Abstract
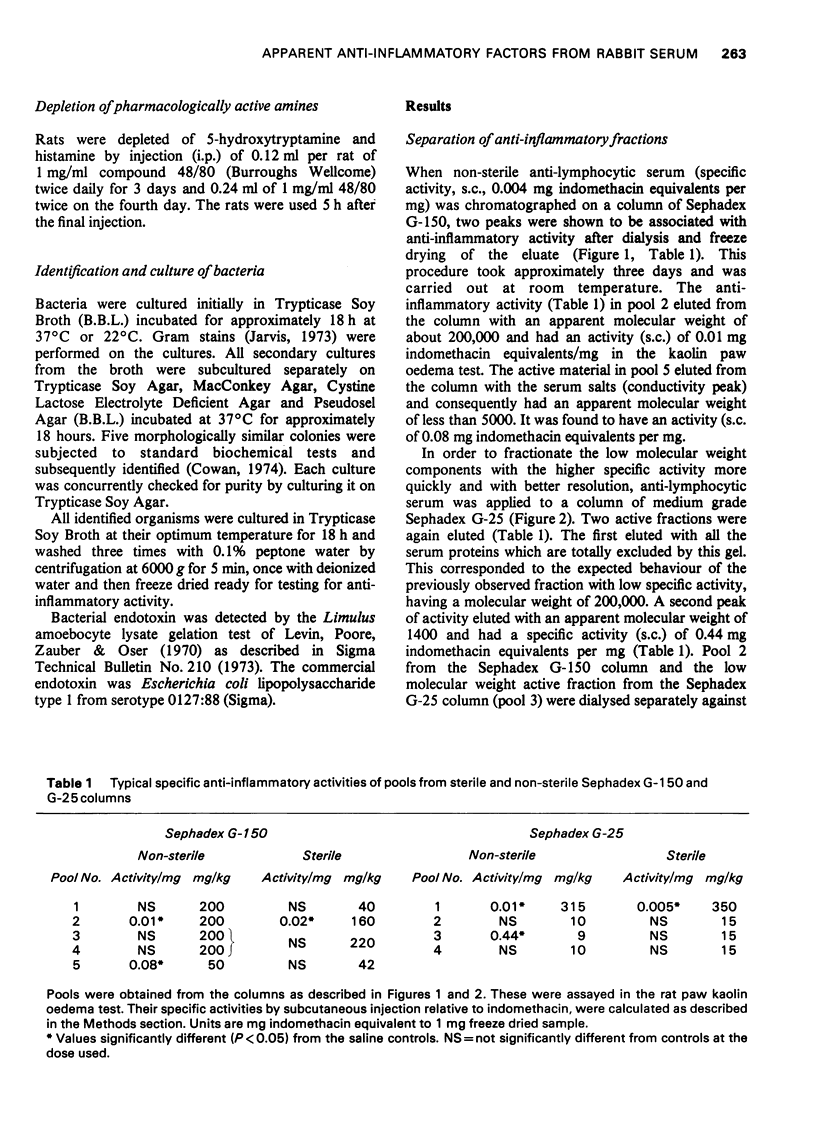
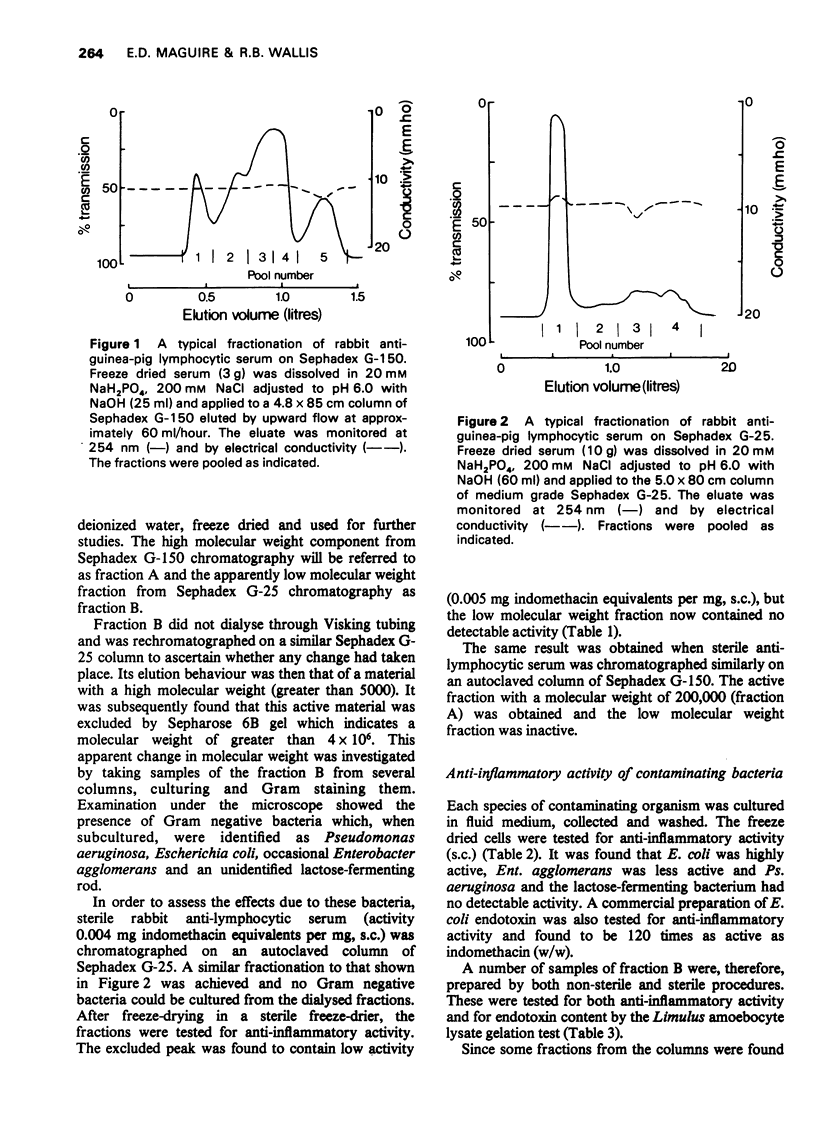
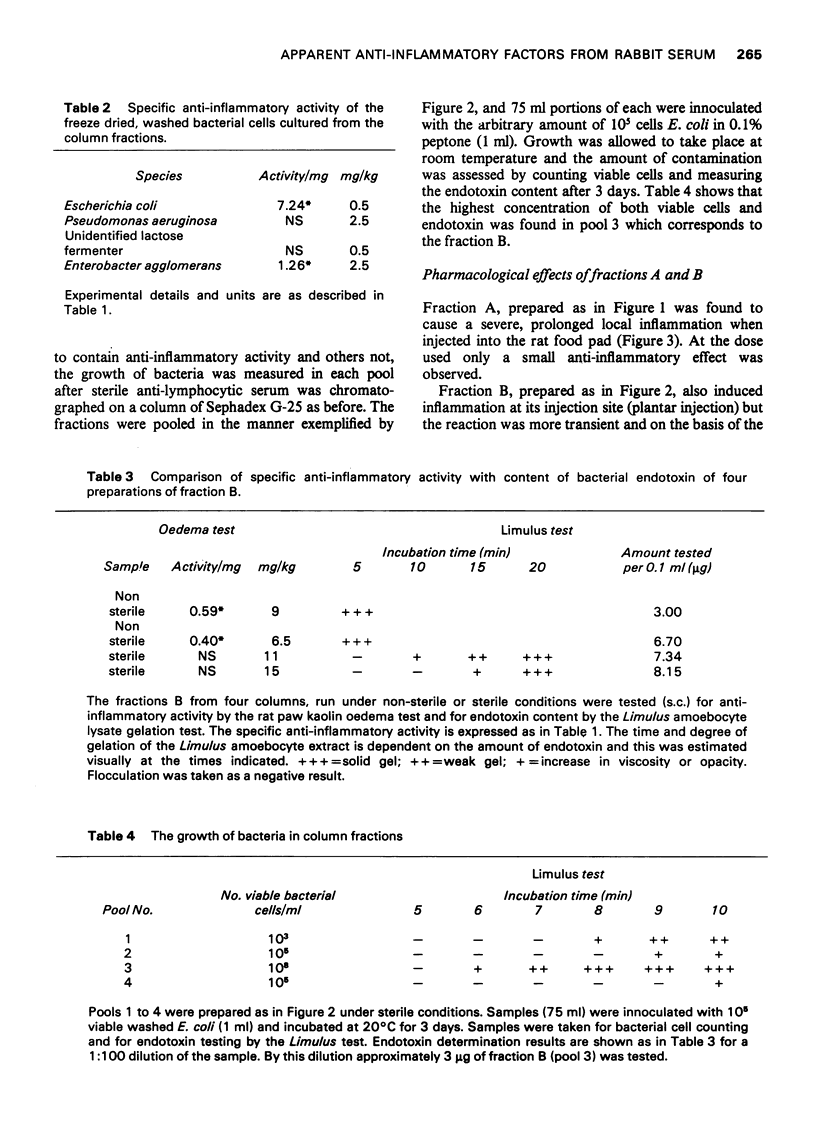
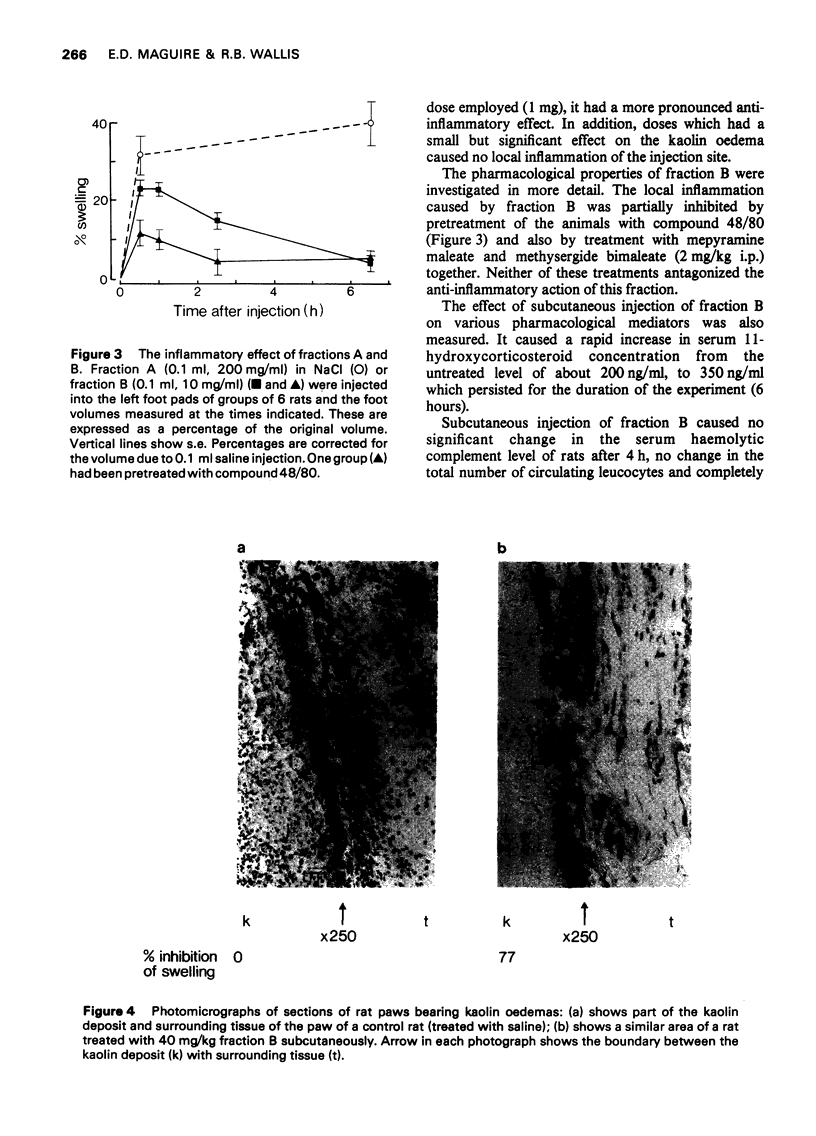
1 Rabbit anti-guinea-pig lymphocytic serum was fractionated by gel filtration to obtain partially purified materials possessing anti-inflammatory activity. The pharmacological properties of these materials were then studied. 2 Two fractions were found which reproducibly contained significant activity. One of these activities caused inflammation at the site of injection and was associated with high molecular weight protein (2008000). The other activity was found in low molecular weight fraction but was shown to be due to small amounts of endotoxin from Gram negative bacteria. These organisms contaminated the fractions in spite of the recommended precautions for gel filtration having been taken. 3 The endotoxin-containing fraction completely abolished leucocyte infiltration into the rat foot which had been injected with kaolin. It had no apparent effect on circulating haemolytic complement. It caused maximal elevation of serum 11-hydroxycorticosteroid concentrations and was found to cause the release of pharmacologically active amines. Many of the previously reported naturally occurring anti-inflammatory substances have similar pharmacological properties to those of the endotoxin-containing fraction. 4 It was concluded that doubt will exist about the presence of anti-inflammatory factors in mammalian body fluids unless stringent precautions are taken to exclude measurable bacterial contamination. 5 These experiments also cast doubt on the validity of accepted procedures for excluding microbial growth from columns used in the fractionation of serum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. C., Hicks R. The possible occurrence of endogenous anti-inflammatory substances in the blood of injured rats. Br J Pharmacol. 1975 Jan;53(1):85–91. doi: 10.1111/j.1476-5381.1975.tb07333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham E. J., Robinson B. V., Robson J. M. Anti-inflammatory properties of human inflammatory exudate. Br Med J. 1969 Apr 12;2(5649):93–96. doi: 10.1136/bmj.2.5649.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham M. E., Robinson B. V., Gaugas J. M. 2 anti-inflammatory components in antilymphocytic serum. Nature. 1970 Jul 18;227(5255):276–277. doi: 10.1038/227276a0. [DOI] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI C. R., CLUFF L. E., SCHEDER E. P. Studies on the pathogenesis of staphylococcal infection. IV. The effect of bacterial endotoxin. J Exp Med. 1961 May 1;113:845–860. doi: 10.1084/jem.113.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIPASQUALE G., GIRERD R. J., BEACH V. L., STEINETZ B. G. ANTIPHLOGISTIC ACTION OF GRANULOMA POUCH EXUDATES IN INTACT OR ADRENALECTOMIZED RATS. Am J Physiol. 1963 Dec;205:1080–1082. doi: 10.1152/ajplegacy.1963.205.6.1080. [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Insley M. Y., Elliott P. N., Sturgess E. A., Smith M. J. Anti-inflammatory activity in human plasma. J Pharm Pharmacol. 1973 Nov;25(11):881–886. doi: 10.1111/j.2042-7158.1973.tb09967.x. [DOI] [PubMed] [Google Scholar]
- LADEN C., BLACKWELL R. Q., FOSDICK L. S. Anti-inflammatory effects of counterirritants. Am J Physiol. 1958 Dec;195(3):712–718. doi: 10.1152/ajplegacy.1958.195.3.712. [DOI] [PubMed] [Google Scholar]
- Leme J. G., Schapoval E. E. Stimulation of the hypothalamo-pituitary-adrenal axis by compounds formed in inflamed tissue. Br J Pharmacol. 1975 Jan;53(1):75–83. doi: 10.1111/j.1476-5381.1975.tb07332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J., Poore T. E., Zauber N. P., Oser R. S. Detection of endotoxin in the blood of patients with sepsis due to gran-negative bacteria. N Engl J Med. 1970 Dec 10;283(24):1313–1316. doi: 10.1056/NEJM197012102832404. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C., Peters J. Properties of a simplified bioassay for adrenocorticotrophic activity using the steroidogenic response of isolated adrenal cells. J Endocrinol. 1973 Oct;59(1):43–55. doi: 10.1677/joe.0.0590043. [DOI] [PubMed] [Google Scholar]
- MUNOZ J. Permeability changes produced in mice by Bordetella pertussis. J Immunol. 1961 Jun;86:618–626. [PubMed] [Google Scholar]
- McArthur J. N., Smith M. J., Freeman P. C. Anti-inflammatory substance in human serum. J Pharm Pharmacol. 1972 Aug;24(8):669–671. doi: 10.1111/j.2042-7158.1972.tb09087.x. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Smith M. J., Ford-Hutchinson A. W., Billimoria F. J. Mode of action of an anti-inflammatory fraction from normal human plasma. Nature. 1975 Apr 3;254(5499):444–446. doi: 10.1038/254444a0. [DOI] [PubMed] [Google Scholar]
- van Gool J., Schreuder J., Ladiges N. C. Inhibitory effect of foetal alpha 2 globulin, an acute phase protein, on carrageenin oedema in the rat. J Pathol. 1974 Apr;112(4):245–262. doi: 10.1002/path.1711120409. [DOI] [PubMed] [Google Scholar]



