DM, Jardine LJ, Meek DW. Critical roles for the serine 20, but not the serine 15, phosphorylation site and for the polyproline domain in regulating p53 turnover. Biochem J. 2001;359:459–464. doi: 10.1042/0264-6021:3590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ. Enzyme distribution between the cortex and the infected region of soybean nodules. J Exp Bot. 1991;42:961–967. [Google Scholar]
- Gordon AJ, Minchin FR, Skøt L, James CL. Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol. 1997;114:937–946. doi: 10.1104/pp.114.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Skøt L, James CL, Minchin FR. Short-term metabolic responses of soybean root nodules to nitrate. J Exp Bot. 2002;53:423–428. doi: 10.1093/jexbot/53.368.423. [DOI] [PubMed] [Google Scholar]
- Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP. Carbon partitioning to cellulose synthesis. Plant Mol Biol. 2001;47:29–51. [PubMed] [Google Scholar]
- Huber SC, Huber JL, Liao PC, Gage DA, McMichael RW, Jr, Chourey PS, Hannah LS, Koch K. Phosphorylation of serine-15 of maize leaf sucrose synthase: occurrence in vivo and possible regulatory significance. Plant Physiol. 1996;112:793–802. doi: 10.1104/pp.112.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E, Huber S. Correlation between apparent activation state of nitrate reductase (NR), NR hysteresis and degradation of NR protein. J Exp Bot. 1997;48:1367–1374. [Google Scholar]
- Konishi T, Nakai T, Sakai F, Hayashi T. Formation of callose from sucrose in cotton fiber microsomal membranes. J Wood Sci. 2001;47:331–335. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom S, Ek P, Muszynska G, Ek B, Szczegielniak J, Engstrom L. Phosphorylation of sucrose synthase from maize seedlings. Acta Biochim Polonica. 1997;44:809–817. [PubMed] [Google Scholar]
- Loog M, Toomik R, Sak K, Muszynska G, Jarv J, Ek P. Peptide phosphorylation by Ca2+-dependent protein kinase form maize seedlings. Eur J Biochem. 2000;267:337–343. doi: 10.1046/j.1432-1327.2000.01002.x. [DOI] [PubMed] [Google Scholar]
- Luo K, Hurley TR, Sefton BM. Cyanogen bromide cleavage and proteolytic peptide mapping of proteins immobilized to membranes. Methods Enzymol. 1991;201:149–152. doi: 10.1016/0076-6879(91)01014-s. [DOI] [PubMed] [Google Scholar]
- Morell M, Copeland L. Sucrose synthase of soybean nodules. Plant Physiol. 1985;78:149–154. doi: 10.1104/pp.78.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T, Konishi T, Zhang XQ, Chollet R, Tonouchi N, Tsuchida T, Yoshinaga F, Mori H, Sakai F, Hayashi T. An increase in apparent affinity for sucrose of mung bean sucrose synthase is caused by in vitro phosphorylation or directed mutagenesis of Ser-11. Plant Cell Physiol. 1998;39:1337–1341. doi: 10.1093/oxfordjournals.pcp.a029339. [DOI] [PubMed] [Google Scholar]
- Ricard B, VanToai T, Chourey P, Saglio P. Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiol. 1998;116:1323–1331. doi: 10.1104/pp.116.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Subbaiah CC, Sachs MM. Altered patterns of sucrose synthase phosphorylation and localization precede callose induction and root tip death in anoxic maize seedlings. Plant Physiol. 2001;125:585–594. doi: 10.1104/pp.125.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J. 1999;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Sung L, Moloney AH, Hunt S, Layzell DB. The effect of excision on O2 diffusion and metabolism in soybean nodules. Physiol Plant. 1991;83:67–74. [Google Scholar]
- Ueno Y, Imanari E, Emura J, Yoshizawa-Kumagaye K, Nakajima K, Inami K, Shiba T, Sakakibara H, Sugiyama T, Izui K. Immunological analysis of the phosphorylation state of maize C4-form phosphoenolpyruvate carboxylase with specific antibodies raised against a synthetic phosphorylated peptide. Plant J. 2000;21:17–26. doi: 10.1046/j.1365-313x.2000.00649.x. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC. Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. 1997;420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber JL, Huber SC. Identification of sucrose synthase as an actin binding protein. FEBS Lett. 1998;430:205–208. doi: 10.1016/s0014-5793(98)00659-0. [DOI] [PubMed] [Google Scholar]
- Winter H, Huber SC. Regulation of sucrose metabolism in higher plants: localization and regulation of activity of key enzymes. Crit Rev Plant Sci. 2000;19:31–67. doi: 10.1080/10409230008984165. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE. Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-Q, Chollet R. Seryl-phosphorylation of soybean nodule sucrose synthase (nodulin-100) by a Ca2+-dependent protein kinase. FEBS Lett. 1997;410:126–130. doi: 10.1016/s0014-5793(97)00537-1. [DOI] [PubMed] [Google Scholar]
- Zhang X-Q, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X-Q, Lund AA, Sarath G, Cerny RL, Roberts DM, Chollet R. Soybean nodule sucrose synthase (nodulin-100): further analysis of its phosphorylation using recombinant and authentic root-nodule enzymes. Arch Biochem Biophys. 1999;371:70–82. doi: 10.1006/abbi.1999.1415. [DOI] [PubMed] [Google Scholar]