Abstract
The extent to which Crassulacean acid metabolism (CAM) plant δ13C values provide an index of the proportions of CO2 fixed during daytime and nighttime was assessed. Shoots of seven CAM species (Aloe vera, Hylocereus monocanthus, Kalanchoe beharensis, Kalanchoe daigremontiana, Kalanchoe pinnata, Vanilla pauciflora, and Xerosicyos danguyi) and two C3 species (teak [Tectona grandis] and Clusia sp.) were grown in a cuvette, and net CO2 exchange was monitored for up to 51 d. In species exhibiting net dark CO2 fixation, between 14% and 73.3% of the carbon gain occurred in the dark. δ13C values of tissues formed inside the cuvette ranged between −28.7‰ and −11.6‰, and correlated linearly with the percentages of carbon gained in the light and in the dark. The δ13C values for new biomass obtained solely during the dark and light were estimated as −8.7‰ and −26.9‰, respectively. For each 10% contribution of dark CO2 fixation integrated over the entire experiment, the δ13C content of the tissue was, thus, approximately 1.8‰ less negative. Extrapolation of the observations to plants previously surveyed under natural conditions suggests that the most commonly expressed version of CAM in the field, “the typical CAM plant,” involves plants that gain about 71% to 77% of their carbon by dark fixation, and that the isotopic signals of plants that obtain one-third or less of their carbon in the dark may be confused with C3 plants when identified on the basis of carbon isotope content alone.
Soon after the discovery that C4 plants differ distinctively from C3 plants in their 13C/12C composition (Bender, 1968, 1971), it was proposed that, in Crassulacean acid metabolism (CAM) plants, the 13C to 12C ratio may be an indicator of the extent to which their biomass was derived from nocturnal CO2 fixation relative to diurnal CO2 fixation (Bender et al., 1973; Osmond et al., 1973; Allaway et al., 1974). The 13C to 12C ratio is an indicator because the enzyme responsible for net CO2 uptake in the dark, PEP carboxylase, discriminates less against 13C than does Rubisco, the enzyme responsible for most net CO2 uptake during the light. CAM plants, which gain CO2 almost exclusively at night via PEP carboxylase, would, thus, be expected to show δ13C values similar to C4 plants, whereas the δ13C values of plants in which dark CO2 fixation contributes only little to carbon gain should resemble those of C3 plants. The postulate was supported by evidence such as that of Nalborczyk et al. (1975) who reported that well-watered young Kalanchoe daigremontiana plants supplied for 24 d with CO2 solely during the light exhibited a δ13C value of −25.5‰, and plants supplied with CO2 only during the dark exhibited a δ13C value of −10.6‰, whereas plants supplied with CO2 during the day and the night exhibited an intermediate δ13C value of −15‰. Subsequent theoretical and experimental evidence demonstrated that fractionation in CAM plants reflects both carboxylation and diffusion limitations (O'Leary and Osmond, 1980; O'Leary, 1981; Holtum et al., 1983, 1984), which, in turn, are modified by temperature (Deleens et al., 1985), environmental stresses (Osmond et al., 1976), and the leakage of CO2 from the tissues (O'Leary, 1988; Broadmeadow et al., 1992; Borland and Griffiths, 1996).
This interpretation of whole-plant carbon isotope contents provided the theoretical basis for the use of δ13C values to screen plants for CAM, particularly in the tropics (Rundel et al., 1979; Winter, 1979; Teeri et al., 1981; Griffiths and Smith, 1983; Winter et al., 1983; Earnshaw et al., 1987; Arroyo et al., 1990; Kluge et al., 1991; Zotz and Ziegler, 1997). In recent analyses on the evolution of CAM in the Bromeliaceae and the closely related, reportedly C3 Rapateaceae, the δ13C values of about two-thirds of the 2,800 known species of bromeliads and 85 of the approximately 100 species of the Rapateaceae were determined (Crayn et al., 2001; D.M. Crayn, J.A.C. Smith, and K. Winter, unpublished data). The survey of Bromeliaceae yielded a bimodal distribution of δ13C values, with frequency peaks around −13‰ (indicating strong CAM) and −26‰ (indicating C3 species). The rapateads exhibited a broad but unimodal distribution of δ13C values between −37.7‰ and −19.8‰. From such and other studies, it has been estimated that more than 6% of vascular plant species have some ability to exhibit CAM (Smith and Winter, 1996; Winter and Smith, 1996).
A shortcoming of using whole-tissue carbon isotope surveys to obtain an integrated value of the contributions of dark and light CO2 fixation to total carbon gain is that, in the absence of measurements of acidity, CO2 exchange, or instantaneous carbon isotope discrimination, it is unclear where C3 ends and CAM begins. In general, surveys of plant 13C/12C composition indicate a least negative limit for C3 species of around −23‰ to −20‰, values that can also indicate some CAM activity. Confirmed CAM species such as Tillandsia usneoides, Didierea madagascariensis H. Baill., and Microsorium punctatum (L.) Copel. have been reported to exhibit values of −19.8‰ (Griffiths and Smith, 1983), −21.2‰ (Winter, 1979), and −22.6‰ (Holtum and Winter, 1999), respectively. Moreover, diel acid fluctuations have been measured in Tillandsia elongata H.B.K. var subimbricata (Baker) L.B. Sm. and Guzmania monostachia (L.) Rusby ex Mez var monostachia, species for which δ13C values of −26.4‰ and −26.5‰, respectively, have been reported (Griffiths and Smith, 1983).
O'Leary (1988) predicted a linear relationship between δ13C values of CAM plants and the proportions of CO2 fixed at night and during the day. Surprisingly, given the number of isotopic surveys of vegetation that have been published, O'Leary's prediction has never been tested experimentally. O'Leary cautioned that his qualitative model did not take into account environmental interactions. If one assumes that, for a given species subject to a given set of conditions, −28‰ is the isotope composition when 100% of the CO2 is fixed by Rubisco in the light then, theoretically, any δ13C value less negative than −28‰ indicates that some dark fixation occurs. However, in the real world, chemical and diffusional processes contribute to the isotopic composition of plants, such that integrated tissue δ13C values are affected by plant biochemistry, plant-environment interactions and the 13C/12C composition of the source air, all of which exhibit variation (O'Leary, 1981; Farquhar et al., 1989; Griffiths, 1992). Osmond et al. (1976) concluded “nothing short of a complete balance sheet of CO2 assimilation throughout the life of the plant is required to adequately predict these (dark versus light fixation) relationships.”
We aim to afford a better interpretation of intermediate δ13C values measured during vegetation surveys and to provide experimentally measured values for the relationship put forward by O'Leary (1988). To this end, a balance sheet was generated of light and dark CO2 assimilation and δ13C compositions for well-watered, nonstressed CAM plants. Shoots exhibiting different proportions of daytime to nighttime CO2 fixation were grown inside a gas-exchange cuvette for up to 51 d, during which time the proportions of CO2 fixed in the light and dark were quantified. The relationships between the proportions of CO2 fixed in the light and dark and the δ13C value of the biomass accumulated were then assessed.
RESULTS
Plant Growth and Carbon Gain
During the 12 to 51 d inside the gas-exchange cuvette, tissues exhibited increases in dry mass of between 2.6- and 10.3-fold, indicating that between 61% and 99% of the material harvested from the cuvette at the end of the experiments was formed during the experiments (Table I). Leaf areas expanded up to 17.3-fold (Table I). The smallest increase in leaf area, a 1.2-fold increase by leaves from Xerosicyos danguyi, was compensated for by an expansion in leaf thickness that was reflected in a 290% increase in dry mass. The length of the stem of Hylocereus monocanthus in the cuvette extended from 0.5 to 17.3 cm.
Table I.
Dry mass, leaf area, and carbon gain during light and dark periods of shoots of CAM and C3 species developing in a gas-exchange cuvette under various temperature regimes for between 12 and 51 d
| Species | Light/Dark Temperature | Time in Cuvette | Dry Mass
|
Leaf Area
|
Cumulative Carbon Gain
|
Carbon Gain during Final 24 h
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Final | Final/Initial | Final | Final/Initial | Light | Dark | Dark | Dark | |||
| °C | d | mg | cm2 | mmol CO2 | % | % | ||||
| A. vera | 28 /22 | 30 | 146 | 2.9 | ND | – | 1.77 | 2.28 | 56.3 | 60.0 |
| Clusia sp. | 28 /22 | 18 | 101 | 2.6 | 13.8 | 3.5 | 4.88 | −0.45 | 0 | 0 |
| H. monocanthus | 28 /22 | 18 | 811 | 90.1 | 82.3a | – | 1.28 | 2.93 | 69.5 | 81.9 |
| K. beharensis | 30 /17 | 28 | 86 | 12.3 | 9.2 | 7.7 | 2.24 | 1.19 | 34.7 | 38.5 |
| K. daigremontiana | 30 /17 | 18 | 188 | 9.9 | 28.2 | 6.0 | 6.00 | 0.97 | 14.0 | 13.4 |
| K. pinnata (a) | 25 /25 | 14 | 155 | 10.3 | 36.4 | 17.3 | 6.95 | −0.65 | 0 | 0 |
| K. pinnata (b) | 25 /21 | 16 | 137 | 9.8 | 26.4 | 7.0 | 5.77 | −0.34 | 0 | 0 |
| K. pinnata (c) | 25 /17 | 14 | 194 | – | 31.3 | – | 4.42 | 2.16 | 32.8 | 34.1 |
| K. pinnata (d) | 30 /17 | 16 | 261 | 8.2 | 49.7 | 4.8 | 8.50 | 1.92 | 18.5 | 20.1 |
| T. grandis | 28 /22 | 12 | 68 | 9.7 | 17.5 | 7.6 | 3.63 | −0.30 | 0 | 0 |
| V. pauciflora | 28 /22 | 51 | 108 | 2.9 | 12.6 | 2.1 | 1.09 | 3.05 | 73.7 | 83.5 |
| X. danguyi | 30 /17 | 21 | 263 | 2.9 | 14.2 | 1.2 | 4.60 | 2.77 | 37.5 | 52.0 |
ND, Not determined.
Total surface area (length 17.3 cm).
Patterns of Net CO2 Exchange
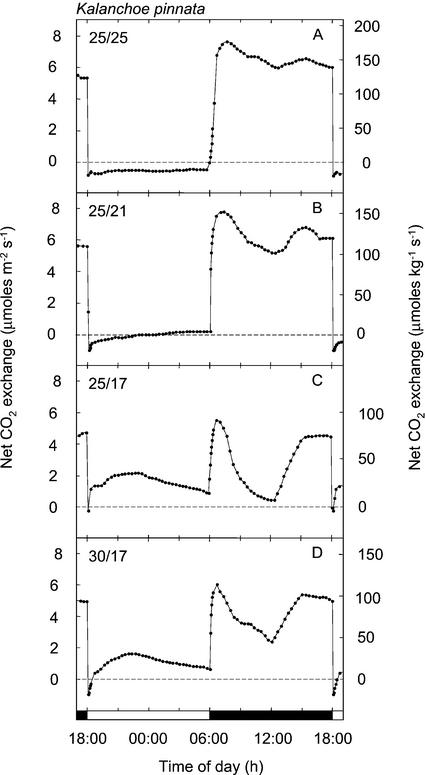
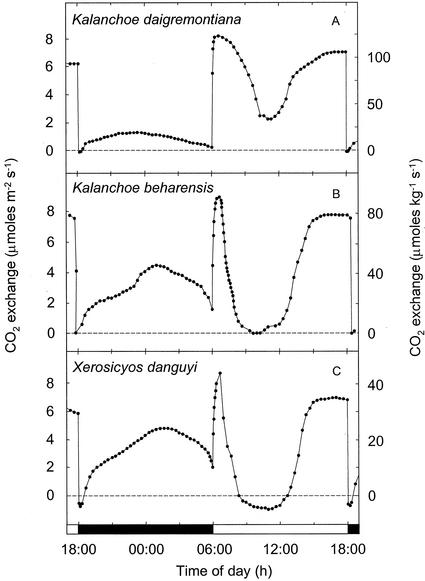
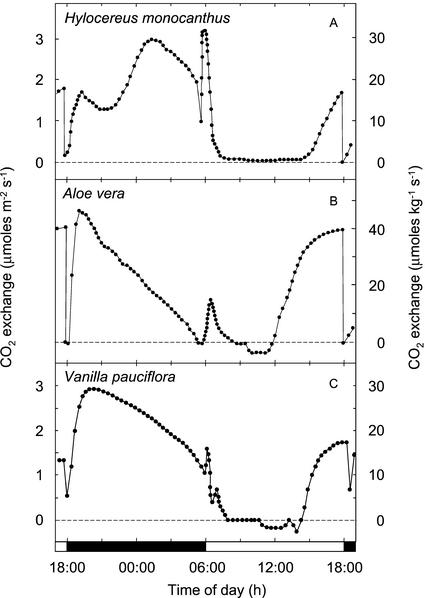
The species examined exhibited a variety of proportions of light and dark CO2 uptake, with net carbon gain in the dark ranging between 0% and 73.7% of the cumulative net carbon gain (Table I). The diversity of C3 and CAM patterns of CO2 exchange observed is depicted during the final 24 h of each experiment (Figs. 1–4). The proportion of dark fixation by K. pinnata responded to the thermoperiod (Fig. 1). At a high night temperature of 25°C, 100% of the net CO2 fixation occurred during the light, whereas at a night temperature of 17°C, up to 33% of the total carbon gain was derived during the dark (Fig. 1; Table I). When the night temperature was decreased from 25°C to 21°C, the 12-h net carbon gain remained negative, although a low rate of net CO2 fixation was observed during the latter one-half of the dark period. Low temperature-associated increases in net carbon gain during the dark by K. pinnata were accompanied by reductions in the amounts of CO2 assimilated during the light and a change in the diurnal patterns of light fixation. A decreasing amount of CO2 incorporated during the light was correlated with a more pronounced depression in CO2 uptake in the middle of the light period, and an increasing proportion of the light fixation that was accounted for by the phase II CO2 uptake burst at the onset of the light period (Osmond, 1978). A. vera, K. beharensis, K. daigremontiana, V. pauciflora, and X. danguyi, all plants known to perform CAM, exhibited varying proportions of dark and light CO2 fixation (Figs. 2 and 3). Patterns characteristic of gas exchange during the light were a phase II CO2 uptake burst, a phase III period of reduced CO2 exchange that in A. vera, V. pauciflora, and X. danguyi resulted in low rates of net CO2 efflux for 2 to 4 h, and phase IV afternoon CO2 fixation.
Figure 1.
Twenty-four-hour net CO2 exchange by shoots of Kalanchoe pinnata exposed to four temperature regimes. A, 25°C, 190 μmol m−2 s−1 light/25°C dark, dew point 18°C; B, 25°C, 180 μmol m−2 s−1 light/21°C dark, dew point 18°C; C, 25°C, 190 μmol m−2 s−1 light/17°C dark, dew point 15°C; and D, 30°C, 190 μmol m−2 s−1 light/17°C dark, dew point 15°C. A through D depict net CO2 exchange during the final 24 h of experiments that lasted 14 to 16 12-h-light/12-h-dark cycles.
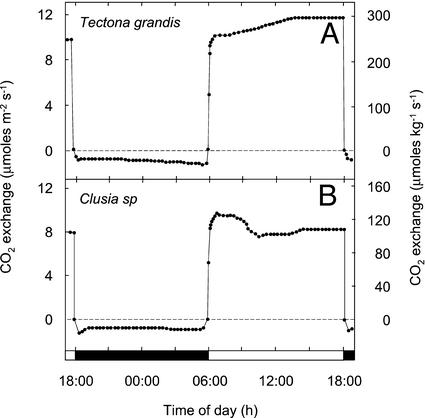
Figure 4.
Twenty-four-hour net CO2 exchange by shoots of two species of C3 plants. A, Teak: 28°C, 360 μmol m−2 s−1 light/22°C dark, dew point 20°C; and B, Clusia sp.: 28°C, 360 μmol m−2 s−1 light/22°C dark, dew point 20°C. The panels depict net CO2 exchange during the final 24 h of experiments that lasted 12 and 18 12-h-light/12-h-dark cycles, respectively.
Figure 2.
Twenty-four-hour net CO2 exchange by shoots of three species of CAM plants. A, K. daigremontiana: 30°C, 185 μmol m−2 s−1 light/17°C dark, dew point 15°C; B, Kalanchoe beharensis: 30°C, 185 μmol m−2 s−1 light/17°C dark, dew point 15°C; and C, Xerosicyos dangyui: 30°C, 200 μmol m−2 s−1 light/17°C dark, dew point 15°C. A through C depict net CO2 exchange during the final 24 h of experiments that lasted between 18 and 28 12-h-light/12-h-dark cycles.
Figure 3.
Twenty-four-hour net CO2 exchange by shoots or cladodes of three species of CAM plants. A, Aloe vera: 28°C, 193 μmol m−2 s−1 light/22°C dark, dew point 15°C; B, H. monocanthus: 28°C, 350 μmol m−2 s−1 light/22°C dark, dew point 20°C; and C, Vanilla pauciflora: 30°C, 304 μmol m−2 s−1 light/22°C dark, dew point 20°C. A through C depict net CO2 exchange during the final 24 h of experiments that lasted between 18 and 51 12-h-light/12-h-dark cycles.
Some peculiarities in the CO2 exchange patterns depicted probably reflect the heterogeneous nature of the tissues in the cuvette. These include the split phase-II CO2 uptake burst in V. pauciflora and the rise and fall of CO2 uptake by H. monocanthus during the early dark period (Fig. 3).
Teak (Tectona grandis), a C3 species, and Clusia sp., exhibited net CO2 uptake only during the light (Fig. 4). The Clusia sp. exhibited a phase II-like CO2 uptake burst similar to that observed for K. pinnata growing in a 25°C/25°C day/night regime.
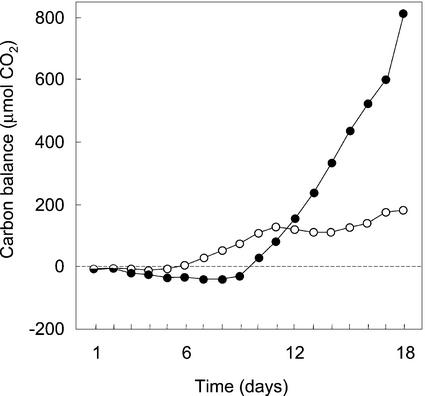
In general, the small amounts of tissues initially placed in the gas-exchange cuvette exhibited net CO2 exchange patterns roughly similar to those expressed in the tissues harvested at the end of the experiments. However, in H. monocanthus the patterns of carbon exchange changed markedly (Fig. 5). The tissue initially exhibited net carbon loss during both the light and the dark. C3-like net carbon gain in the light and carbon loss in the dark occurred after d 6, whereas CAM-like net carbon gain during the dark began after d 10. By d 18, dark CO2 fixation accounted for 81.9% of the CO2 fixed.
Figure 5.
Net CO2 balances for 18 12-h-light (○) and dark (●) cycles during which the stem succulent H. monocanthus grew inside the gas-exchange cuvette.
Carbon Isotope Compositions
The δ13C composition of tissues harvested after exposure in the gas-exchange cuvette, defined as total tissue, ranged from −11.6‰ to −28.7‰ (Table II). The carbon isotope values of newly formed tissue that had been corrected for the isotopic compositions and dry weights of the tissues initially placed in the cuvette, defined as new tissue, were similar to the values for the total tissues harvested after 12 to 51 d, differing by only between −0.9‰ and +0.3‰. It should be noted that Equation 2 (see “Materials and Methods”), which was used to calculate the δ13C of new tissues, is only valid if the bulk of the carbon in the new growth in the cuvette is supplied from CO2 fixed by the plant material in the cuvette. If the observation that carbon constitutes, on average, about 45% of the dry weight of plant tissues holds for succulents (Epstein, 1972), then for eight of the nine species examined, the cumulative carbon gains by the tissues inside the cuvette were sufficient to sustain between 110% and 191% of the increases of biomass inside the cuvette (Table I). The exception was the stem-succulent H. monocanthus for which only 14% of the 90-fold increase in the dry weight was accounted for by the assimilation of CO2 inside the cuvette.
Table II.
The δ13C compositions of carbon in the tissues initially placed in the gas-exchange cuvette, in the tissues harvested from the cuvette, and calculated to have been incorporated during the experimental periods (per Eq. 2) in shoots of CAM and C3 species exposed to various temperature regimes for between 12 and 51 d
| Species | δ13C
|
||
|---|---|---|---|
| Initial | Final | New | |
| ‰ | |||
| A. vera | −15.5 ± 0.0 | −16.1 ± 0.0 | −16.4 |
| Clusia sp. | −29.2 ± 0.0 | −28.7 ± 0.0 | −28.4 |
| H. monocanthus | −9.8 ± 0.0 | −11.6 ± 0.0 | −11.6 |
| K. beharensis | −20.1 ± 0.5 | −20.7 ± 0.1 | −20.8 |
| K. daigremontiana | −25.1 ± 0.0 | −24.5 ± 0.1 | −24.4 |
| K. pinnata (a) | −17.1 ± 0.4 | −25.7 ± 0.1 | −26.6 |
| K. pinnata (b) | −24.8 ± 0.1 | −25.1 ± 0.0 | −25.1 |
| K. pinnata (c) | ND | −21.3 ± 0.1 | – |
| K. pinnata (d) | −24.3 ± 0.0 | −22.5 ± 0.1 | −22.3 |
| T. grandis | −27.3 ± 0.4 | −27.6 ± 0.0 | −27.6 |
| V. pauciflora | −13.6 ± 0.3 | −14.0 ± 0.1 | −14.3 |
| X. danguyi | −18.9 ± 0.0 | −20.1 ± 0.5 | −20.8 |
See Table I for light/dark temperature regimes and lengths of treatments. ND, Not determined.
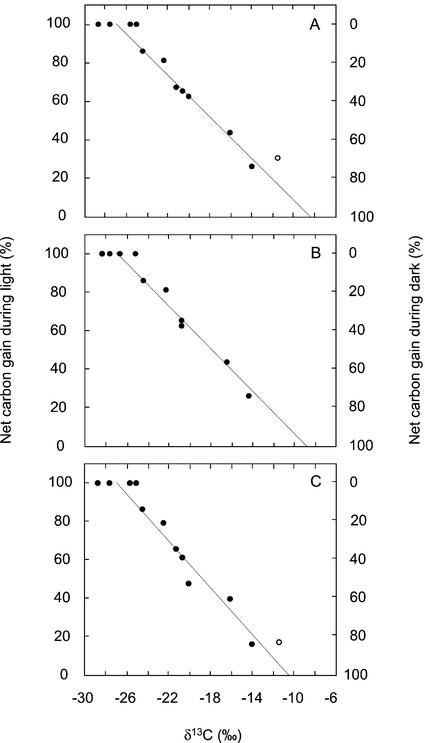
The δ13C values of the total and the new tissues were correlated in a linear manner with the cumulative proportions of carbon gained during the dark and the light (Fig. 6, A and B). The linear regressions, which exhibited r2 values of 0.95 and 0.96, respectively, predicted δ13C values of −8.3‰ and −8.7‰ after CO2 fixation exclusively in the dark by the total and new tissues, respectively. A value of −26.9‰ was predicted for 100% light fixation in both tissues.
Figure 6.
Relationships between the cumulative net carbon gain in the light and the dark and the δ13C values of tissues harvested after between 12 and 51 d (A) or the δ13C values of tissues formed (calculated per Eq. 2) after between 12 and 51 d (B). C, Relationship between δ13C values of tissues harvested after between 12 and 51 d in the gas-exchange cuvette and the proportions of CO2 fixed in the light and the dark during the final 24 h of exposure. Extrapolation of the fitted regression (y = −5.3734x − 44.63; r2 = 0.95) in A indicates δ13C values of −8.3‰ for 100% dark CO2 uptake and −26.9‰ for 100% CO2 uptake during the light. Extrapolation of the fitted regression (y = −5.4909x − 47.94; r2 = 0.96) in B indicates δ13C values of −8.7‰ for 100% dark CO2 uptake and −26.9‰ for 100% CO2 uptake during the light. In C, the fitted regression (y = −6.0772x − 63.54; r2 = 0.94) indicates δ13C values of −10.5‰ for 100% dark CO2 uptake and −26.9‰ for 100% CO2 uptake during the light. The equivalent values in C for newly formed tissue were −10.9‰ and −27.0‰, respectively (y = −6.2039x − 67.43; r2 = 0.93). The value for H. monocanthus (○) was not included in the regressions of A and C as approximately 86% of the carbon was imported from tissues outside the gas-exchange cuvette.
The δ13C values of the total and new tissues were also correlated in a linear manner with the proportions of carbon gained in the light and the dark during the final 24 h within the gas-exchange cuvette (Fig. 6C). The linear regressions predicted δ13C values of −26.9‰ and −27.0‰ after exclusively CO2 fixation in the light by the total and new tissues, respectively. The predicted values for 100% dark fixation were −10.5‰ and −10.9‰, respectively. The δ13C values for H. monocanthus were excluded from the regressions because the assumptions of Equation 2 were not met.
DISCUSSION
The proportion of CO2 fixed during the light and dark was the major contributor to variation in whole-tissue carbon isotope composition of the C3 and CAM plants examined. A range of 18.6‰ was estimated for the difference between tissues that assimilated carbon exclusively during the dark or the light (Fig. 6). Because the relationship between the contributions of light and dark CO2 uptake and whole-tissue δ13C was linear, each 10% contribution of dark CO2 fixation to net CO2 exchange should shift δ13C values by about +1.8‰.
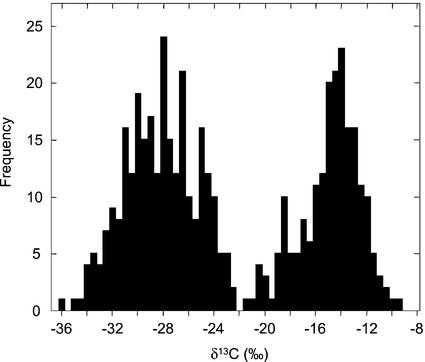
The above observations have major implications for the interpretation of the bimodal distributions of δ13C values characteristic of isotope-based C3-CAM vegetation surveys and define a “typical” CAM plant growing in the field (Rundel et al., 1979; Winter, 1979; Teeri et al., 1981; Griffiths and Smith, 1983; Winter et al., 1983; Earnshaw et al., 1987; Arroyo et al., 1990; Kluge et al., 1991; Zotz and Ziegler, 1997; Crayn et al., 2001). In surveys such as those summarized in Figure 7, the individuals in the more positive isotopic cluster, which usually has a mode of around −13‰ to −14‰ and ranges between about −9‰ and −19‰, are generally categorized as CAM. On the basis of the regression presented in Figure 6B, one could conclude that the typical CAM plant with a δ13C value of −13‰ to −14‰ obtained around 71% to 77% of its carbon from dark fixation. A plant at the more negative edge of the CAM cluster, at around −19‰, would gain 44% of its carbon from dark fixation. The more negative of the two clusters, which generally exhibits a mode around −27‰ and a range between about −21‰ and −32‰, is typically designated as a C3 cluster but, according to our observations, may contain both C3 species and plants that obtain up to 33% of their carbon via dark CO2 fixation. That is, the carbon isotope method alone does not distinguish between field-grown C3 species and plants that obtain up to one-third of their carbon during the dark, which include weak and many facultative CAM plants. Such plants can only be distinguished by careful measurements of diel titratable acidity changes or dark CO2 uptake. Measurements of low levels of dark CO2 uptake in the field may be at the limits of resolution of currently available portable gas-exchange systems.
Figure 7.
δ13C values of 506 species from the Asclepiadaceae, Bromeliaceae, Crassulaceae, Cucurbitaceae, Didiereaceae, Euphorbiaceae, Orchidaceae, Polypodiaceae, and Vittariaceae plant families known to contain both C3 and CAM members. Values were selected from Winter (1979), Teeri et al. (1981), Griffiths and Smith (1983), Winter et al. (1983), Earnshaw et al. (1987), and Kluge et al. (1991).
It has been suggested that in some CAM species, PEP carboxylase may be active for parts of the photoperiod (Winter and Tenhunen, 1982). The isotopic compositions of the CAM species that exhibited 100% total carbon gain in the light were approximately 2‰ more positive than those of C3 species, but because we were unable to distinguish between PEP carboxylase or diffusional effects in the light and PEP carboxylase-based recycling of CO2 in the dark, we can neither rule out nor confirm the possibility that PEP carboxylase may be fixing external CO2 during the light. However, if PEP carboxylase fixation was occurring during the light in the CAM species, the linearity of the relationship between isotopic signature and the proportions of light and dark CO2 uptake is consistent with a constant proportional contribution of PEP carboxylase-fixed carbon to carbon gain during the light for the species in our study.
Although the proportion of CO2 fixed during the light and dark was the major contributor to variation in carbon isotope composition of newly fixed carbon in our study of well-watered plants, the range in δ13C values measured in the field is greater than that observed in our study (compare Figs. 6B and 7). The extent to which diffusion or carboxylation limit CO2 fixation in the light and in the dark contributes to this difference. The isotopic compositions for carbon fixed in the light or in the dark estimated in this study, −26.9‰ and −8.7‰, respectively, support the postulate that both light and dark CO2 fixation in well-watered CAM plants are colimited by diffusion and carboxylation (O'Leary and Osmond, 1980; O'Leary, 1981, 1988). If one assumes an average source CO2 isotopic composition of −8.7‰ (see “Materials and Methods”), then the expected Rubisco- and diffusion-limited isotopic compositions of carbon fixed in the light would be −35.7‰ and −13.1‰, respectively, and the corresponding values for dark-fixed carbon would be −3‰ and −13.1‰, respectively (Farquhar et al., 1989). Uncertainty in estimating the contributions of dark- and light-fixed CO2 using the correlations developed in Figure 6 can arise when plants are collected from environments that affect the relative limitations of diffusion and carboxylation (Farquhar et al., 1989), e.g. from extreme habitats characterized by edaphic stress (Farquhar et al., 1982), along altitudinal gradients (Körner et al., 1988; Friend et al., 1989; Crayn et al., 2001), or from forest understoreys with different levels of canopy closure and CO2 stratification (Holtum and Winter, 2001). It is good field practice to collect, as a reference, material from sympatric C3 plants. When collecting in forests or close to the ground, air samples should also be taken if practicable.
Other processes that complicate the isotopic signal as an index of light and dark fixation include the translocation of carbon into developing photosynthetic organs, variations in the amounts of respiratory CO2 that are refixed, loss of isotopically heavy CO2 in the light during phase III, and short-term variations in the δ13C content of ambient CO2. In our experiments, with the exception of the stem-succulent H. monocanthus, translocation of carbon into the growing tissues was small because the plants had little or no photosynthesizing tissue outside the cuvette. Net carbon gains were more than sufficient to account for the increases in the dry masses of the tissues inside the cuvette, indicating that the incubated tissues were carbon sources rather than sinks. For H. monocanthus, it was calculated, on the basis of measured net CO2 exchange and a determination that 43% of the dry matter was carbon, that 86% of the 90-fold increase in dry mass observed during 18 d in the gas-exchange cuvette was due to the reallocation of resources from mature stem outside the cuvette to the enclosed developing stem. Because of the massive importation of carbon into the developing stem enclosed within the cuvette, the isotopic composition of the 17.3 cm of the stem inside the cuvette did not reflect the proportions of CO2 incorporated by that tissue during the light and dark, suggesting that meaningful isotopic compositions of such stem-succulents in the field will only be obtained if sampling is restricted to mature regions well behind the growing tip. Moreover, the reasonably rapid developmental change from C3 to CAM in H. monocanthus resulted in a gas-exchange pattern for the final 24 h inside the cuvette that, in contrast to the other species studied, correlated poorly with the cumulative proportions of dark and light carbon gain measured throughout the period that the tissue was enclosed (Table I; Figs. 3 and 5).
The observations for H. monocanthus are of relevance to interpreting the isotopic signatures of tissues undergoing developmental change. The shifts between C3 and CAM in leaves of facultative CAM species like Mesembryanthemum crystallinum and Clusia spp. may be accompanied by changes in the proportions of 13C-enriched or -depleted metabolites that are exchanged with the rest of the plant. In such circumstances the interpretation of analyses of single leaves collected during vegetation surveys will be superficial until more extensive sampling is undertaken.
During phase III in the light, X. danguyi, A. vera, and V. pauciflora evolved 1.6%, 0.5%, and 1.4%, respectively of total carbon fixed. Although the proportion of the daily carbon gain lost during phase III tends to be small, it may be considerably enriched in 13C. In Tillandsia utriculata, a 1% loss of net carbon uptake during phase III was sufficient to change the plant δ13C value by −0.64‰ (Griffiths et al., 1990). In vegetation surveys, such a shift is unlikely to change the interpretation of strong CAM that would be attributed to plants such as A. vera (−16.1‰) and V. pauciflora (−14.0‰) and even to X. danguyi, which exhibited an intermediate δ13C value of −20.1‰. In well-watered plants, the net loss of CO2 during phase III tends to be observed in strong CAM species, whereas in plants that exhibit less developed CAM, such as the K. pinnata plants depicted in Figure 1, CO2 uptake tends to remain positive during phase III.
The δ13C values of the limits for the regressions depicted in Figure 6 will vary with the isotopic composition of atmospheric CO2. However, the slope of the regression should remain similar. Variation due to the annual shift of about −0.05‰ generated by the combustion of fossil-fuels and the small annual seasonal fluctuation of about 0.2‰ (Mook et al., 1983), can be predicted. Correction of field data will be easier if one collects sympatric C3 species. To our knowledge, few δ13C values reported from herbarium-based surveys have been corrected for the atmospheric δ13C composition, which has changed from about −6.4‰ to about −8.7‰ since 1750 (Mook et al., 1983; Friedli et al., 1986). The δ13C values of herbarium specimens of 12 C3 species collected between 1750 and 1988 changed from −25.8‰ ± 0.25‰ to −26.4‰ ± 0.29‰ (Peñuelas and Azcón-Bieto, 1992). The shift is small probably because the associated increase in [CO2] has reduced stomatal apertures, thereby increasing diffusive resistance to CO2 and reducing the contribution of the Rubisco-based fractionation to the isotopic signal.
Diel variations, generally associated with the vertical stratification of CO2 beneath forest canopies, can add significant uncertainty to CAM isotopic signals. δ13C values about 2‰ more negative during the night than during the day, as has been reported for a semi-evergreen seasonal forest in Trinidad (Broadmeadow et al., 1992), would result in tissues becoming 0.2‰ more negative for every 10% CO2 fixed during the dark. Because the vertical gradients of up to 5‰ that have been reported for CO2 under forest canopies (Vogel, 1978; Medina and Minchin, 1980; Francey et al., 1985; Medina et al., 1986; Sternberg et al., 1989; Broadmeadow et al., 1992) are usually associated with changes in CO2 concentration that reflect soil, root, and leaf respiration, correction for the effects of the position of the plant under the canopy requires at least isotopic knowledge of the CO2 concentration of ambient atmosphere and the δ13C value of leaf litter (Sternberg et al., 1989; Broadmeadow et al., 1992). In this study in which the gas-exchange cuvette was supplied with well-mixed air sampled from above a 5-storey building, the diel variation of atmospheric δ13C was 1.3‰. CO2 sampled at dawn (7 am) was −9.4‰ ± 0.06‰ (n = 5), whereas CO2 sampled at dusk (6 pm) was −8.1‰ ± 0.04‰ (n = 7).
In conclusion, we have provided experimental confirmation for the hypothesis of O'Leary (1988) that the relationship between carbon isotope ratio and the proportion of dark and light CO2 fixation in plants that exhibit CAM is linear and have established the slope and limits of the relationship for well-watered plants exposed to an ambient CO2 concentration. Extrapolation of our observations to plants surveyed under natural conditions suggests first, that the most commonly expressed version of CAM in the field, the typical CAM plant, involves plants that gain about 71% to 77% of their carbon by dark fixation, and second, that the isotopic signals of plants that obtain less than one-third of their carbon in the dark may be confused with C3 plants when identified on the basis of carbon isotope content alone. This uncertainty in the interpretation of isotopic signals begs the question of whether the isotopic distribution of CAM plants in the field is unimodal or bimodal. Does the isotopic distribution of terrestrial CAM plants exhibit a mode around −13‰ to −14‰ with a skewed margin that tails out to values close to −27‰? Or does the so-called C3 isotopic cluster conceal a second peak of abundance indicative of ecological niches for plants, such as some tropical epiphytic ferns (e.g. Holtum and Winter, 1999), with low capacities for dark CO2 fixation? The striking observation that, in mature source tissues, the proportions of carbon incorporated during the light and dark in a single day correlates with the δ13C composition (Fig. 6C) should, in conjunction with measurements of titratable acidity, greatly assist in sorting out these relationships.
MATERIALS AND METHODS
Plants and Gas Exchange
Aloe vera L. (syn. Aloe barbadensis Mill.) (Asphodelaceae), Hylocereus monocanthus (Lem.) Br. & R. (Cactaceae), Kalanchoe beharensis Drake Del Castillo (Crassulaceae), Kalanchoe daigremontiana Hamet et Perr. (Crassulaceae), Kalanchoe pinnata (Lam.) Persoon (Crassulaceae), Vanilla pauciflora Dressler (Orchidaceae), Xerosicyos danguyi Humbert (Cucurbitaceae), teak (Tectona grandis L.f.) (Verbenaceae), and Clusia aff. grandiflora (Clusiaceae) were cultivated in pots in a naturally ventilated shade-house at the Tupper Center of the Smithsonian Tropical Research Institution (Panama City, Republic of Panama).
Net CO2 exchange by intact shoots exposed to different light/dark temperature regimes under a 12-h-light/12-h-dark cycle was measured using a through-flow gas-exchange system (Walz, Effeltrich, Germany) operating at 1 or 2 L min−1 air. The CO2 concentration of the gas supply, ambient air sourced 16 m above ground level and passed through a 2 m3 buffer, varied between 360 and 430 μL L−1.
Developing shoots were sealed inside a 1.2-L Plexiglas cuvette of the gas-exchange system in a controlled environment cabinet (GEC, Chagrin Falls, OH). Duplicates of similar shape and size were harvested at the beginning of each experiment. Root systems still attached to the plants were outside the cuvette but still in the cabinet. Lower leaves on the stem outside the cuvette were removed except for X. danguyi. For K. beharensis, K. daigremontiana, and K. pinnata, the apex including the top leaf pair about 4 cm high was used. For A. vera, an entire four-leaf 2.5-cm-high juvenile shoot was used. For H. monocanthus, approximately 0.5 cm of a developing stem was sealed in the cuvette. For X. danguyi, the cuvette initially contained four small leaves attached to about 3 cm of cane from which the apex had been removed. During the study, the four leaves increased in size. For V. pauciflora, the cuvette contained an initiating leaf and a developing leaf shoot attached to a 20-cm defoliated shoot that was outside the cuvette. For teak and Clusia sp., the upper leaf pair of about 4-cm-tall seedlings was inserted in the cuvette.
H. monocanthus, X. danguyi, teak, Clusia sp., and V. pauciflora were grown in potting mix during the gas-exchange measurements, whereas the roots of A. vera, K. beharensis, K. daigremontiana, and K. pinnata were maintained in one-half-strength Johnson's solution (Winter, 1973).
Tissue harvested at either d 0 or between 12 and 51 d growth in the gas-exchange cuvette was dried for 48 h at 65°C, weighed, and ground to a fine powder using a mortar and pestle. 13C/12C analyses were performed on three aliquots of the powdered extracts of tissue harvested on d 0, and on five to eight aliquots of the extracts of tissue grown in the gas-exchange system.
Carbon-Isotope Determinations
Carbon isotope ratios were determined for CO2 derived from 2- to 4-mg samples of dried tissue. Material analyzed at the Duke University Phytotron (Durham, NC) was combusted under oxygen (DUMAS combustion) in an elemental analyzer (NA1500 Series 1, Carlo Erba Instrumentazione, Milan) and analyzed using a SIRA Series II isotope ratio mass spectrometer (Micromass, Manchester, UK) operating in automatic trapping mode. V. pauciflora tissue, similarly treated, was analyzed at the Institute of Ecology and Conservation Biology, University of Vienna, using a Finnigan MAT Deltaplus in continuous flow mode (Finnigan, Bremen, Germany).
Following the appropriate corrections for other isotopes, the abundance of 13C in each sample was calculated relative to the abundance of 13C in standard CO2 that had been calibrated against Pee Dee belemnite (Belemnitella americana). Relative abundance was determined using the relationship
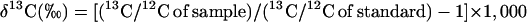 |
1 |
The δ13C content of tissue formed during the period of growth in the cuvette was calculated assuming the relationship
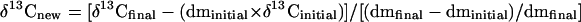 |
2 |
where δ13Cinitial, δ13Cfinal, and δ13Cnew are the δ13C values, in per mil, of the initial, final, and new material, respectively. Similarly, dminitial and dmfinal are the dry masses, in grams, of the initial and harvested tissues.
Carbon isotope ratios of CO2 from 12 0.5-L samples of air collected at dawn and dusk in August, 2001, from the inlet of the gas-exchange cuvette were determined at the Institute of Arctic and Alpine Research, University of Colorado (Boulder), using a Micromass Optima dual gas inlet spectrometer.
ACKNOWLEDGMENTS
Larry Giles (Duke University), Wolfgang Wanek (University of Vienna, Vienna), and Bruce Vaughn (University of Colorado) performed the measurements of δ13C, and V. pauciflora was identified by Neal Smith (Smithsonian Tropical Research Institute, Panama) under conditions of stress.
Footnotes
This research was supported by the Andrew W. Mellon Foundation through the Smithsonian Institution, by the Smithsonian Tropical Research Institute, by the Australian Research Council, and by Dr. Rosemary G. Dunn.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002915.
LITERATURE CITED
- Allaway WG, Osmond CB, Troughton JH. Environmental regulation of growth, photosynthetic pathway and carbon isotope discrimination ratio in plants capable of Crassulacean acid metabolism. In: Bieleski RL, Ferguson AR, Cresswell MM, editors. Mechanisms of Regulation of Plant Growth (Bulletin 12). Wellington: The Royal Society of New Zealand; 1974. pp. 195–202. [Google Scholar]
- Arroyo MK, Medina E, Ziegler H. Distribution and δ13C values of Portulacaceae species in the high Andes of northern Chile. Bot Acta. 1990;103:291–295. [Google Scholar]
- Bender MM. Mass spectrometric studies of carbon 13 variations in corn and other grasses. Radiocarbon. 1968;10:468–472. [Google Scholar]
- Bender MM. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry. 1971;10:1239–1244. [Google Scholar]
- Bender MM, Rouhani I, Vines HM, Black CC. 13C/12C ratio changes in Crassulacean acid metabolism plants. Plant Physiol. 1973;52:427–430. doi: 10.1104/pp.52.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland AM, Griffiths H. Variations in the phases of Crassulacean acid metabolism and regulation of carboxylation patterns determined by carbon-isotope-discrimination techniques. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Heidelberg: Springer; 1996. pp. 230–249. [Google Scholar]
- Broadmeadow MSJ, Griffiths H, Maxwell C, Borland AM. The carbon isotope ratio of plant organic material reflects temporal and spatial variations in CO2 partial pressure and δ13C within tropical forest formations in Trinidad. Oecologia. 1992;89:435–441. doi: 10.1007/BF00317423. [DOI] [PubMed] [Google Scholar]
- Crayn DM, Smith JAC, Winter K. Carbon-isotope ratios and photosynthetic pathways in the Rapateaceae. Plant Biol. 2001;3:569–576. [Google Scholar]
- Deleens E, Reichel I, O'Leary MH. Temperature dependence of carbon isotope fractionation in CAM plants. Plant Physiol. 1985;79:202–206. doi: 10.1104/pp.79.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw MJ, Winter K, Ziegler H, Stichler W, Cruttwell NEG, Kerenga K, Cribb PJ, Wood J, Croft JR, Carver KA et al. Altitudinal changes in the incidence of Crassulacean acid metabolism in vascular epiphytes and related life forms in Papua New Guinea. Oecologia. 1987;73:566–572. doi: 10.1007/BF00379417. [DOI] [PubMed] [Google Scholar]
- Epstein E. Mineral Nutrition of Plants: Principles and Perspectives. New York: John Wiley & Sons; 1972. [Google Scholar]
- Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z. Effects of salinity and humidity on δ13C values of halophytes: evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia. 1982;52:121–124. doi: 10.1007/BF00349020. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- Francey RJ, Gifford RM, Sharkey TD, Weir B. Physiological influences on carbon isotope discrimination in huon pine (Lagarostrobos franklinii) Oecologia. 1985;66:211–218. doi: 10.1007/BF00379857. [DOI] [PubMed] [Google Scholar]
- Friedli H, Lötscher H, Oeschger H, Siegenthaler U, Stauffer B. Ice core record of the 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature. 1986;324:28–31. [Google Scholar]
- Friend AD, Woodward FI, Switsur VR. Field measurements of photosynthesis, stomatal conductance, leaf nitrogen, and δ13C along altitudinal gradients in Scotland. Funct Ecol. 1989;3:117–136. [Google Scholar]
- Griffiths H. Carbon isotope discrimination and the integration of carbon assimilation pathways in terrestrial CAM plants. Plant Cell Environ. 1992;15:1051–1062. [Google Scholar]
- Griffiths H, Broadmeadow MSJ, Borland AM, Hetherington CS. Short-term changes in carbon-isotope discrimination identify transitions between C3 and C4 carboxylation during Crassulacean acid metabolism. Planta. 1990;181:604–610. doi: 10.1007/BF00193017. [DOI] [PubMed] [Google Scholar]
- Griffiths H, Smith JAC. Photosynthetic pathways in the Bromeliaceae of Trinidad: relations between life-forms, habitat preference and the occurrence of CAM. Oecologia. 1983;60:176–184. doi: 10.1007/BF00379519. [DOI] [PubMed] [Google Scholar]
- Holtum JAM, O'Leary MH, Osmond CB. Effect of varying CO2 partial pressure on photosynthesis and on carbon isotope composition of carbon-4 of malate from the Crassulacean acid metabolism plant Kalanchoe daigremontiana Hamet et Perr. Plant Physiol. 1983;71:602–609. doi: 10.1104/pp.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtum JAM, Summons R, Roeske CA, Comins HN, O'Leary MH. Oxygen-18 incorporation into malic acid during nocturnal carbon dioxide fixation in Crassulacean acid metabolism plants. J Biol Chem. 1984;259:6870–6881. [PubMed] [Google Scholar]
- Holtum JAM, Winter K. Degrees of Crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Aust J Plant Physiol. 1999;26:749–757. [Google Scholar]
- Holtum JAM, Winter K. Are plants growing close to the floors of tropical forests exposed to markedly elevated concentrations of carbon dioxide? Aust J Bot. 2001;49:629–636. [Google Scholar]
- Kluge M, Brulfert J, Ravelomanana D, Lipp J, Ziegler H. Crassulacean acid metabolism in Kalanchoe species collected in various climatic zones of Madagascar: a survey of δ13C analysis. Oecologia. 1991;88:407–414. doi: 10.1007/BF00317586. [DOI] [PubMed] [Google Scholar]
- Körner C, Farquhar GD, Rocksandic Z. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia. 1988;74:623–632. doi: 10.1007/BF00380063. [DOI] [PubMed] [Google Scholar]
- Medina E, Minchin P. Stratification of δ13C values of leaves in Amazonian rain forests. Oecologia. 1980;45:377–378. doi: 10.1007/BF00540209. [DOI] [PubMed] [Google Scholar]
- Medina E, Montes G, Cuevas E, Rokzandic Z. Profiles of CO2 concentration and δ13C values in tropical rain forests of the upper Rio Negro Basin, Venezuela. J Trop Ecol. 1986;2:207–217. [Google Scholar]
- Mook WG, Koopmans M, Carter AF, Keeling CD. Seasonal, latitudinal and secular variations in the abundance of isotopic ratios of atmospheric carbon dioxide: 1. Results from land stations. J Geophys Res. 1983;88:10915–10933. [Google Scholar]
- Nalborczyk E, La Croix LJ, Hill RD. Environmental influences on light and dark CO2 fixation by Kalanchoe daigremontiana. Can J Bot. 1975;53:1132–1138. [Google Scholar]
- O'Leary MH. Carbon isotope fractionation in plants. Phytochemistry. 1981;20:553–567. [Google Scholar]
- O'Leary MH. Carbon isotopes in photosynthesis. BioScience. 1988;38:328–335. [Google Scholar]
- O'Leary MH, Osmond CB. Diffusional contribution to carbon isotope fractionation during dark CO2 fixation in CAM plants. Plant Physiol. 1980;66:931–934. doi: 10.1104/pp.66.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB. Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol. 1978;29:379–414. [Google Scholar]
- Osmond CB, Allaway WG, Sutton BG, Troughton JH, Queiroz O, Lüttge U, Winter K. Carbon isotope discrimination in photosynthesis of CAM plants. Nature. 1973;246:41–42. [Google Scholar]
- Osmond CB, Bender MM, Burris RH. Pathways of CO2 fixation in the CAM plant Kalanchoe daigremontiana: III. Correlation with δ13C value during growth and water stress. Aust J Plant Physiol. 1976;3:787–799. [Google Scholar]
- Peñuelas J, Azcón-Bieto J. Changes in leaf Δ 13C of herbarium plant species during the last 3 centuries of CO2 increase. Plant Cell Environ. 1992;15:485–489. [Google Scholar]
- Rundel PW, Rundel JA, Ziegler H, Stichler W. Carbon isotope ratios of central Mexican Crassulaceae in natural and glasshouse environments. Oecologia. 1979;38:45–50. doi: 10.1007/BF00347823. [DOI] [PubMed] [Google Scholar]
- Smith JAC, Winter K. Taxonomic distribution of Crassulacean acid metabolism. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Berlin: Springer; 1996. pp. 427–436. [Google Scholar]
- Sternberg LDL, Mulkey SS, Wright SJ. Ecological interpretation of leaf carbon isotope ratios: influence of respired carbon dioxide. Ecology. 1989;70:1317–1324. [Google Scholar]
- Teeri JA, Tonsor SJ, Turner M. Leaf thickness and carbon isotope composition in the Crassulaceae. Oecologia. 1981;50:367–369. doi: 10.1007/BF00344977. [DOI] [PubMed] [Google Scholar]
- Vogel JC. Recycling of carbon in a forest environment. Oecol Plant. 1978;13:89–94. [Google Scholar]
- Winter K. CO2-Fixierungsreaktionen bei der Salzpflanze Mesembryanthemum crystallinum unter variierten Auβenbedingungen. Planta. 1973;114:75–85. doi: 10.1007/BF00390286. [DOI] [PubMed] [Google Scholar]
- Winter K. δ13C values of some succulent plants from Madagascar. Oecologia. 1979;40:103–112. doi: 10.1007/BF00388814. [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC. An introduction to Crassulacean acid metabolism: biochemical principles and ecological diversity. In: Winter K, Smith JAC, editors. Crassulacean Acid Metabolism. Heidelberg: Springer; 1996. pp. 1–13. [Google Scholar]
- Winter K, Tenhunen JD. Light-stimulated burst of carbon dioxide uptake following nocturnal acidification in the Crassulacean acid metabolism plant Kalanchoe daigremontiana. Plant Physiol. 1982;70:1718–1722. doi: 10.1104/pp.70.6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Wallace BJ, Stocker GC, Roksandic Z. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia. 1983;57:129–141. doi: 10.1007/BF00379570. [DOI] [PubMed] [Google Scholar]
- Zotz G, Ziegler H. The occurrence of Crassulacean acid metabolism among vascular epiphytes from Central Panama. New Phytol. 1997;137:223–229. doi: 10.1046/j.1469-8137.1997.00800.x. [DOI] [PubMed] [Google Scholar]