Abstract
1 The membrane/buffer partition coefficient of [14C]-pentobarbitone has been determined as a function of the lipid composition of bilayer membranes. 2 A new technique based on ultrafiltration gave comparable results to conventional techniques but required less time for equilbration. 3 The membrane/buffer coefficient was independent of pentobarbitone concentration in the range studies. 4 The apparent partition coefficient varied with pH and was a linear function of the degree of dissociation of pentobarbition. 5 Both the charged and uncharged forms of pentobarbitone partitioned into the membrane, the latter to a much greater extent than the former. 6 At low pH the highest partition coefficient observed was in egg phosphatidylcholine bilayer membranes. 7 Incorporation of cholesterol or phosphatidic acid into phosphatidylcholine membranes greatly reduced the partition coefficient. 8 High pressures do not greatly change these partition coefficients.
Full text
PDF

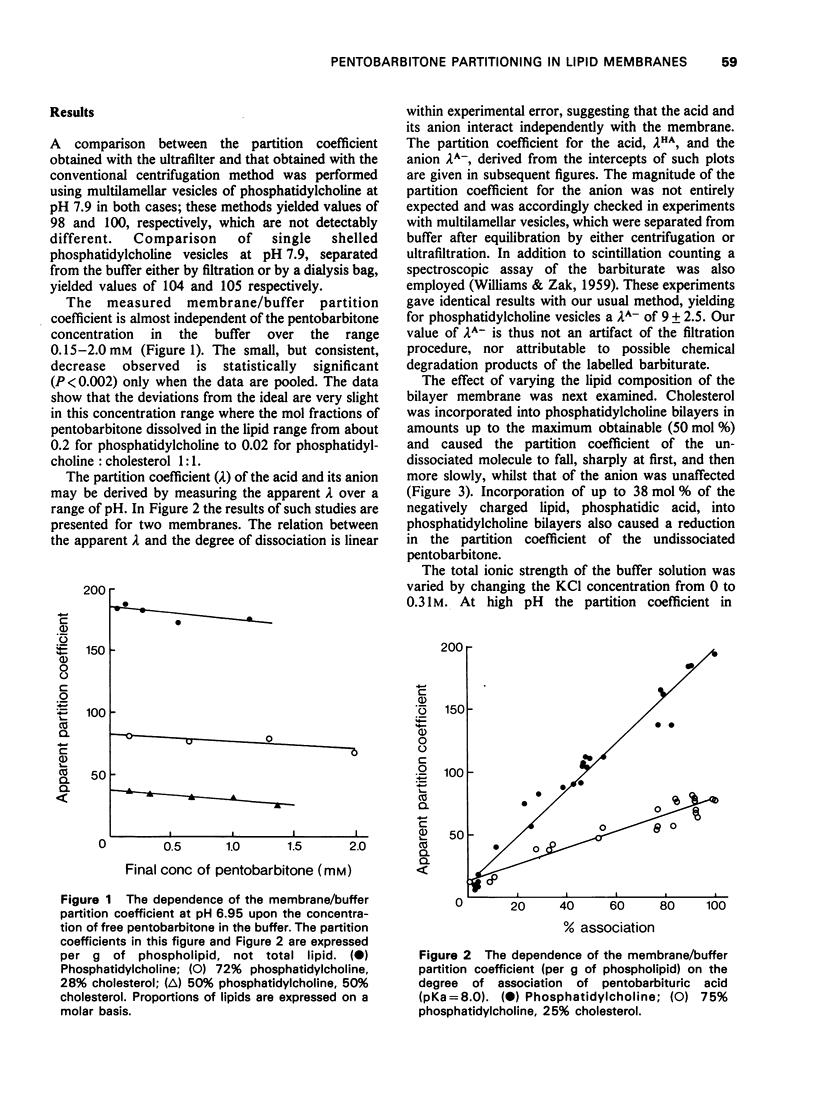
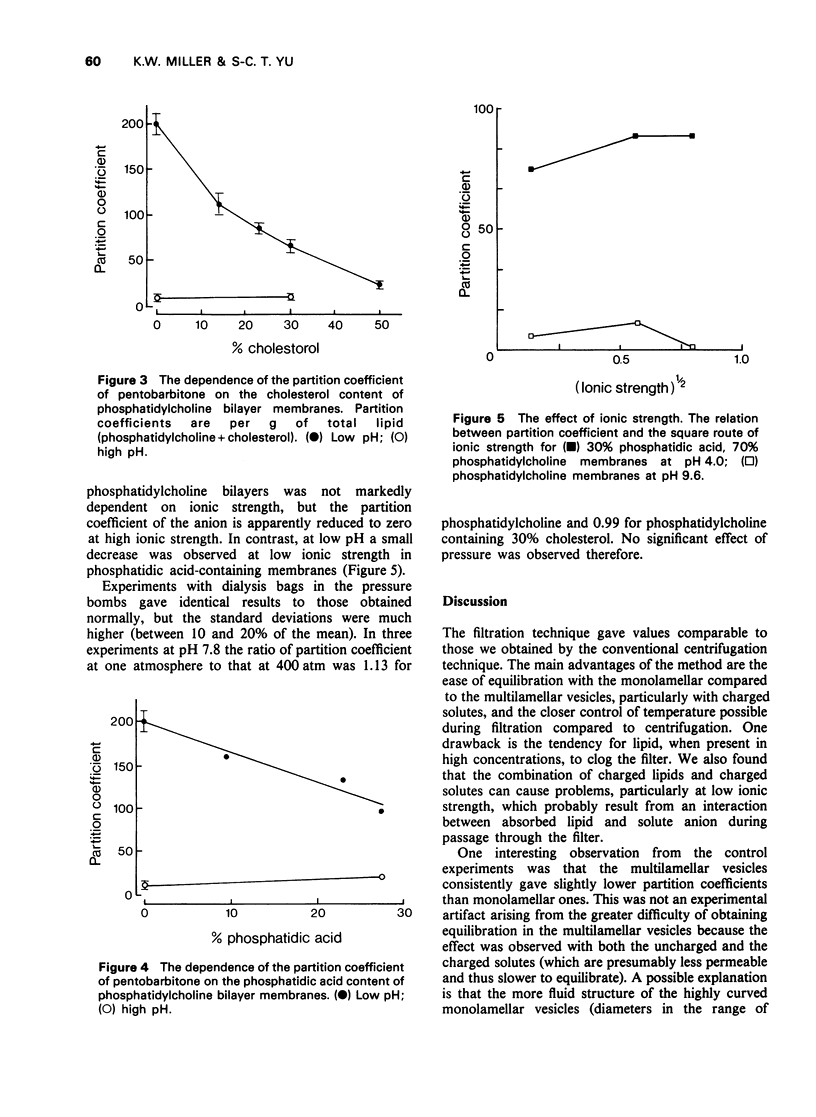



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE B. B., KURZ H., SCHANKER L. S. The importance of dissociaton constant and lipid-solubility in influencing the passage of drugs into the cerebrospinal fluid. J Pharmacol Exp Ther. 1960 Sep;130:20–25. [PubMed] [Google Scholar]
- Bangham A. D. Model membranes. Chem Phys Lipids. 1972 May;8(4):386–392. doi: 10.1016/0009-3084(72)90069-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Action of anionic and cationic nerve-blocking agents: experiment and interpretation. Science. 1966 Jul 22;153(3734):429–432. doi: 10.1126/science.153.3734.429. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Membrane structure: some general principles. Science. 1973 Aug 17;181(4100):622–629. doi: 10.1126/science.181.4100.622. [DOI] [PubMed] [Google Scholar]
- Colley C. M., Metcalfe J. C. The localisation of small molecules in lipid bilayers. FEBS Lett. 1972 Aug 15;24(3):241–246. doi: 10.1016/0014-5793(72)80364-8. [DOI] [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V. The asymetric arrangement of phospholipids in the human erythrocyte membrane. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1027–1031. doi: 10.1016/0006-291x(73)91509-x. [DOI] [PubMed] [Google Scholar]
- Guidotti G. The composition of biological membranes. Arch Intern Med. 1972 Feb;129(2):194–201. [PubMed] [Google Scholar]
- Hansch C., Anderson S. M. The structure-activity relationship in barbiturates and its similarity to that in other narcotics. J Med Chem. 1967 Sep;10(5):745–753. doi: 10.1021/jm00317a001. [DOI] [PubMed] [Google Scholar]
- Heap R. B., Symons A. M., Watkins J. C. Steroids and their interactions with phospholipids: solubility, distribution coefficient and effect on potassium permeability of liposomes. Biochim Biophys Acta. 1970 Dec 15;218(3):482–495. doi: 10.1016/0005-2760(70)90011-1. [DOI] [PubMed] [Google Scholar]
- Johnson S. M. The effect of charge and cholesterol on the size and thickness of sonicated phospholipid vesicles. Biochim Biophys Acta. 1973 Apr 25;307(1):27–41. doi: 10.1016/0005-2736(73)90022-9. [DOI] [PubMed] [Google Scholar]
- Kakemi K., Arita T., Hori R., Konishi R. Absorption and excretion of drugs. XXXI. On the relationship between partition coefficients and chemical structures of barbituric acid derivatives. Chem Pharm Bull (Tokyo) 1967 Nov;15(11):1705–1712. doi: 10.1248/cpb.15.1705. [DOI] [PubMed] [Google Scholar]
- Klein R. A. The detection of oxidation in liposome preparations. Biochim Biophys Acta. 1970 Sep 8;210(3):486–489. doi: 10.1016/0005-2760(70)90046-9. [DOI] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Miller K. W. Inert gas narcosis and animals under high pressure. Symp Soc Exp Biol. 1972;26:363–378. [PubMed] [Google Scholar]
- Miller K. W., Pang K. Y. General anaesthetics can selectively perturb lipid bilayer membranes. Nature. 1976 Sep 16;263(5574):253–255. doi: 10.1038/263253a0. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Paton W. D., Smith R. A., Smith E. B. The pressure reversal of general anesthesia and the critical volume hypothesis. Mol Pharmacol. 1973 Mar;9(2):131–143. [PubMed] [Google Scholar]
- Narahashi T., Frazier D. T., Deguchi T., Cleaves C. A., Ernau M. C. The active form of pentobarbital in squid giant axons. J Pharmacol Exp Ther. 1971 Apr;177(1):25–33. [PubMed] [Google Scholar]
- Roth S., Seeman P. The membrane concentrations of neutral and positive anesthetics (alcohols, chlorpromazine, morphine) fit the Meyer-Overton rule of anesthesia; negative narcotics do not. Biochim Biophys Acta. 1972 Jan 17;255(1):207–219. doi: 10.1016/0005-2736(72)90023-5. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- WILLIAMS L. A., ZAK B. Determination of barbiturates by automatic differential spectrophotometry. Clin Chim Acta. 1959 Mar;4(2):170–174. doi: 10.1016/0009-8981(59)90126-3. [DOI] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. The role of cholesterol in lipid membranes. Biochim Biophys Acta. 1969 Jan 28;173(1):143–145. doi: 10.1016/0005-2736(69)90045-5. [DOI] [PubMed] [Google Scholar]


