Abstract
The Golgi apparatus behaves as a bona fide Ca2+ store in animal cells and yeast (Saccharomyces cerevisiae); however, it is not known whether this organelle plays a similar role in plant cells. In this work, we investigated the presence of an active Ca2+ accumulation mechanism in the plant cell Golgi apparatus. Toward this end, we measured Ca2+ uptake in subcellular fractions isolated from the elongating zone of etiolated pea (Pisum sativum) epicotyls. Separation of organelles using sucrose gradients showed a strong correlation between the distribution of an ATP-dependent Ca2+ uptake activity and the Golgi apparatus marker enzyme, xyloglucan-fucosyltransferase. The kinetic parameters obtained for this activity were: the rate of maximum Ca2+ uptake of 2.5 nmol mg min−1 and an apparent Km for Ca2+ of 209 nm. The ATP-dependent Ca2+ uptake was strongly inhibited by vanadate (inhibitor concentration causing 50% inhibition [I50] = 126 μm) and cyclopiazonic acid (I50 = 0.36 nmol mg protein−1) and was not stimulated by calmodulin (1 μm). Addition of Cd2+ and Cu2+ at nanomolar concentration inhibited the Ca2+ uptake, whereas Mn2+, Fe2+, and Co2+ had no significant effect. Interestingly, the active calcium uptake was inhibited by thapsigargin (apparent I50 = 88 nm), a well-known inhibitor of the endoplasmic reticulum and Golgi sarco-endoplasmic reticulum Ca2+ ATPase from mammalian cells. A thapsigargin-sensitive Ca2+ uptake activity was also detected in a cauliflower (Brassica oleracea) Golgi-enriched fraction, suggesting that other plants may also possess thapsigargin-sensitive Golgi Ca2+ pumps. To our knowledge, this is the first report of a plant Ca2+ pump activity that shows sensitivity to low concentrations of thapsigargin.
Ca2+ plays an important role in plant growth, development, and signal transduction. Many of the functions mediated by Ca2+ depend upon a fine-tuning of its concentration in different organelles. Therefore, the spatial regulation of Ca2+ concentration is essential for the proper functioning of plant cells. Ca2+ pumps and Ca2+/H+ antiporters appear to be the main mechanisms by which plant cells accumulate Ca2+ in the organelles (Fox and Guerinot, 1998; Sze et al., 2000). Ca2+ pumps are vital in regulating the cytosolic Ca2+ concentration in plant cells, and are widely distributed on membranes, including the plasma membrane (PM), vacuole, and endoplasmic reticulum (ER; Sze et al., 2000).
In animal cells, the Golgi apparatus is also able to accumulate Ca2+. This organelle has been reported to contain two Ca2+ pumps, one sensitive and the other insensitive to thapsigargin (Taylor et al., 1997; Pinton et al., 1998; Rojas et al., 2000). Studies in yeast (Saccharomyces cerevisiae) have also shown that a Ca2+ pump is located at the Golgi apparatus, and, interestingly, mutations in the gene encoding this Ca2+ pump lead to alterations in the glycosylation pattern of secreted proteins (Rudolph et al., 1989). In plants, even though some studies suggested that Ca2+ pump(s) may be located in the Golgi apparatus (Canut et al., 1993; Logan and Venis, 1995), no further evidence has been provided yet that this organelle may play a role in Ca2+ uptake in plant cells.
Plant Ca2+ pumps have been classified in two different classes based on biochemical studies: their comparison with animal Ca2+ pumps, and the effect of inhibitors and activators on their transport activities. These are: type IIA (ER-type) Ca2+-ATPases, and type IIB (PM-type) Ca2+-ATPases (Sze el al., 2000). However, regardless of their classification (ER or PM type), none of the pumps described until now have been shown to be sensitive to low concentration of the animal sarco-endoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin.
In this paper, we analyzed the presence of a Ca2+ pump in the plant Golgi apparatus by carrying out Ca2+ uptake assays on subcellular fractions from pea (Pisum sativum) etiolated epicotyls. Upon identifying a Ca2+ active uptake in Golgi fractions, we performed functional (kinetic and pharmacological) studies of the active Ca2+ transport in an enriched vesicle fraction of the organelle. Here, we provide evidence of a Ca2+ active accumulation into Golgi apparatus vesicles driven by a Ca2+ pump that is inhibited in the nanomolar range by thapsigargin, a feature not previously found in other plant Ca2+-ATPases. Finally, a thapsigargin-sensitive Ca2+ active uptake was also detected in cauliflower (Brassica oleracea) Golgi fractions, suggesting that this calcium uptake mechanism is not pea specific.
RESULTS
An ATP-Dependent Ca2+ Uptake Activity Colocalizes with the Golgi Apparatus
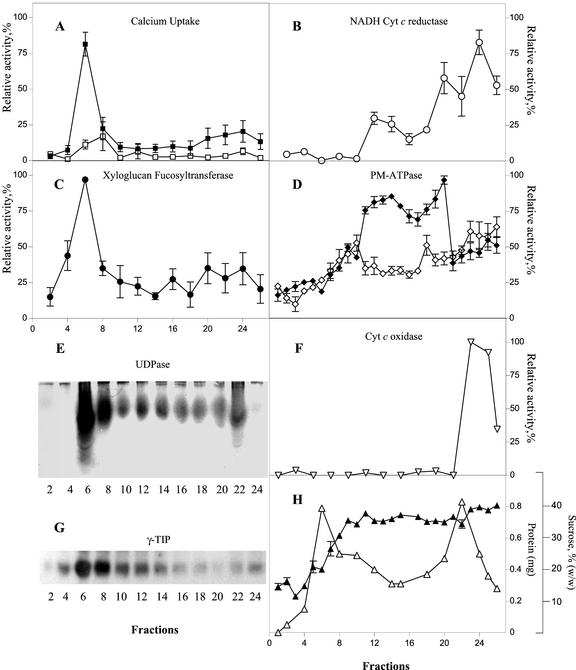
To investigate whether the plant Golgi apparatus contains an active Ca2+ accumulation mechanism, we measured the 45Ca2+ uptake in subcellular fractions obtained from the elongating zone of etiolated pea epicotyls. To separate Golgi vesicles from other organelles (in particular ER membranes), we used Suc step gradients (0.25/1.1/1.3 m) as described in “Materials and Methods.” This procedure allowed a good separation of the Golgi marker enzymes xyloglucan fucosyltransferase (XG-FucTase; Fig. 1C; Wulff et al., 2000) and UDPase (Fig. 1E; Orellana et al., 1997), from ER (Fig. 1B), PM (1D), and mitochondrial (Fig. 1F) markers; however, the tonoplast marker γ-TIP showed a broad distribution and some degree of overlapping with the Golgi marker was observed (Fig. 1G). Measurement across the gradient of the ATP-dependent Ca2+ uptake showed a narrow peak of activity that comigrated with the distribution of the Golgi marker XG-FucTase (Fig. 1A). In addition, a broad peak of ATP-dependent Ca2+ uptake activity was detected toward denser fractions that contained ER and PM membranes.
Figure 1.
Active Ca2+ uptake in subcellular fractions of etiolated pea epicotyls. A, 45Ca2+ uptake by subcellular fractions measured in a reaction mixture containing 1 μm free Ca2+ in the presence (▪) and in the absence (□) of ATP. B, NADH cytochrome c (Cyt c) reductase activity insensitive to antimycin A. C, XG-FucTase activity. D, PM-ATPase activity measured in the presence (♦) and in the absence (⋄) of 70 μm vanadate. E, UDPase activity measured in native gels. F, Cyt c oxidase activity. G, Detection of γ-tonoplast intrinsic protein (γ-TIP) in subcellular fractions using western blots. H, Suc concentration and total protein across the gradient, determined by refractometry and the bicinchoninic acid method, respectively. The results are the average of three independent gradients measured by triplicates. The average relative activity and its deviation are plotted. The relative activity was standardized by setting the highest value obtained from the measurements at 100% relative activity. All subsequent values were then adjusted accordingly and the deviation was calculated. The highest value on each case correspond to: A, 1.95 pmol min−1 μL−1; B, 196 nmol min−1 μL−1; C, 0.52 pmol min−1 μL−1; D, 6.13 nmol min−1 μL−1; and F, 3.92 μmol min−1 μL−1.
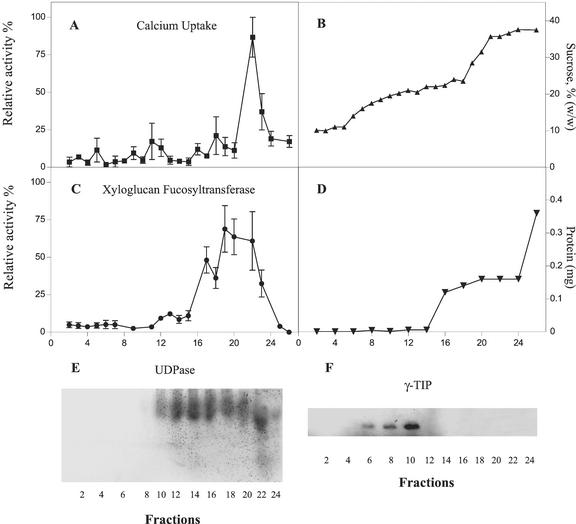
Golgi apparatus membranes were separated from tonoplast by centrifugation in a discontinuous 0.67/1.3 m Suc gradient as described in “Materials and Methods.” As shown in Figure 2, this procedure allowed a significant separation of the XG-FucTase and UDPase activities (Fig. 2, C and E) from the tonoplast marker γ-TIP (Fig. 2F). Although the XG-FucTase and UDPase activities were located toward denser fractions, γ-TIP was detected toward light density fractions. Overexposure of the western blots showed some minor amount of γ-TIP present at high-density fractions. This may correspond to γ-TIP associated to the Golgi apparatus, probably in transit to the tonoplast (Jauh et al., 1999). Measurement of ATP-dependent Ca2+ uptake showed that most of the activity was detected around the more dense fractions of the gradient (1.14 g mL−1) that also contained XG-FucTase and UDPase activities (Fig. 2). In contrast, fractions containing tonoplast did not significantly accumulate Ca2+ under our experimental conditions. The distribution of the Ca2+ uptake activity did not follow the same pattern of XG-FucTase and UDPase activities distribution; however, this result could be explained by the compartments found within the Golgi apparatus (Dupree and Sherrier, 1998) that can lead to differential patterns of enzymes distribution upon separation in density gradients. These findings strongly suggest that Golgi apparatus vesicles contain an active Ca2+ uptake mechanism. Because Ca2+ uptake by the tonoplast was not significant, to avoid the loss of Golgi vesicles due to the fractionation procedure, most of the following experiments were done in an enriched Golgi fraction that contained some level of tonoplast.
Figure 2.
Separation of Golgi apparatus membranes and active Ca2+ uptake from tonoplast. A, ATP-dependent Ca2+ uptake in subcellular fractions measured in the presence of 1 μm free Ca2+. B, Suc concentration across the gradient determined by refractometry. C, XG-FucTase activity. D, Total protein across the gradient. E, UDPase activity measured in native gels. F, Detection of γ-TIP using western blots. The measurements were done in triplicate. The average relative activity and its deviation were plotted. The relative activity was standardized by setting the highest value obtained from the measurements at 100% relative activity. All subsequent values were then adjusted accordingly and the deviation was calculated. The highest value on each case correspond to: A, 0.314 pmol min−1 μL−1; and C, 0.13 pmol min−1 μL−1.
The Active Ca2+ Uptake Mechanism Has a High Affinity for Ca2+ and Is Inhibited by Cd2+ and Cu2+
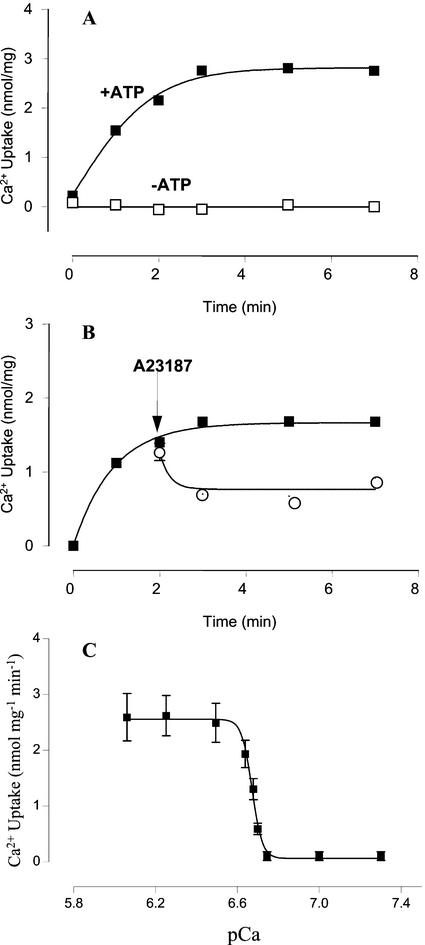
To functionally characterize this Ca2+ transport system, 45Ca2+ uptake assays (Fig. 3) were carried out using an enriched Golgi apparatus-containing vesicle fraction. As shown in Figure 3A, Ca2+ uptake was stimulated by ATP and was negligible in its absence. The Ca2+ accumulated in the vesicles was only partially released (50%) by the Ca2+ ionophore A23187 (Fig. 3B), probably because Ca2+ binds to lumenal macromolecules. The kinetic parameters of pea Golgi apparatus active Ca2+ transport were determined measuring the initial rates of 45Ca2+ uptake at different free Ca2+ concentrations (Fig. 3C). The calculated apparent Km for Ca2+ was 209 nm (pCa 6.68) and the rate of maximum Ca2+ uptake was 2.5 nmol mg−1 min−1.
Figure 3.
Active Ca2+ uptake by Golgi vesicles. A, Time course of Ca2+ incorporation into Golgi apparatus vesicles measured in the incubation buffer containing 300 nm free Ca2+ in the presence (▪) and in the absence (□) of ATP. B, Time course of Ca2+ uptake and release by the Ca2+ ionophore A23187. ▪, Control; ○, ionophore. Free Ca2+ concentration was 210 nm. Values are mean ± se. The arrows indicate the time when the ionophore was added. C, Ca2+ dependence of active Ca2+ uptake in Golgi apparatus-enriched fraction. Ca2+ uptake initial rates were measured at different free Ca2+ concentrations that were estimated with the WinMaxC 2.05 computer program (Chris Patton, Hopkins Marine Station, Stanford University, CA). The experimental points were fitted by a nonlinear fitting program (GraphPad Prism 2.0, GraphPad Software, Inc., San Diego). Apparent Km value of 0.21 μm (pCa 6.68) was obtained from by interpolation in nonlinear fit. Experiments were performed in triplicate. Values are mean ± se.
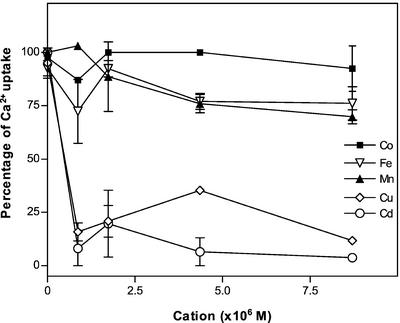
To test the interaction of other divalent cations with the pea Golgi Ca2+ pump we carried out experiments of Ca2+ uptake in the presence of Mn2+, Cd2+, Co2+, Cu2+, and Fe2+ (Fig. 4). As shown in Figure 4, Cu2+ and Cd2+ strongly inhibited Ca2+ uptake, whereas Mn2+ and Fe2+ had only a slight effect even at concentrations as high as 8.7 μm. On the other hand, Co2+ did not inhibit the pump. These results suggest that Mn2+ and Fe2+ did not appreciably compete with Ca2+ for the pump Ca2+-binding sites.
Figure 4.
Ca2+ uptake in the presence of other divalent cations. The 45Ca2+ uptake by the pea Golgi vesicles was measured at 25°C for 1.5 min, in an incubation buffer containing 870 nm free Ca2+ (4 times pump Km), 2 mm ATP, and 3 mm MgCl2. Experiments were carried out in the absence and in the presence of increasing concentrations (870 nm, 1.47 μm, 4.35 μm, and 8.70 μm) of each of the following divalent cations: Cu2+, Co2+, Mn2+, Fe2+, and Cd2+. Values of the Ca2+ uptake at 1.5 min in the presence of different concentrations of the divalent cations are expressed as a percentage of the Ca2+ uptake measured in the absence of other cations (6.3 ± 0.2 nmol mg−1).
Ca2+ Uptake in Golgi Apparatus Is Inhibited by Vanadate and Cyclopiazonic Acid But Not Stimulated by Calmodulin
To identify the Ca2+ uptake mechanism, we tested the effect of some compounds that have been widely used to characterize Ca2+ uptake processes (Inesi and Sagara, 1994; Sze et al., 2000). Because the Golgi apparatus is involved in active membrane-trafficking processes with the ER (Boevink et al., 1998), an organelle where Ca2+ uptake process is well characterized, we performed pharmacological studies in parallel using both Golgi apparatus and ER vesicle fractions. When Golgi apparatus vesicles were incubated in the presence of 200 μm vanadate, the calcium uptake was greatly reduced (Table I), suggesting that the Ca2+ transport activity was driven by a P-type Ca2+-ATPase. As expected, this compound also inhibited active Ca2+ uptake in ER vesicles.
Table I.
Effect of vanadate, cyclopiazonic acid, and calmodulin on active Ca2+ uptake
| Vesicle | Na Vanadate (200 μm)
|
Cyclopiazonic Acid (100 nmol mg−1 protein)
|
Calmodulin (1 μm)
|
|||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| nmol mg−1 | ||||||
| Golgi | 2.6 ± 0.3 | 0.5 ± 0.05 | 2.7 ± 0.1 | 0.0 | 2.4 ± 0.1 | 2.7 ± 0.1 |
| ER | 2.9 ± 0.2 | 0.4 ± 0.1 | 3.2 ± 0.2 | 0.0 | 2.8 ± 0.1 | 6.5 ± 0.4 |
Net ATP-stimulated 45Ca2+ uptake by GA vesicles at 3 min was determined as described in “Materials and Methods.” Free Ca2+ concentration was 300 nm. Values are mean ± se of three different experiments in triplicate.
The effect of cyclopiazonic acid, a specific inhibitor of animal SERCA-type (Inesi and Sagara, 1994) and (IIA) plant Ca2+-ATPases (Sze et al., 2000) was also studied. As shown in Table I, the addition of this compound at 100 nmol mg−1 protein completely inhibited active Ca2+ accumulation by both Golgi apparatus and ER vesicles, suggesting that the Golgi apparatus contains a SERCA-type or a (IIA) Ca2+-ATPase. This result also indicates that Ca2+ uptake driven by a hypothetical H+/Ca2+ antiporter present in Golgi apparatus was negligible under our experimental conditions. This result was confirmed by the finding that both bafilomycin A and carbonyl cyanide 3-chlorophenylhydrazone did not affect Ca2+ uptake (not shown).
It has been reported that calmodulin stimulates plant PM-type (IIB) Ca2+ ATPase activity present in ER (Hsieh et al., 1991; Chen et al., 1993; Hwang et al., 1997; Hong et al., 1999), PMs (Thomson et al., 1993; Askerlund, 1997; Bonza et al., 2000), and tonoplast (Gavin et al., 1993; Malmstrom et al., 1997), but does not activate plant ER-type (IIA) Ca2+ pumps located in ER (Liang and Sze, 1998). We found that 1 μm calmodulin had no effect on the active Ca2+ uptake by Golgi apparatus vesicles (Table I). In contrast, active Ca2+ accumulation in ER vesicles increased 2- to 3-fold in the presence of 1 μm calmodulin.
The Golgi Apparatus Ca2+ Pump Is Sensitive to Thapsigargin
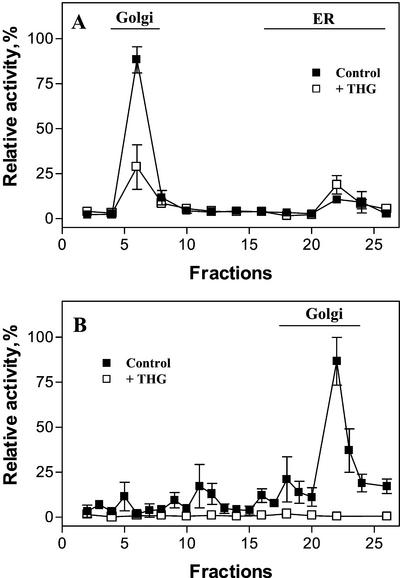
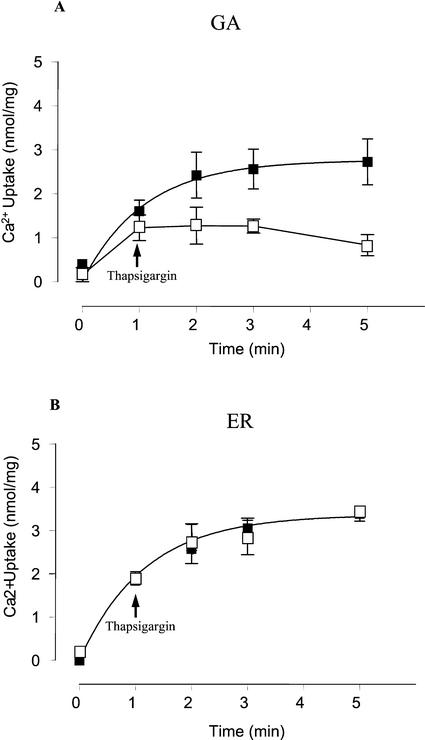
Thapsigargin is a specific inhibitor of most animal intracellular SERCA-type Ca2+ pumps present in the sarcoplasmic/ER (Sagara and Inesi, 1991; Treiman et al., 1998) and Golgi apparatus (Taylor et al., 1997; Zhong and Inesi, 1998; Rojas et al., 2000). However, to our knowledge, no Ca2+ pump sensitive to this compound has been described in plant cells. In pea stems, thapsigargin had a clear effect on the active calcium uptake of subcellular fractions that correlated with the Golgi marker (Fig. 5). In addition, thapsigargin inhibited Ca2+ uptake by Golgi apparatus vesicles, whereas thapsigargin did not block the Ca2+ uptake by pea ER vesicles (Fig. 6). To further characterize the effects of this inhibitor on the Golgi apparatus Ca2+ pump, we studied the concentration dependence of transport inhibition by thapsigargin. Results showed that the apparent inhibitor concentration causing 50% inhibition (I50) value for thapsigargin was 88 nm at 600 μg protein mL−1 (Table II).
Figure 5.
Effect of thapsigargin on the Ca2+ uptake by subcellular fractions from etiolated pea stems. Organelles were separated as described in Figures 1A or 2B, and the uptake of 45Ca2+ by subcellular fractions was measured in a reaction mixture containing 1 μm free Ca2+ in the presence (□) and absence of 2 μm thapsigargin (▪). The lines named Golgi in A and B indicate the distribution in the gradient of the Golgi marker XG-FucTase. The line named ER in A indicates the distribution in the gradient of the ER marker, NADH Cyt c reductase activity insensitive to antimycin A. The activity of the ER marker was negligible in B. THG, Thapsigargin.
Figure 6.
Thapsigargin inhibits the uptake of Ca2+ in Golgi but not in ER vesicles. Uptake of Ca2+ was measured using Golgi apparatus-enriched vesicles (A) and ER-enriched vesicles (B). After 1 min of incubation, 2 μm thapsigargin (THG; □) or the vehicle (▪) were added to the incubation medium and the Ca2+ uptake was determined for another 4 min. The free Ca2+ concentration in the medium was 300 nm. Values are mean ± se.
Table II.
Inhibitor concentrations that cause 50% inhibition (I50) of Ca2+ uptake in Golgi vesicles
Protein concentration in Golgi apparatus fraction was 600 μg mL−1. The range of inhibitor concentrations tested were: thapsigargin, from 0.1 to 3,000 nm; cyclopiazonic acid, between 0.01 and 100 nmol mg−1; and vanadate, from 0.01 to 10,000 μm. Free Ca2+ concentration was 870 nm. Values are mean ± se of three different experiments in triplicate.
A Thapsigargin-Sensitive Ca2+ Uptake Mechanism Is Also Present in Cauliflower
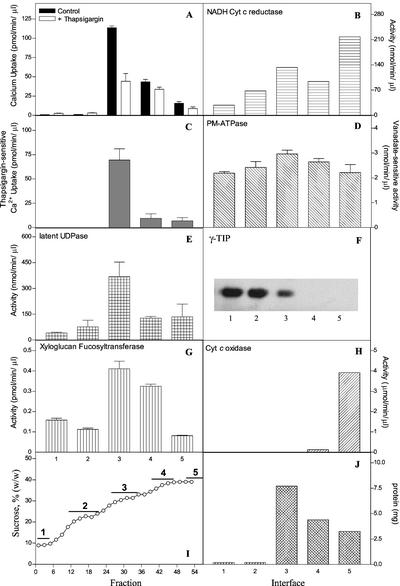
To test whether a thapsigargin-sensitive Golgi Ca2+ pump activity was also present in other plants, we measured Ca2+ uptake in subcellular fractions obtained from the floral meristem of cauliflower. To this end, we fractionated the organelles in a four-step Suc gradient where, in addition to a pellet, four membranes bands were observed and collected. Ca2+ uptake, the effect of thapsigargin, and different marker enzymes were measured. The results (Fig. 7) showed that the distribution of a thapsigargin-sensitive Ca2+ uptake activity correlated with Golgi markers and not with ER, PM, tonoplast, and mitochondrial markers.
Figure 7.
A thapsigargin-sensitive Ca2+ pump is present in Golgi-enriched cauliflower subcellular fractions. Membrane fractions (1–5, depicted in I) were obtained from cauliflower as described in “Materials and Methods.” Ca2+ uptake was measured in the presence and absence of thapsigargin (A). The thapsigargin-sensitive Ca2+ uptake component is shown in C. The Golgi markers Latent UDPase and XG-FucTase are shown in E and G, respectively. B, NADH Cyt c reductase activity insensitive to antimycin A (ER marker). D, Vanadate-sensitive PM-ATPase activity (PM marker). F, Detection of γ-TIP in membrane fractions using western blots. H, Cyt c oxidase activity. I, Suc concentration across the gradient determined by refractometry. The lines named 1 through 5 indicate the interfaces from where the membrane fractions were collected. J, Total protein measured in the membrane interfaces.
DISCUSSION
In the present study, we functionally identified and characterized a novel Ca2+ pump activity present in enriched Golgi apparatus vesicles isolated from the elongation zone of etiolated pea epicotyl. We carried out a detailed subcellular fractionation of pea etiolated pea epicotyl, and measured Ca2+ uptake activity across the gradient. The results show that the main Ca2+ uptake activity present in etiolated pea stems strongly correlated with the Golgi apparatus marker enzyme XG-FucTase. The measurement of another enzymatic marker for this organelle, the Golgi UDPase, using native gels, was also in good agreement with the distribution of XG-FucTase. This confirms the correlation between this Ca2+ uptake activity and the Golgi markers. Subcellular fractionation studies also show that this Ca2+ pump activity was not localized at the ER or in the PM, and it was clearly separated from the tonoplast marker γ-TIP (Jauh et al., 1999). Measurements of a thapsigargin Ca2+ uptake activity in subcellular fractions from cauliflower floral meristem suggested that Golgi apparatus from other plants might have Ca2+ pumps with similar characteristics. The pea Golgi apparatus active Ca2+ accumulation showed high affinity for Ca2+ (a Km of 209 nm). This activity was almost completely inhibited by vanadate and cyclopiazonic acid and was not stimulated by calmodulin, indicating that it was driven by a P-type calcium pump rather than by a Ca2+/H+ antiporter. Ca2+-ATPases with these properties have been found in several endomembranes in different plant tissues and have been classified as type IIA (ER type) to indicate that they share similar characteristics to the SERCA animal Ca2+-ATPases (Sze et al., 2000). The calcium uptake was not inhibited by Mn2+, suggesting that this cation does not compete with the uptake of Ca2+. Therefore, in contrast to other plant Ca2+ pumps, this Ca2+ pump would not transport Mn2+. The inhibition caused by Cd2+ and Cu2+ could be due to competition for the Ca2+ uptake; however, the decrease in Ca2+ transport may be also explained by a lock of the pump, or alternatively due to alterations in the redox state of key residues of the pump (Zhang et al., 1990).
One of the most striking findings of this work was the fact that the Golgi apparatus Ca2+ pump was inhibited by thapsigargin, the most widely used specific inhibitor of SERCA-type Ca2+-ATPases, with an apparent I50 of 88 nm at a protein concentration of 600 μg mL−1. This apparent I50 value could be lower if less protein is used in the assay because Sagara and Inesi (1991) reported that the thapsigargin concentration dependence of Ca2+ transport inhibition is an apparent function of the concentration of protein in the reaction mixture. To our knowledge, this is the first report of a plant Ca2+ pump inhibited by lownanomolar concentrations of this compound. Other studies in plants had shown no effect of thapsigargin on Ca2+ pumps (Liang and Sze, 1998), or an effect at high concentrations that are nonselective (Thomson et al., 1994). In the animal Golgi apparatus membrane, the presence of two Ca2+ pumps has been reported: one sensitive and the other insensitive to thapsigargin (Taylor et al., 1997; Pinton et al., 1998; Rojas et al., 2000). Our results suggest that the plant Golgi-localized Ca2+ pump may have some relationship to the thapsigargin-sensitive animal Golgi calcium pump.
Studies on ER-localized Ca2+ pumps in other plants suggested the presence of two classes of Ca2+ pumps classified as IIB (PM type) and IIA (ER type; Liang and Sze, 1998; Hong et al., 1999). These two types of pumps are thapsigargin insensitive. Our results indicate that Ca2+ uptake by pea ER vesicles is thapsigargin insensitive, stimulated in the presence of calmodulin, and inhibited by cyclopiazonic acid. These results suggest that pea ER contain the IIA and IIB Ca2+ pumps, which are different from the Golgi-localized Ca2+ pump.
Recently, Liang et al. (1997) showed that the Arabidopsis Ca2+-ATPase ECA1 was able to complement the yeast mutant defective in Golgi (Pmr1) and vacuolar (Pmc1) Ca2+ pumps. Even though most of the ECA1 protein fractionated along with the ER, they suggest that some of this protein could be localized in the Golgi. Because ECA1 is not inhibited by thapsigargin, we think it is unlikely that the pea ECA1-orthologous gene encodes the pea Golgi Ca2+-ATPase that we have identified in this work.
In this work, we have kinetically and pharmacologically characterized this Ca2+-ATPase. This work sets the stage whereby a molecular analysis of this Golgi-localized thapsigargin-sensitive Ca2+ ATPase activity can be molecularly characterized. Future investigation will reveal whether this Ca2+ pump is orthologous to one of the members of the P-type ion pump subfamilies recently identified in the Arabidopsis genome (Axelsen and Palmgren, 2001).
What is the role of this Golgi Ca2+ pump? There are several potential roles for the Ca2+ accumulated by the pump in the Golgi apparatus lumen. One possibility is that similar to what occurs in yeast, calcium may play a role in protein glycosylation, and secretion of both proteins and polysaccharides. This may suggest that the role of Ca2+ in protein glycosylation and secretion of proteins/polysaccharides may be an evolutionary conserved mechanism among yeast, mammals, and plants. In addition, Ca2+ is likely to participate in the fusion and fission of vesicles that are in transit in the secretory pathway. On the other hand, the Golgi may serve as a calcium store, like the ER and vacuole. Eventually, this Ca2+ may be released upon activation of signaling pathways. Ca2+ may also be required by lumenally located enzymes that use it as a cofactor. Alternatively, Ca2+ may also bind to polysaccharides that are synthesized in the Golgi and then secreted to the cell wall, like pectins. All of these hypotheses about the role of Ca2+ in the Golgi, as well as the molecular nature of the Ca2+ transporters, remain to be analyzed. Identification of the gene(s) encoding for this (these) transporter(s) will aid in clarify the putative roles of the thapsigargin-sensitive Ca2+ pump located in the Golgi apparatus of etiolated pea epicotyls and cauliflower.
MATERIALS AND METHODS
Plant Material
Pea (Pisum sativum var Alaska) seedlings were grown in moist vermiculite for 7 to 8 d in the dark at 25°C. Stem segments of 1 to 3 cm were excised from the elongating epicotyls, and kept on ice until homogenization. Cauliflower (Brassica oleracea) was obtained from local markets.
Subcellular Fractionation of Pea Stems and Preparation of Golgi and ER Vesicles
Pea stem segments (40–70 g) were minced by hand using razor blades in the presence of 1 volume of 0.5 m Suc (Suc), 0.1 m KH2PO4 (pH 6.65), 5 mm MgCl2, and 1 mm dithiothreitol (added fresh). After the tissue was completely chopped, it was homogenized for 3 min in a mortar. Golgi apparatus vesicles were obtained by step Suc gradient centrifugation following the method described by Muñoz et al. (1996). All isolation procedures were done on ice. The tissue homogenate was filtered through Miracloth (Calbiochem-Novabiochem, San Diego) and centrifuged at 1,000g for 5 min. The supernatant was layered on 8 mL of 1.3 m Suc cushion and centrifuged at 100,000g for 90 min. The upper phase was removed without disturbing the interface fraction and Suc layers of 1.1 and 0.25 m were overlaid on the membrane pad. The Suc gradient was then centrifuged at 100,000g for 100 min. Fractions of 1 mL were collected from the top of the gradient and immediately used in enzymatic and 45Ca2+ uptake assays. To prepare Golgi and ER vesicles for transport assays, fractions containing the maximum enzymatic activity for Golgi and ER markers were collected. These membrane fractions were collected and diluted with cold distilled water to twice the volume. Both suspensions were centrifuged at 100,000g for 50 min. The pellets were gently resuspended using a Dounce homogenizer in a buffer containing 0.25 m Suc, 1 mm MgCl2, and 10 mm Tris-HCl (pH 7.5). The vesicles were aliquoted and stored at −70°C until used.
Golgi Apparatus and Vacuole Membrane Separation
To further separate Golgi apparatus vesicles from the vacuole contaminating vesicles we took the membrane interface collected from the 0.25/1.1 m Suc interface of the discontinuous gradient previously described, and loaded on top of a 5-mL 1.3 m Suc cushion. Then, 10 mL of 0.67 m Suc and 5 mL of 0.25 m Suc were laid on top of the membranes. The gradient was centrifuged for 90 min at 100,000g. One-milliliter fractions were collected and immediately used for Ca2+ uptake assays, enzymatic determinations, and western blotting.
Cauliflower Subcellular Fractionation
The floral meristem was homogenized in 1 volume of 0.5 m Suc, 0.1 m KH2PO4 (pH 6.65), 5 mm MgCl2, and 1 mm dithiothreitol (added fresh) using a blender. The homogenate was filtrated through Miracloth and centrifuged at 1,000g for 5 min. The supernatant was layered on 8 mL of 1.3 m Suc cushion and centrifuged at 100,000g for 90 min. The membranes located on top of the 1.3 m layer were collected, adjusted to 1.1 m Suc, and layered on top of a new 1.3 m Suc cushion. Then, 0.74 and 0.25 m Suc solutions were layered respectively on top of the 1.1 m layer; then, the Suc gradient was centrifuged at 100,000g for 100 min. After centrifugation, four membrane bands and a pellet were observed throughout the gradient. These were collected, spun down at 100,000g, and resuspended in a buffer containing 0.25 m Suc, 1 mm MgCl2, and 10 mm Tris-HCl (pH 7.5). The membrane fractions were named 1 through 4 relative to their position in the tube, from top to bottom, respectively. Fraction 5 corresponds to the pellet resuspended in the same solution.
Subcellular Marker Assays
XG-FucTase activity was measured as a Golgi marker in the presence of 0.1% (v/v) Triton X-100, as described by Wulff et al. (2000). NADH Cyt c reductase insensitive to antimycin A (ER marker) and Cyt c oxidase (mitochondrial marker) were assayed as described by Briskin et al. (1987). Vanadate-sensitive ATPase activity (PM marker) was measured as described by Lanzetta et al. (1979). As a vacuole membrane marker, we used a polyclonal antibody (kindly donated by Dr. John C. Rogers, Institute of Biological Chemistry, Washington State University, Pullman, WA) against the pea tonoplast intrinsic protein γ-TIP. Immunoblotting using anti-γ-TIP antibody was carried out as described by Jauh et al. (1999). Proteins from subcellular fractions were denatured in sample buffer at 60°C, separated by SDS-PAGE, and transferred onto polyvinylidene difluoride membranes. The anti-γ-TIP antibody was used at 0.1 ng mL−1. Goat anti-rabbit IgG conjugated to peroxidase (diluted 1:10,000 [v/v]) was used as secondary antibody. Latent UDPase and UDPase activity in native gels (Golgi markers) were measured as described by Orellana et al. (1997). Proteins were measured with the bicinchoninic acid method (Pierce Chemical, Rockford, IL) with bovine serum albumin as standard.
Ca2+ Uptake Assays
Ca2+ uptake by Golgi apparatus and ER fractions was assayed measuring 45Ca2+ accumulation by the vesicles using a filtration assay. Experiments were performed using triplicates each time, and each experiment was done at least twice. Either Golgi apparatus or ER vesicles were suspended (0.3 or 0.6 mg mL−1 final concentration) in a buffer solution containing 100 mm KCl and 20 mm MOPS-Tris (pH 7.2). Free Ca2+ concentration in the incubation buffer was fixed at desired values using variable amounts of CaCl2 (containing 4.5 mCi 45Ca2+ mmol−1 CaCl2, NEN Life Science Products, Boston) and EGTA. Free Ca2+ concentrations were calculated by means of the WinMAXC 2.05 computer program. Vesicles were incubated in the buffer at 25°C for 1 min and transport was initiated (time zero) by adding a mixture of ATP and MgCl2 to final concentrations of 2 and 3 mm, respectively. Samples of 250 μL in triplicate or quadruplicate were taken at different times and filtered through 0.45-μm nitrocellulose membranes (Millipore, Bedford, MA). The filters were immediately rinsed with 5 mL of ice-cold buffer (100 mm KCl and 20 mm MOPS-Tris [pH 7.2]), then dried and counted in a scintillation counter. ATP-dependent Ca2+ uptake (nmol mg−1 min−1) was defined as the difference between the 45Ca2+ retained in the filters following incubations in the presence and absence of ATP-Mg.
When needed, orthovanadate (200 μm), cyclopiazonic acid (100 nmol mg protein−1), thapsigargin (2 μm), calmodulin (1 μm), carbonyl cyanide 3-chlorophenylhydrazone (5 μm), Cd2+, Cu2+, Co2+, Fe2+, Mn2+, or the Ca2+ ionophore A23187 (10 μm) were added to the incubating solution.
ACKNOWLEDGMENTS
We thank Dr. John C. Rogers for providing the γ-TIP antibody, Dr. Lee Meisel for a helpful discussion of the results and the manuscript, and Lorena Norambuena for advice with subcellular fractionation.
Footnotes
This work was supported in part by Fondo Nacional de Desarrollo Científico y Tecnológico (grant nos. 1970494, 1000675, and ICM P 99–031–F to A.O.) and by Comisión Nacional de Investigación Científica y Tecnológica (doctoral fellowship to V.R.O.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002055.
LITERATURE CITED
- Askerlund P. Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower. Plant Physiol. 1997;114:999–1007. doi: 10.1104/pp.114.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 2001;126:696–706. doi: 10.1104/pp.126.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- Bonza MC, Morandini P, Luoni L, Geisler M, Palmgren MG, De Michelis MI. At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol. 2000;123:1495–1505. doi: 10.1104/pp.123.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin DP, Leonard RT, Hodges TK. Isolation of the plasma membrane: membrane markers and general principles. Methods Enzymol. 1987;148:542–558. [Google Scholar]
- Canut H, Carrasco A, Rossignol M, Ranjeva R. Is vacuole the richest store of IP3-mobilizable calcium in plant cells? Plant Sci. 1993;90:135–143. [Google Scholar]
- Chen FH, Ratterman DM, Sze H. A plasma membrane-type Ca2+-ATPase of 120 kilodaltons on the endoplasmic reticulum from carrot (Daucus carota) cells: properties of the phosphorylated intermediate. Plant Physiol. 1993;102:651–661. doi: 10.1104/pp.102.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P, Sherrier DJ. The plant Golgi apparatus. Biochim Biophys Acta. 1998;1404:259–270. doi: 10.1016/s0167-4889(98)00061-5. [DOI] [PubMed] [Google Scholar]
- Fox TC, Guerinot ML. Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:669–696. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- Gavin O, Pilet PE, Chanson A. A tonoplast localization of a calmodulin-stimulated Ca-pump from maize roots. Plant Sci. 1993;92:143–150. [Google Scholar]
- Hong B, Ichida A, Wang Y, Gens JS, Pickard BG, Harper JF. Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 1999;119:1165–1176. doi: 10.1104/pp.119.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh W-L, Pierce WS, Sze H. Calcium-pumping ATPases in vesicles from carrot cells. Stimulation by calmodulin or phosphatidylserine and formation of a 120 kD phosphoenzyme. Plant Physiol. 1991;97:1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between ER-type and PM-type Ca pumps: partial purification of a 120 kD Ca-ATPase from endomembranes. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPase. J Membr Biol. 1994;141:1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Jauh G-Y, Phillips TE, Rogers JC. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell. 1999;11:1867–1882. doi: 10.1105/tpc.11.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8586. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Sze H. A high-affinity Ca2+ pump, ECA1, from the endoplasmic reticulum is inhibited by cyclopiazonic acid but not by thapsigargin. Plant Physiol. 1998;118:817–825. doi: 10.1104/pp.118.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Venis MA. Characterisation and immunological identification of a calmodulin-stimulated Ca2+-ATPase from maize shoots. J Plant Physiol. 1995;145:702–710. [Google Scholar]
- Malmstrom S, Askerlund P, Palmgren MG. A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Lett. 1997;400:324–328. doi: 10.1016/s0014-5793(96)01448-2. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Norambuena L, Orellana A. Evidence for a UDP-glucose transporter in Golgi apparatus-derived vesicles from pea and its possible role in polysaccharide biosynthesis. Plant Physiol. 1996;112:1585–1594. doi: 10.1104/pp.112.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana A, Neckelmann G, Norambuena L. Topography and function of Golgi uridine-5′-diphosphatase from pea stems. Plant Physiol. 1997;114:99–107. doi: 10.1104/pp.114.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Pozzan T, Rizzuto R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 1998;17:5298–5308. doi: 10.1093/emboj/17.18.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas P, Surroca A, Orellana A, Wolff D. Kinetic characterization of calcium uptake by the rat liver Golgi apparatus. Cell Biol Int. 2000;24:229–233. doi: 10.1006/cbir.2000.0496. [DOI] [PubMed] [Google Scholar]
- Rudolph HK, Anteví A, Fink ER, Buckley CM, Dorman TE, Le Vitre JA, Davidow LS, Mao J-I, Moir DT. The yeast secretory pathway is perturbed by mutation in PMRI, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Jones SM, Dahl RH, Nordeen MH, Howell KE. Characterization of the Golgi complex cleared of proteins in transit and examination of calcium uptake activities. Mol Biol Cell. 1997;8:1911–1931. doi: 10.1091/mbc.8.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LJ, Xing T, Hall JL, Williams LE. Investigation of the calcium-transporting ATPases at the endoplasmic reticulum and plasma membrane of red beet (Beta vulgaris) Plant Physiol. 1993;102:553–564. doi: 10.1104/pp.102.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LJ, Hall JL, Williams LE. Study of the effect of inhibitors of the animal sarcoplasmic/endoplasmic reticulum-type calcium pumps on the primary calcium ATPases of red beet. Plant Physiol. 1994;104:1295–1300. doi: 10.1104/pp.104.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiman M, Caspersen C, Christensen SB. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- Wulff C, Norambuena L, Orellana A. GDP-fucose uptake into the golgi apparatus during xyloglucan biosynthesis requires the activity of a transporter-like protein other than the UDP-glucose transporter. Plant Physiol. 2000;122:867–877. doi: 10.1104/pp.122.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Yamaguchi M, Kimura S, Higham S, Kraus-Friedmann N. Effects of heavy metal on rat liver microsomal Ca2+-ATPase and Ca2+ sequestering. Relation to SH groups. J Biol Chem. 1990;265:2184–2189. [PubMed] [Google Scholar]
- Zhong L, Inesi G. Role of the S3 stalk segment in the thapsigargin concentration dependence of sarco-endoplasmic reticulum Ca2+ ATPase inhibition. J Biol Chem. 1998;273:12994–12998. doi: 10.1074/jbc.273.21.12994. [DOI] [PubMed] [Google Scholar]