Abstract
Dinucleotide repeat DNA with the pattern (GA)n/(TC)n, so-called GAGA elements, control gene expression in animals, and are recognized by a specific regulatory protein. Here, a yeast one-hybrid screen was used to isolate soybean (Glycine max) cDNA encoding a GAGA-binding protein (GBP) that binds to (GA)n/(CT)n DNA. Soybean GBP was dissimilar from the GAGA factor of Drosophila melanogaster. Recombinant GBP protein did not bind to dinucleotide repeat sequences other than (GA)n/(CT)n. GBP bound to the promoter of the heme and chlorophyll synthesis gene Gsa1, which contains a GAGA element. Removal of that GAGA element abrogated binding of GBP to the promoter. Furthermore, insertion of the GAGA element to a nonspecific DNA conferred GBP-binding activity on that DNA. Thus, the GAGA element of the Gsa1 promoter is both necessary and sufficient for GBP binding. Gbp mRNA was expressed in leaves and was induced in symbiotic root nodules elicited by the bacterium Bradyrhizobium japonicum. In addition, Gbp transcripts were much higher in leaves of dark-treated etiolated plantlets than in those exposed to light for 24 h. Homologs of GBP were found in other dicots and in the monocot rice (Oryza sativa), as well. We suggest that interaction between GAGA elements and GBP-like proteins is a regulatory feature in plants.
Repetitive DNA sequences are found throughout the genomes of higher eukaryotes. Satellite, minisatellite, and microsatellite DNAs are tandemly repeated sequences, with the latter comprising repeats of two to five nucleotides (Charlesworth et al., 1994). Although repeat DNA is primarily associated with heterochromatin, microsatellite sequences can be found within or near genes, and in some cases have been shown to affect gene expression. In particular, so-called GAGA elements comprising the dinucleotide repeat sequence (GA)n/(CT)n have been found in the promoters of numerous genes in animals (Gilmour et al., 1989; Kerrigan et al., 1991; Li et al., 1998; Simar-Blanchet et al., 1998; Bevilacqua et al., 2000; Melfi et al., 2000; Wyse et al., 2000; Busturia et al., 2001; Hodgson et al., 2001; Mishra et al., 2001). GAGA elements have been most thoroughly examined in Drosophila melanogaster, where they are involved in the regulation of numerous developmental genes. In those cases, GAGA elements repress gene expression by stabilizing nucleosomes, and thereby preventing transcription (Croston et al., 1991; Lu et al., 1993). A protein called GAGA factor, encoded by the trithorax-like gene in D. melanogaster, binds to GAGA elements in promoters and, in most cases, relieves repression. GAGA factor binding to the element results in local nucleosome disruption to allow gene expression (Tsukiyama et al., 1994; Tsukiyama and Wu, 1995).
Dinucleotide repeat sequences and other microsatellite DNA are also found in higher plants (Lagercrantz et al., 1993; Bell and Ecker, 1994; Struss and Plieske, 1998; Cardle et al., 2000; Casacuberta et al., 2000). The soybean (Glycine max) Gsa1 gene encoding the chlorophyll and heme synthesis enzyme Glu 1-semialdehyde aminotransferase has a (GA)9/(CT)9 GAGA element in its promoter that is implicated to control that gene (Frustaci et al., 1995). Glu 1-semi-aldehyde aminotransferase catalyzes the formation of the tetrapyrrole precursor δ-aminolevulinic acid (ALA); thus, Gsa1 is highly expressed in leaves and symbiotic root nodules for chlorophyll and heme synthesis, respectively (Sangwan and O'Brian, 1993; Frustaci et al., 1995). Leghemoglobin is an abundant plant heme protein in nodules that is not expressed in nonsymbiotic root tissue (for review, see O'Brian, 2000). In accordance, Gsa1 is expressed at a very low level in uninfected roots and GAGA-binding activity is absent in nuclear extracts of that tissue (Frustaci et al., 1995).
In the present study, we identify a regulated gene from soybean that encodes a protein that binds specifically to (GA)n/(CT)n DNA, including the Gsa1 promoter. The soybean GAGA-binding protein (GBP) is dissimilar to the animal protein, but expressed homologs are found in other plants. It is likely that interaction between GAGA elements and its cognate protein is a regulatory feature in gene expression in higher plants.
RESULTS
Isolation of a Soybean Nodule cDNA That Encodes a GBP
A yeast one-hybrid screen allows the isolation of cDNAs that encode proteins that bind to a cis-acting element. A perfect dinucleotide repeat element [(GA)27/(CT)27] was cloned upstream of the HIS3 selectable marker gene in pHISi and subsequently integrated into the genome of yeast (Saccharomyces cerevisiae) strain YM4271. The HIS3 gene confers His prototrophy on the strain, and is not expressed to high levels unless the GAL4-activation domain (AD) is brought to the promoter. The heme and chlorophyll gene Gsa1 from soybean contains a GAGA element in its promoter, and this gene is expressed in leaves and root nodules (Frustaci et al., 1995). Thus, a unidirectional soybean nodule cDNA library was constructed in pGAD424, which results in protein fusions of yeast GAL4-AD with the product of the inserted cDNA. Thus, a fusion between a soybean nodule GAGA-binding domain with GAL4-AD will result in recruitment of the AD to the GAGA element at the HIS3 promoter and activate transcription.
The one-hybrid screen yielded a nodule cDNA clone named pNPGAD3 that conferred His prototrophy on yeast strain YM4271. Introduction of pNPGAD3 into a yeast strain that had the GAGA sequence upstream of the lacZ gene conferred a blue cell phenotype in the presence of X-gal, indicative of reporter gene activity (Fig. 1A). Northern-blot analysis using the nodule cDNA as probe yielded an RNA about 1.2 kb in size, which was substantially longer than the 776-bp cDNA insert of pNPGAD3. An additional 5′ sequence was obtained by PCR using a nodule cDNA library constructed in pUC18 as template and primers complementary to the vector and to a portion of the pNPGAD3 insert cDNA.
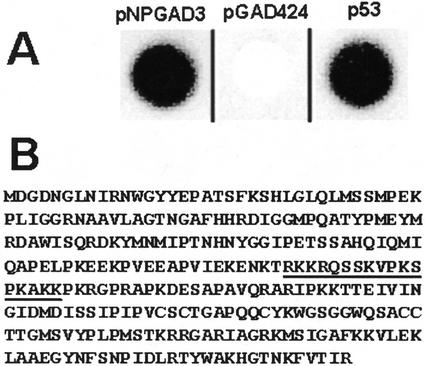
Figure 1.
Identification of GBP. A, Activation of lacZ by pNPGAD3 in a yeast one-hybrid system. Yeast strain YM4271(placZGAGA) harboring pNPGAD3 was spotted on a plate and grown, and then the plate was flooded with X-gal. Blue color formation (dark color in black and white image) indicates β-galactosidase activity (left spot). pNPGAD3 encodes a GAL4-GBP fusion protein, indicating that GBP binds to the GAGA element in the lacZ promoter. The vector pGAG424 did not activate the lacZ gene under the control of the GAGA element (middle spot). As a positive control, pGAD53 m, which encodes a GAL4-p53 fusion protein, was introduced into strain YM4271(p53Blue), which harbors a p53-binding site in the lacZ promoter (right spot). B, The deduced protein sequence of GBP. The underlined segment denotes a putative nuclear localization signal.
The cloned cDNA encoded a protein 282 amino acids in size (Fig. 1B) and contained a long 5′-untranslated region of 237 nucleotides in length. The encoded protein contained no substantial homology to GAGA factor from D. melanogaster, nor was it similar to any characterized protein in the databases. However, it was similar to unknown proteins from other plants (see below). The protein contained a putative nuclear localization signal (Fig. 1B), consistent with its ability to bind DNA. Thus, this screen identified a heretofore uncharacterized gene and protein that we designated Gbp and GBP, respectively (GenBank accession no. AF502431).
GBP Binds (GA)n/(CT)n Dinucleotide Repeat DNA But Not Other Dinucleotide Repeat DNA
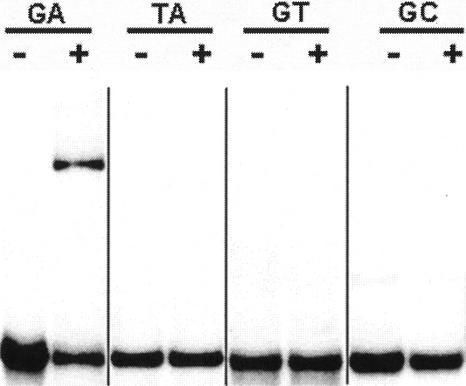
Dinucleotide repeat DNA has been reported to take on unique conformations (Hentschel, 1982); thus, it was possible that GBP recognizes an overall DNA conformation that may not be strictly sequence specific. To address this question, we measured GBP binding to (GA)27/(CT)27 double-stranded DNA and other dinucleotide repeat DNA by electrophoretic mobility shift assays (EMSA). Initial experiments indicated that pure recombinant GBP formed multiple complexes with the (GA)27/(CT)27 DNA as observed by multiple bands and an overall smear pattern on the EMSA gels (data not shown). This was probably the result of multiple binding sites on the DNA for protein. However, a GBP-maltose-binding protein (MBP) fusion gave a single band (Fig. 2), perhaps because steric affects of the larger protein did not allow multiple protein complexes to form on the DNA. MBP alone did not form a complex (data not shown); thus, the binding was specific to GBP. The binding of GBP to (GA)27/(CT)27 DNA in vitro corroborated the yeast one-hybrid data. Electrophoretic mobility shifts were not observed using other dinucleotide repeat DNA; specifically, (GT)27/(CA)27, (AT)27/(TA)27, or (GC)27/(CG)27 double-stranded DNA (Fig. 2). Thus, GBP is specific for (GA)n/(CT)n DNA and is not a general dinucleotide repeat DNA-binding protein.
Figure 2.
Interaction of GBP with dinucleotide repeat DNA. EMSA were carried out with a 54-bp double-stranded DNA comprising 27 repeating units of GA/CT (GA), TA/AT (TA), GT/CA (GT), and GC/CG (GC). The GC/CG-unbound DNA ran faster than the other unbound DNA fragments, and the image was moved for direct comparison with the other free DNAs. The DNAs were run either free (−) or with MBP-GBP fusion protein (+). The mobility of the DNAs were unaffected in the presence of MBP alone (data not shown).
GBP Binds to the Soybean Gsa1 Promoter in Vitro
The soybean Gsa1 promoter contains a perfect dinucleotide repeat sequence of (GA)9/(CT)9 (Frustaci et al., 1995; Fig. 3A). This GAGA element is immediately downstream of a (TA)5/(AT)5 sequence that is presumably a TATA element. We addressed the binding of GBP to the Gsa1 promoter by EMSA as described above using a 60-bp DNA fragment corresponding to the Gsa1 promoter that includes the GAGA element. Purified recombinant GBP bound to the Gsa1 promoter DNA as seen by mobility shifts, whereas a control 60-bp fragment corresponding to a region of pBluescript SK (pSK) did not (Fig. 3C). GBP-MBP fusion protein also bound to this element as well (data not shown), which was consistent with its binding to the longer dinucleotide (Fig. 2). The 18-bp GAGA element sequence within the Gsa1 fragment was removed and replaced with an 18-bp sequence corresponding to a portion of pSK (Fig. 3B). This fragment did not bind to GBP (Fig. 3C), showing that the dinucleotide repeat was necessary for GBP binding to the Gsa1 promoter. It also demonstrated the GBP did not bind to the TATA box in the Gsa1 promoter, which is consistent with the inability to bind to a (TA)27/(AT)27 DNA sequence (Fig. 2). Finally, introduction of the GAGA element into the pSK fragment resulted in binding of the DNA to GBP. The data show that GBP binds to the Gsa1 promoter in vitro and that the GAGA element is both necessary and sufficient for binding.
Figure 3.
Binding of GBP to the promoter of the Gsa1 gene. A, The promoter region of Gsa1, including the GAGA element. The underlined region was used in the gel shift assays. The bolded nucleotide shows the transcription start site determined previously (Frustaci et al., 1995). The italicized codon shows the translation start site. B, The four probes used in the analysis are as follows: I, 60-bp DNA fragment corresponding to the multiple cloning site of pBluescript SK used as a negative control; II, 60-bp DNA fragment within the Gsa1 promoter that includes the GAGA element, underlined in A; III, probe II, except that the 18-bp GAGA element was removed and replaced with an 18-bp sequence from pBluescript SK; and IV, probe I, except 18 bp was removed and replaced with the GAGA element. C, EMSA were carried out with the four probes and purified GBP. −, Free probe; +, presence of GBP in the binding reaction.
The Gbp Gene Is Expressed in Soybean Leaves and Is Induced in Symbiotic Root Nodules
Expression of the Gbp gene was assessed at the mRNA level by northern blots using a Gbp fragment as a probe (Fig. 4). Gbp transcripts were observed in leaves of 24-d-old plants (Fig. 4A). Gbp mRNA was also examined in leaves of etiolated plantlets either grown in the dark completely or exposed to light for 24 h immediately before harvesting the plants. Gbp message levels were high in the dark-treated plants, but expressed to a much lesser extent in those exposed to light, indicating that Gbp is a light-responsive gene in etiolated plantlets. The expression of Gbp in leaves of mature plants, which were harvested in the light, indicates that the effects of light differ in the plants grown under different conditions.
Figure 4.
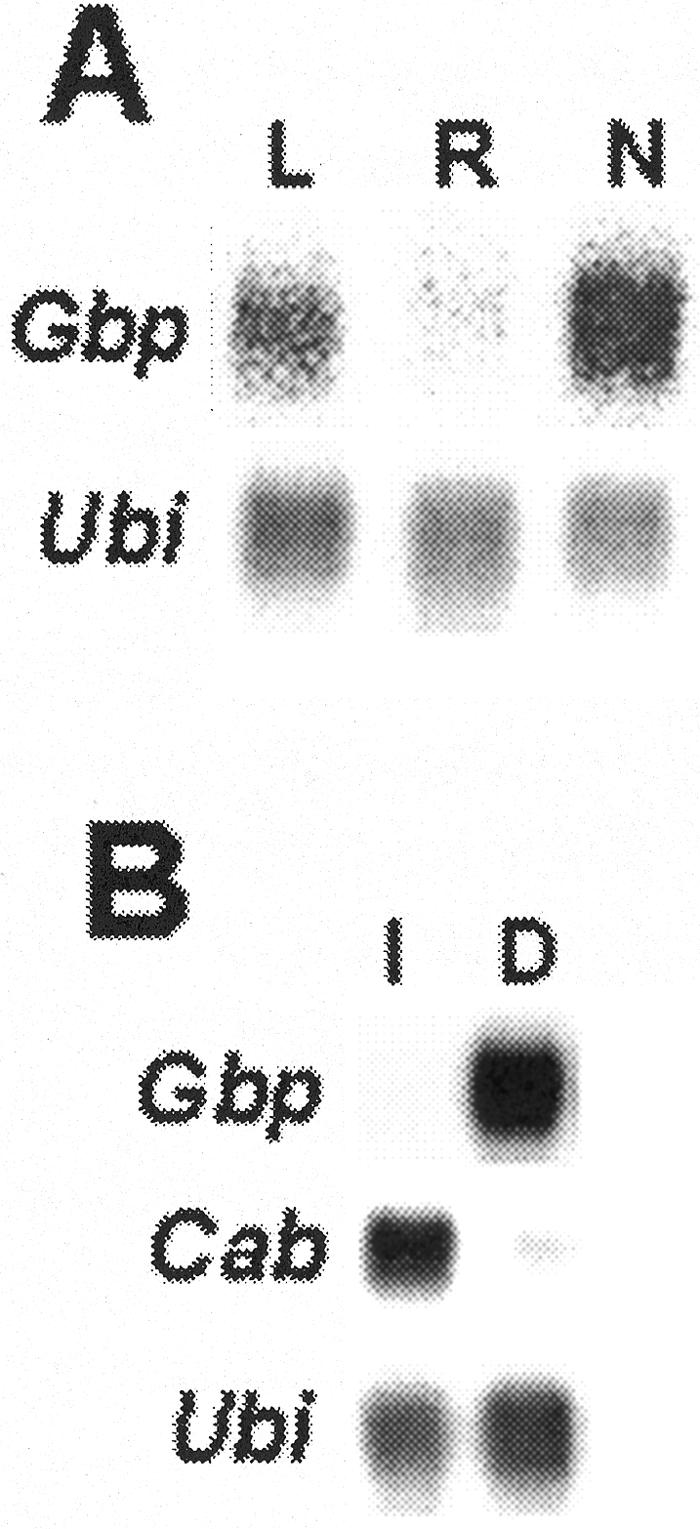
Northern-blot analysis of Gbp mRNA in soybean tissues. A, Gbp mRNA was analyzed in poly(A+) RNA from leaves (L), roots (R), and nodules (N). Ubiquitin (Ubi) was used as a control for a constitutively expressed gene. B, Leaves from illuminated (I) or dark-treated (D) etiolated plantlets were analyzed for Gbp. Cab is a control for a light-regulated gene.
Soybean root nodules from 24-d-old plants elicited by infection with the bacterial endosymbiont Bradyrhizobium japonicum expressed Gbp mRNA at a level comparable with leaves and substantially higher than that found in uninfected roots (Fig. 4A). Thus, Gbp is activated in symbiotic root nodules. The data show that Gbp is a regulated gene in soybean.
Gbp Gene Homologs Are Found in Other Plants
We searched for Gbp gene homologs in other plants in GenBank (http://www.ncbi.nlm.nih.gov) and from expressed sequence tag (EST) cDNA databases compiled by The Institute for Genomics Research (TIGR; http://www.tigr.org/tdb) using BLAST (blastp and tblastn algorithms; Altschul et al., 1990). Genes encoding proteins with high similarity to GBP were found in the dicots Arabidopsis, potato (Solanum tuberosum), and tomato (Lycopersicon esculentum) and in the monocot rice (Oryza sativa; Fig. 5). Soybean GBP had the greatest similarity to the predicted amino acid sequence of homologs in potato and tomato (54% identity, 67% similarity) and the least to that found in rice (42% identity, 53% similarity). Furthermore, the proteins from the dicots were homologous over the entire length, but the rice GBP homolog was less similar at the N-terminal portion and is predicted to contain an additional 53 amino acids at that end. Arabidopsis contains two GBP homologs with 72% identity to each other. Finally, the Gbp gene homologs have corresponding ESTs; therefore, they are expressed genes.
Figure 5.
Alignment of soybean GBP with homologs from other plants. The GBP homologs were identified by BLAST searches of databases. Arabidopsis sequences (protein identification nos. AAF63172 and AAF18588) and the rice sequence (protein identification no. AAK52535) were identified in the GenBank database using a BLAST search. Corresponding ESTs have been found for these sequences; thus, they are annotated as unknown proteins. The potato and tomato sequences were derived from EST consensus sequences found in the TIGR database (http://www.tigr.org/tdb) using a BLAST search (identification nos. TC85862 and TC23396 for tomato and potato, respectively). The sequences were aligned using Clustal W (version 1.81). The stars represent identity at that position in all six sequences. The colon represents similarity at that position.
The Arabidopsis genome contains two additional genes that encode hypothetical proteins (GenBank accession nos. AAC36166 and AAF18661) with high similarity (56% and 48% identity) to soybean GBP at the C terminus (amino acids 166–282), but are dissimilar at the N terminus. This made it difficult to predict whether the numerous plant ESTs in the databases that corresponded only to the 3′ end of Gbp gene actually encode homologs of GBP. However, ESTs corresponding to the 5′ end were identified in soybean (BE660059), Medicago truncatula (TIGR no. TC33063), and Lotus japonicus (GenBank no. AW719515), raising the possibility of an additional Gbp homolog in soybean as well as in other legumes. The Gbp-like M. truncatula cDNAs were from nodules, nodulated roots, and leaves, and the single similar cDNA from L. japonicus was from nodules, indicating that Gbp homologs are expressed similarly among legumes. It is likely that Gbp is an expressed gene in many plants.
DISCUSSION
In the present study, we identified a soybean cDNA encoding GBP, a protein that specifically binds the dinucleotide repeat DNA (GA)n/(CT)n and binds to the GAGA element of the Gsa1 gene. The findings implicate a functional interaction between the dinucleotide repeat DNA and a specific protein in plants; hence, this phenomenon is not confined to animals, but rather it occurs in higher eukaryotes more generally. Dinucleotide repeat DNA can form cruciform structures (Hentschel, 1982). However, GBP recognized only (GA)n/(CT)n repeat DNA and not other dinucleotide repeats (Fig. 2); hence, the basis of recognition is not a general feature of dinucleotide repeat sequences. Thus, GBP is not expected to bind to the TATA box dinucleotide repeat element found in most eukaryotic genes and does not bind to the putative TATA box of Gsa1. Furthermore, the GAGA element of Gsa1 was both necessary and sufficient for binding by GBP (Fig. 3), suggesting that presence of the element in a gene promoter can be taken as prima facie evidence for recognition by GBP.
The Gbp gene was expressed at a low level in roots, but was elevated substantially in symbiotic root nodules (Fig. 4); thus, Gbp is a regulated gene with a likely role in nodule function. The GAGA element of the Gsa1 gene has been implicated in control of that gene, and the expression pattern of Gbp is qualitatively similar to that of Gsa1 (Sangwan and O'Brian, 1993; Frustaci et al., 1995). Furthermore, GBP binds to the Gsa1 promoter at the GAGA element. Collectively, the data suggest that GBP is involved in the positive control of the Gsa1 gene.
High expression of Gsa1 is required for chlorophyll synthesis in green tissues for synthesis of the tetrapyrrole precursor ALA. In accordance, ALA formation in those tissues is controlled by, or coordinated with, factors related to photosynthesis, particularly light (Bougri and Grimm, 1996; Kumar et al., 1996; Tanaka et al., 1996). Nodules are unusual in that a high level of tetrapyrrole synthesis occurs in non-photosynthetic tissue for heme formation, requiring induction of ALA synthesis genes in the absence of light (Frustaci et al., 1995; Sangwan and O'Brian, 1999). Similarly, Gsa1 is expressed in leaves of etiolated plants for synthesis of chlorophyll precursors (Frustaci et al., 1995). Here, we found that Gbp expression does not require light, and is actually higher in etiolated plants exposed to light compared with the light-treated plants. Thus, it is plausible that GBP compensates for the lack of light or some other factor normally associated with photosynthesis to allow expression of Gsa1 in nodules and in etiolated plant leaves.
Gbp gene homologs were identified in other dicots and in a monocot as well; therefore, the gene may be common in higher plants. Analysis of the Arabidopsis chromosome using the Patmatch program (http://www.arabidopsis.org) revealed 813 perfect GA/CT dinucleotide repeats of 18 nucleotides [(GA)9/(CT)9)] or longer in the genome. Although the analysis did not allow a practical determination of the location of all the elements with respect to genes, we could readily find (GA)n/(CT)n sequences in the upstream regions of numerous genes. The gene encoding geranylgeranyl reductase (Keller et al., 1998) has a (GA)9/(CT)9 element immediately upstream of the transcription start site, and the same element is found in the promoter of the GPA1 gene encoding a G protein α-subunit (Ma et al., 1990). Similarly, genes encoding unknown proteins were found that have (GA)n/(CT)n sequences in their upstream regions (e.g. protein identification nos. AAF26463, AAG51765, and AAL08240). The present work shows that soybean GBP binds to (GA)n/(CT)n repeat sequences independent of the genetic context (Fig. 3); therefore, GBP would very likely bind to promoters containing that element, at least in vitro. From this, we speculate that interactions between GBP-like proteins and GAGA elements are a regulatory feature in higher plants.
MATERIALS AND METHODS
Plants and Bacteria
Soybeans (Glycine max cv Essex) were inoculated with Bradyrhizobium japonicum strain I110 and grown in a growth chamber under a 16-h-light/8-h-dark regime at 25°C and harvested after 24 d. Leaves, roots, and nodules were taken for RNA extraction and analysis. Etiolated plantlets were grown in a growth chamber in complete darkness for 10 d and either left in the dark or exposed to direct light to green for the final 24 h before leaves were harvested for RNA isolation. Escherichia coli strains TB1 and DH5α were used for propagation and handling of plasmids. Strains harboring plasmids used in this study were grown in Luria-Bertani (LB) medium supplemented with 50 to 200 μg mL−1 ampicillin.
RNA Isolation and Analysis
RNA was isolated from tissues of 24-d-old plants or from leaves of etiolated seedlings. Tissues were excised, frozen in liquid N2, and homogenized in a blender with buffer and phenol (2:2:3 [w/v/v]). The homogenized buffer contained 500 mm Tris (pH 8), 10 mm EDTA, 100 mm NaCl, 0.5% (w/v) deoxycholate, and 1 mm β-mercaptoethanol. Total RNA was isolated from the homogenate and poly(A+) RNA prepared as described (Frustaci et al., 1995). Northern-blot analysis of poly(A+) RNA was carried out as described previously (Sangwan and O'Brian, 1999) using cDNAs as probes. Five micrograms of RNA was used in each lane.
Construction of a Soybean Nodule cDNA Library for Yeast One-Hybrid Screening
Five micrograms of poly(A+) RNA isolated from 24-d-old nodules was used for cDNA synthesis using a cDNA synthesis kit according to the manufacturer's instructions (Stratagene, La Jolla, CA). The resultant cDNA had an EcoRI site at the 5′ end and a XhoI site at the 3′ end for unidirectional cloning. cDNA greater than 400 bp in size was ligated into the EcoRI/SalI site of the GAL4-AD vector pGAD424 (CLONTECH Laboratories, Palo Alto, CA), and ligated DNA was used to transform E. coli strain XL1-Blue MRF′, and transformants were selected on LB plates containing 200 μg mL−1 ampicillin. Approximately 106 colonies were scraped off the plates and plasmids isolated from the cells. Over 90% of the plasmids contained inserts as estimated from miniplasmid preps of 20 clones. The library should encode fusion protein of the GAL4-AD with products of the nodule cDNA.
Construction of Yeast Strains and Selection for cDNA Encoding GBP Using a One-Hybrid Screen
Double-stranded DNA containing (GA)27/(CT)27 flanked by EcoRI and SalI on the 5′ and 3′ ends [with respect to the (GA)27 strand] were constructed by annealing commercially synthesized oligonucleotides together. The DNA was ligated into the EcoRI/SalI of pLacZi or the EcoRI/MluI site of pHISi, which contain a lacZ and HIS3 gene, respectively. In the latter case, the MluI and SalI sites of the insert and vector, respectively, were filled in with the Klenow fragment of DNA polymerase before ligation. The plasmids were linearized by digestion with XhoI for pHISiGAGA or NcoI for pLacZGAGA, introduced and integrated into the genome of yeast (Saccharomyces cerevisiae) strain YM4271.
The nodule cDNA library was screened for clones encoding proteins that interacted with GAGA element DNA by introducing the library into YM4271(pHISiGAGA) and selecting for colonies that grew in the absence of Leu and His and in the presence of 50 mm 3-amino-1,2,4-triazole. 3-Amino-1,2,4-triazole is a competitive inhibitor of the HIS3 gene and eliminates leaky expression. Strain YM4271 is a His auxotroph; thus, growth requires recruitment of the GAL4-AD to the HIS3 promoter as a result of interaction of fusion protein with the GAGA element in the HIS3 promoter. Colonies arising after 2 d were restreaked on the selective media, and plasmids were isolated and transformed into YM4271(pLacZGAGA) to test for the ability to activate another gene by development of blue color in the presence of X-gal. As a positive control, YM4271(p53BLUE), which contains three tandem copies of a p53-binding site upstream of lacZ, was activated by pGAD53 m, which encodes the mouse p53. The selection identified three identical clones that strongly activated the reporter genes; one of them, pNPGAD3, was used in this study.
pNPGAD3 did not contain the entire cDNA as judged by comparing the insert size with the mRNA size on northern blots. Thus, an additional 5′ sequence was obtained by PCR using the pGAD424 nodule library as template, and primers corresponding to the vector and to the insert. The vector primer used was 5′-GCGATAACGCGTTTGGAAT-3′ and the insert primer used was 5′-GGCCAGATGACCATAGAGGA-3′. A BstXI restriction site was present in the DNA that overlapped the original pNPGAD3 and the PCR product, which was used to construct a single, complete cDNA that contained the complete open reading frame and flanking DNA.
Overexpression of Gbp cDNA in E. coli and Purification of the Recombinant Protein
The coding region of the GBP was amplified by PCR using primers that added restriction sites of BamHI and SalI to the 5′ and 3′ ends, respectively. The forward and reverse primers were 5′-ATCAGTTGGTGGATCCATGGA-TGGTGATAA-3′ and 5′-CCATAGAGTCGACCTACCTGATAGTGACAA-3′. The PCR product was ligated into the BamHI/SalI site of pMalC2 (New England Biolabs, Beverly, MA) and transformed into E. coli strain TB1. The plasmid encodes a fusion of MBP with GBP. The cells were grown at 37°C in LB medium to an optical density of 600 of 0.5, then put on ice for 30 min. Afterward, 2% (v/v) ethanol was added to cultures, and the cells were induced with 0.5 mm isopropylthio-β-galactoside and continued to incubate with shaking at 20°C overnight. The cells were broken with a French pressure cell and cleared by centrifugation at 8,000g for 20 min. The fusion protein was purified from the extract using an amylose resin according to the manufacturer's instructions. The protease Xa was used to cleave the MBP from GBP, and GBP was purified from the other proteins by the amylose resin and size fractionation.
EMSA
Binding of GAGA protein to various DNA elements was carried out by EMSA as described previously (Frustaci et al., 1995). The binding buffer reaction mixture contained 10 mm bis tris borate (pH 7.5), 1 mm MgCl2, 40 mm KCl, 5% (v/v) glycerol, 0.1% (v/v) Nonidet P-40, and 1 mm dithiothreitol. To a 25-μL reaction mix, the following was added: 2.5 μg of bovine serum albumin, 1 μg of unlabeled poly dI-dC as nonspecific competitor DNA, 50 fmol of 32P-labeled DNA (approximately 6 × 106 becquerels), and 10 or 100 pmol of GBP. Samples were run on 5% (w/v) non-denaturing PAGE and exposed to autoradiography as described previously (Frustaci et al., 1995).
Footnotes
This work was supported by the National Science Foundation (grant no. MCB–0089928).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002618.
LITERATURE CITED
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker J. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bevilacqua A, Fiorenza MT, Mangia F. A developmentally regulated GAGA box-binding factor and Sp1 are required for transcription of the hsp70.1 gene at the onset of mouse zygotic genome activation. Development. 2000;127:1541–1551. doi: 10.1242/dev.127.7.1541. [DOI] [PubMed] [Google Scholar]
- Bougri O, Grimm B. Members of a low-copy number gene family encoding glutamyl-tRNA reductase are differentially expressed in barley. Plant J. 1996;9:867–878. doi: 10.1046/j.1365-313x.1996.9060867.x. [DOI] [PubMed] [Google Scholar]
- Busturia A, Lloyd A, Bejarano F, Zavortink M, Xin H, Sakonju S. The MCP silencer of the DrosophilaAbd-B gene requires both pleiohomeotic and GAGA factor for the maintenance of repression. Development. 2001;128:2163–2173. doi: 10.1242/dev.128.11.2163. [DOI] [PubMed] [Google Scholar]
- Cardle L, Ramsay L, Milbourne D, Macaulay M, Marshall D, Waugh R. Computational and experimental characterization of physically clustered simple sequence repeats in plants. Genetics. 2000;156:847–854. doi: 10.1093/genetics/156.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casacuberta E, Puigdomenech P, Monfort A. Distribution of microsatellites in relation to coding sequences within the Arabidopsis thalianagenome. Plant Sci. 2000;157:97–104. doi: 10.1016/s0168-9452(00)00271-5. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371:215–220. doi: 10.1038/371215a0. [DOI] [PubMed] [Google Scholar]
- Croston GE, Kerrigan LA, Lira LM, Marshak DR, Kadonaga JT. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991;251:643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Frustaci JM, Sangwan I, O'Brian MR. gsa1is a universal tetrapyrrole synthesis gene in soybean and is regulated by a GAGA element. J Biol Chem. 1995;270:7387–7393. doi: 10.1074/jbc.270.13.7387. [DOI] [PubMed] [Google Scholar]
- Gilmour DS, Thomas GH, Elgin SCR. Drosophilanuclear proteins bind to regions of alternating C and T residues in gene promoters. Science. 1989;245:1487–1490. doi: 10.1126/science.2781290. [DOI] [PubMed] [Google Scholar]
- Hentschel CC. Homocopolymer sequences in the spacer of a sea urchin histone repeat are sensitive to S1 nuclease. Nature. 1982;295:714–716. doi: 10.1038/295714a0. [DOI] [PubMed] [Google Scholar]
- Hodgson JW, Argiropoulos B, Brock HW. Site-specific recognition of a 70-base-pair element containing d(GA)(n) repeats mediates bithoraxoid polycomb group response element-dependent silencing. Mol Cell Biol. 2001;21:4528–4543. doi: 10.1128/MCB.21.14.4528-4543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller Y, Bouvier F, d'Harlingue A, Camara B. Metabolic compartmentation of plastid prenyllipid biosynthesis: evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur J Biochem. 1998;251:413–417. doi: 10.1046/j.1432-1327.1998.2510413.x. [DOI] [PubMed] [Google Scholar]
- Kerrigan LA, Croston GE, Lira LM, Kadonaga JT. Sequence-specific transcriptional antirepression of the DrosophilaKruppel gene by the GAGA factor. J Biol Chem. 1991;266:574–582. [PubMed] [Google Scholar]
- Kumar AM, Csankovszki G, Söll D. A second and differentially expressed glutamyl-tRNA reductase gene from Arabidopsis thaliana. Plant Mol Biol. 1996;30:419–426. doi: 10.1007/BF00049321. [DOI] [PubMed] [Google Scholar]
- Lagercrantz U, Ellegren H, Andersson L. The abundance of various polymorphic microsatellite motifs differs between plants and vertebrates. Nucleic Acids Res. 1993;21:1111–1115. doi: 10.1093/nar/21.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liang VC, Sedgwick T, Wong J, Shi YB. Unique organization and involvement of GAGA factors in transcriptional regulation of the Xenopusstromelysin-3 gene. Nucleic Acids Res. 1998;26:3018–3025. doi: 10.1093/nar/26.12.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Wallrath LL, Granok H, Elgin SC. (CT) n (GA) n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophilahsp26 gene. Mol Cell Biol. 1993;13:2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Yanofsky MF, Meyerowitz EM. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci USA. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melfi R, Palla F, Di Simone P, Alessandro C, Cali L, Anello L, Spinelli G. Functional characterization of the enhancer blocking element of the sea urchin early histone gene cluster reveals insulator properties and three essential cis-acting sequences. J Mol Biol. 2000;304:753–763. doi: 10.1006/jmbi.2000.4273. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Mihaly J, Barges S, Spierer A, Karch F, Hagstrom K, Schweinsberg SE, Schedl P. The iab-7 polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol Cell Biol. 2001;21:1311–1318. doi: 10.1128/MCB.21.4.1311-1318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian MR. Heme biosynthesis and function in the Rhizobium-legume symbiosis. In: Triplett EW, editor. Prokaryotic Nitrogen Fixation. Model System for the Analysis of a Biological Process. Norfolk, UK: Horizon Scientific Press; 2000. pp. 509–528. [Google Scholar]
- Sangwan I, O'Brian MR. Expression of the soybean (Glycine max) glutamate 1-semialdehyde aminotransferase gene in symbiotic root nodules. Plant Physiol. 1993;102:829–834. doi: 10.1104/pp.102.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I, O'Brian MR. Expression of a soybean gene encoding the tetrapyrrole synthesis enzyme glutamyl-tRNA reductase in symbiotic root nodules. Plant Physiol. 1999;119:593–598. doi: 10.1104/pp.119.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simar-Blanchet AE, Legraverend C, Thissen JP, Le Cam A. Transcription of the rat serine protease inhibitor 2.1 gene in vivo: correlation with GAGA box promoter occupancy and mechanism of cytokine-mediated down-regulation. Mol Endocrinol. 1998;12:391–404. doi: 10.1210/mend.12.3.0080. [DOI] [PubMed] [Google Scholar]
- Struss D, Plieske J. The use of microsatellite markers for detection of genetic diversity in barley populations. Theor Appl Genet. 1998;97:308–315. [Google Scholar]
- Tanaka R, Yoshida K, Nakayashiki T, Masuda T, Tsuji H, Inokuchi H, Tanaka A. Differential expression of two hemA mRNAs encoding glutamyl-tRNA reductase proteins in greening cucumber seedlings. Plant Physiol. 1996;110:1223–1230. doi: 10.1104/pp.110.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T, Becker PB, Wu C. ATP-dependent nucleosome disruption at a heat-shock promoter mediated by binding of GAGA transcription factor. Nature. 1994;367:525–532. doi: 10.1038/367525a0. [DOI] [PubMed] [Google Scholar]
- Tsukiyama T, Wu C. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell. 1995;83:1011–1020. doi: 10.1016/0092-8674(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Wyse BD, Linas SL, Thekkumkara TJ. Functional role of a novel cis-acting element (GAGA box) in human type-1 angiotensin II receptor gene transcription. J Mol Endocrinol. 2000;25:97–108. doi: 10.1677/jme.0.0250097. [DOI] [PubMed] [Google Scholar]