Abstract
Our objective was to determine the sensitivity of components of the photosynthetic apparatus of maize (Zea mays), a C4 plant, to high temperature stress. Net photosynthesis (Pn) was inhibited at leaf temperatures above 38°C, and the inhibition was much more severe when the temperature was increased rapidly rather than gradually. Transpiration rate increased progressively with leaf temperature, indicating that inhibition was not associated with stomatal closure. Nonphotochemical fluorescence quenching (qN) increased at leaf temperatures above 30°C, indicating increased thylakoid energization even at temperatures that did not inhibit Pn. Compared with CO2 assimilation, the maximum quantum yield of photosystem II (Fv/Fm) was relatively insensitive to leaf temperatures up to 45°C. The activation state of phosphoenolpyruvate carboxylase decreased marginally at leaf temperatures above 40°C, and the activity of pyruvate phosphate dikinase was insensitive to temperature up to 45°C. The activation state of Rubisco decreased at temperatures exceeding 32.5°C, with nearly complete inactivation at 45°C. Levels of 3-phosphoglyceric acid and ribulose-1,5-bisphosphate decreased and increased, respectively, as leaf temperature increased, consistent with the decrease in Rubisco activation. When leaf temperature was increased gradually, Rubisco activation acclimated in a similar manner as Pn, and acclimation was associated with the expression of a new activase polypeptide. Rates of Pn calculated solely from the kinetics of Rubisco were remarkably similar to measured rates if the calculation included adjustment for temperature effects on Rubisco activation. We conclude that inactivation of Rubisco was the primary constraint on the rate of Pn of maize leaves as leaf temperature increased above 30°C.
It has long been recognized that C4 plant species have a higher temperature optimum for photosynthesis than C3 plants due to the operation of a CO2-concentrating system that inhibits Rubisco oxygenase activity (Berry and Björkman, 1980; Edwards and Walker, 1983). In C3 plants, inhibition of net photosynthesis (Pn) at moderately high temperatures has usually been ascribed to an increase in the ratio of Rubisco oxygenase:Rubisco carboxylase activities. As temperature increases, the ratio of dissolved O2/CO2 and the specificity of Rubisco for O2 increase, thus favoring oxygenase activity (Monson et al., 1982; Jordan and Ogren, 1984; Sage and Sharkey, 1987) and resulting in inhibition of Pn. As a consequence, when C3 plants are exposed to high CO2 and/or low O2, i.e. conditions that reduce oxygenase activity, the temperature optimum for Pn is increased (Berry and Björkman, 1980; Edwards and Walker, 1983).
For C3 and C4 plants, the temperature range for optimum Pn is broad, and at temperatures above this range, Pn decreases (Edwards and Walker, 1983). Temperature-induced decreases in Pn in C3 species are closely associated with inactivation of Rubisco (Law and Crafts-Brandner, 1999), and when the activation state of Rubisco and gas solubilities are taken into account, the rate of Pn at any given temperature or level of atmospheric CO2 or O2 reflects Rubisco kinetics (Crafts-Brandner and Salvucci, 2000). The temperature-induced decrease in Rubisco activation, and the associated inhibition of Pn, in C3 plants results in large part from the inability of Rubisco activase activity to keep pace with a faster rate of Rubisco inactivation as temperature is increased (Crafts-Brandner and Salvucci, 2000). Activase kinetics and physical denaturation of activase appear to be causative factors contributing to the decrease in Rubisco activation at high temperature (Crafts-Brandner and Salvucci, 2000; Salvucci et al., 2001).
Although C4 plants have a higher temperature optimum than C3 plants, Pn is usually inhibited when leaf temperatures exceed about 38°C (Berry and Björkman, 1980; Edwards and Walker, 1983). Although the C4 photosynthetic system is more complex than the C3 system, the ultimate limitation to CO2 fixation for both photosynthetic types is the activity of Rubisco (von Caemmerer et al., 1997; Edwards et al., 2001). Low temperature effects on C4 photosynthesis have been frequently examined (Labate et al., 1991; Long, 1998). Studies pertaining to the effects of high temperature on C4 photosynthetic metabolism are less common, and we hypothesized that high temperature may inactivate Rubisco and limit Pn in a similar manner as for C3 plants. However, it seemed feasible that heat stress might also impact C4-specific processes such as fixation of CO2 by phosphoenolpyruvate (PEP) carboxylase, shuttling of C4 acids from mesophyll to bundle sheath cells, or energy balance due to the differential localization of PSII and the Calvin cycle. Therefore, our objective was to probe the effect of heat stress on photosynthetic processes, including Rubisco activation, activities of PEP carboxylase, and pyruvate phosphate dikinase (PPDK), and PSII stability, in maize (Zea mays) leaves. We report that the most heat-sensitive process in maize leaves is the activation of Rubisco by Rubisco activase, and that acclimation to heat stress is associated with the expression of novel form of activase.
RESULTS
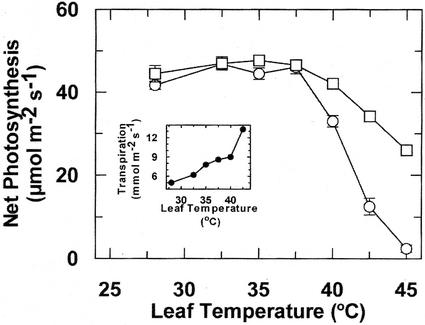
Pn in maize exhibited a broad temperature optimum between 28°C and 37.5°C (Fig. 1). Pn was inhibited as temperature exceeded 37.5°C, and the relative inhibition was much greater when the leaf temperature was increased rapidly compared with gradually. For example, at 45°C, Pn was inhibited >95% and 50% for the rapid and gradual heat stress treatments, respectively. Increasing the level of atmospheric CO2 3-fold above ambient did not alter the temperature response of Pn (data not shown). Consistent with reports for other plant species (Jiao and Grodzinski, 1996; Law and Crafts-Brandner, 1999), the inhibition of Pn by heat stress was not associated with stomatal closure, as evidenced by progressive increases in transpiration (Fig. 1, inset) and stomatal conductance (data not shown) as leaf temperature was increased.
Figure 1.
Effect of leaf temperature on Pn and transpiration (inset) of maize leaves. After attaining steady-state net Pn and transpiration at 28°C, leaf temperature was increased rapidly at 1°C min−1 (○) or gradually at 2.5°C h−1 (□). Pn and transpiration were determined after 1 h at the indicated temperature. Different plants were used for each rapid temperature increase treatment, whereas the same leaf was used for the gradual heat stress treatment. Each data point represents the mean ± se of three independent measurements.
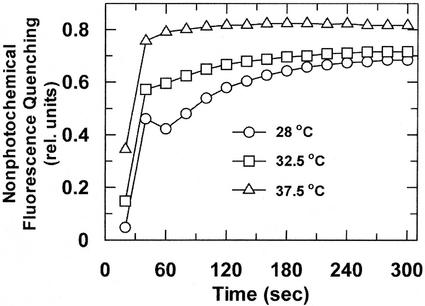
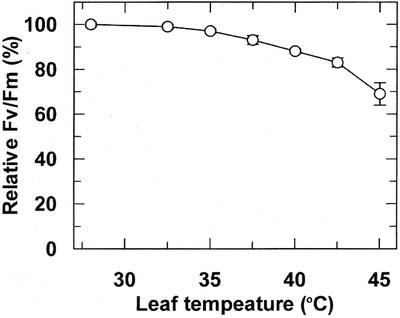
Nonphotochemical fluorescence quenching (qN) was quite sensitive to leaf temperature, with detectable increases occurring at 32.5°C to 37.5°C (Fig. 2), well before detectable heat inhibition of Pn (Fig. 1), and progressively increasing at temperatures up to 45°C (data not shown). In contrast to qN, the maximum quantum yield of PSII (Fv/Fm) was relatively insensitive to leaf temperatures up to 42.5°C (Fig. 3). As leaf temperature was increased above 42.5°C, Fv/Fm decreased below 80% relative to the 28°C control. However, even at 45°C, Fv/Fm was still 70% of the 28°C control.
Figure 2.
Effect of leaf temperature on qN of maize leaves. An attached leaf was dark adapted for 1 h at 28°C prior to conducting nonphotochemical quenching analysis. Subsequent measurements were made on the same leaf tissue after increasing the leaf temperature at 1°C min−1 in the dark to 32.5°C for 1 h and then to 37.5°C for 1 h. Each curve represents the mean ± se of three independent measurements. At 28°C, qN was the same for plants that were dark adapted for 1 and 3 h (data not shown).
Figure 3.
Effect of leaf temperature on the Fv/Fm of maize leaves. Intact leaves were dark adapted for 1 h at 28°C, Fv/Fm was measured, leaf temperature was increased at 1°C min−1 to the indicated temperature, and Fv/Fv was measured again. Different plants were used for each temperature treatment, and the data points represent the mean ± se of three independent measurements. The average Fv/Fm for the 28°C treatment was 0.801 ± 0.005.
The effect of leaf temperature on two key enzymes of C4 photosynthesis, PPDK and PEP carboxylase, was assessed. We first determined if assay temperature equivalent to the highest leaf temperature treatment inhibited either enzyme. When extracted from leaves sampled at 28°C and assayed at 30°C and 45°C for several minutes, the activity of PEP carboxylase was higher at 45°C, and the activity of PPDK was similar at 30°C and 45°C (data not shown). Thus, when assayed in vitro, both enzymes were tolerant of high temperature.
Although the leaf tissue was rapidly extracted and assayed, the recovered activity of PPDK was low relative to the rate of photosynthesis. However, when extracted from leaves heated to 45°C, PPDK activity was similar to activity extracted from leaves treated at 28°C, when both were assayed at 30°C (Table I). Likewise, PEP carboxylase activity, assayed under optimal conditions to reflect total potential enzyme activity, was insensitive to leaf temperature up to at least 45°C (Table I). However, when assayed at limiting substrate levels and in the presence of the inhibitor malate, conditions designed to reflect the in vivo activation state (Giglioli-Guivarc'h et al., 1996), the enzyme extracted from the heated leaf was significantly more sensitive to inhibition by malate (Table I). For example, malate inhibited PEP carboxylase activity by about 50% when leaf temperature was 28°C, whereas this inhibition was 59% and 66% when leaf temperature was 40°C and 45°C, respectively.
Table I.
Effect of leaf temperature on the activities of PPDK, PEP carboxylase, and Rubisco
| Leaf Temperature | PPDKa | PEP
Carboxylase
|
Rubisco
|
||
|---|---|---|---|---|---|
| Vmaxb | Malate sensitivityc | Initiald | Totale | ||
| °C | μmol m−2 s−1 | % Inhibition | μmol m−2 s−1 | ||
| 28 | 15.7 ± 1 | 308 ± 14 | 51 ± 3 | 32 ± 2 | 31 ± 1 |
| 40 | – | – | 59 ± 1 | – | – |
| 42.5 | – | – | 63 ± 3 | – | – |
| 45 | 13.4 ± 1 | 295 ± 10 | 66 ± 2 | 6 ± 1 | 29 ± 3 |
All assays were conducted at 30°C. Values represent the mean ± se of four replicates for PPDK and PEP carboxylase, and three replicates for Rubisco.
PPDK assayed under conditions to preserve the in vivo activation state.
Assayed under optimal conditions to indicate total potential activity.
Assayed using limiting substrate in the presence or absence of 0.5 mm l-malate; indicates the in vivo activation state.
Initial activity measured immediately after extraction; indicates in vivo activity.
Total activity measured after incubating in CO2 and Mg2+ to fully activate Rubisco.
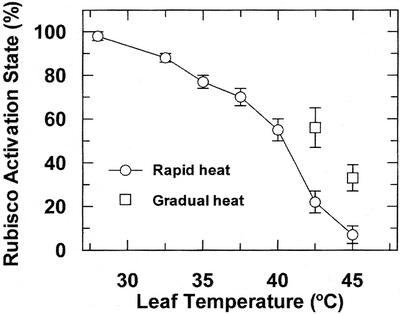
Similar to the two C4 enzymes, and consistent with results for C3 plant species (Weis, 1981a, 1981b; Crafts-Brandner and Law, 2000), the total activity of Rubisco was not affected by leaf temperatures up to at least 45°C (Table I). However, the activation state of Rubisco was decreased progressively as leaf temperature was increased above 28°C (Fig. 4). When leaf temperature was increased rapidly to 40°C, activation state was decreased to a level that was 58% relative to the 28°C control. Rapidly increasing the leaf temperature to 45°C led to a nearly complete inactivation of Rubisco. In contrast, when leaf temperature was increased gradually, Rubisco inactivation was much less severe at 42.5°C or 45°C, indicating acclimation of the activation process.
Figure 4.
Effect of leaf temperature on the activation state of Rubisco in maize leaves. Leaves were allowed to attain steady-state Pn (1 h) under high light and ambient CO2 at a given temperature prior to sampling. Leaf temperature was increased rapidly at 1°C min−1 or gradually at 2.5°C h−1, and samples were taken after 1 h at the indicated temperature. Different plants were used for each temperature treatment, and the data points represent the mean ± se of three independent measurements.
The temperature-induced inactivation of Rubisco was associated with markedly decreased levels of 3-phosphoglyceric acid (3-PGA) and moderate increases in ribulose-1,5-bisphosphate (RuBP; Table II). These data are consistent with decreased carboxylation of RuBP by Rubisco as leaf temperature increased (Weis, 1981a; Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999).
Table II.
Effect of leaf temperature on abundances of 3-PGA and RuBP in maize
| Leaf Temperature | 3-PGA | RuBP |
|---|---|---|
| °C | μmol m−2 | |
| 28 | 427 ± 14 | 51 ± 5 |
| 37 | 228 ± 16 | 60 ± 12 |
| 28 | 440 ± 44 | 56 ± 6 |
| 42 | 82 ± 8 | 96 ± 20 |
Portions of each leaf were sampled after treatment first at 28°C and again after leaf temperature was rapidly increased to the higher temperature. Values represent the mean ± se of four replicates for each treatment.
The effect of heat stress on decreasing Rubisco activation in C3 plants has been attributed to an effect on Rubisco activase (Crafts-Brandner and Salvucci, 2000). In some plant species, including maize, heat stress induces the synthesis of constitutive and apparent novel activase polypeptides (Sánchez de Jiménez et al., 1995; Law and Crafts-Brandner, 2001; Law et al., 2001). Under control conditions, maize leaves contain a 42-kD activase polypeptide that has sequence homology to the short activase polypeptide found in many species (Ayala-Ochoa et al., 1998). Similar to the report by Sánchez de Jiménez et al. (1995), when maize plants were subjected to 2 d of heat stress, there was significant accumulation of a putative activase polypeptide that was larger than the constitutive activase polypeptide (Fig. 5). This putative new activase polypeptide was visible on western blots within 8 h of applying the heat stress gradually. Sequencing of this polypeptide revealed an N terminus of AKEV, a sequence identical to maize activase (Ayala-Ochoa et al., 1998).
Figure 5.
Western-blot analysis of the effect of heat stress on abundance of Rubisco activase in maize leaves. Controls were treated for two diurnal cycles of 14 h of light at 28°C and 10 h of dark at 24°C, and leaf tissue was sampled at the end of the dark period of the 2nd d (control). Other plants were treated for two diurnal cycles of 14 h of light at 40°C and 10 h of dark at 34°C, and leaf tissue was sampled at the end of the dark period on the 2nd d (48 h heat). In addition, for some plants, the leaf temperature was increased at 2.5°C h−1 in the light until reaching 42.5°C, and after 1.5 h at 42.5°C, leaf tissue was sampled (8 h of heat). Just above the constitutive 42-kD activase polypeptide, the arrow indicates a polypeptide that was immunoreactive to activase antibodies and that contained an N-terminal sequence identical to the constitutive maize activase.
DISCUSSION
Pn
As is typical in C4 species (Berry and Björkman, 1980; Edwards and Walker, 1983), maize Pn was tolerant of relatively high leaf temperatures, with inhibition not observed until leaf temperature exceeded 37.5°C (Fig. 1). In addition, there was significant acclimation of Pn when leaf temperature was increased gradually such that over 50% of the maximum rate was retained even at the extreme leaf temperature of 45°C. This degree of acclimation was greater than was observed for the C3 plants cotton (Gossypium hirsutum) and wheat (Triticum aestivum) that were grown and analyzed under similar conditions (Law and Crafts-Brandner, 1999). The general temperature response of Pn in maize was similar to the response of C3 plants that were analyzed under high atmospheric CO2 (Crafts-Brandner and Salvucci, 2000). This similarity suggests that the CO2-concentrating mechanism of maize provided substantial compensation for the photosynthetic inhibition by high temperature normally observed in C3 plants under ambient conditions.
Chlorophyll Fluorescence
The effect of heat stress on chlorophyll fluorescence traits of maize leaves was similar to results reported for C3 plants (Bilger et al., 1987; Feller et al., 1998; Law and Crafts-Brandner, 1999), with perturbations in qN occurring well before any detrimental effects on Fv/Fm (Figs. 2 and 3). At the moderate leaf temperatures of 32.5°C to 37.5°C, increased qN (Fig. 2) indicated an increased thylakoid energization associated with an increased ratio of ATP:ADP. For C3 plants, this increase in qN induced by heat stress is correlated with decreased Pn and it is considered to indicate inhibition of Calvin cycle activity (Bilger et al., 1987; Feller et al., 1998; Law and Crafts-Brandner, 1999). However, for maize there was no inhibition of Pn up to 37.5°C. As discussed below, we suggest that under moderate heat stress, the energy supply available for photosynthetic metabolism was increased but did not lead to higher rates of Pn because of compensatory limitations in the activation state of Rubisco.
Heat stress did lead to decreases in Fv/Fm, but this inhibition was marginal until leaf temperature exceeded 42.5°C (Fig. 3). PSII is well known to be sensitive to high temperature, and it is often cited as the most heat-sensitive component of photosynthesis (Berry and Björkman, 1980; Havaux, 1993; Heckathorn et al., 1998). At very high leaf temperatures, i.e. 45°C, there was a marked decline in Fv/Fm, and, therefore, it is likely that damage to PSII contributed to the inhibition of Pn at this temperature. However, with maize and several C3 species that we have examined (Crafts-Brandner and Salvucci, 2000), qN perturbations, and also decreases in Rubisco activation state, consistently occur at leaf temperatures lower than required to inhibit Fv/Fm.
C4 Enzymes
PEP carboxylase and PPDK are key C4 enzymes, and both are subject to light/dark regulation (Ashton et al., 1990). Inhibition of either enzyme by heat stress, especially PPDK due to its low activity (Furbank et al., 1997), may decrease the supply of C4 acids available for decarboxylation, thereby limiting the supply of CO2 to Rubisco. Under conditions designed to preserve the activation state of the enzyme in vivo (Ashton et al., 1990), we could not detect any inhibition of PPDK (Table I) even at leaf temperatures that completely inhibited Pn (Fig. 1).
Although total PEP carboxylase activity was insensitive to leaf temperatures up to 45°C, there was evidence that the activation state of the enzyme was marginally sensitive to temperatures of 40°C or higher (Table I). This apparent inactivation of PEP carboxylase could be explained if temperature differentially affected the activities of the regulatory kinase or the phosphatase, thereby decreasing the extent of phosphorylation of PEP carboxylase in the light (Jiao and Chollet, 1991). It is not known how high temperature affects the levels of oxaloacetic acid, malic acid, and Asp, but an increased level of these metabolites, coupled with the increased sensitivity of PEP carboxylase to inhibition, would decrease the activity of PEP carboxylase (Ashton et al., 1990) and could potentially impact Pn at leaf temperatures above 40°C.
Rubisco Activation
Although Pn was constant between 28°C and 37.5°C (Fig. 1), Rubisco activation decreased over this temperature range (Fig. 4) in a similar manner as reported for C3 species measured under ambient levels of CO2 and O2 (Weis, 1981a, 1981b; Kobza and Edwards, 1987; Law and Crafts-Brandner, 1999; Crafts-Brandner and Salvucci, 2000). However, as leaf temperature was rapidly increased to 40°C or higher, activation state and Pn of maize declined to levels approaching zero. Thus, of all the traits that were measured, loss of Rubisco activation was most closely associated with inhibition of Pn at high leaf temperatures.
The fact that loss of Rubisco activation was not accompanied by a decrease in Pn at leaf temperatures ranging from 28°C to 37.5°C (Fig. 1) can be explained by the CO2-concentrating mechanism of C4 plants. In C3 plants, Rubisco activity is limited by the CO2 concentration and, as temperature increases, the affinity of the enzyme for CO2 and the solubility of CO2 decrease. In addition, Rubisco deactivation occurs at a faster rate as temperature is increased (Crafts-Brandner and Salvucci, 2000). Because of these limitations, the catalytic turnover rate of Rubisco increases only minimally with temperature, even at low O2. As a consequence, Pn declines at leaf temperatures greater than about 32°C in C3 plants because this slight increase in catalytic turnover is offset by a decrease in Rubisco activation state (Crafts-Brandner and Salvucci, 2000). In maize, Rubisco activation also decreased at leaf temperatures above 32°C, but the high level of CO2 in the mesophyll chloroplasts allowed for a substantial temperature-dependent increase in the catalytic turnover rate of activated Rubisco, thus counteracting the effects of decreased activation state until temperature approached 40°C. Thus, Pn of maize was relatively constant between 28°C and 37.5°C, a result that was similar to C3 plants that were measured under elevated atmospheric CO2 (Crafts-Brandner and Salvucci, 2000).
Although the CO2-concentrating system of maize alleviated inhibition of Pn between 28°C and 37.5°C, the progressive inactivation of Rubisco prevented the potential increases in Pn predicted based on Rubisco kinetics, gas solubilities, and an internal CO2 concentration of 2,500 μbar (Fig. 6). In support of this, when the predicted rates of Pn were adjusted for temperature effects on Rubisco activation, the temperature response (Fig. 6) was closely related to the experimentally determined values (Fig. 1) over the entire temperature range that was evaluated. Therefore, similar to C3 plants (Crafts-Brandner and Salvucci, 2000), decreased activation state of Rubisco appeared to be the major limitation to the rate of Pn in maize as leaf temperature increased.
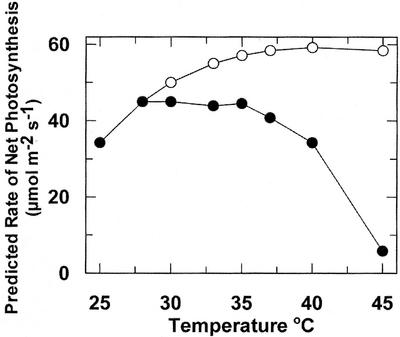
Figure 6.
The effect of temperature on the rates of net Pn of maize leaves predicted based on the kinetics of Rubisco. Net Pn at 28°C was assumed to be equal to the maximum activity of Rubisco at 28°C. Net Pn at each temperature was estimated assuming full activation of the enzyme at each temperature (○) or after adjusting the predicted rate for experimentally determined activation state at each temperature (●; from Fig. 4, rapid heat). The adjusted rates of Pn are closely related to the measured rates of Pn for rapid heat stress treatments (see Fig. 1).
We propose that the mechanism of Rubisco inactivation associated with moderate increases in leaf temperature in maize is the same as reported for C3 plants, namely, that as temperature increases, the rate of inactivation occurs faster than the ability of Rubisco activase to mediate reactivation (Crafts-Brandner and Salvucci, 2000). The increase in qN at moderately high leaf temperatures (Fig. 3) indicated that ATP supply was not a limitation to Pn at moderate leaf temperatures. In addition, ATP production via noncyclic photophosphorylation appears to be stimulated during heat stress (Bukhov et al., 1999, 2000) and, in isolated chloroplasts, heat stress led to an increase in the ratio of ATP:ADP (Weis, 1981a). Consistent with this, the level of RuBP was increased even when the temperature exceeded 40°C (Table II). The results presented here indicate that energy supply for photosynthetic carbon metabolism, including metabolite shuttling between bundle sheath and mesophyll cells and regeneration of RuBP, was not limiting and that the inability of Rubisco activase to maintain a high Rubisco activation state was the primary limitation to Pn over the temperature range of 28°C to 37.5°C. Thus, our results detailing the effects of high temperature on the biochemistry of C4 photosynthesis differ from those proposed by Sage (2002), who used biochemical analysis and modeling to predict that RuBP or PEP regeneration, and not Rubisco capacity, would be the major limitations to C4 Pn at high temperature.
As leaf temperature was increased above 37.5°C, the increased catalytic turnover of activated Rubisco was apparently not sufficient to overcome the substantial inactivation of the enzyme, leading to inhibition of Pn. As noted above, inactivation of PEP carboxylase and damage to PSII also may contribute to inhibition of Pn at very high leaf temperatures. However, we suspect that direct effects of extremely high temperature on the physical stability of activase may be the major factor limiting Rubisco activation. We have reported that activase denatures in vivo and in vitro at a temperature of about 40°C (Feller et al., 1998; Salvucci et al., 2001).
At 28°C, the activation state of Rubisco approached 100%. These data are interesting because the estimated level of CO2 around Rubisco in C4 plants (Edwards et al., 2001) would cause substantial inactivation of the enzyme in leaves of C3 plants (Sage et al., 1988, 1989; Crafts-Brandner and Salvucci, 2000). Furthermore, increasing the level of CO2 around maize leaves to 1,200 μbars did not affect the Rubisco activation state or alter the effect of high temperature (data not shown). The effect of high CO2 on Rubisco activation in C3 plants was postulated to result from unfavorable ATP:ADP ratios (Crafts-Brandner and Salvucci, 2000). It is apparent that the ATP:ADP ratios are adequate for full Rubisco activation even though the CO2 levels are elevated.
Acclimation to Heat Stress
Acclimation of Pn and Rubisco activation to heat stress was significant when the leaf temperature was increased gradually (Figs. 1 and 4), and this acclimation was associated with the appearance of a new activase polypeptide (Fig. 5). Increased synthesis of constitutive activase polypeptides, or induction of novel polypeptides, has been implicated in the mechanism of response to heat stress in wheat and cotton (Law and Crafts-Brandner, 2001; Law et al., 2001). Thus, the induction of new activase polypeptides in response to heat stress appears to be widespread among plant species.
Conclusions
Taken together, our results indicate that maize leaves responded to heat stress in a similar manner as C3 plants. For both photosynthesis types, inactivation of Rubisco occurred after only moderate temperature increases, and at high temperature, inactivation was nearly complete. The increased temperature optimum for Pn of C4 versus C3 plants can be attributed to the CO2-concentrating system of C4 plants, which compensates for Rubisco inactivation by increasing the CO2 level around Rubisco. As for C3 plants (Crafts-Brandner and Salvucci, 2000), the experimental rate of Pn in maize could be predicted solely from the kinetics of Rubisco if adjustments were made for temperature effects on Rubisco activation. We conclude that as leaf temperature increases, Rubisco activation state declines due to decreased Rubisco activase activity, eventually constraining Pn.
MATERIALS AND METHODS
Plant Material
Kernels of maize (Zea mays var. Pioneer brand 33A14) were planted in 15- × 15-cm pots containing a commercial potting mixture (Summer Winds Garden Center, Boise, ID). Plants were grown in an air-conditioned greenhouse maintained at 28°C for 14 h and at 24°C for 10 h. Natural light intensity peaked at an average of 1,900 μmol m−2 s−1 photosynthetically active radiation (PAR) on most days. Plants were fertilized three times per week with 750 mL of solution containing 2 g L−1 20-20-20 fertilizer (Grow More, Gardena, CA) supplemented with 0.5 mL L−1 of a micronutrient solution containing 2 mm MnCl2, 10 mm H3BO3, 0.4 mm ZnSO4, 0.2 mm CuSO4, 0.4 mMNa2MoO4, and 0.1 mm NiCl2. In addition, 750 mg of chelated Fe [sodium ferric ethylenediamine, di-(o-hydroxyphenyl acetate)] and 3.8 g of Hi Yield 0-45-0 superphosphate fertilizer (Voluntary Purchasing Groups, Bonham, TX) were incorporated into the upper 2 cm of the potting soil. For all experiments, the fifth or sixth leaf was used.
Gas Exchange
Pn, transpiration, and leaf conductance were measured with a portable photosynthesis system (model 6400; Li-Cor, Lincoln, NE) using a light intensity of 1,800 μmol m−2 s−1 PAR and a constant 350 μbar partial pressure of CO2 in the sample chamber. For some experiments, the partial pressure of CO2 was increased above 350 μbar using the built-in CO2 injection system of the photosynthesis unit. Plants were moved from the greenhouse to a plant growth chamber prior to determining gas exchange traits. By maintaining a high relative humidity in the growth chamber, and by using the internal heating system of the photosynthesis unit in conjunction with the growth chamber heating system, the leaf temperature could be increased from 28°C to 45°C. For rapid heat stress treatments, leaf temperature was increased at 1°C min−1 to the desired temperature and was then held at constant temperature for 1 h prior to making gas exchange measurements. For gradual heat stress treatments, leaf temperature was increased in increments of 2.5°C (at 1°C min−1) once every 60 min until the desired leaf temperature was attained. Gas-exchange measurements were made 1 h after attaining the desired temperature. In all cases, steady-state conditions were attained prior to measuring gas-exchange traits. Measurements for each treatment were repeated three times using different plants.
Chlorophyll Fluorescence
Fv/Fm and qN were determined using a portable fluorometer (PAM-2000; Walz, Effeltrich, Germany) as described previously (Law and Crafts-Brandner, 1999). Plants were dark adapted in a plant growth chamber for 1 h at 28°C prior to fluorescence measurements. The leaf temperature was then increased to the desired level, and after 1 h, measurements were taken from the same leaf tissue. Controls kept at 28°C and measured after 1 and 3 h of dark indicated that fluorescence traits were not significantly altered by the amount of time in the dark (data not shown). Leaf temperature was manipulated by changing the temperature in the high humidity growth chamber, and the temperature was verified using a thermocouple. Measurements for each treatment were repeated three times using different plants.
Enzyme Activities
For PEP carboxylase and PPDK assays, fresh leaf tissue was extracted in buffer and was assayed immediately after a particular temperature treatment. Controls were sampled after 1 h of treatment at 28°C, 1,800 μmol m−2 s−1 PAR, and a constant 350 μbar partial pressure of CO2 in the sample chamber of the photosynthesis system (Li-Cor). For high temperature treatments, leaf temperature was increased rapidly at 1°C min−1 to the desired temperature, and leaf tissue was sampled after 1 h. Leaf temperature was controlled using the portable photosynthesis unit and plant growth chamber.
PEP carboxylase was extracted in ice-cold 100 mm HEPES, pH 7.5, 10 mm MgCl2, 1 mm EDTA, 1% (w/v) casein, 1% (w/v) polyvinylpyrrolidone, 5 mm 2-mercaptoethanol, and 0.05% (v/v) Triton X-100. The extraction buffer for PPDK was the same as for PEP carboxylase with the following modifications: The buffer was 50 mm HEPES, pH 8.0, and 5 mm dithiothreitol was used in place of 2-mercaptoethanol. The extraction buffer for PPDK was maintained at room temperature.
PEP carboxylase was assayed spectrophotometrically at 30°C as described by Giglioli-Guivarc'h et al. (1996). Assays conducted at pH 8.0 under optimal conditions provided an estimate of the maximum potential enzyme activity, and assays conducted at pH 7.3 using limiting levels of the substrate PEP in the presence or absence of 0.5 mm l-malate provided an indication of the phosphorylation status of the enzyme.
PPDK was assayed spectrophotometrically at 30°C as described by Ashton et al. (1990). The assay mixture contained 12.5 μm Cibacron blue F3GA to preserve the in vivo activation state of the enzyme (Ashton et al., 1990).
To test the effect of assay temperature per se on enzyme activity, PEP carboxylase (optimal assay) and PPDK were extracted from leaves of control plants and were assayed at 30 and 45°C.
Immediately after temperature treatment, leaf samples to be used for Rubisco activation assays were rapidly frozen between two pieces of metal cooled to the temperature of liquid N2 and stored at −80°C prior to determination of enzyme activity. Leaf temperature was increased rapidly or gradually using a light intensity of 1,800 μmol m−2 s−1 PAR and a constant 350 μbar partial pressure of CO2 in the sample chamber of the photosynthesis system (Li-Cor). Leaf tissue was extracted and assayed for initial and total Rubisco activity as described in detail by Crafts-Brandner and Salvucci (2000) except that total activities were determined after incubation in CO2 and Mg2+ for 5 min, which was determined to be adequate for full activation of extracted Rubisco (data not shown). Rubisco activity was determined by incorporation of 14CO2 into acid-stable products at 30°C (Salvucci, 1992). The activation state, or percentage of activation (Perchorowicz et al., 1981), of Rubisco was determined by the ratio of initial total enzyme activities.
For C4 enzymes, activities were determined using extracts from leaves of four different plants. For Rubisco activity, activities were determined using extracts from leaves of three different plants, and each of the three extracts was assayed in duplicate.
Western-Blot Analysis
Leaf tissue was homogenized in 100 mm potassium phosphate, pH 7.5, and 14 mm 2-mercaptoethanol, and aliquots were immediately mixed with SDS-PAGE sample buffer and boiled for 3 min as described by Feller et al. (1998). Samples representing equal amounts of leaf area were electrophoresed in 10% (w/v) SDS-PAGE gels according to the method of Chua (1980). Polypeptides were electrophoretically transferred to nitrocellulose and were probed with a monospecific polyclonal antibody against isolated recombinant tobacco (Nicotiana tabacum) Rubisco activase (Feller et al., 1998) according to procedures described by Salvucci et al. (1998).
Metabolite Analysis
Leaf temperature was manipulated using the portable photosynthesis system (Li-Cor) as described above. Leaf tissue was sampled after attaining steady-state photosynthesis at 28°C, 1,800 μmol m−2 s−1 PAR, and a constant 350 μbar partial pressure of CO2 in the sample chamber. Using a different section of the same leaf, the leaf temperature was subsequently rapidly increased at 1°C min−1 to the desired level and, after attaining steady-state Pn, leaf tissue was sampled. Leaf tissue was flash frozen by pressing the tissue between two pieces of metal cooled to temperature of liquid N2. RuBP and 3-PGA were extracted in ice-cold 5% (v/v) trifluoroacetic acid and were determined as described in detail in Law and Crafts-Brandner (1999). At least four replicates were sampled for each temperature treatment. For a given plant, portions of the same leaf were sampled after treatment first at 28°C and then after treatment at a higher temperature.
Isolation of Activase for N-Terminal Sequencing
Activase was partially purified from heat-stressed maize leaves by rapid extraction and ammonium sulfate precipitation (Law et al., 2001). The partially purified preparation was fractionated by SDS-PAGE and the polypeptides were transferred to polyvinylidene difluoride membrane (Law et al., 2001). Bands corresponding to polypeptides of 44 and 42 kD were excised and sequenced at the Protein Sequencing Facility at Arizona State University (Tempe; Law et al., 2001). Immunoblotting showed that these polypeptides were recognized by antibodies to activase (see above).
Predicted Rates of Pn
Photosynthetic rates were predicted based on the kinetics of Rubisco with or without adjustment for Rubisco activation state using the full equations of Laing et al. (1974) as described previously (Crafts-Brandner and Salvucci, 2000) and assuming saturating levels of RuBP at all temperatures. Rubisco activity at 28°C was based on the measured rate of Pn at this temperature assuming an activation state of 100%. Rubisco activity was adjusted for temperature and changes in gas solubilities as described previously (Crafts-Brandner and Salvucci, 2000). A CO2 concentration of 2,500 μbar (Edwards et al., 2001) and published kinetic constants for the maize enzyme (Jordan and Ogren, 1981) were used for the calculations.
ACKNOWLEDGMENT
The authors acknowledge the excellent technical support provided by Donald L. Brummett.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002170.
LITERATURE CITED
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. Enzymes of C4 photosynthesis. In: Lea P, editor. Methods in Plant Biochemistry. Vol. 3. London: Academic Press; 1990. pp. 39–72. [Google Scholar]
- Ayala-Ochoa A, Loza-Tavera H, Sánchez de Jiménez E. A cDNA from maize encoding ribulose-1,5-bisphosphate carboxylase/oxygenase activase (accession no. AFO84478) Plant Physiol. 1998;118:1533. [Google Scholar]
- Berry JA, Björkman O. Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol. 1980;31:491–543. [Google Scholar]
- Bilger W, Schreiber U, Lange OL. Chlorophyll fluorescence as an indicator of heat induced limitation of photosynthesis in Arbutus unedoL. In: Tenhunen JD, Catarino FM, Lange OL, editors. Plant Response to Stress. Berlin: Springer-Verlag; 1987. pp. 391–399. [Google Scholar]
- Bukhov NG, Samson G, Carpentier R. Nonphotosynthetic reduction of the intersystem electron transport chain of chloroplasts following heat stress: steady-state rate. Photochem Photobiol. 2000;72:351–357. [PubMed] [Google Scholar]
- Bukhov NG, Wiese C, Neimanis S, Heber U. Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res. 1999;59:81–93. [Google Scholar]
- Chua N-H. Electrophoretic analysis of chloroplast proteins. Methods Enzymol. 1980;69:434–446. [Google Scholar]
- Crafts-Brandner SJ, Law RD. Effect of heat stress on the inhibition and recovery of the ribulose-1,5-bisphosphate carboxylase/oxygenase activation state. Planta. 2000;212:67–74. doi: 10.1007/s004250000364. [DOI] [PubMed] [Google Scholar]
- Crafts-Brandner SJ, Salvucci ME. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc Natl Acad Sci USA. 2000;97:13430–13435. doi: 10.1073/pnas.230451497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Walker D. C3,C4: Mechanisms and Cellular and Environmental Regulation of Photosynthesis. Berkeley: University of California Press; 1983. [Google Scholar]
- Edwards GE, Furbank RT, Hatch MD, Osmond CB. What does it take to be C4? Lessons from the evolution of C4 photosynthesis. Plant Physiol. 2001;125:46–49. doi: 10.1104/pp.125.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U, Crafts-Brandner SJ, Salvucci ME. Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 1998;116:539–546. doi: 10.1104/pp.116.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion S, von Caemmerer S, Ashton AR. Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Aust J Plant Physiol. 1997;24:477–485. [Google Scholar]
- Giglioli-Guivarc'h N, Pierre J-N, Brown S, Chollet R, Vidal J, Gadal P. The light-dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell. 1996;8:573–586. doi: 10.1105/tpc.8.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M. Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ. 1993;16:461–467. [Google Scholar]
- Heckathorn SA, Downs CA, Sharkey TD, Coleman JS. The small, methionine-rich chloroplast heat-shock protein protects photosystem II electron transport during heat stress. Plant Physiol. 1998;116:439–444. doi: 10.1104/pp.116.1.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Chollet R. Post-translational regulation of phosphoenolpyruvate carboxylase in C4 and crassulacean acid metabolism plants. Plant Physiol. 1991;95:981–985. doi: 10.1104/pp.95.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Grodzinski B. The effect of leaf temperature and photorespiratory conditions on export of sugars during steady-state photosynthesis in Salvia splendons. Plant Physiol. 1996;111:169–178. doi: 10.1104/pp.111.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Ogren WL. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- Jordan DB, Ogren WL. The CO2specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase: dependence on ribulose-bisphosphate concentration, Ph and temperature. Planta. 1984;161:308–313. doi: 10.1007/BF00398720. [DOI] [PubMed] [Google Scholar]
- Kobza J, Edwards GE. Influences of leaf temperature on photosynthetic carbon metabolism in wheat. Plant Physiol. 1987;83:69–74. doi: 10.1104/pp.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate CA, Adcock MD, Leegood RC. Effects of temperature on the regulation of photosynthetic carbon assimilation in leaves of maize and barley. Planta. 1991;181:547–554. doi: 10.1007/BF00193009. [DOI] [PubMed] [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. Regulation of soybean net photosynthetic CO2 fixation by the interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiol. 1974;54:678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ. Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1999;120:173–181. doi: 10.1104/pp.120.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ. High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys. 2001;386:261–267. doi: 10.1006/abbi.2000.2225. [DOI] [PubMed] [Google Scholar]
- Law RD, Crafts-Brandner SJ, Salvucci ME. Heat stress induces the synthesis of a new form of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in cotton leaves. Planta. 2001;214:117–125. doi: 10.1007/s004250100592. [DOI] [PubMed] [Google Scholar]
- Long S. Ecology of C4 photosynthesis: environmental responses. In: Sage RR, Monson RK, editors. C4 Plant Biology. New York: Academic Press; 1998. [Google Scholar]
- Monson RK, Stidham MA, Williams GJ, III, Edwards GE, Uribe EG. Temperature dependence of photosynthesis in Agropyron smithii Rybd: factors affecting net CO2 uptake in intact leaves and contribution from ribulose-1,5-bisphosphate carboxylase measured in vivo and in vitro. Plant Physiol. 1982;69:921–928. doi: 10.1104/pp.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz JT, Raynes DA, Jensen RG. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci USA. 1981;78:2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot. 2002;53:609–620. doi: 10.1093/jexbot/53.369.609. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD. The effect of temperature on the occurrence of O2 and CO2insensitive photosynthesis in field grown plants. Plant Physiol. 1987;84:658–664. doi: 10.1104/pp.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seeman JR. The in vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgarisL. Planta. 1988;174:407–416. doi: 10.1007/BF00959528. [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seeman JR. Acclimation of photosynthesis to elevated CO2in five C3 species. Plant Physiol. 1989;89:590–596. doi: 10.1104/pp.89.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME. Subunit interactions of Rubisco activase: Polyethylene glycol promotes self-association, stimulates ATPase and activation activities, and enhances interactions with Rubisco. Arch Biochem Biophys. 1992;298:688–696. doi: 10.1016/0003-9861(92)90467-b. [DOI] [PubMed] [Google Scholar]
- Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol. 2001;127:1053–1064. [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Wolfe GR, Hendrix DL. Purification and properties of an unusual NADPH-dependent ketose reductase from the silverleaf whitefly. Insect Biochem Mol Biol. 1998;28:357–363. [Google Scholar]
- Sánchez de Jiménez E, Medrano L, Martínez-Barajas E. Rubisco activase, a possible new member of the molecular chaperone family. Biochemistry. 1995;34:2826–2831. doi: 10.1021/bi00009a012. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentisleads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol. 1997;113:469–477. doi: 10.1104/pp.113.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis E. Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta. 1981a;151:33–39. doi: 10.1007/BF00384234. [DOI] [PubMed] [Google Scholar]
- Weis E. The temperature sensitivity of dark-inactivation and light-activation of the ribulose-1,5-bisphosphate carboxylase in spinach chloroplasts. FEBS Lett. 1981b;129:197–200. [Google Scholar]