Abstract
F forms stable complexes with Al at conditions found in the soil. Fluoroaluminate complexes (AlFx) have been widely described as effective analogs of inorganic phosphate (Pi) in Pi-binding sites of several proteins. In this work, we explored the possibility that the phytotoxicity of AlFx reflects their activity as Pi analogs. For this purpose, 32P-labeled phosphate uptake by excised roots and plasma membrane H+-ATPase activity were investigated in an Al-tolerant variety of maize (Zea mays L. var. dwarf hybrid), either treated or not with AlFx. In vitro, AlFx competitively inhibited the rate of root phosphate uptake as well as the H+-ATPase activity. Conversely, pretreatment of seedlings with AlFx in vivo promoted no effect on the H+-ATPase activity, whereas a biphasic effect on Pi uptake by roots was observed. Although the initial rate of phosphate uptake by roots was inhibited by AlFx pretreatment, this situation changed over the following minutes as the rate of uptake increased and a pronounced stimulation in subsequent 32Pi uptake was observed. This kinetic behavior suggests a reversible and competitive inhibition of the phosphate transporter by fluoroaluminates. The stimulation of root 32Pi uptake induced by AlFx pretreatment was tentatively interpreted as a phosphate starvation response. This report places AlF3 and AlF4− among Al-phytotoxic species and suggests a mechanism of action where the accumulation of Pi-mimicking fluoroaluminates in the soil may affect the phosphate absorption by plants. The biochemical, physiological, and environmental significance of these findings is discussed.
Gaseous and particulate F that is emitted by fertilizer and smelter plants are assumed to play an important role in forest decline and soil sterility (Klumpp et al., 1996a, 1996b). It was possible to trace the fluorine pollution of soil and soil solution for more than 30 km from one of the pollution sources (Arnesen et al., 1995). Even in regions not influenced by fluorine or F emission, F burden of soils may result from their natural content (geological origin), or from the admixture with harvest and groundwater (water leakage), as well as from the F input via continuous fertilization of soils, which can increase F contents to levels that much exceed its natural abundance in agricultural soils (e.g. Sikora et al., 1992a, 1992b; Stevens et al., 1997). Once in the soil, this very reactive halogen complexes tightly with Al over a wide range of pH values, forming fluoroaluminate complexes (AlFx, where x = 1–6; Lindsay, 1979; Elrashidi and Lindsay, 1986; Elrashidi et al., 1998; Arnesen, 1998). Actually, it has been shown that Al3+ binds F more strongly than 60 other metal ions (Martin, 1996).
Al phytotoxicity is one of the major factors limiting the productivity of crops on acid soils (Foy et al., 1978). The identity of rhyzotoxic species of Al is controversial (Kinraide, 1991, 1997). For a long time, the main rhyzotoxic species of Al were thought to be Al3+, Al(OH)2+, and Al(OH)2+ (Wright et al., 1987). Afterward, the status of Al-OH was altered because its toxicity was supposed to be only a consequence of relief of Al3+ toxicity by H+ (Kinraide et al., 1992; Kinraide, 1997). Thus, the trivalent cation was considered to be the main mononuclear toxic species, in addition to the very toxic polynuclear Al13 tridecamer species (Parker et al., 1989; Kochian, 1995). On the other hand, high concentrations of F can occur in acid soils as a consequence of precipitation of atmospheric pollutants (Supharungsun and Wainwright, 1982). Early reports have established that complexation of Al with F could alleviate the toxic effects of Al, suggesting that AlFx either were not phytotoxic or were less toxic than Al3+ (Cameron et al., 1986; MacLean et al., 1992). Nevertheless, uptake of Al and F into whole tissues from AlFx-containing solutions has been reported (Takmaz-Nisancioglu and Davison, 1988; Nagata et al., 1993; Rai et al., 1998). Moreover, when F was added to uncontaminated soils, most of the F and Al in soil solution were in the form of AlFx complexes (Arnesen, 1997, 1998), and Al concentration in plants was positively correlated with F concentration, suggesting a putative AlFx uptake (Arnesen, 1997; Elrashidi et al., 1998). Later experimentation has confirmed that at least some AlFx species (e.g. AlF2+ and AlF2+) are toxic to plants (Kinraide, 1997; Stevens et al., 1997).
Although significant progress has been made toward understanding the mechanisms of Al3+ toxicity (e.g. Jones and Kochian, 1995; MacDiarmid and Gardner, 1998; Plieth et al., 1999; Sivaguru et al., 2000; Taylor et al., 2000; Pineros and Kochian, 2001), relatively little attention has been directed toward the AlFx phytotoxicity mechanism. On the other hand, in enzymology, the properties of fluoroaluminates have been explored extensively during the last two decades. AlFx (namely AlF30 and AlF4−) were characterized as potent inhibitors of several ATPases (Lunardi et al., 1988; Missiaen et al., 1988; Troullier et al., 1992) and they also have been widely used as activators of G proteins (Sternweis and Gilman, 1982; Bigay et al., 1987). Both effects were related to the ability of these fluorometallic complexes to act as analogs of inorganic phosphate (Pi), binding with high affinity, but reversibly, either directly in phosphate-binding sites of several proteins or in nucleotide-binding sites of some enzymes by simulating the γ-phosphate of GTP and ATP molecules (for review, see Chabre, 1990; Wittinghofer, 1997). In a previous work, we established that AlFx can inhibit, in vitro, the plasma membrane H+-ATPase of corn roots via a similar mechanism (Façanha and de Meis, 1995). However, because in vivo experimentation was not tried, the physiological significance of this finding remains to be seen.
In this work, we explore the mechanism of AlFx action in vivo. To address this issue, we have studied the plasma membrane H+-ATPase activity as well as the phosphate uptake in roots of an Al-tolerant variety of maize (Zea mays L. var. dwarf hybrid), either pretreated or not with AlFx. The results suggest that AlFx treatment of seedlings does not directly affect the P-type plasma membrane H+-ATPase, whereas it promotes a striking influence on phosphate uptake by roots of this maize variety. AlFx modification of Pi uptake kinetics is consistent with a competitive inhibition of phosphate transport. Our speciation analysis (using GEOCHEM-PC, http://envisci.ucr.edu/faculty/dparker/default.htm; Parker et al., 1995) highlights AlF30 and AlF4− as the most probable species involved in this mechanism. Implications for F pollution and Al phytotoxicity are discussed.
RESULTS
Speciation Calculation
Speciation analysis using the GEOCHEM-PC program (Parker et al., 1995) showed that AlF30 was the dominant Al species in our experiments (Table I). This species and AlF4− are well known for their ability to mimic Pi (Chabre, 1990). It was estimated that in the presence of 1 mm NaF and 0.1 mm AlCl3, Al was complexed completely with about 30% of the F present, consistent with the predominance of AlF3 species (Table I). In addition, we calculated the ionic species present when a range of Pi concentrations was used that covered the composition of the Pi uptake media (0.2 mm CaSO4 and 0.005–0.1 mm KH2PO4). No complexation was found involving Al-P species, even in the presence of 0.1 mm Pi, yet 100% of Al was complexed with F and the AlFx speciation was very similar to that presented in Table I (e.g. AlF3 0.0715 mm even at 0.1 mm Pi). This is in agreement with predictions of Lindsay (1979), where the distribution of different AlFx complexes depended mainly on the balance of Al and F concentrations and the pH of the medium. Although Al has a strong tendency to form complexes with Pi, Al has strongest affinity for F. Fluorine is the most electronegative element and the most chemically active of the nonmetallic elements. The association constants (log Ka) for AlF3 and AlHPO4 are 16.8 and 8.1, respectively (for Ka of other complexes, see Façanha and de Meis, 1995). In the absence of F, the predicted Al3+ activity was 43 μm and about 46% of Al species were present as hydroxides [mainly Al(OH)2+, Al(OH)2+, and Al(OH)30].
Table I.
Al speciation using the GEOCHEM-PC program was determined for complete hydroponic medium (pH 4.3) supplemented with 0.1 mm AlCl3 and 1 mm NaF, except where specified by an asterisk
| Species | Concentration | Activity | Percentage |
|---|---|---|---|
| m | % | ||
| A13+ | 0 | 0 | – |
| A13+* | 43.3 × 10−6 | 43.3 × 10−6 | 43a |
| F− | 45.8 × 10−3 | 45.8 × 10−3 | 45b |
| HF | 13.0 × 10−6 | 12.9 × 10−6 | 13b |
| AlF2+ | 1.06 × 10−7 | 6.79 × 10−8 | <0.1a |
| AlF2+ | 15.3 × 10−6 | 13.8 × 10−6 | 15a |
| AlF30 | 71.2 × 10−6 | 71.0 × 10−6 | 71a |
| AlF4− | 13.3 × 10−6 | 11.9 × 10−6 | 13a |
| AlF52− | 1.19 × 10−7 | 7.60 × 10−8 | <0.1a |
| AlF63− | 8.68 × 10−11 | 3.16 × 10−11 | <0.0001a |
An asterisk indicates Al3+ in the presence of 0.1 mm AlCl3, but without NaF addition.
Percentage of complex was calculated in relation to the total Al (0.1 mm Al = 100%).
Percentage of complex was calculated in relation to the total F (1 mm F = 100%).
Even though hydroponic medium contained 33 μm KH2PO4, the GEOCHEM-PC analysis revealed that 89.55% of Pi was present in solid form with Fe+3 and the orthophosphate (H2PO4− and HPO42−) activity was predicted to be only 3 μm.
Effects of Al and F on Root Elongation
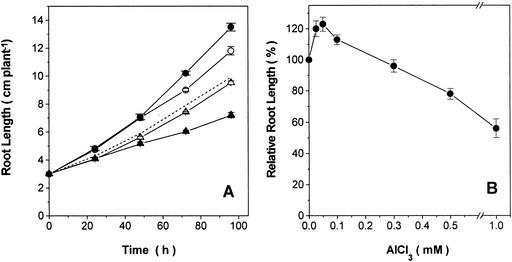
Growth response of primary roots either treated or not with aluminum chloride, sodium fluoride, or a combination of both was studied in an Al-tolerant variety of maize. The treatment of seedlings with 0.1 mm AlCl3 resulted in a small but consistent stimulation of root growth (Fig. 1). In contrast, root growth was markedly inhibited in seedlings treated with 1 mm NaF and the inhibition was intensified by the presence of both Al and F, suggesting that AlFx species may be more toxic than F itself (triangles in Fig. 1A). Alternatively, it is possible that the growth inhibition obtained with 1 mm NaF plus 0.1 mm AlCl3 may represent an additive effect of AlFx species along with that promoted by the excess of F present in the medium as a free ligand (predicted activity 0.45 mm). In agreement with this hypothesis, when a higher Pi concentration (0.1 mm Pi) was used in a nutrient medium containing AlFx, the root growth inhibition was reduced to a level close to that promoted by F alone (dashed curve in Fig. 1A). To assess the threshold of Al rhyzotoxicity in this Al-tolerant variety of maize, seedlings were treated with a range of AlCl3 concentrations (0–1 mm), revealing that root growth is inhibited as AlCl3 concentrations are raised to values exceeding 0.3 mm (Fig. 1B).
Figure 1.
Effects of Al, F, and AlFx on root elongation in an Al-tolerant maize. A, Time course of root elongation of 4-d-old seedlings selected for similar root length (approximately 3 cm) exposed to a hydroponic medium without additions (–○–) or supplemented with either 0.1 mm AlCl3 (–●–), 1 mm NaF (–▵–), or a combination of both: 0.1 mm AlCl3 plus 1 mm NaF (AlFx, –▴–). Dashed line shows the effect of AlFx in the presence of a higher Pi concentration (0.1 mm Pi). se values (n = 5, 18 plants per treatment in five independent experiments) are shown as vertical bars. For data obtained with 72 and 94 h of treatment, there is 95% confidence that root lengths are significantly different from the control using Student's t distribution. B, Relative root lengths of seedlings grown for 72 h in the hydroponic medium supplemented with various AlCl3 concentrations. Root length obtained in the absence of AlCl3 was assigned as 100%. se values (n = 3, 10 plants per Al concentration in three independent experiments) are shown as vertical bars.
Effects of Al and F on Phosphate Uptake
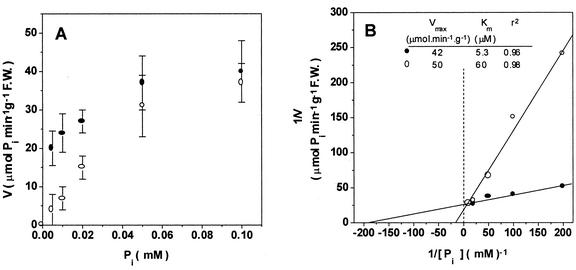
Addition of 0.1 mm AlCl3 plus 1 mm NaF to the uptake medium containing 10 μm Pi promoted a clear inhibition of uptake by root segments and this effect was antagonized by increasing the concentration of phosphate (Fig. 2A). The Km obtained in the presence of AlFx increased more than 10-fold, whereas the Vmax was not significantly different from the values obtained with control roots (Fig. 2B). This result indicates that AlFx competitively inhibits phosphate uptake, suggesting a common binding site for both phosphate and fluoroaluminate species in a high-affinity phosphate transporter (taking into account the calculated Km ≈ 5.3 μM).
Figure 2.
Kinetics of 32Pi uptake by excised corn roots carried out in the presence (–○–) or in the absence (–●–) of AlFx. A, Specific activity (V) of Pi uptake versus phosphate concentration. B, Double reciprocal (Lineweaver-Burk) plot of Pi uptake in A. The inset shows the values of Vmax, Km, and correlation coefficient (r2) for each condition. The reaction medium contained 1.7 MBq μmol−1 [32P] KH2PO4 adjusted to pH 4.3, and 0.2 mm CaSO4 supplemented with 1 mm NaF plus 0.1 mm AlCl3 (AlFx). The uptake assay was started by immersion of 0.5 g fresh weight root segments into uptake medium. After 35 min of incubation, the roots sections were washed with 0.2 mm CaSO4 and the amount of 32P absorbed was counted as described in “Materials and Methods.” Values are the means of four independent experiments ± se.
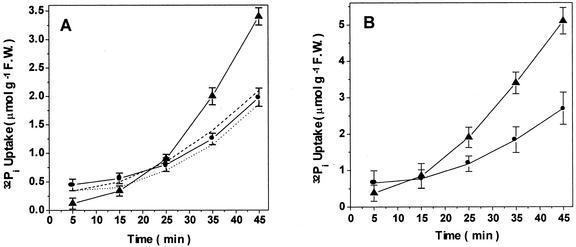
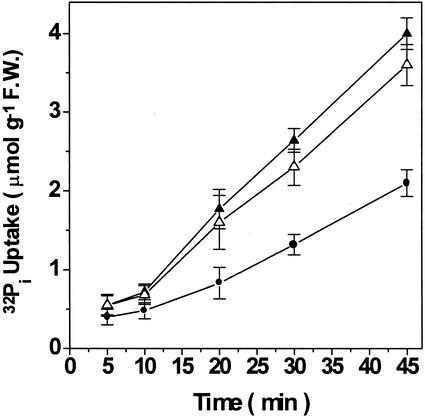
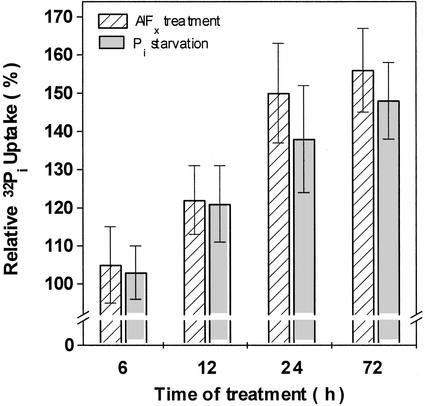
The time course of 32Pi uptake by root segments in the presence of 0.1 mm KH2PO4 from seedlings grown in the presence of AlFx also exhibited a clear inhibition of the initial rate of uptake (Fig. 3A). However, this situation changed over the following minutes of incubation, as the rate of uptake increased and a significant stimulation in 32Pi uptake was observed after 30 min (Fig. 3A). This biphasic effect may reflect an AlFx ⇔ Pi exchange taking place at the phosphate absorption sites on the root surface: At first, the Pi-binding sites would be occupied by AlFx, and then, as AlFx began to be displaced from these sites in exchange for Pi from the medium, all Pi-binding sites would gradually lose the competing analogous species. Supporting this hypothesis, an increase in the Pi concentration of the uptake medium to 1 mm led to a time course of 32Pi uptake with an earlier stimulatory effect, consistent with a faster AlFx ⇔ Pi exchange (Fig. 3B). In addition, when AlFx-pretreated roots were rinsed with deferoxamine, a powerful Al-chelating agent, to displace the AlFx from root surface before the uptake assay, the inhibitory effect did not occur, and only a stimulation of 32Pi uptake was detected, regardless of the time of reaction (Fig. 4). The same effect was observed when using citrate, a natural chelator of Al, suggesting that both chelators were able to induce displacement of AlFx from its binding sites (triangles in Fig. 4). AlFx-induced stimulation of the 32Pi uptake can be compared with the stimulation exhibited by Pi-starved roots (Fig. 5), which has been shown to involve an overexpression of the phosphate transporters (Muchhal and Raghothama, 1999). Note that no significant change in 32Pi uptake was found in excised corn roots pretreated with only Al or F (dashed and dotted lines in Fig. 3A).
Figure 3.
Time course of 32Pi uptake with 0.1 mm KH2PO4 (A) and 1 mm KH2PO4 (B) at pH 4.3, by excised corn roots. Four-day-old seedlings were exposed for 72 h to hydroponic medium alone (control, –●–), or containing an additional 0.1 mm AlCl3 (dotted line), 1 mm NaF (dashed line; symbols are omitted for clarity in these two curves), or a combination of both: 0.1 mm AlCl3 plus 1 mm NaF (AlFx, –▴–). Values represent the means ± se of four (A) or three (B) independent experiments.
Figure 4.
Time course of 32Pi uptake (0.1 mm KH2PO4, at pH 4.3) by excised corn roots pretreated or not with AlFx and washed with Al chelators. Four-day-old seedlings were exposed for 72 h to either a hydroponic medium alone (control, –●–), or containing an additional 0.1 mm AlCl3 plus 1 mm NaF (AlFx, –▵–, –▴–). Afterward, root segments were incubated for 2 min (under strong agitation at 30°C) with either 0.5 mm deferoxamine (–●–, –▴–), or citrate (–▵–) before 32Pi uptake assay. Values represent the means ± se of three independent experiments.
Figure 5.
Stimulation of 32Pi uptake by excised corn roots promoted by AlFx or Pi starvation. Four-day-old seedlings were transferred to a nutrient medium containing Pi and supplemented additionally with 0.1 mm AlCl3 and 1 mm NaF (AlFx treatment), or to nutrient medium lacking KH2PO4 (Pi starvation). Seedlings were removed at times indicated and excised root segments were assayed for 32Pi uptake in the presence of 0.1 mm Pi. Stimulation of 32Pi uptake was calculated as a percentage of uptake by roots grown in complete nutrient medium and tested in the presence of 0.1 mm Pi. Values represent the means ± se of three independent experiments. There is no significant difference between column pairs (Student's t test, P ≤ 0.05). From 12 h onward, Pi uptake for each treatment is significantly different from the relevant control (100%, not shown; Student's t test, P ≤ 0.05).
Effects of Al and F on the Plasma Membrane H+-ATPase Activity
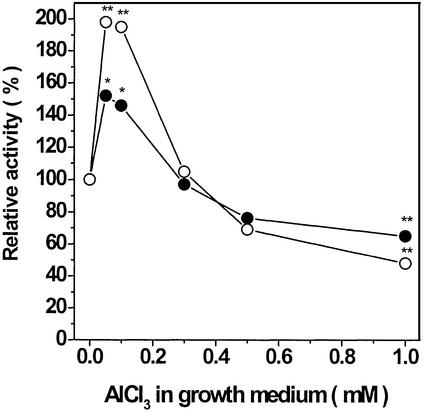
Phosphorus is acquired by plant roots in an energy-mediated cotransport process driven by a proton gradient generated by the plasma membrane H+-ATPase (Ullrich-Eberius et al., 1981). Therefore, it is possible that the previously described AlFx-induced inhibition (in vitro) of plasma membrane H+-ATPase (Façanha and de Meis, 1995) may account for the inhibition of the 32Pi uptake assay. To test this possibility, plasma membranes were isolated from corn roots that had been grown for 72 h with Al, F, or both (AlFx), and the effects of this treatment were examined using the isolated P-type H+-ATPase. No effect was observed in either ATPase activity or H+ transport in plasma membrane vesicles isolated from corn roots treated with either F alone or AlFx compared with control (Table II). Surprisingly, pretreatment of seedlings with 0.1 mm AlCl3 promoted a stimulation of the ATP hydrolysis rate as well as of the initial velocity of ATP-dependent proton gradient formation (Table II). Some inhibition was found only when seedlings were grown in concentrations above 0.3 mm AlCl3 (Fig. 6).
Table II.
Effects of treating roots with Al, F, and AlFx on the plasma membrane H+-ATPase activity
| Parameter | Treatment
|
|||
|---|---|---|---|---|
| Control | AlCl3 | NaF | AlFx | |
| H+ gradient | ΔF (%) | |||
| Steady state | 25 ± 6 (n = 5) | 34 ± 8 (n = 4) | 22 ± 9 (n = 4) | 25 ± 5 (n = 5) |
| Initial velocity | F min−1 | |||
| 120 ± 16 (n = 5) | **235 ± 30 (n = 4) | 125 ± 27 (n = 4) | 127 ± 22 (n = 5) | |
| Hydrolysis | ||||
| Initial velocity | nmol mg−1 min−1) | |||
| 130 ± 16 (n = 5) | *191 ± 23 (n = 3) | 128 ± 26 (n = 3) | 132 ± 19 (n = 5) | |
H+-ATPase activity was determined in plasma membrane vesicles isolated from roots of seedlings grown in hydroponic medium alone (control), and supplemented either with 0.1 mm AlCl3, 1 mm NaF, or both (AlFx). Values are the means of n experiments ± se. *, Significance at P ≤ 0.01 confidence (Student's t test), compared with control membranes. **, Significance at P ≤ 0.001 confidence (Student's t test), compared with control membranes.
Figure 6.
Effects of Al on the H+-ATPase activities. Plasma membrane vesicles were isolated from roots of seedlings grown in hydroponic medium alone (control), or treated for 72 h with 0.05 to 1 mm AlCl3. The initial rates of vanadate-sensitive ATP hydrolysis (–●–) and ATP-dependent H+ transport (–○–), obtained when growth medium did not contain Al, were assigned as 100%. Each point is the average of at least three independent experiments. One or two asterisks indicate significance at P ≤ 0.05 and P ≤ 0.01 (Student's t test), respectively.
The addition of AlFx directly into the reaction medium has confirmed that these complexes nevertheless are able to inhibit the plasma membrane H+-ATPase activity in vitro (Table III). This suggests that if a phosphate-like AlFx species could gain access to the cytoplasm this enzyme certainly would be an important target. In agreement with the hypothesis that AlFx mimics phosphate at the Pi-binding sites, the inhibition of ATPase activity was also alleviated by raising the Pi concentration of the medium (Table III). The apparent Pi affinity in this effect, however, appears to be much lower than was found for Pi uptake activity by root segments (compare Fig. 2 with Table III).
Table III.
Phosphate antagonizes AlFx-induced inhibition of ATP hydrolysis
| H2PO4 | ATP Hydrolysis
|
||
|---|---|---|---|
| Control | AlFx | Inhibition | |
| mm | nmol mg−1 min−1 | % | |
| 0 | 120 ± 10.8 | 18 ± 6.6 | 85 |
| 1 | 100 ± 9.1 | 22 ± 8.3 | 78 |
| 10 | 76.6 ± 10 | 38.3 ± 6.6 | 50 |
| 20 | 70 ± 13.3 | 50 ± 11.6 | 29 |
Vanadate-sensitive ATP hydrolysis was assayed in plasma membrane vesicle preparations in the presence of 1 mm NaF plus 0.1 mm AlCl3 (AlFx) and different H2PO4 concentrations. The reaction media also contained 50 mm MOPS-Tris, pH 6.5; 1 mm ATP; 3 mm MgSO4; and 30 μg mL−1 plasma membrane protein. Values are the means ± se of four experiments.
DISCUSSION
The Proposed Model
Contrary to prior expectations, Al-F complexes have been shown to be toxic to plants (Kinraide, 1997; Stevens et al., 1997; Fig. 1). Although several hypotheses for the mechanism of Al-F toxicity have been considered, so far all of them have been rejected (Kinraide, 1997). The present study focuses on the description of an alternative mechanism for the toxicity of AlFx through a well-known phosphate-mimicking property attributed to these complexes. Phosphorus is acquired by plant roots primarily via high-affinity Pi transporters (for recent review, see Raghothama, 2000). Several pieces of evidence support a model where AlFx complexes can mimic the tetrahedral phosphate group competing with it for the same binding sites on the Pi carriers and possibly stabilizing an inactive conformation. First, AlFx-induced inhibition of Pi uptake was antagonized by raising the Pi concentration in the reaction medium (Fig. 2). Second, the stimulation of Pi uptake in corn roots after AlFx pretreatment is similar to that observed after Pi starvation (Fig. 5). Third, in contrast with in vitro assays of the ATPase activity (Façanha and de Meis, 1995; Table III), pretreatment of corn seedlings in vivo with AlFx had no effect on the activity of the plasma membrane H+-ATPase (Table III). This supports the model where the fluoroaluminates act as physiological Pi analogs by competing directly for the same binding sites of Pi transport rather than any indirect effect on the proton motive force of the process. Finally, our speciation calculations using GEOCHEM-PC (Parker et al., 1995) show that AlF30 [AlF3 (OH)−] is the major Al species present in our experiments, followed by the AlF4− complex. Both are reputed to be very effective orthophosphate (H2PO4− and HPO42−) analogs, and have been shown to block Pi-binding sites of diverse proteins (Wittinghofer, 1997).
These evidences support the proposal that the property of AlFx to mimic Pi may describe the most important mechanism of AlFx toxicity whenever AlF3 and AlF4 are the dominant species. On the other hand, in view of the ubiquity of phosphate in cell metabolism, it is possible that the competitive inhibition of Pi transport can be only one of many mechanisms by which these Pi analogs can affect the plant growth. Theoretically, these species could interact with another Pi-binding sites present on plant cell surfaces including membrane receptors, channels, and apoplast enzymes.
Biochemical Significance
AlFx have been shown to bind with high affinity, but reversibly, in phosphate-binding sites of several proteins in plant, fungal, and mammalian cells. This observation has proven to be tremendously useful for studying the activation of heterotrimeric G proteins in vivo (Chabre, 1990; Wittinghofer, 1997), for elucidation of three-dimensional structures of GTPases (e.g. Sondek et al., 1994) and ATPases (e.g. Braig et al., 2000), and for understanding the biochemical mechanism of GTP and ATP hydrolysis, including the role of GTPase-activating proteins (e.g. Xu et al., 1997). Our functional analysis of Pi uptake suggests that AlFx complexes may act as phosphate analogs, reversibly blocking the Pi-binding sites of phosphate transporters. As far as we know, this is the first description of fluoroaluminates acting as competitive inhibitors of phosphate transporters, and these compounds may prove to be useful in expanding our knowledge of the structure, regulation, and function of these carriers, which often share gene homology to each other, particularly among the plant (Raghothama, 2000) and fungi (Harrison and VanBuuren, 1995; Yompakdee et al., 1996) isoforms.
Physiological Consideration
In plant systems, AlF30 and AlF4− complexes were already described to inhibit in vitro the plasma membrane H+-ATPase from corn roots (Facanha and de Meis, 1995) and the cabbage (Brassica capitata) phospholipase D (Li and Fleming, 1999), in both cases by simulating the Pi anion. However, although AlF2+ and AlF2+ have been identified as toxic to the plants and probably gain access to the cytoplasm, several pieces of evidence have shown that AlF30 and AlF4− complexes are not readily taken up by plant roots; thus, it was concluded that these species were not likely to be phytotoxic (Nagata et al., 1993; Kinraide, 1997; Stevens et al., 1997). On the contrary, although our data from ATPase activity are consistent with the inaccessibility of these complexes to the cytoplasm (Table II), the treatment of plants with AlFx containing >80% AlF30 and AlF4− species (Table I) clearly promoted an inhibitory effect on both the root elongation (Fig. 1A) and the 32P uptake by corn roots (Figs. 2 and 3A). Apparently, AlFx may exert its toxicity even externally to the cell membrane and at least part of this effect is due to a blockage of Pi transporters.
Recently, genes encoding phosphate transporters have been isolated from a number of plant species, and their transcripts were found to be highly inducible upon Pi starvation, resulting in enhanced Pi uptake when Pi was resupplied (Raghothama, 2000). Our data suggest that AlFx treatment of plants may elicit a similar Pi starvation response because the corn roots increase their capacity for Pi uptake after exposure to fluoroaluminates for a period of more than 12 h (Fig. 5). Likewise, the increase in Pi uptake rate by the AlFx-pretreated roots correlates well with the profile exhibited by Pi-starved roots (Fig. 5; see also data from Clarkson and Scattergood, 1982; Goldstein et al., 1989) and is consistent with the time required for induction of phosphate transporter proteins in response to Pi starvation (Muchhal and Raghothama, 1999).
The stimulation of root elongation (Fig. 1) and the plasma membrane H+-ATPase activity (Table II; Fig. 6) in response to Al treatment of seedlings with concentrations below 0.3 mm appear at first glance to contradict common knowledge of Al rhyzotoxicity. Although regarded as a toxic element, Al frequently stimulates growth at concentrations lower than the threshold of Al phytotoxicity (e.g. Mullette, 1975; Clark, 1977; Foy et al., 1978; Kinraide, 1993; Malkanthi et al., 1995; Clune and Copeland, 1999). There is substantial evidence that in most cases these beneficial effects occur through the alleviation of H+ toxicity by Al3+ (Kinraide, 1993). Kinraide (1988) showed that 0.1 mm Al3+ (in wheat [Triticum aestivum] roots) increased cell membrane electrical polarity and stimulated H+ extrusion, which was shown to be essential for continued root growth at low pH (Yan et al., 1992). Later, it was demonstrated that the plasmalemma H+-ATPase contributes significantly to this process (Yan et al., 1998). Nevertheless, an inhibition of ATPase activity was promoted by root treatment with AlCl3 at concentrations >0.3 mm (Fig. 6), in consonance with data from Matsumoto et al. (1992). In maize, it was proposed that an ATPase-dependent increase of H+ extrusion could induce cell wall plasticity, in accordance with the acid growth theory (Hager et al., 1991; Frias et al., 1996). Although limited information on the effects of Al treatment on H+-ATPase activity using Al-tolerant species and cultivars makes it difficult to relate these changes to Al and low pH resistance, it seems to be clear that there is a fairly consistent relationship among Al effects on the H+-ATPase and root elongation (compare Fig. 1B with Fig. 6).
Environmental Impact
Environmental problems have to be assessed holistically; otherwise, solving one problem may create a new one at a different level (Sibbesen and Runge-Metzger, 1995). For instance, early reports have established that complexation of Al with F could even alleviate the toxic effects of Al (Cameron et al., 1986; MacLean et al., 1992), leading several agricultural groups all over the world to test the possibility of using NaF as an acid soil additive (Keerthisinghe et al., 1991). This practice was not widespread, most likely due to the adverse effects caused by toxicity of F itself and/or of AlFx species. Nevertheless, such AlFx toxicity has not been easily detected in previous work because high Pi concentrations were used in nutrient and assay media (usually 0.1 mm Pi). Another problem in these studies was the frequent use of Al-sensitive species, where the harmful effects of Al3+ can mask the inhibition promoted by AlFx. However, in the field, the Pi concentrations barely exceed 0.01 mm (Raghothama, 2000) and various hybrid varieties selected for Al3+ resistance have been used by farmers. Our data suggest that under these conditions, if AlF3 and AlF4 species are present in the soil, they certainly would compete with Pi for absorption sites on the root surface.
In addition, sustainability of conventional agriculture is still based upon a high input of agrochemicals. Soil amendments such as phosphate fertilizers, which contain high concentrations of F as impurities (up to 3.5%), also may cause an inadvertent and hazardous increase of F in soils (e.g. Keerthisinghe et al., 1991; Sikora et al., 1992a, 1992b). Our data, along with those previously described on chemical behavior of AlFx, strengthen the possibility that both the conspicuous presence of Al in the earth's crust and the environmental pollution by fluorine may interact leading to exacerbation of the problem of phosphorus availability in the soils. This unique mechanism for AlFx phytotoxicity warns us against the indiscriminate massive application of fertilizers and other F-containing soil amendments worldwide.
In summary, the present report places AlF30 and AlF4− among Al-phytotoxic species and describes a mechanism of action where the accumulation of these Pi-mimicking AlFx in the soil may affect the phosphate absorption by plants. In addition, AlFx sensitivity of this maize Al-tolerant variety brings into question the validity of current protocols for crop selection based only on their Al+3 tolerance. Further studies on the effects of F complexation on Al phytotoxicity would be suitable to guide successful breeding programs as well as development of transgenic lines adapted to Al stress and/or Pi deficiency.
MATERIALS AND METHODS
Plant Growth and AlFx Treatment
Seeds of an Al-tolerant variety of maize (Zea mays L. var. dwarf hybrid), provided by Sementes Agroceres S.A. (Uberlandia-MG, Brazil), were surface sterilized by soaking in 0.5% (w/v) NaClO solution and then placed in water for 6 h after rinsing. Afterward, the seeds were sown on wet filter paper and germinated in the dark at 28°C. Four-day-old seedlings with approximately 3-cm-long roots were transferred into hydroponic solution containing 810 mg L−1 Ca(NO3)2·4H2O, 100 mg L−1 NH4NO3, 40 mg L−1 KCl, 97 mg L−1 K2SO4, 54 mg L−1 KNO3, 214 mg L−1 Mg(NO3)2·6H2O, 4.4 mg L−1 KH2PO4, 17 mg L−1 Fe-EDTA, 1.64 mg L−1 MnCl2·4H2O, 1.43 mg L−1 H3BO3, 0.62 mg L−1 ZnSO4·7H2O, 0.14 mg L−1 CuSO4·5H2O, and 0.18 mg L−1 Na2MoO4·2H2O. Only for the experiment shown in dashed line in Figure 1, the hydroponic solution contained 13.7 mg L−1 KH2PO4 (approximately 0.1 mm) instead of 4.4 mg L−1 (approximately 0.03 mm). The nutrient medium was supplemented with 0.1 mm AlCl3 only, 1 mm NaF only, or a combination of both (AlFx treatment). The solution pH was monitored and adjusted when necessary during the growth to oscillate between pH 4.2 and 4.3. Root lengths were measured with a ruler at determined times, as shown in Figure 1. After 96 h of treatment, roots were collected and used for further experiments.
Plasma Membrane-Enriched Vesicles
Plasma membrane vesicles were isolated from roots using differential centrifugation essentially as described by De Michelis and Spanswick (1986), with some modifications. About 100 g (fresh weight) of corn roots was homogenized using a mortar and pestle in 2 mL g−1 of ice-cold buffer containing 250 mm Suc, 10% (w/v) glycerol, 0.5% (v/v) polyvinylpyrrolidone (polyvinylpyrrolidone-40, 40 kD), 2 mm EDTA, 0.5% (w/v) bovine serum albumin, and 0.1 m Tris-HCl buffer, pH 8.0. Just before use, 150 mm KI, 2 mm dithiothreitol (DTT), and 1 mm phenylmethylsulfonyl fluoride were added to the buffer. The homogenate was strained through four layers of cheesecloth and centrifuged at 8,000g for 10 min. The supernatant was recovered and centrifuged at 100,000g for 40 min. The pellet was resuspended in a small volume of ice-cold buffer containing 10 mm Tris-HCl, pH 7.6; 15% (v/v) glycerol; 1 mm DTT; 1 mm phenylmethylsulfonyl fluoride; and 1 mm EDTA. The suspension containing the root vesicles was layered over a 20%/30%/42% (w/w) discontinuous Suc gradient that contained, in addition to Suc, 10 mm Tris-HCl buffer, pH 7.6; 1 mm DTT; and 1 mm EDTA. After centrifugation at 100,000g for 3 h in a swinging bucket, the vesicles that sedimented at the interface between 30%/42% (w/v) Suc were collected, diluted with 50 mL of ice-cold buffer containing 10 mm Tris-HCl, pH 7.6; 10% (v/v) glycerol; 1 mm DTT; and 1 mm EDTA, and centrifuged at 100,000g for 40 min. The pellet was resuspended in the same medium and these plasma membrane vesicles were either used immediately or frozen under liquid N2 and stored at −70°C until use. Protein concentrations were determined by the method of Lowry et al. (1951).
ATPase Activity
ATPase activity was determined by measuring the release of Pi, either colorimetrically (Fiske and Subbarow, 1925) or using [γ-32P]ATP (0.34 MBq μmol−1), as previously described by de Meis (1988). The reaction medium contained 50 mm HEPES-KOH (pH 6.5), 5 mm MgSO4, 100 mm KCl, and 1 mm ATP, with or without 0.1 mm Na3VO4. In some experiments the medium was supplemented with 0.1 mm AlCl3 and/or 1 mm NaF as indicated in the Table III. The reaction was started by addition of 0.03 mg L−1 vesicle protein and stopped with ice-cold 5% (w/v) trichloracetic acid after 30 min of incubation at 30°C. Before the hydrolysis assay, vesicles were always frozen and thawed twice. Plasma membrane vesicles were approximately 70% inside-out in freeze/thaw vesicles. In all experiments, the ATPase activity was measured with and without vanadate, and the difference between these two activities was attributed to the plasma membrane H+-ATPase. ATPase activity of plasma membrane vesicles was unaffected by either bafilomycin A1 (50 nm), an inhibitor of V-type H+-ATPase, or oligomycin (10 nm), an inhibitor of mitochondrial ATPase.
ATPase H+ Pumping
The electrochemical H+ gradient generated by the H+-ATPase was estimated from the initial rate of quenching of the fluorescent pH probe 9-amino-6-chloro-2-methoxyacridine (415/485 nm excitation/emission). The assay medium contained 10 mm HEPES-KOH (pH 6.5), 100 mm KCl, 5 mm MgCl2, 2.5 μm 9-amino-6-chloro-2-methoxyacridine, and 0.05 mg L−1 vesicle protein. The reaction was triggered by addition of 1 mm ATP and was carried out at 30°C and the proton gradient formed was dissipated by addition of the protonophore carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone [3 μm p-(trifluoromethoxy)phenylhydrazone]. More than 90% of the vesicle H+ gradient measured at pH 6.5 was inhibited by orthovanadate (0.1 mm Na3VO4), a very effective inhibitor of the plasma membrane P-type H+-ATPase (Sze, 1985).
[32P] Phosphate Uptake by Excised Corn Roots
The experimental procedure followed essentially the method of Sentenac and Grignon (1985) with some modifications. In brief, root segments (approximately 0.5 g fresh weight) cut from the root apex were incubated in uptake medium containing 0.2 mm CaSO4 and 0.01 to 0.1 mm KH2PO4 labeled with 32Pi (1.7 MBq μmol−1), adjusted at pH 4.3 with 0.1 m HCl. After the incubation time (5–45 min at 30°C in a rotary shaker), solution was removed under vacuum and root segments were washed in continuous flux of 2 mm CaSO4 (250 mL). In experiments of Figure 4, root segments were pre-incubated with either 0.5 mm deferoxamine or sodium citrate during 2 min (at 30°C in a rotary shaker) before incubation in the uptake medium. Afterward, the segments were dried with filter paper, weighed again, and treated for 12 h with 2% (w/v) Triton X-100 solution. The extract obtained was counted then for the presence of 32Pi using Cerenkov radiation. To estimate the amount of 32Pi associated with the cell wall, a sample of roots was pretreated with Triton X-100 before incubation in the uptake medium and the radioactivity obtained in these conditions was subtracted in all experiments.
ACKNOWLEDGMENTS
We gratefully acknowledge the helpful advice of Dr. Leopoldo de Meis about methodological aspects of our work, Dr. Martha M. Sorenson and Dr. Lev A. Okorokov for revision and helpful discussion of the manuscript, and Dr. Andrew Smith for critical reading and useful suggestions. We are also thankful to Dr. Luciano P. Canellas for helpful hints on speciation calculation using the GEOCHEM-PC program and to André L. Silva for technical assistance.
Footnotes
This work was supported by the Conselho Nacional de Desenvolvimento Científìco e Tecnológico (grant no. 465918/00–0), by the Fundação de Amparo à Pesquisa do Estado de Rio de Janeiro (grant no. E–26/172.333/00), and by the Fundação Estadual do Norte Fluminense. Part of this work was presented in the 11th Workshop on Plant Membrane Biology (Cambridge, 1998) and in the Gordon Research Conference on Water and Salt Stress in Plants (Oxford, 1998).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001651.
LITERATURE CITED
- Arnesen AKM. Availability of fluoride to plant growth in contaminated soils. Plant Soil. 1997;191:13–25. [Google Scholar]
- Arnesen AKM. Effect of fluoride pollution on pH and solubility of Al, Fe, Ca, Mg, K and organic matter in soil from Ardal (Western Norway) Water Air Soil Pollut. 1998;103:375–388. [Google Scholar]
- Arnesen AKM, Abrahamsen G, Sandvik G, Krogstad T. Aluminum smelters and fluoride pollution of soil and soil solution in Norway. Sci Total Environ. 1995;163:39–53. [Google Scholar]
- Bigay J, Deterre P, Pfister C, Chabre M. Fluoride complexes of aluminum or beryllium act on G-proteins as reversibly bound analogs of the γ-phosphate of GTP. EMBO J. 1987;6:2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig K, Menz RI, Montgomery MG, Leslie AGW, Walker JE. Structure of bovine mitochondrial F1-ATPase inhibited by Mg2+ADP and aluminum fluoride. Struct Fold Des. 2000;8:567–573. doi: 10.1016/s0969-2126(00)00145-3. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Ritchie GSP, Robson AD. Relative toxicities of inorganic aluminum complexes to barley. Soil Sci Soc Am J. 1986;50:1231–1236. [Google Scholar]
- Chabre M. Aluminofluoride and beryllofluoride complexes: new phosphate analogs in enzymology. Trends Biochem Sci. 1990;15:6–10. doi: 10.1016/0968-0004(90)90117-t. [DOI] [PubMed] [Google Scholar]
- Clark RB. Effect of aluminum on growth and mineral elements of A1-tolerant and A1-intolerant corn. Plant Soil. 1977;47:653–662. [Google Scholar]
- Clarkson DT, Scattergood CB. Growth and phosphate transport in barley and tomato plants during the development of, and recovery from, phosphate stress. J Exp Bot. 1982;33:865–875. [Google Scholar]
- Clune TS, Copeland L. Effects of aluminium on canola roots. Plant Soil. 1999;216:27–33. [Google Scholar]
- de Meis L. Approaches to studying the mechanisms of ATP synthesis in sarcoplasmic reticulum. Methods Enzymol. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]
- De Michelis MI, Spanswick RM. H+-pumping driven by the vanadate-sensitive ATPase in membrane-vesicles from corn roots. Plant Physiol. 1986;81:542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrashidi MA, Lindsay WL. Solubility of aluminum fluoride, fluorite, and fluorophlogopite minerals in soils. Soil Sci Soc Am J. 1986;50:594–598. [Google Scholar]
- Elrashidi MA, Persaud N, Baligar VC. Effect of fluoride and phosphate on yield and mineral composition of barley grown on three soils. Commun Soil Sci Plant Anal. 1998;29:269–283. [Google Scholar]
- Facanha AR, de Meis L. Inhibition of maize root H+-ATPase by fluoride and fluoroaluminate complexes. Plant Physiol. 1995;108:241–246. doi: 10.1104/pp.108.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CF, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- Foy CD, Chaney RL, White MC. Physiology of metal toxicity in plants. Annu Rev Plant Physiol. 1978;29:511–566. [Google Scholar]
- Frias I, Caldeira MT, Perez Castineira JR, Navarro Avino JP, Culianez Macia FA, Kuppinger O, Stransky H, Pages M, Hager A, Serrano R. A major isoform of the maize plasma membrane H+-ATPase: characterization and induction by auxin in coleoptiles. Plant Cell. 1996;8:1533–1544. doi: 10.1105/tpc.8.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AH, Mayfield SP, Danon A, Tibbot BK. Phosphate starvation-inducible metabolism in Lycopersicon esculentum: III. Changes in protein secretion under nutrient stress. Plant Physiol. 1989;91:175–182. doi: 10.1104/pp.91.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. Auxin-induced exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta. 1991;185:527–537. doi: 10.1007/BF00202963. [DOI] [PubMed] [Google Scholar]
- Harrison MJ, VanBuuren ML. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature. 1995;378:626–629. doi: 10.1038/378626a0. [DOI] [PubMed] [Google Scholar]
- Jones DL, Kochian LV. Aluminum inhibition of the inositol 1,4,5-trisphosphate signal-transduction pathway in wheat roots-a role in aluminum toxicity. Plant Cell. 1995;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe G, McLaughlin MJ, Freney JR. Use of gypsum, phosphogypsum and fluoride to ameliorate subsurface acidity in a pasture soil. In: Wright RJ, Baligar VC, Murrmann RP, editors. Plant-Soil Interactions at Low pH. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1991. pp. 509–517. [Google Scholar]
- Kinraide TB. Proton extrusion by wheat roots exhibiting severe aluminum toxicity symptoms. Plant Physiol. 1988;88:418–423. doi: 10.1104/pp.88.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide TB. Identity of the rhyzotoxic aluminum species. Plant Soil. 1991;134:167–178. [Google Scholar]
- Kinraide TB. Aluminum enhancement of plant-growth in acid rooting media-a case of reciprocal alleviation of toxicity by two toxic cations. Physiol Plant. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Kinraide TB. Reconsidering the rhyzotoxicity of hydroxyl, sulphate, and fluoride complexes of aluminum. J Exp Bot. 1997;48:1115–1124. [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 1992;99:1461–1468. doi: 10.1104/pp.99.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp A, Domingos M, Klumpp G. Assessment of the vegetation risk by fluoride emissions from fertilizer industries at Cubatão, Brazil. Sci Total Environ. 1996a;192:219–228. [Google Scholar]
- Klumpp A, Klumpp G, Domingos M, da Silva MD. Fluoride impact on native tree species of the Atlantic forest near Cubatão, Brazil. Water Air Soil Pollut. 1996b;87:57–71. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance on plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Li L, Fleming N. Aluminum fluoride inhibition of cabbage phospholipase D by a phosphate-mimicking mechanism. FEBS Lett. 1999;461:1–5. doi: 10.1016/s0014-5793(99)01414-3. [DOI] [PubMed] [Google Scholar]
- Lindsay WL. Chemical Equilibria in Soil. New York: John Wiley & Sons Inc., Wiley-Interscience Eds; 1979. pp. 41–43. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lunardi J, Dupuis A, Garin J, Issartel JP, Michel L, Chabre M, Vignais PV. Inhibition of H+-transporting ATPase by formation of a tight nucleoside diphosphate fluoroaluminate complex at the catalytic site. Proc Natl Acad Sci USA. 1988;85:8958–8962. doi: 10.1073/pnas.85.23.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- MacLean DC, Hansen KS, Schneider RE. Amelioration of aluminum toxicity in wheat by fluoride. New Phytol. 1992;121:81–88. [Google Scholar]
- Malkanthi DRR, Yokoyama K, Yoshida T, Moritsugu M, Matsushita K. Effects of low pH and Al on growth and nutrient-uptake of several plants. Soil Sci Plant Nutr. 1995;41:161–165. [Google Scholar]
- Martin RB. Ternary complexes of Al+3 and F− with a third ligand. Coord Chem Rev. 1996;149:23–32. [Google Scholar]
- Matsumoto H, Yamamoto Y, Kasai M. Changes of some properties of plasma membrane-enriched fraction of barley roots related to aluminum stress: membrane-associated ATPase, aluminum and calcium. Soil Sci Plant Nutr. 1992;38:411–419. [Google Scholar]
- Missiaen L, Wuytack F, Desmedt H, Vrolix M, Casteels R. AlF4− reversibly inhibits P-type cation-transport ATPases, possibly by interacting with the phosphate-binding site of the ATPase. Biochem J. 1988;253:827–833. doi: 10.1042/bj2530827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchhal US, Raghothama KG. Transcriptional regulation of plant phosphate transporters. Proc Natl Acad Sci USA. 1999;96:5868–5872. doi: 10.1073/pnas.96.10.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullette KJ. Stimulation of growth in eucalyptus due to aluminum. Plant and Soil. 1975;42:495–499. [Google Scholar]
- Nagata T, Hayatsu M, Kosuge N. Aluminum kinetics in the tea plant using Al-27 and F-19 NMR. Phytochemistry. 1993;32:771–775. [Google Scholar]
- Parker DR, Kinraide TB, Zelazny LW. On the phytotoxicity of polynuclear hydroxy-aluminum complexes. Soil Sci Soc Am J. 1989;53:789–796. [Google Scholar]
- Parker DR, Norwell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In: Loeppert RH, editor. Soil Chemical Equilibrium and Reaction Models, Soil Science Society of America Special Publication, No. 42. Madison, WI: American Society of Agronomy; 1995. pp. 253–270. [Google Scholar]
- Pineros MA, Kochian LV. A patch-clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiol. 2001;125:292–305. doi: 10.1104/pp.125.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieth C, Sattelmacher B, Hansen UP, Knight MR. Low-pH-mediated elevations in cytosolic calcium are inhibited by aluminium: a potential mechanism for aluminium toxicity. Plant J. 1999;18:643–650. doi: 10.1046/j.1365-313x.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- Raghothama KG. Phosphate transport and signaling. Curr Opin Plant Biol. 2000;3:182–187. [PubMed] [Google Scholar]
- Rai LC, Husaini Y, Mallick N. pH-altered interaction of aluminium and fluoride on nutrient uptake, photosynthesis and other variables of Chlorella vulgaris. Aquat Toxicol. 1998;42:67–84. [Google Scholar]
- Sentenac H, Grignon C. Effect of pH on orthophosphate uptake by corn roots. Plant Physiol. 1985;77:136–141. doi: 10.1104/pp.77.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen E, Runge-Metzger A. Phosphorus balance in European agriculture: status and policy options. In: Tiessen H, editor. SCOPE 54: Phosphorus in The Global Environment: Transfers, Cycles and Management. Chichester, UK: John Wiley & Sons; 1995. pp. 43–57. [Google Scholar]
- Sikora FJ, Copeland JP, Dillard EF, Burnell JR. Corn growth as affected by suspension fertilizers containing fluorosilicic acid. Soil Sci Soc Am J. 1992a;56:961–966. [Google Scholar]
- Sikora FJ, Copeland JP, Mullins GL. Apparent solubility products of phosphorus impurity compounds in commercial monoammonium phosphate fertilizers. Soil Sci Soc Am J. 1992b;56:402–407. [Google Scholar]
- Sivaguru M, Fujiwara T, Samaj J, Baluska F, Yang Z, Osawa H, Maeda T, Mori T, Volkmann D, Matsumoto H. Aluminum-induced 1→3-β-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata: a new mechanism of aluminum toxicity in plants. Plant Physiol. 2000;124:991–1006. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. GTPase mechanism of G proteins from the 1.7-Å crystal structure of transducin α-GDP-AlF4−. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- Sternweis PC, Gilman AG. Aluminum: a requirement for activation of the regulatory component of adenylate-cyclase by fluoride. Proc Natl Acad Sci USA. 1982;79:4888–4891. doi: 10.1073/pnas.79.16.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DP, McLaughlin MJ, Alston AM. Phytotoxicity of aluminium-fluoride complexes and their uptake from solution culture by Avena sativa and Lycopersicon esculentum. Plant Soil. 1997;192:81–93. [Google Scholar]
- Supharungsun S, Wainwright M. Determination, distribution, and absorption of fluoride in atmospheric-polluted soils. Bull Environ Toxicol. 1982;28:632–636. doi: 10.1007/BF01605597. [DOI] [PubMed] [Google Scholar]
- Sze H. H+-translocating ATPases: advances using membrane-vesicles. Annu Rev Plant Physiol. 1985;36:175–208. [Google Scholar]
- Takmaz-Nisancioglu S, Davison AW. Effects of aluminum on fluoride uptake by plants. New Phytol. 1988;109:149–155. [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troullier A, Girardet JL, Dupont Y. Fluoroaluminate complexes are bifunctional analogs of phosphate in sarcoplasmic-reticulum Ca2+-ATPase. J Biol Chem. 1992;267:22821–22829. [PubMed] [Google Scholar]
- Ullrich-Eberius CI, Novacky A, Fischer E, Lüttge U. Relationship between energy-dependent phosphate-uptake and the electrical membrane-potential in (Lemna-Gibba) G1. Plant Physiol. 1981;67:797–801. doi: 10.1104/pp.67.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittinghofer A. Signaling mechanistics: aluminum fluoride for molecule of the year. Curr Biol. 1997;7:R682–R685. doi: 10.1016/s0960-9822(06)00355-1. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Baligar VC, Wright SF. Estimation of phytotoxic aluminum in soil solution using three spectrophotometric methods. Soil Sci. 1987;144:224–233. [Google Scholar]
- Xu YW, Morera S, Janin J, Cherfils J. AlF3 mimics the transition state of protein phosphorylation in the crystal structure of nucleoside diphosphate kinase and MgADP. Proc Natl Acad Sci USA. 1997;94:3579–3583. doi: 10.1073/pnas.94.8.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Feuerle R, Schaffer S, Fortmeier H, Schubert S. Adaptation of active proton pumping and plasmalemma ATPase activity of corn roots to low root medium pH. Plant Physiol. 1998;117:311–319. doi: 10.1104/pp.117.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Schubert S, Mengel K. Effect of low root medium pH on net proton release, root respiration, and growth of corn (Zea mays L.) and broad bean (Vicia faba L.) Plant Physiol. 1992;99:415–421. doi: 10.1104/pp.99.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yompakdee C, Ogawa N, Harashima S, Oshima Y. A putative membrane protein, Pho88p, involved in inorganic phosphate transport in Saccharomyces cerevisiae. Mol Gen Genet. 1996;251:580–590. doi: 10.1007/BF02173648. [DOI] [PubMed] [Google Scholar]