Abstract
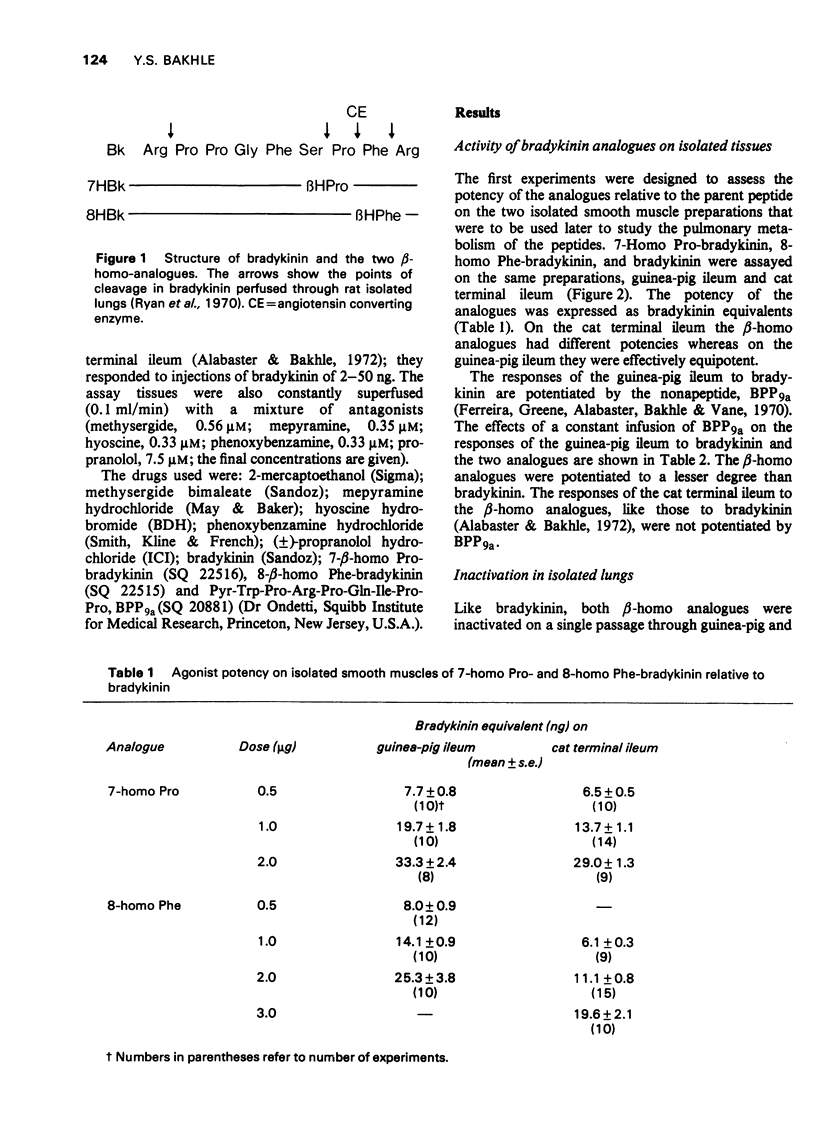
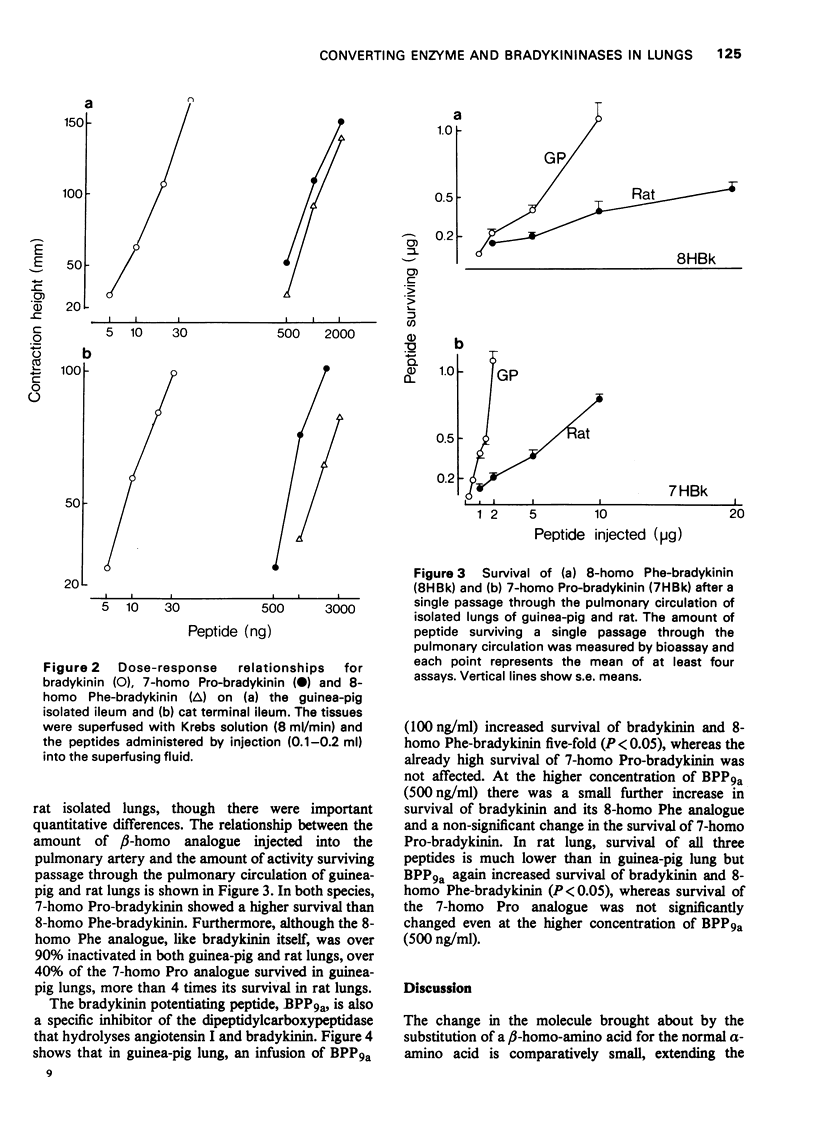
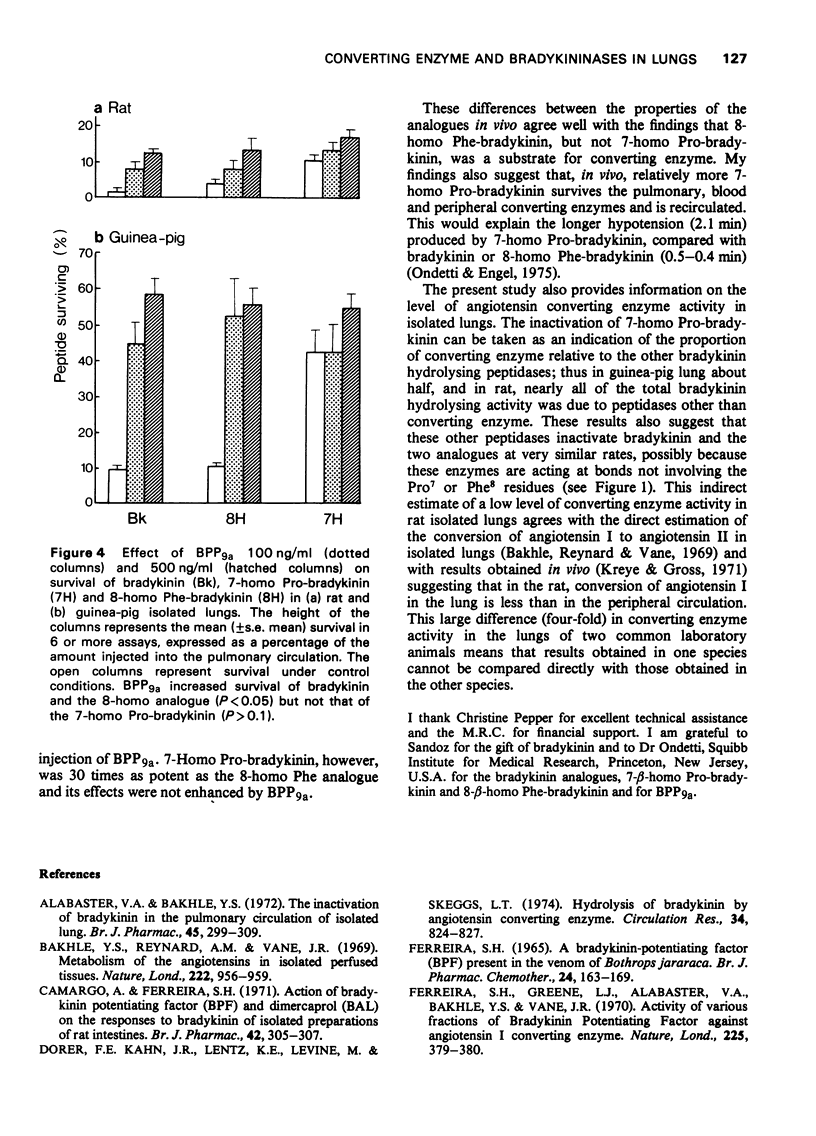
1 The activity and pulmonary metabolism of two peptides, 7-homo Pro-bradykinin and 8-homo Phe-bradykinin were studied in isolated systems. 2 Both analogues were about 50-70 times less active than bradykinin on the guinea-pig ileum and 70-160 times less active on isolated strips of cat terminal ileum. 3 The action of both analogues on guinea-pig ileum was potentiated (2.5-3.0 fold) by a bradykinin potentiating peptide (BPP9a) but less so than the action of bradykinin (4-5 fold). 4 Like bradykinin, the 8-homo Phe analogue was extensively inactivated (greater than 90%) in a single passage through the pulmonary circulation of guinea-pig or rat isolated lungs and this inactivation was prevented by pre-treatment of the lungs with BPP9a. 5 The 7-homo Pro analogue was inactivated to a lesser degree in guinea-pig lungs (58%) and in rat lungs (89%) and its inactivation was not affected by BPP9a. 6 It is concluded that the 8-homo Phe analogue is a substrate for the dipeptidylcarboxypeptidase (angiotensin I converting enzyme) of lung, whereas the 7-homo Pro analogue is not a substrate. 7 There is about four times as much dipeptidylcarboxypeptidase activity in guinea-pig isolated lungs as there is in rat isolated lungs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alabaster V. A., Bakhle Y. S. The inactivation of bradykinin in the pulmonary circulation of isolated lungs. Br J Pharmacol. 1972 Jun;45(2):299–310. doi: 10.1111/j.1476-5381.1972.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhle Y. S., Reynard A. M., Vane J. R. Metabolism of the angiotensins in isolated perfused tissues. Nature. 1969 Jun 7;222(5197):956–959. doi: 10.1038/222956a0. [DOI] [PubMed] [Google Scholar]
- Camargo A., Ferreira S. H. Action of bradykinin potentiating factor (BPF) and dimercaprol (BAL) on the responses to bradykinin of isolated preparations of rat intestines. Br J Pharmacol. 1971 Jun;42(2):305–307. doi: 10.1111/j.1476-5381.1971.tb07113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorer F. E., Kahn J. R., Lentz K. E., Levine M., Skeggs L. T. Hydrolysis of bradykinin by angiotensin-converting enzyme. Circ Res. 1974 Jun;34(6):824–827. doi: 10.1161/01.res.34.6.824. [DOI] [PubMed] [Google Scholar]
- FERREIRA S. H. A BRADYKININ-POTENTIATING FACTOR (BPF) PRESENT IN THE VENOM OF BOTHROPS JARARCA. Br J Pharmacol Chemother. 1965 Feb;24:163–169. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Greene L. H., Alabaster V. A., Bakhle Y. S., Vane J. R. Activity of various fractions of bradykinin potentiating factor against angiotensin I converting enzyme. Nature. 1970 Jan 24;225(5230):379–380. doi: 10.1038/225379a0. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. The disappearance of bradykinin and eledoisin in the circulation and vascular beds of the cat. Br J Pharmacol Chemother. 1967 Jun;30(2):417–424. doi: 10.1111/j.1476-5381.1967.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreye V. A., Gross F. Conversion of angiotensin I to angiotensin II in peripheral vascular beds of the rat. Am J Physiol. 1971 May;220(5):1294–1296. doi: 10.1152/ajplegacy.1971.220.5.1294. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Engel S. L. Bradykinin analogs containing beta-homoamino acids. J Med Chem. 1975 Jul;18(7):761–763. doi: 10.1021/jm00241a024. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Angiotensin-converting enzyme and the regulation of vasoactive peptides. Annu Rev Biochem. 1976;45:73–94. doi: 10.1146/annurev.bi.45.070176.000445. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Abinko T., Endo N., Kameyama T., Sasaki K. Biologically active synthetic fragments of bradykinin. Jpn J Pharmacol. 1969 Jun;19(2):325–327. doi: 10.1254/jjp.19.325. [DOI] [PubMed] [Google Scholar]


