Abstract
This paper reports the expression and localization of linamarase in roots of two cassava (Manihot esculenta Crantz) cultivars of low and high cyanide. Two different patterns of linamarase activity were observed. In the low-cyanide type, young leaves displayed very high enzyme activity during the early plant growing stage (3 months), whereas in root peel, the activity increased progressively to reach a peak in 11-month-old plants. Conversely, in the high-cyanide cultivar (HCV), root peel linamarase activity decreased during the growth cycle, whereas in expanded leaves linamarase activity peaked in 11-month-old plants. The accumulation of linamarin showed a similar pattern in both cultivars, although a higher concentration was always found in the HCV. Linamarase was found mainly in laticifer cells of petioles and roots of both cultivars with no significant differences between them. At the subcellular level, there were sharp differences because linamarase was found mainly in the cell walls of the HCV, whereas in the low-cyanide cultivar, the enzyme was present in vacuoles and cell wall of laticifer cells. Reverse transcriptase-PCR on cassava tissues showed no expression of linamarase in cassava roots, thus, the transport of linamarase from shoots to roots through laticifers is proposed.
Cassava (Manihot esculenta Crantz) is an important source of calories in tropical countries and ranks 10th among all crops in worldwide production (McMahon et al., 1995). Because of the presence of cyanoglycosides, cassava is potentially toxic to human populations that subsist on cassava-based diets and low ingestion of protein. Almost all of the tissues of cassava contain large amounts of cyanogenic glycosides, such as linamarin and lotoaustralin. However, linamarin accounts for 95% of the total cyanoglycosides, therefore, most of the research on cassava cyanoglycosides has been focused on the biochemistry and metabolism of such compounds (Padmaja, 1995).
Even though tuberous roots are the most commercially important part of the plant, little information is available on the synthesis of linamarin in such tissues because most of the data is limited to seedlings. The content of cyanoglycosides in cassava roots is dependent on the cultivar and the growth conditions (Grace, 1977). Most cassava cultivars are incorrectly called non-cyanogenic because the cyanoglycoside content is less than 100 mg kg−1 fresh roots; but there are cyanogenic cultivars, also called bitter cassavas, which may contain cyanoglycosides of up to 500 mg kg−1 fresh roots (Wheatley et al., 1993). There is not presently an acyanogenic cassava cultivar reported, and several studies on cassava linamarin synthesis suggest that the cyanoglucosides accumulated in roots are synthesized in shoots and then transported to roots where they are stored. However, McMahon and Sayre (1995) demonstrated that secondary roots were capable of synthesizing linamarin at rates equivalent to leaves.
In cassava, the production of cyanide or cyanogenesis is the result of the hydrolysis of linamarin by linamarase to form an acetone cyanydrin, which is either spontaneously or enzymatically transformed by α-hydroxynitrile lyase to release hydrogen cyanide (HCN). Cassava linamarase and hydroxynitrile lyase have been purified and characterized, and their cDNAs have been isolated (Cooke et al., 1978; Eksittikul and Chulavatnatol, 1988; Hughes et al., 1992, 1994; McMahon et al., 1995; White et al., 1998). Even though linamarin and linamarase are present in most of the plant tissues, no HCN is detected under physiological conditions, suggesting that the enzymes and their substrate exist in two different compartments.
Previous studies on compartmentalization of cyanogenic glycosides and their degrading enzymes have shown that in leaves, 50% to 70% of the linamarase activity was apoplastic and located in cell walls (Mkpong et al., 1990; Gruhnert et al., 1994). Pancoro and Hughes (1992) demonstrated that leaf laticifer cells were enriched with linamarase using an antisense linamarase riboprobe. This isoform of linamarase was purified and characterized by Elias et al. (1997), which confirmed the location of the enzyme in laticifers and in cell walls of leaves. However, very little information is available on the location and expression of the linamarin-degrading enzymes in cassava roots. This paper reports the expression and immunolocalization of linamarase in roots of two cassava cultivars during the growth cycle.
RESULTS
Distribution of Linamarase Activity and Linamarin Content in Cassava Tissues
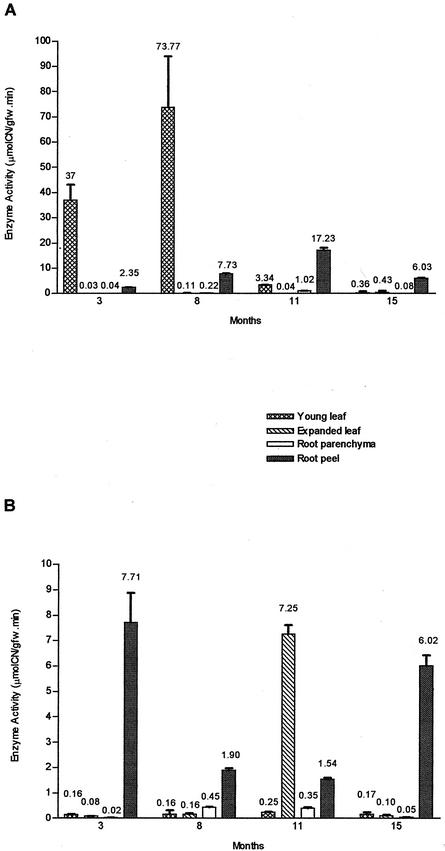
Two cassava varieties, cv V47 (high-cyanide cultivar [HCV]) and cv V56 (low-cyanide cultivar [LCV]), with high- and low-cyanide root content, respectively, were selected from preliminary studies because they showed optimal development and a stable phenotype under different environmental growth conditions. Figure 1 shows that linamarase activity displayed different patterns of expression in these cassava cultivars during the growth cycle, with root peel as the root tissue with the highest linamarase activity (Fig. 1, A and B). However, there was a sharp contrast in the enzyme activity of the root peel of either variety through the growth cycle, because in LCV, the linamarase activity increased gradually from 3-month-old plants until reaching a peak of 17.23 ± 0.9 μmol CN− g−1 fresh weight min−1 in 11-month-old plants. HCV root peel conversely displayed a decrease in its linamarase activity from an initial high of 7.71 ± 1.16 μmol CN− g−1 fresh weight in 3-month-old plants to attain its lowest level in 11-month-old plants with 1.54 ± 0.07 μmol CN− g−1 fresh weight min−1. A second peak of activity appeared late in the growth cycle at 15-month-old plants, in contrast with a decrease in the linamarase activity of the LCV counterpart, although at this stage, both cultivars displayed similar levels of enzyme activity.
Figure 1.
Linamarase activity in cassava tissues during the growth cycle. A, Low-cyanide content cassava (cv V56-LCV). B, Cyanogenic cassava (cv V47-HCV). Values are expressed as mean of enzyme activity (micromoles of cyanide per gram fresh weight per minute) ± sd (n = 3). The means are shown on the top of each column. The variation in assay values were in general less than 10%. gfw, Grams of fresh weight tissue.
The above results showed that at harvest (9- to 11-month-old plants), the linamarase activity of LCV root peel is about 10 times that of the corresponding to HCV. Such developmental differences were also manifested in the leaves of the two types of cultivars, because in LCV, there was a very high peak of activity in young leaves (third from apex) of 8-month-old plants (73.77 ± 20.18 μmol CN− g−1 fresh weight min−1), whereas in HCV, the enzyme activity of young leaves remained very low (0.16–0.25 μmol CN− g−1 fresh weight min−1). In expanded leaves (seventh from apex) of this variety, the highest peak of linamarase activity occurred late in the growth cycle, in 11-month-old plants (7.25 ± 0.35 μmol CN− g−1 fresh weight min−1), again in sharp contrast to LCV, where such activity was barely measurable. Interestingly enough, in both cultivars, the peak of linamarase activity in leaves was followed 3 to 4 months later by an increase of the enzyme activity of the root peel. Not only was the pattern of linamarase activity different between cultivars throughout the growth cycle, but the absolute values of such enzyme activities in leaf and root peel was 10 times higher in LCV than in HCV.
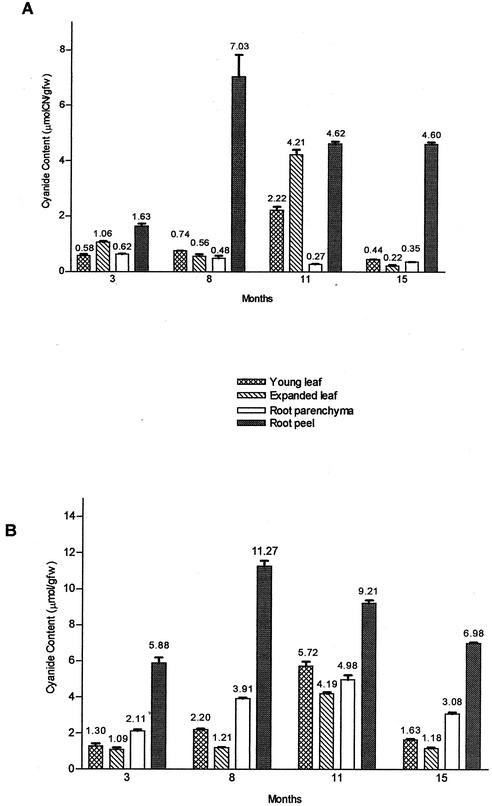
The linamarin content of cassava tissues during the growth cycle is shown in Figure 2, where we observe that the overall pattern of linamarin accumulation seemed very similar for both varieties. The highest concentration of linamarin for either type was observed in root peel 8 months after sowing the plants. HCV, however, showed 1.6 times the amount of cyanide in root peel as compared with LCV. As expected, the accumulation of cyanide during the growth cycle in root parenchyma of HCV was higher (18-fold) than in LCV with a peak of accumulation at 11-month-old plants. In leaves, the highest linamarin concentration occurred, as well, in 11-month-old plants of either type, but in HCV, young leaves (third from apex) were the most cyanogenic in contrast to LCV where the highest linamarin concentrations appeared in the expanded leaves (seventh from apex).
Figure 2.
Linamarin content of cassava tissues during the growth cycle. A, Low-cyanide content cassava (cv V56-LCV). B, Cyanogenic cassava (cv V47-HCV). Values expressed as mean of cyanide content (micromoles of cyanide per gram fresh weight) ± sd (n = 3). The means are shown on the top of each column. The variation in assay values were in general less than 10%. gfw, Grams of fresh weight tissue.
Immunoblot Analysis
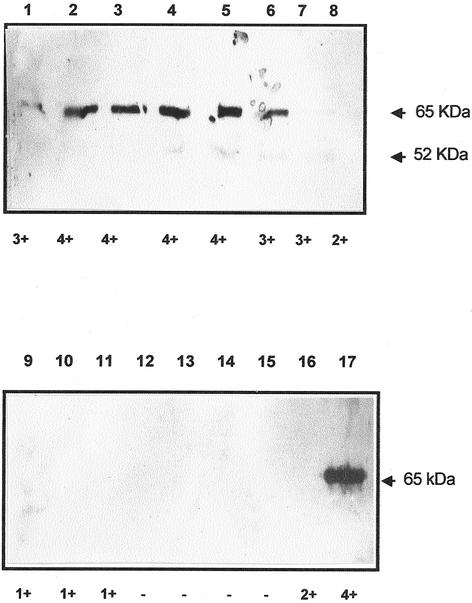
Figure 3 shows immunoblots of different chromatographic fractions obtained from the purification procedure applied to linamarase. We observe that after electrophoresis, the immunological reaction occurred only with a major polypeptide band of Mr = 65,000, corresponding to that of the pure enzyme, and a minor one of Mr = 52,000, which was present in fractions containing β-glucosidase activity (lanes 2–6). We also note that those chromatographic fractions with low or no enzyme activity (lanes 7–16) did not react with the antibody. The purified enzyme used to raise the antibodies gave a very strong reaction with a single polypeptide band of Mr = 65,000 (lane 17), coinciding with that reported for linamarase (Hughes et al., 1992). Given the appearance of the Mr = 52,000 band in fractions with linamarase activity (lanes 4–7), it is likely that it may have corresponded to a proteolytic fragment of linamarase, or it might have been simply the result of a nonspecific reaction. However, it is noteworthy that such polypeptide band was not present in the fraction used to raise the antibodies.
Figure 3.
Immunoblot analysis of fractions separated by molecular exclusion during linamarase purification. Total soluble protein (15 μL fraction−1) was loaded on a 10% (w/v) polyacrylamide gel. After separation, proteins were transferred to a nitrocellulose membrane, and the blot challenged with rabbit antiserum raised against cassava linamarase (1:5,000). Horseradish peroxidase goat anti-rabbit antibodies (1:10,000) were used as secondary antibody. 3,3′,5,5′-tetramethyl-benzidene was used as a substrate for the peroxidase. Lane 1, f4; lane 2, f5; lane 3, f6; lane 4, f7; lane 5, f8; lane 6, f9; lane 7, f10; lane 8, f11; lane 9, f16; lane 10, f20; lane 11, f24; lane 12, f52; lane 13, f61; lane 14, f62; lane 15, f63; lane 16, f64; lane 17, 1.5 μg of purified linamarase used to raise the antibodies. Activity of each fraction is indicated under each lane. 4+, High activity; 1+, low activity; and −, no activity. f, Fraction. Arrowheads indicate Mr = 65,000 and 52,000.
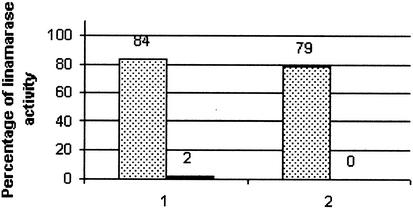
The specificity of the rabbit immune serum raised against the commercial linamarase from cassava was further tested by immunoprecipitation of the crude enzyme activity with the antibody prepared against the purified enzyme. Linamarase activity of the leaf extracts incubated with the normal serum was compared with those incubated with immune serum. Figure 4 shows that a decrease in linamarase activity of 98.6% (n = 4) was obtained with the immune serum, indicating that the rabbit antiserum recognized the linamarase present in the cassava leaf extracts. This result was confirmed by immunoblot analysis using plant extracts of both cultivars.
Figure 4.
Linamarase antiserum specificity: immunoprecipitation of linamarase. Total soluble protein (5–40 μg) of plant leaf crude extracts was incubated with 1:10 diluted serum. Reactions were incubated overnight at 37°C and then incubated for 1 h with protein A-Sepharose. After precipitation of protein A-Sepharose-antibody complexes, linamarase activity was measured on supernatants. The figure shows the result of two of the different experiments. Linamarase activity in the crude extract previous immunoprecipitation was 10.96 ± 0.15 32 μmol CN− g−1 fresh weight min−1. First columns represent the percentage of linamarase activity in the extract after incubation with normal serum (dots). Second columns represent the percentage of linamarase activity in the extract after incubation with linamarase antiserum. An average decrease in linamarase activity of 98.6% (n = 4) was calculated from the results obtained.
Immunolocalization of the Enzyme in Cells and Tissues
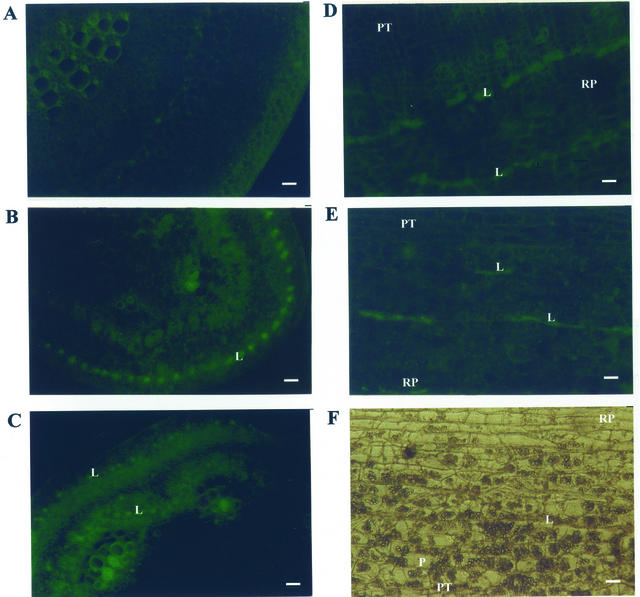
In the normal rabbit serum-treated section leaves, no fluorescence was observed throughout all the tissue. More than 2 min was required for film to capture the natural fluorescence of plant leaf tissues (Fig. 5A). However, in antiserum-treated sections of cassava leaves, specific fluorescence corresponding to linamarase was located mainly in laticifers of both cultivars (Fig. 5, B and C). In HCV expanded leaf petiole sections (Fig. 5B), the specific fluorescence included as well parenchymatous cells and cells associated with the cambium, suggesting that more than one cellular type is involved in the synthesis of linamarase in leaves. In root sections, high concentrations of starch granules were observed (Fig. 5D). To reduce the side effects of a high concentration of starch in the root sections, α-amylase (8 mg mL−1) was added to the antiserum preparation. In antiserum-treated sections of cassava roots, specific fluorescence corresponding to linamarase was mainly located in the laticifers of both cultivars (Fig. 5, E and F).
Figure 5.
In situ immunofluorescence of linamarase in cassava tissues. A, Petiole section of mature leaf of low-cyanide content cassava (LCV), incubated with normal serum. As expected, a background tissue fluorescence is observed. B, Petiole section of young leaf of LCV after incubation with anti-linamarase serum. A specific fluorescence corresponding to linamarase was located mainly in laticifers. C, Petiole section of mature leaf of HCV after incubation with anti-linamarase serum. A specific fluorescence corresponding to linamarase was located mainly in laticifers, cambium, and cells surrounding them. D, Root section of LCV. Root peel (RP) and parenchyma tissue (PT) is observed. Parenchyma (P) cells and laticifer (L) cells are indicated. E, Root section of HCV after incubation with anti-linamarase serum. A specific fluorescence corresponding to linamarase was located mainly in laticifers. F, Root section of LCV after incubation with anti-linamarase serum. A specific fluorescence corresponding to linamarase was located mainly in laticifers. Bars = 100 μm.
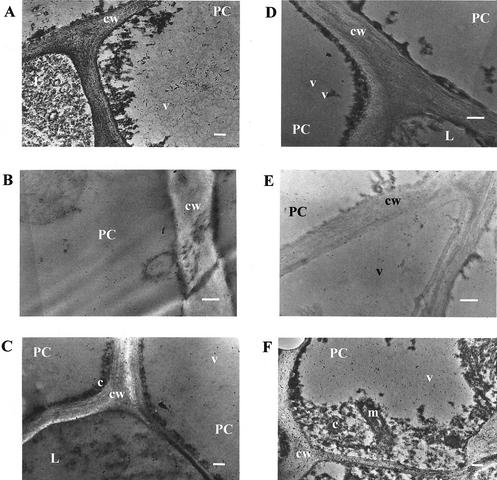
The subcellular location of linamarase was examined by electron microscopy using gold-conjugated goat anti-rabbit IgG. Figure 6 shows some of the pictures obtained from sections that were challenged with the linamarase antiserum. Pictures were taken at different magnifications of the tissues. Full-cell pictures were chosen for the statistical analysis (STATISTICA for Windows v5.0, StatSoft, Tulsa, OK). Gold particles were counted, and the number of particles in different subcellular compartments was averaged. For each experiment, a control with preimmune serum was included. Table I shows the estimated means of gold particle density obtained for each cell compartment of petiole and root tissues. The estimated means for each compartment were compared using the null hypothesis μ1 − μ2 = 0 and the alternative hypothesis μ1 − μ2 ≠ 0. The results show that in both cultivars, the particle density of cytosol and cell wall of parenchyma cells was significantly different when compared with the means for vacuole and organelles of root peel, parenchyma tissue, or petiole. However, for LCV, the null hypothesis was accepted when densities of particles in the cell wall and modified vacuole of laticifer cells were compared. In HCV, the null hypothesis was conversely rejected, suggesting that cell wall of laticifer cells had a higher concentration of linamarase. In addition, the gold particle density for each cell type of petiole and root peel was averaged and compared. The estimated means for each cellular type were compared using the null hypothesis μ1 − μ2 = 0 and the alternative hypothesis μ1 − μ2 ≠ 0. The results show that in both cultivars, parenchyma cells and laticifer cells of petiole had the same particle density, whereas in root peel, the null hypothesis was rejected, indicating a higher particle density in laticifer cells of this tissue (Table II). In short, linamarase was found in roots mainly in cell walls and modified vacuoles of laticifer cells and in the cytosol and cell walls of leaf cells. No significant differences were observed between the two cassava cultivars.
Figure 6.
Electron micrographs of cassava leaf and root sections. Sections were incubated with anti-linamarase serum followed by incubation with 10-nm gold-conjugated goat anti-rabbit antibodies. In non-cyanogenic cassava: A, root peel; B, root parenchyma; and C, petiole. In cyanogenic cassava: D, root peel; E, root parenchyma; and F, petiole. Bars = 2 μm. cw, Cell wall; v, vacuole; c, citosol; m, mitochondria; pc, parenchymatous cell; L, laticifer cell. Arrows indicate a gold particle.
Table I.
Immunogold labeling of linamarase in different cellular compartment of parenquimatous and laticifer cells
| Cell Type | Vacuole | Cell Wall | Cytoplasm | Organelles |
|---|---|---|---|---|
| Petiole of LCV (nonparametric test) | ||||
| Parenchyma (8) | 6.66 ± 4.24 | 8.77 ± 8.21 | 31.77 ± 27.07* | 9.90 ± 4.43 |
| Laticifers (4) | 6.74 ± 3.28 | 6.00 ± 3.38 | Not founded | Not founded |
| Parenchymatous tissue of LCV(parametric test) | ||||
| Parenchyma (9) | 6.41 ± 3.11 | 7.50 ± 5.36* | 20.67 ± 16.44* | 6.61 ± 4.50 |
| Root peel of LCV (parametric test) | ||||
| Parenchyma (5) | 2.69 ± 1.01 | 5.63 ± 2.82* | 8.72 ± 4.63* | 4.26 ± 3.41 |
| Laticifer (4) | 5.41 ± 3.84 | 6.37 ± 2.80 | Not founded | Not founded |
| Petiole of HCV (nonparametric test) | ||||
| Parenchyma (15) | 2.74 ± 2.26 | 30.49 ± 19.96* | 22.36 ± 10.97* | 11.29 ± 4.07 |
| Laticifer (4) | 13.31 ± 4.04* | 10.01 ± 12.38* | Not founded | Not founded |
| Parenchymatous tissue of HCV(nonparametric test) | ||||
| Parenchyma (15) | 4.63 ± 1.05 | 12.98 ± 6.62* | 6.22 ± 4.36 | 4.53 ± 2.70 |
| Root peel of HCV (nonparametric test) | ||||
| Parenchyma (10) | 3.43 ± 1.10 | 24.74 ± 11.63* | 8.52 ± 4.06* | Not founded |
| Laticifers (4) | 2.79 ± 0.29 | 10.34 ± 6.41* | Not founded | Not founded |
The results are the mean of gold particles per μm2 ± sd. Means were compared using one-way ANOVA. No. of cells analyzed is in parentheses. An asterisk indicates significant differences (P = 0.05).
Table II.
Immunogold labeling of linamarase in different cell type and tissues of low- and high-cyanide content cassava
| Cassava Varieties | Tissue | Cell Type | Density |
|---|---|---|---|
| part μm−2 | |||
| LCV | Petiole | Parenchyma (8) | 13.32 ± 10.38 |
| Laticifer (4) | 12.18 ± 6.30 | ||
| Root peel | Parenchyma (5) | 10.31 ± 4.44*1 | |
| Laticifer (4) | 20.28 ± 6.47*1 | ||
| HCV | Petiole | Parenchyma (15) | 26.40 ± 11.17 |
| Laticifer (4) | 32.04 ± 29.49 | ||
| Root peel | Parenchyma (10) | 3.09 ± 0.63*2 | |
| Laticifer (4) | 3.77 ± 0.63*2 |
The results are the mean of gold particles per μm2 ± sd. Means were compared using Student's t test. No. of cells analyzed is in parentheses. An asterisk indicates significant differences (P1 = 0.01; P2 = 0.05).
Detection of Linamarase mRNA in Cassava Tissues
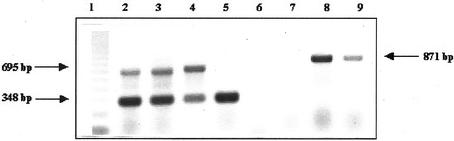
Results of reverse transcriptase (RT)-PCR performed to detect specific mRNAs were similar in both cassava varieties (Fig. 7). A band of 871 bp corresponding to linamarase mRNA was amplified in cassava leaves, but not in root tissues, indicating that linamarase mRNA is present in young leaves but not in root peel of 9-month-old plants. As a control for integrity of the isolated root peel total RNA, cassava GADPH was amplified. An expected band of 348 bp corresponding to cassava GADPH mRNA was obtained. In some of the samples, a 695-bp band corresponding to the GADPH gene was also amplified. However, a band corresponding to linamarase gene (1,504 bp) was not amplified in any of the samples under the experimental conditions used.
Figure 7.
RT-PCR analysis of linamarase in cassava tissues. Lane 1, 123-bp ladder; lanes 2 through 5, using specific cassava glyceraldehyde diphosphate hydrogenase (GADPH) primers; lanes 6 through 9, using specific cassava linamarase primers. Lanes 2 and 8; Using 100 ng of total RNA from young leaves of high-cyanide content cassava (HCV); lanes 3 and 9, using 100 ng of total RNA from low-cyanide content cassava (LCV); lanes 4 and 6, using 1,000 ng of total RNA from root peel of HCV; lanes 5 through 7, using 1,000 ng of total RNA from root peel of LCV.
DISCUSSION
In developing countries, cassava provides an important source of calories, mainly in marginal areas with low levels of income and limited access to farm technologies. However, cassava production is also an important source of employment and income in these countries. In this sense, IMPACT projections suggest that cassava demand in the next 20 years is expected to increase considerably, therefore, special efforts should be made to improve cassava root yield and address the problems with cassava consumption such as cyanogenesis (Pinstrup-Andersen et al., 1999).
Cultivar-dependent differences in the cyanogenic components of cassava have been very controversial. Extensive research has been done to understand the physiological basis to account for these differences. Several factors, intrinsic and extrinsic, including the genotype and the environmental conditions such as dryness and soil composition have been assigned for the differences observed (Grace, 1977). In the present work, two different cassava cultivars have been studied. Both differ in the expression of linamarase and accumulation of linamarin as it was shown in “Results.”
The two cultivars under study have a differential pattern of linamarase activity during the growth cycle. Special care had to be taken at the moment of the analysis of the data and of triplicate measurements with different dilutions of the extracts. An analysis of more than two plants of each cultivar of each age, sharing the same organ sampling for different determinations was needed too. Because it is very difficult to control the environmental factors, the mode of several plants were taken into account at the moment of the analysis. This, indicates that if the genotype is important, the environment conditions are also important modulators of the expression of the enzymes involved in both the synthesis of linamarin and the expression of linamarase. However, despite the differences observed in the absolute values from one field assay to the other (preliminary data not shown), the general tendency was always the same, and the results were consistent with the observations made by other authors in similar studies (McMahon et al., 1995). Clearly, the linamarase activity distribution varied between different organs and tissues of the same plant and between cultivars as is shown in the results presented. However, a generalization cannot be ascertained for every cassava cultivar. In the present work, the low-cyanide cassava showed very high expression of the enzyme in young leaves of 3- and 8-month-old plants, whereas the activity in root peel increased through the growth cycle. The HCV conversely showed a decrease in the root peel linamarase activity during the growth cycle. In 11-month-old plants, at a time in the growth cycle when the starch stored in roots starts to be used for new growth, the activity in expanded leaves increased, and 4 months later, there was a peak of linamarase activity in the root peel of this cultivar. Because both cassava cultivars had differential characteristic pattern of linamarase expression, they can be used as a model to study the physiological differences between cyanogenic and non-cyanogenic cassavas.
Linamarase has been studied previously in leaves at the subcellular levels. White et al. (1994) and Gruhnert et al. (1994) showed that in more than 50% of the cassava leaves, linamarase was present in the apoplast. The apoplastic location was confirmed by Mkpong et al. (1990) who showed that most of the immunogold labeling was present in the cell wall of cassava leaves. Pancoro and Hughes (1992) demonstrated the presence of mRNA in laticifer cells of petiole and leaves, and Elias et al. (1997) purified and characterized the laticifer isozyme. However, this is the first report, to our knowledge, in which the immunolocalization of linamarase in cassava roots is shown and the location of the enzyme is compared in low- and high-cyanide cassava cultivars. The results of immunofluorescence presented here showed clearly the immunolocalization of linamarase in laticifers of petioles and roots of both cultivars. The results obtained support the hypothesis, proposed first by Pancoro and Hughes (1992), that linamarase may be transported from shoots to roots throughout the branched laticifer network in the cassava plant.
The immunolabeling results also showed that in the high-cyanide plants, linamarase was located in the cell wall and in the cytosol, whereas in the low-cyanide cassava cultivar, linamarase was mainly located in modified vacuoles of laticifer cells and cytosol of parenchyma cells. This may suggest differences in the linamarase efficiency and/or mechanisms of transport between the cultivars studied. However, there is no report of differences at molecular levels of the linamarase genes in cassavas that may be responsible for the differential locations observed.
One of the most discussed aspects of cyanogenesis in cassava is the biosynthesis and transport of linamarin (McMahon et al., 1995). Early studies indicated that linamarin was transported from shoots to primary roots, which were not able to synthesize linamarin (Koch et al., 1992; Bokanga et al., 1993). The enzymes involved in the biosynthesis have been characterized, and a vacuolar location of linamarin has been demonstrated (White et al., 1994; McMahon and Sayre, 1995). Two mechanisms for the transport of linamarin to roots have been proposed; either symplastically via the phloem or apoplastically as a non-hydrolyzable cyanoglucoside. In addition to results indicating the contribution of the shoots to the root linamarin, McMahon and Sayre (1995) and Du et al. (1995) demonstrated that roots were also capable of synthesizing linamarin at rates equivalent to those of leaves. Therefore, the linamarin present in root parenchyma may have two biosynthetic sources. Our work indicates that, in general, all the tissues of cyanogenic cassava have more cyanide than the low-cyanide cassava cultivar, indicating a higher rate of synthesis and accumulation of linamarin in these cultivars. However, in the low-cyanide cassava cultivar, the expression of linamarase is higher in most of the tissues.
The most important finding of the results here presented is the absence of linamarase mRNA in the roots of the two cultivars under study. Since no linamarase mRNA has been detected in cassava roots, the transport of linamarase from leaves to roots is suggested. Our work also indicates that there must be a differential rate of linamarase expression and transport that accounts for the differences in the enzyme activity observed in roots. Further molecular genetic investigation of linamarase genes and their promoters may be extremely useful for designing a more efficiently strategy to release the cyanide in cyanogenic cassava cultivars.
MATERIALS AND METHODS
Plant Material
Two cassava (Manihot esculenta Crantz) cultivars, cv V47 and cv V56 from Instituto de Estudios Avanzados germplasm bank, were grown from in vitro plants. Both cultivars varied in their root cyanoglucoside content; cv V47 (HCV) is a bitter cassava and cv V56 is an LCV. Plants were grown outdoors from stem cuttings of these in vitro plants for 3 to 15 months before use as a source of plant tissue. Tissue extracts for enzyme assays and western analysis were prepared by grinding liquid nitrogen frozen plant tissues in 100 mm sodium phosphate buffer (pH 6.8) using a mortar with a pestle. Plant extracts were centrifuged, and supernatants were frozen at −20°C until needed. A fraction of the same tissue used for enzyme activity was employed for cyanide quantification. These extracts were prepared using standard procedures as described by Ikediobi et al. (1980) consisting in tissue disruption in liquid nitrogen followed by fast grinding (<30 s) in 100 mm HCl acid, and the homogenate was immediately adjusted to pH 6.8 with base. The resulting supernatant was stored at −20°C until analyzed.
Linamarase Activity and Analytical Methods
Linamarase activity was determined by estimating the HCN liberated during the reaction. Reactions were performed at 37°C with 13 mm of linamarin in the presence of 100 mm sodium phosphate buffer, pH 6.8, in a closed vessel. A time course of 30 min with different dilutions of the extract was used for measurements of activity. Excess cassava linamarase (BDH, Poole, Dorset, UK) was used for quantification of tissue cyanoglycosides, and the cyanide was measured spectrophotometrically at 585 nm using a cyanide determination kit (Spectroquant Cyanide 14800, Merck, Darmstadt, Germany; Hughes et al., 1992). Potassium cyanide was used as a standard. All assays were carried out with three different plants of the same age, grown under the same conditions. At least three measurements of different dilutions of the same extract were performed. Protein was determined by triplicate with a protein determination kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions, using bovine serum albumin (BSA) as the standard (Bradford, 1976).
Preparation of Antibodies
Linamarase was purified from a cassava BDH extract subjected to molecular exclusion by using column chromatography with Sephadex G200. Fractions with β-activity were then electrophoresed (Laemmli, 1970) in a 10% (w/v) SDS-PAGE gel and stained with the silver stain kit from Bio-Rad. Fractions showing a single electrophoretic protein band of linamarase (Mr = 65,000) were pooled, freeze-dried, and desalted before resuspension in 10 mm potassium phosphate buffer (pH 7.0). The purified protein (100 μg) was emulsified with an equal volume of Freund's complete adjuvant for the first challenge and with Freund's incomplete adjuvant for the second and third injections. Injections were administered subcutaneously to a Dutch rabbit at two sites. The second and third injections were performed 28 and 42 d after the first injection. Blood (20 mL/bleed) was collected before the first injection and at d 35 and 56 after the first injection. The blood was incubated at 37°C for 1 h and then left overnight at 4°C. Clotted blood cells were decanted, and the remaining serum was centrifuged at 5,000g for 10 min and stored at −20°C (Harlow and Lane, 1988).
Antibody Specificity
The specificity of the rabbit antibodies against linamarase was checked by their ability to immunoprecipitate the activity of this enzyme. Cassava leaf extracts containing 5 to 40 μg of protein were incubated with either anti-linamarase polyclonal antibody or normal serum, at a dilution 1:10 in a 50-μL final volume. Incubations were carried out overnight in a water bath at 37°C. Then 2.5 mg of protein A-Sepharose in 10 μL of phosphate buffered saline (PBS) was added, and the mixture was incubated at 37°C for 1 h with agitation and centrifuged at 10,000g for 1 min to precipitate the antigen-antibody-protein A-Sepharose complex. The supernatants were collected, and enzyme activity was measured in triplicate. Preimmune serum (normal serum) used for controls was obtained from the same rabbit used to prepare the antibody before being immunized.
Immunoblot Analysis
Protein extracts were subjected to electrophoresis on 10% (w/v) polyacrylamide gels in the presence of 1% (w/v) SDS and then transferred onto nitrocellulose membranes using a Trans-Blot Semidry Bio-Rad system. Proteins were visualized with Ponceau S and washed in dilution buffer containing 10 mm sodium phosphate buffer, pH 7.4, 0.9% (w/v) NaCl, and 0.1% (w/v) Tween 20. Membranes were blocked overnight with 3% (w/v) nonfat milk in dilution buffer. Blots were challenged with antiserum raised against linamarase diluted 1:5,000 in dilution buffer supplemented with 1% (w/v) nonfat milk. Bound antibodies were visualized with goat anti-rabbit antibodies conjugated to horseradish peroxidase. The ECL detection kit from Amersham (Buckinghamshire, UK) was used according to the manufacturer's instructions.
In Situ Immunofluorescence Labeling of Linamarase
The method reported by Hattersley et al. (1977) was followed with the following modifications. Leaves and roots were cut by hand with razor blades and immediately immersed in 70% (v/v) ethanol for 2 h. Sections were rinsed in PBS and then blocked for 1 to 2 h in blocking solution (PBS-1% [w/v] BSA) with gentle agitation. After blocking, sections were incubated overnight with anti-linamarase serum diluted 1:8 in PBS. The cuts were then washed three times in PBS for 20 min and incubated with a solution containing a dilution 1:10 of conjugated goat anti-rabbit IgG coupled with fluorescein isothiocyanate. After a 1.5-h incubation in darkness, sections were washed as above and then mounted on slides in a solution of 50% (v/v) glycerol-1% (v/v) thymol. All procedures were performed at 22°C to 25°C. Sections were observed using a microscope with a blue excitation (450–490 nm) two-position filter system that allows excitation of fluorescein (Zeiss, Jena, Germany). Pictures were taken with a Konica camera adapted to the microscope using the automatic exposure mode and with Kodak Gold color film (400 ISO, Eastman-Kodak, Rochester, NY).
Immunogold Electron Microscopy
Cassava tissues (1 × 1 × 2 mm) were fixed in 2% (w/v) paraformaldehyde and 1% (v/v) glutaraldehyde in 50 mm PIPES buffer (pH 7.2) for 2 h at 4°C under vacuum to facilitate infiltration (Mohan et al., 1993). To remove the fixative, tissues were washed twice for 30 min at 4°C with PIPES buffer. The tissues were dehydrated at 4°C successively in solutions of 15%, 30%, 50%, and 80% (v/v) ethanol and then embedded in 25%, 40%, 50%, and 100% (v/v) LR White resin at 4°C for 15 d under vacuum. Polymerization was performed at 50°C for 24 h. Blocks were cut with a ultramicrotome (Ultracut, Reichert-Jung, NuBlock, Germany), and sections were placed on 300-mesh gold grids. The grids were floated, sections side down, on a drop of blocking buffer (0.8% [w/v] BSA, 0.1% [w/v] gelatin, 100 mm PBS, pH 7.2, 0.05% [w/v] sodium azide, and 5% [v/v] fetal calf serum) for 45 min at RT and then incubated for 16 h at 4°C with a dilution 1:5 of the linamarase antibody in the same buffer. Sections were rinsed in blocking buffer and incubated for 3 h at 22°C to 25°C with goat antirabbit-IgG conjugated to 10-nm colloidal gold diluted 1:50 in the same buffer. After three washes in washing buffer (0.8% [w/v] BSA, 0.1% [w/v] gelatin, 100 mm PBS, pH 7.2, and 0.05% [w/v] sodium azide) and water, sections were stained with 4% (w/v) uranyl acetate and 0.4% (w/v) lead citrate for 10 min each and then rinsed with water. Controls were done with preimmune serum and secondary antibodies. Electron microscopy was carried out on an EM-400 electron microscope (Philips, Eindhoven, The Netherlands).
Detection of Linamarase mRNA in Cassava Tissues
The RT-PCR technique (Promega, Madison, WI) was used to investigate the presence of mRNAs in cassava tissues. Plant tissues were obtained from 9-month-old plants. Extraction of total RNA was carried out by the CsCl gradient method as described by Lewinsohn et al. (1994). RNA integrity was checked by the OD ratio and by formaldehyde denaturing gels. The RT-PCR reaction was performed with the Access RT-PCR System (Promega) in a PCR System 9600 (Perkin-Elmer Applied Biosystems, Foster City, CA). Oligonucleotides sequences used were 5′-GCA ATG GAG ACG TTG CAG TTG-3′ and 5′-GTC GCA TTA ACA CCA CTA TCA-3′ for cassava linamarase and 5′-CAG AAG ACT GTT GAC GGC CC-3′ and 5′-CAA TTC CAG CCT TGG CGT C-3′ for cassava GADPH. RT-PCR reactions were done in a final volume of 50 μL containing 1× avian myeloblastosis virus/Thermus flavus buffer (Promega), 200 μm of each dNTP, 50 pmol of each primer, 1 mm MgSO4, 5 units of T. flavus DNA polymerase, 5 units of avian myeloblastosis virus RT, and different amounts of total RNA. The mixture was incubated at 48°C for 45 min to synthesize the first cDNA chain and at 94°C for 105 s to inactivate the RT enzyme. Thermal cycling reactions were carried out for 26 cycles, consisting each of denaturation at 94°C, 30 s; annealing at 52°C, 30 s; extension at 72°C, 60 s; and ending with a 72°C, 7-min extension step. PCR samples were separated on a 1.5% (w/v) agarose gel (Sigma, St. Louis). PCR fragments amplified were cloned with the Topo2.1 kit from Invitrogen (Carlsbad, CA) and then sequenced.
ACKNOWLEDGMENTS
We thank Dr. Tatiana Mérida for interpretation of micrograph pictures. We thank Dr. Gloria Villegas, Francisco Noria, Mirtha Romano, and Dr. Paola Tonino for their contribution to the electron microscopy. We also thank Luis Hidalgo and Sonia Schwarz for helping with the bleeding of rabbits and Elio Estevez for his contribution in preliminary studies. We thank Antonio Bonelli for the photographing processing. We especially thank Dr. Gloria Villegas and Katalin Hudak for proofreading the manuscript.
Footnotes
This work was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (grant no. S1–97000558), by Instituto de Estudios Avanzados, and by Universidad Simón Bolívar.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.000927.
LITERATURE CITED
- Bokanga M, Halkier B, Moller B. Studies on the biosynthesis of cyanogenic glucosides in cassava. In: Roca WM, Thro AM, editors. Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. Centro Internacional de Agricultura Tropical, Cali, Colombia. 1993. pp. 418–423. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooke R, Blake GG, Battershill JM. Purification of cassava linamarase. Biochemistry. 1978;17:381–383. [Google Scholar]
- Du L, Bokanga M, Moller BL, Halkier BA. The biosynthesis of cyanogenic glucosides in roots of cassava. Phytochemistry. 1995;39:323–326. [Google Scholar]
- Eksittikul T, Chulavatnatol M. Characterization of cianogenic β-glucosidase (Linamarase) from cassava (Manihot esculenta Crantz) Arch Biochem Biophys. 1988;266:263–269. doi: 10.1016/0003-9861(88)90257-3. [DOI] [PubMed] [Google Scholar]
- Elias M, Nambisan B, Sudhakaran PR. Characterization of linamarase of latex and its localization in petioles in cassava. Arch Biochem Biophys. 1997;341:222–228. doi: 10.1006/abbi.1997.9924. [DOI] [PubMed] [Google Scholar]
- Grace MR. Elaboración de la Yuca. Colección FAO: Producción y Protección Vegetal. Organización de las Naciones Unidas para la Agricultura y la Alimentación, Italy: Rome; 1977. pp. 1–162. [Google Scholar]
- Gruhnert CH, Biel B, Selmar D. Compartmentalization of cyanogenic glucosides and their degrading enzymes. Planta. 1994;195:36–42. [Google Scholar]
- Harlow E, Lane D. Antibodies. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1988. [Google Scholar]
- Hattersley PW, Watson L, Osmond CB. In situ immunofluorescent labeling of ribulose-1,5-bisphosphate carboxylase in leaves of C3 and C4 plants. Aust J Plant Physiol. 1977;4:523–539. [Google Scholar]
- Hughes J, Carvalho FJP, Hughes MA. Purification, characterization, and cloning of α-hydroxynitrile lyase from cassava (Manihot esculenta) Arch Biochem Biophys. 1994;311:496–502. doi: 10.1006/abbi.1994.1267. [DOI] [PubMed] [Google Scholar]
- Hughes MA, Brown K, Pancoro A, Murray BS, Oxtoby E, Hughes J. A molecular and biochemical analysis of the structure of the cyanogenic β-glucosidase (linamarase) from cassava (Manihot esculenta Crantz) Arch Biochem Biophys. 1992;295:273–279. doi: 10.1016/0003-9861(92)90518-2. [DOI] [PubMed] [Google Scholar]
- Ikediobi CO, Onyia GOC, Eluwah CE. A rapid and inexpensive enzymatic assay for total cyanide in cassava (Manihot esculenta Crantz) Agric Biol Chem. 1980;44:2803–2809. [Google Scholar]
- Koch B, Nielsen VS, Halkier BA, Olsen CE, Moller B. The biosynthesis of cyanogenic glucosides in seedlings of cassava (Manihot esculenta Crantz) Arch Biochem Biophys. 1992;292:141–150. doi: 10.1016/0003-9861(92)90062-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Steel CL, Croteau R. Simple isolation of functional RNA from woody stems of gymnosperms. Plant Mol Biol Rep. 1994;12:20–25. [Google Scholar]
- McMahon JM, Sayre RT. Regulation of cyanogenesis in cassava (Manihot esculenta Crantz) In: Roca WM, Thro AM, editors. Proceedings of the Second International Scientific Meeting of the Cassava Biotechnology Network, Bogor, Indonesia. Centro Internacional de Agricultura Tropical, Cali, Colombia. 1995. pp. 423–431. [Google Scholar]
- McMahon JM, White WLB, Sayre RT. Cyanogenesis in cassava (Manihot esculenta Crantz) J Exp Bot. 1995;46:731–741. [Google Scholar]
- Mkpong OE, Yang H, Chism G, Sayre R. Purification, characterization, and localization of linamarase in cassava. Plant Physiol. 1990;93:176–181. doi: 10.1104/pp.93.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Philip T, Knox RB. Special preparation methods for immunocytochemistry of plant cells. In: Beesley J, editor. Immunocytochemistry, a Practical Approach. UK: Oxford University Press; 1993. pp. 77–102. [Google Scholar]
- Padmaja G. Cyanide detoxification in cassava for food and feed uses. Crit Rev Food Sci Nutr. 1995;35:299–339. doi: 10.1080/10408399509527703. [DOI] [PubMed] [Google Scholar]
- Pancoro A, Hughes MA. In-situ localization of cyanogenic β-glucosidase (linamarase) gene expression in leaves of cassava (Manihot esculenta Crantz) using non-isotopic riboprobes. Plant J. 1992;2:821–827. [Google Scholar]
- Pinstrup-Andersen P, Pandia-Lorch R, Rosegrant MW. World Food Prospects: Critical Issues for the Early Twenty-First Century. Food Policy Report. Washington, DC: International Food Policy Research Institute; 1999. pp. 1–32. [Google Scholar]
- Wheatley CC, Orrego JI, Sanchez T, Granados E. Quality evaluation of the cassava core collection at CIAT. In: Roca WM, Thro AM, editors. Proceedings of the First International Scientific Meeting of the Cassava Biotechnology Network. Centro Internacional de Agricultura Tropical, Cali, Colombia. 1993. pp. 379–383. [Google Scholar]
- White WLB, Arias-Garzon D, McMahon JM, Sayre RT. Cyanogenesis in cassava: the role of hydroxynitrile lyase in root cyanide production. Plant Physiol. 1998;116:1219–1225. doi: 10.1104/pp.116.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WLB, McMahon JM, Sayre RT. Regulation of cyanogenesis of cassava. Acta Hortic. 1994;375:69–78. [Google Scholar]