Abstract
We have identified a new plant gene, AtCOX17, encoding a protein that shares sequence similarity to COX17, a Cu-binding protein from yeast (Saccharomyces cerevisiae) and vertebrates that mediates the delivery of Cu to the mitochondria for the assembly of a functional cytochrome oxidase complex. The newly characterized Arabidopsis protein has six Cys residues at positions corresponding to those known to coordinate Cu binding in the yeast homolog. Moreover, we show that the Arabidopsis COX17 cDNA complements a COX17 mutant of yeast restoring the respiratory deficiency associated with that mutation. These two lines of evidence indicate that the plant protein identified here is a functional equivalent of yeast COX17 and might serve as a Cu delivery protein for the plant mitochondria. COX17 was identified by investigating the hypersensitive response-like necrotic response provoked in tobacco (Nicotiana tabacum) leaves after harpin inoculation. AtCOX17 expression was activated by high concentrations of Cu, bacterial inoculation, salicylic acid treatment, and treatments that generated NO and hydrogen peroxide. All of the conditions inducing COX17 are known to inhibit mitochondrial respiration and to produce an increase of reactive oxygen species, suggesting that gene induction occurs in response to stress situations that interfere with mitochondrial function.
Plants have evolved complex defense mechanisms to protect themselves against environmental challenges. Frequently, these responses are accompanied by a certain degree of cellular damage due in part to the generation of an oxidative burst that is triggered together with several other processes that make up the general stress defense. Stress-associated interruptions or alterations in normal metabolic processes can accelerate the accumulation of reactive oxygen species (ROS) such as superoxide (O2−) and hydrogen peroxide (H2O2). These oxygen intermediates can play a role as signals to activate the production of stress defense products, but due to their strong ability to react with membranes, nucleic acids, and proteins, must be carefully regulated to avoid unwanted toxicity. To this end, plants have developed a number of nonenzymatic and enzymatic defenses, including antioxidants such as ascorbate and glutathione, as well as ROS-scavenging enzymes such as superoxide dismutases, catalases, and peroxidases (Scandalios, 1990).
The generation of reactive oxygen intermediates (ROIs) is an important component in the response of plants to pathogen invasion (Grant and Loake, 2000). Infection by avirulent pathogens often results in the formation of a hypersensitive response (HR), a programmed cell death process that is associated with the defense of plants against invading microorganisms (for review, see Dangl et al., 1996). Recent studies on the cellular signals mediating the HR response have revealed that ROIs act together with nitric oxide (NO) and salicylic acid (SA) to induce cell death (Delledonne et al., 1998). In addition, these signals mediate the activation of specific defense-related genes whose encoded products combine to limit the spread of the pathogen in the infected tissue (for review, see McDowell and Dangl, 2000). Often, the interaction with virulent pathogens is also associated with the generation of ROIs, activation of defense-related genes, and cellular injury. However, the timing and the intensity of the plant response to virulent bacteria differs from that induced by an avirulent pathogen (Grant and Loake, 2000).
Pathogen-induced HR cell death shares features in common with animal apoptosis, including a special role involving mitochondria (for review, see Lam et al., 2001). The animal mitochondrion is sensitive to damage provoked by a diverse array of stress processes. When the level of damage reaches a critical threshold, this organelle initiates a death execution pathway by altering the permeability of its outer membrane and releasing a number of cell death activators into the cytoplasm (Green and Reed, 1998). Furthermore, the disruption of the mitochondrial membrane inhibits electron transport, resulting in the generation of ROS and in a drop in ATP production (Blackstone and Green, 1999).
During our investigations of HR-like cell death induced in tobacco (Nicotiana tabacum) leaves by the bacterial polypeptide harpin, we identified a new cDNA that is expressed whenever biotic and abiotic stresses inhibit the operation of the mitochondria. Sequence analysis of a partial cDNA from tobacco and that of the corresponding Arabidopsis cDNA revealed significant homology with COX17, a gene from yeast (Saccharomyces cerevisiae) and vertebrates encoding a Cu shuttle protein that delivers Cu to the mitochondria for the assembly of cytochrome oxidase (Glerum et al., 1996; Amaravadi et al., 1997). Furthermore, this plant cDNA complemented a yeast COX17 mutation, indicating that the encoded protein might similarly function as a Cu chaperone in plants. The significance of these results in the context of ROI-mediated mitochondrial damage during periods of stress is discussed.
RESULTS
Identification of a COX17 Homolog in Tissues Responding to Harpin Inoculation
We used differential mRNA display to identify plant genes induced after inoculation with HrpN, a protein elicitor from Erwinia amylovora that elicits an HR-like cell death response in non-host tobacco leaves (Wei et al., 1992). The recombinant HrpN protein was produced in Escherichia coli and infiltrated into the apoplast of tobacco leaves as described before (Sanz et al., 1998). Changes in gene expression associated with HrpN inoculation was examined by comparing cDNA prepared from total RNA extracted from leaves 4 h after protein treatment with that prepared from RNA extracted from healthy untreated leaves. From these analyses, a 192-bp cDNA fragment, which was found to be induced reproducibly in response to protein infiltration (not shown), was selected for further characterization. The DNA sequence of this partial cDNA, designated H26-10, is shown in Figure 1A. The larger open reading frame (42 nucleotides) of H26-10 was present at its 5′end and encoded a peptide of 14 amino acids. This putative amino acid sequence shows high similarity (86% identity) to the C-terminal sequence of the protein encoded by an Arabidopsis EST clone of unknown function (GenBank accession no. AF505654) and 64% identity to the terminal sequence of the rice protein encoded by the cDNA clone C28929 (GenBank accession no. AU101012). In addition to these plant genes, the deduced amino acid sequence of the tobacco cDNA shares sequence similarity with the C terminus of COX17, a Cu shuttle protein of yeast and vertebrates that delivers Cu ions to the mitochondria for insertion into the cytochrome oxidase enzyme (Glerum et al., 1996; Amaravadi et al., 1997; Beers et al., 1997; Srinivasan et al., 1998). To examine the significance of this similarity further, the complete sequence of the Arabidopsis clone was determined and its predicted amino acid sequence was compared with that of different COX17 homologs. The Arabidopsis cDNA sequence was 474 bp long (not shown) and its larger open reading frame (222 bp) encodes a polypeptide with a predicted Mr of approximately 8,000. Sequence alignment of the Arabidopsis protein with that from rice, yeast (Glerum et al., 1996), and humans (Amaravadi et al., 1997) is shown in Figure 1B. Overall, the Arabidopsis protein shares approximately 60% identity with the rice protein, and 33% and 51% with COX17 from yeast and humans, respectively. Of particular significance, the six Cys residues, which are known to be involved in Cu binding in the yeast and human COX17 proteins, were present in the Arabidopsis sequence at similar positions. Five of these conserved cysteines were also present in the rice sequence, but the Cys-57 of yeast COX17 was replaced by a Pro in the rice protein.
Figure 1.
A, Nucleotide and deduced amino acid sequence of the tobacco H26-10 cDNA. Nucleotides are numbered from the first nucleotide of the cloned cDNA. The stop codon is marked with an asterisk. B, Sequence alignment of tobacco H26-10. Sequences shown correspond to the tobacco protein, an Arabidopsis protein encoded by the entire expressed sequence tag (EST) clone 192D11T7 (GenBank accession no. AF505654), a rice (Oryza sativa) protein encoded by the EST clone C28929 (GenBank accession no. AU101012), yeast COX17 (Glerum et al., 1996), and human COX17 (Amaravadi et al., 1997). Conserved cysteinyl residues are marked by an asterisk.
AtCOX17 Complements a Yeast COX17 Null Mutant
To determine if the Arabidopsis AtCOX17 cDNA was functionally similar to yeast COX17, we analyzed its ability to complement the respiratory deficiency associated with the yeast mutation. Disruption of COX17 in the yeast strain W303 (W303ΔCOX17) resulted in its inability to grow in a medium containing a non-fermentable substrate such as glycerol (Glerum et al., 1996). AtCOX17 was cloned into the yeast expression vector pYPGE15 (Brunelli and Pall, 1993), downstream of the PGK promoter, in both sense and antisense orientations and introduced into W303ΔCOX17, along with the original pYPGE15 vector. Individual clones were isolated under the appropriated selection conditions, maintained on Glc-containing synthetic dextrose media, and compared with both wild-type yeast and the W303ΔCOX17 strain transformed with a yeast COX17 cDNA (W303ΔCOX17/ST8). As shown in Figure 2, all the strains examined were able to grow in Glc media. When grown on glycerol-containing plates, the AtCOX17 sense construction was able to rescue yeast from its respiratory deficiency, whereas the AtCOX17 antisense construct and the pYPGE15 vector could not. In addition, the two yeast strains used as positive controls (W303ΔCOX17/ST8 and wild-type W303) were able to grow on glycerol-containing plates. Even though the expression of AtCOX17 complemented the yeast COX17 mutation, the growth of the strain transformed with the plant cDNA was slower than that expressing the endogenous yeast protein, indicating that the plant protein was less efficient in complementing the mutation. Despite this growth difference, our results demonstrated that this plant protein might act as a Cu chaperone capable of delivering the metal to mitochondria.
Figure 2.
Functional complementation of the COX17 yeast mutant. Yeast strains are as follows: 1, wild-type W303; 2, respiratory-deficient mutant W303ΔCOX17; 3, respiratory-deficient mutant W303ΔCOX17 transformed with a wild-type COX17 gene from yeast; 4, empty pYPGE15 yeast expression vector; 5, respiratory-deficient mutant (W303ΔCOX17) transformed with the AtCOX17 cDNA cloned in sense orientation into pYPGE15; and 6, respiratory-deficient mutant (W303ΔCOX17) transformed with the AtCOX17 cDNA cloned in antisense orientation into pYPGE15. Yeast strains were grown in yeast peptone dextrose Glc medium (left plate) or in yeast extract peptone dextrose glycerol containing medium (right plate). Plates were photographed at 3 d after being grown at 30°C.
Expression of AtCOX17 Is Induced Early in Response to Pathogen Infection
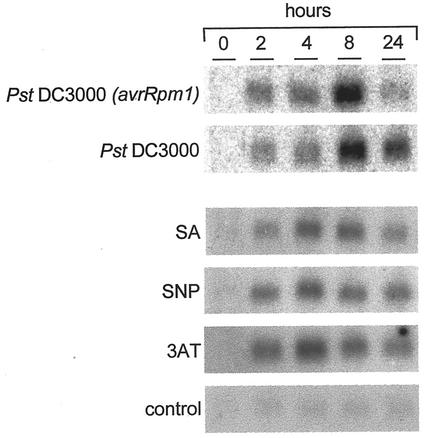
As mentioned above, the COX17 plant cDNA was initially identified in tissues responding to inoculation with a cell death inducing bacterial protein. Therefore, it was of interest to determine whether expression of this cDNA was normally induced in plants infected with a pathogen. To this end, expression of AtCOX17 was investigated at different time intervals in response to the HR-inducing bacterium Pst DC3000 avrRpm1. In addition, and to examine the specificity of this response, expression of the Arabidopsis COX17 cDNA was similarly analyzed in leaves responding to inoculation with Pst DC3000, a bacterial strain that establishes a compatible interaction with Arabidopsis ecotype Columbia-0. Northern-blot analysis shown in Figure 3 revealed that AtCOX17 hybridized to a single transcript in RNA samples extracted 2 h after treatment with the HR-inducing bacterium. Maximum accumulation of AtCOX17 mRNA peaked 8 h after inoculation and decreased 24 h after bacterial treatment. A similar pattern of transcript accumulation was detected in RNA samples obtained from leaves treated with the compatible bacterial strain, although the level of RNA found at the initial intervals was slightly lower than that observed in response to the incompatible strain. Furthermore, transcript accumulation declined more slowly in this interaction; thus, significant levels of AtCOX17 mRNA were still detected in samples prepared 24 h after Pst DC3000 inoculation.
Figure 3.
Analysis of gene expression. RNA was extracted at different intervals from Arabidopsis leaves inoculated with the incompatible bacterium Pst DC3000 avrRpm1, the virulent strain Pst DC3000 (106 colony forming units mL−1), salicylic (SA), sodium nitroprusside (SNP; a generator of NO), and 3-amino-1,2,4-triazole (3AT). Inoculation with water was used as a control. Blots were hybridized with riboprobes derived from the AtCOX17 cDNA. The amount of RNA loaded was verified by ethidium bromide staining.
In addition to pathogen induction, the expression of AtCOX17 was examined in leaves responding to cellular signals known to mediate the plant response to Pseudomonas syringae pv tomato infection. As shown in Figure 3, AtCOX17 RNA levels increased within 2 h after SA injection. Expression reached its maximum accumulation 4 h after treatment and then declined over the next 20 h. A similar expression pattern was observed when leaves were treated with SNP (0.5 mm), a compound known to generate NO in the treated tissues (Delledonne et al., 1998). A weak increase of AtCOX17 mRNA was detected 2 h after infiltration that reached its maximum accumulation 4 h after SNP application.
Among the ROI generating chemicals used, 3AT injection, which generates intracellular H2O2, provoked a strong induction of AtCOX17 mRNA within 2 h after treatment that continued to increase over the next 2 h, and then began to decrease by 8 h of treatment. In contrast to these results, no induction of gene expression was observed when plants were subjected to chemical treatment with Glc-Glc oxidase, which produces extracellular H2O2. Similarly, no induction of AtCOX17 expression was observed when plants were treated with either paraquat or xanthine-xanthine oxidase, which generate intracellular and extracellular O2−, respectively (data not shown). A very weak activation of AtCOX17 expression was observed in water-treated tissues used as a control in these experiments.
Expression of AtCOX17 Is Induced by Metals
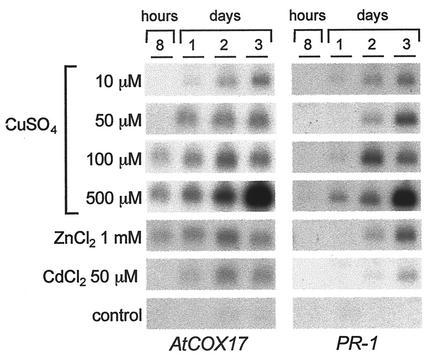
Transcription of genes encoding Cu-binding proteins has been shown to respond to changes in the concentration of Cu (Zhou and Goldsbrough, 1994). To evaluate if this could be also the case for AtCOX17, gene expression was examined after immersing the plants into a Cu solution for different periods of time. As shown in Figure 4, expression of AtCOX17 was induced with all the concentrations examined (from 10–500 μm CuSO4). However, the level of transcript accumulation varied according to the concentration of Cu. A weak level of gene activation was observed by treating the plant with 10 μm CuSO4, whereas maximum mRNA accumulation was detected when plants were immersed for 3 d in 500 μm CuSO4. In addition to Cu, activation of gene expression was examined after treating the plants with metals such as Zn (ZnCl2, 1 mm) and Cd (CdCl2, 50 μm). Results shown in Figure 4 revealed that AtCOX17 was also induced in response to these metals, although the accumulation of transcripts was significantly lower than that detected after Cu treatment. In contrast to these results, no induction of gene expression was observed when plants were immersed in water used as a control in these experiments.
Figure 4.
Metal-regulated expression of AtCOX17. RNA was extracted at different intervals from plants submerged in water solutions containing different concentrations of metals as indicated. RNA from water-immersed plants was used as a control. Blots were hybridized to AtCOX17 (left) and PR-1 (right). The amount of RNA loaded was verified by ethidium bromide staining.
Results from Pontier et al. (1999) indicated that Cu treatment induces a cell death pathway that may resemble HR, and that PR-1 expression, an HR-inducible gene, was expressed in tobacco plants responding to Cu. Accordingly, we found that the expression of PR-1 in Arabidopsis was activated by treatment with Cu and that the accumulation of transcripts increased with increasing concentrations of Cu. PR-1 responded in a similar fashion to the concentration of Cu, and like AtCOX17, was only weakly induced with Zn and Cd, and not induced at all by treatments with water alone.
DISCUSSION
We have characterized a new plant gene, COX17, encoding a protein that shares sequence similarity to COX17, a Cu-binding protein from yeast and vertebrates that mediates the delivery of Cu to the mitochondria for the assembly of the cytochrome oxidase complex (Amaravadi et al., 1997; Beers et al., 1997). We have shown that the Arabidopsis COX17 cDNA complements the mutation of COX17 in yeast restoring the respiratory deficiency associated with this mutation. These data, combined with the conservation of the six Cys residues known to mediate Cu binding in yeast (Srinivasan et al., 1998), strongly suggest that the plant protein identified here is a functional equivalent of yeast COX17 and, therefore, might play a similar role as a Cu delivery system for plant mitochondria. The yeast COX17 is localized in the cytoplasm and in the mitochondrial intermembrane space (Beers et al., 1997). Unlike most proteins targeted to that compartment, it lacks a classical mitochondrial import sequence and might be translocated directly into this organelle through a process based on the interaction of Lys residues with outer membrane phospholipids (Srinivasan et al., 1998). Like the yeast homolog, the plant COX17 proteins lack a mitochondrial import sequence. However, our complementation analyses and the sequence conservation shared with the yeast protein could be indicative of a similar cellular localization.
The cytochrome c oxidase complex accepts electrons from cytochrome c and transfers them to oxygen that is then reduced to water. Disruption of this mitochondrial process results in the generation of ROS, cessation of ATP synthesis, and, in case of animal cells, apoptosis (Bossy-Wetzel et al., 1998; Green and Reed, 1998). In this context, the fact that COX17 was identified first through a differential screen for plant proteins induced in response to treatment with harpin is likely a reflection of the loss of mitochondrial activity provoked by this elicitor (Xie and Chen, 2000). Thus, increased production of COX17 could be a physiological reflex triggered to stabilize the cytochrome oxydase complex and reduce ROS generation. This model could also account for the induction of AtCOX17 in both compatible and incompatible bacterial infections. Because there were no major differences in timing of RNA accumulation in plants infected with Pst DC3000 or Pst DC3000 avrRPM1, it is unlikely that COX17 plays a specific role in the HR cell death program. Instead, it is more likely that induction of AtCOX17 is a necessary part of any response to stress situations that provoke mitochondrial damage. This idea is supported by our results showing that AtCOX17 expression was also stimulated by SA and NO treatments. Both molecules not only act as signaling intermediates in plant defense responses (Durner et al., 1997, 1998; Delledonne et al., 1998), but also interfere with mitochondrial electron transport leading to the generation of ROS, including H2O2, and to a reduction of ATP synthesis (Brown, 1999; Xie and Chen, 1999). Furthermore, because the highest induction of AtCOX17 resulted from treatment with 3AT, production of COX17 could be critical to cells accumulating H2O2.
It is possible that Cu induces AtCOX17 for similar reasons. Application of Cu at high concentrations has been found to provoke an increase of H2O2, cell necrosis, and activation of genes such as PR-1 that are otherwise associated with pathogen infection (Mhiri et al., 1997; Pontier et al., 1998, 1999). Cu is an essential redox cofactor for a wide variety of Cu-dependent enzymes, but the reactivity of Cu makes it highly toxic because of its ability to participate in chemical reactions that generate hydroxyl radicals (Bolwell and Wojtaszek, 1997). To prevent this, and yet provide sufficient Cu for essential biochemical processes, cells have evolved homeostatic mechanisms that bind and deliver Cu to various cellular compartments (for recent review, see Labbé and Thiele, 1999; Harrison et al., 2000). Two Arabidopsis genes, RAN1 and CCH, have been found recently to encode the first components of the intracellular Cu delivery system identified in plants (Himelblau and Amasino, 2000). This pathway is required to create functional ethylene receptors and to facilitate the transport of Cu from decaying organs (Himelblau et al., 1998; Hirayama et al., 1999; Mira et al., 2001). Despite their role in Cu homeostasis, neither CCH nor RAN1 are induced by Cu treatment, indicating that they might be more important in helping cells cope with Cu deficit than with Cu excess. In contrast, activation of gene expression in response to Cu treatment might be an indication that AtCOX17 function much like metallothioneins, which are also induced by high concentrations of metals (Zhou and Goldsbrough, 1994). Metallothioneins, once thought to protect cells from excess amounts of heavy metals, are now believed to play a bigger role as chaperones for zinc-dependent enzymes and transcription factors (Ogra and Suzuki, 2000; Ye et al., 2001). Working in the same way, AtCOX17 could provide cells with a means to donate Cu to cytochrome oxidases molecules being made to repair ROS-induced damage done to the electron transport pathway. Moreover, COX17 could donate Cu to cytosolic enzymes such as Cu/Zn superoxide dismutase that are critical for restricting the accumulation of some ROS. In this manner, COX17 would contribute to the increase in activity of specific enzymes that are required to preserve organelle functionality in a number of biotic and abiotic stress situations.
MATERIALS AND METHODS
Plant Material and Growth
The plants used in this study were tobacco (Nicotiana tabacum cv Petit Havana SR1) and Arabidopsis ecotype Columbia-0. Plants were grown in a grown in chamber (22°, 70% relative humidity, 200 μE m−2 s−1 fluorescent illumination) under a 14-h-light/10-h-dark photoperiod.
Differential Display
Differential display analyses (Liang and Pardee, 1992) were performed on total RNA extracted from tobacco leaves inoculated with a purified maltose-binding protein-hairpin fusion protein as described before (Sanz et al., 1998), and using the RNA map kit (GenHunter Corporation, Brookline, MA). The tobacco cDNA selected for this study was obtained as a 192-bp fragment by using the T12MA primer for reverse transcription of the mRNA and both the AP2 and T12MA primers for further amplification of the cDNA. The induced band in the two independent RNA samples was excised and reamplified according to the manufacture's protocol. The amplified fragment was purified on agarose gel, cloned into the pBSK+ vector (Stratagene, La Jolla, CA), and sequenced. Standard DNA techniques were carried out as described by Ausubel et al. (1988) and Sambrook et al. (1989). Sequence data were analyzed using the Genetic Computer Group (Madison, WI) Package (version 10.1). Searches on the databases were done with the BLAST and FASTA programs. Alignment of the protein sequences was done using the Clustal method and the DNASTAR program (Madison, WI).
Yeast (Saccharomyces cerevisiae) Strains and Construction of the Expression Vector
The yeast strains used in this study were: W303–1A, W303ΔCOX17/ST8, and W303ΔCOX17 (Glerum et al., 1996). The yeast expression vector pYPGE15 (Brunelli and Pall, 1993) was used for cloning the Arabidopsis COX17 cDNA in both sense and antisense orientation, behind the PGK promoter present in the plasmid. Constructs were introduced in yeast by the LiOAc method (Gietz and Woods, 1994). Transformed cells were plated onto selective synthetic dextrose media lacking uracil, and incubated at 30°C until colonies appeared. Selected yeast transformants were tested for functional complementation by growing on yeast extract peptone Glc media (3% [w/v] glycerol, 2% [w/v] ethanol, and 1% [w/v] yeast extract) for 3 d at 30°C, as described by Glerum et al. (1996).
Analysis of Gene Expression and Plant Treatments Examined
RNA was prepared according to Logemann et al. (1987). RNA (10 μg lane−1) was analyzed in agarose-formaldehyde gels, transferred to Hybond N+ membranes (Amersham, Buckinghamshire, UK), and hybridized to single-stranded riboprobes following standard procedures (Sambrook et al., 1989). The amount of loaded RNA was verified by addition of ethidium bromide to the samples and photography under UV light after electrophoresis. Bacterial inoculation was done by injecting leaves with a late-logarithmic culture using a syringe as described by Sanz et al. (1998). Bacterial species used were: Pseudomonas syringae pv tomato DC3000 (Whalen et al., 1991) and P. syringae pv tomato DC3000 avrRpm1 (Debener et al., 1991). For chemical treatment, leaves were injected with SA (1 mm), 3AT (4 mm), paraquat (10 μm), xanthine-xanthine oxidase (2 mm–0.1 units mL−1), and Glc-Glc oxidase (2.5 mm–2.5 units mL−1) or SNP (1 mm). For metal ion treatment, plants were carefully taken out from soil, and roots were washed with distilled water and submerged in a water solution containing different concentrations of metals. Experiments were performed independently at least three times, and representative data are shown.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material.
ACKNOWLEDGMENTS
We thank Alan Caplan for critical reading of the manuscript. We thank Dr. Alexander Tzagoloff for the yeast strains used in this study. We also thank Tomas Cascón for excellent technical assistance, and Ines Poveda and Christiane Germonprez for expert photography and preparation of figures. The ESTs were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus).
Footnotes
This work was supported by the Ministry of Science and Technology (Comisión Interministerial de Ciencia y Tecnología, grant no. BIO97–0656) and by Comunidad Autónoma de Madrid (grant no. 07B/0033/1998 and fellowship to T.B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010963.
LITERATURE CITED
- Amaravadi R, Glerum DM, Tzagoloff A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet. 1997;99:329–333. doi: 10.1007/s004390050367. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols of Molecular Biology, 1987-1988. New York: John Wiley and Sons; 1988. [Google Scholar]
- Beers J, Glerum DM, Tzagoloff A. Purification, characterization, and localization of yeast Cox17p, a mitochondrial copper shuttle. J Biol Chem. 1997;272:33191–33196. doi: 10.1074/jbc.272.52.33191. [DOI] [PubMed] [Google Scholar]
- Blackstone NW, Green DR. The evolution of a mechanism of cell suicide. BioEssays. 1999;21:84–88. doi: 10.1002/(SICI)1521-1878(199901)21:1<84::AID-BIES11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol Mol Plant Pathol. 1997;51:347–366. [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondrial respiration. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Brunelli JP, Pall ML. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast. 1993;9:1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8:1793–1809. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener T, Lehnackers H, Arnold M, Dangl JL. Identification and molecular mapping of a single Arabidopsis thaliana locus determining resistance to a phytopathogenic Pseudomonas syringae isolate. Plant J. 1991;1:289–302. doi: 10.1046/j.1365-313X.1991.t01-7-00999.x. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Durner J, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Trends Plant Sci. 1997;2:266–274. [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. High efficiency transformation in yeast. In: Johnston JA, editor. Molecular Genetics of Yeast: Practical Approaches. Oxford: Oxford University Press; 1994. pp. 121–134. [Google Scholar]
- Glerum DM, Shtanko A, Tzagoloff A. Characterization of COX17, a yeast gene involved in copper metabolism and assembly of cytochrome oxidase. J Biol Chem. 1996;271:14504–14509. doi: 10.1074/jbc.271.24.14504. [DOI] [PubMed] [Google Scholar]
- Grant JJ, Loake GJ. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000;124:21–29. doi: 10.1104/pp.124.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Harrison MD, Jones CE, Solioz M, Dameron CT. Intracellular copper routing: the role of copper chaperones. Trends Biol Sci. 2000;25:29–32. doi: 10.1016/s0968-0004(99)01492-9. [DOI] [PubMed] [Google Scholar]
- Himelblau E, Mira H, Lin SJ, Culotta VC, Peñarrubia L, Amasino RM. Identification of a functional homolog of the yeast copper homeostasis gene ATX1 from Arabidopsis. Plant Physiol. 1998;117:1227–1234. doi: 10.1104/pp.117.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama T, Kieber JJ, Hirayama N, Kogan M, Guzman P, Nourizadeh S, Alonso JM, Dailey WP, Dancis A, Ecker JR. RESPONSIVE-TO-ANTOGANIST1, a Menkes/Wilson disease-related copper transporter, is required for ethylene signaling in Arabidopsis. Cell. 1999;987:383–393. doi: 10.1016/s0092-8674(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Labbé S, Thiele DJ. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends Microbiol. 1999;7:500–505. doi: 10.1016/s0966-842x(99)01638-8. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992;257:967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Logemann J, Shell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Mira H, Martinéz-García F, Peñarrubia L. Evidence for the plant-specific intercellular transport of the Arabidopsis copper chaperone CCH. Plant J. 2001;25:521–528. doi: 10.1046/j.1365-313x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- McDowell JM, Dangl JL. Signal transduction in the plant immune response. Trends Biol Sci. 2000;25:79–82. doi: 10.1016/s0968-0004(99)01532-7. [DOI] [PubMed] [Google Scholar]
- Mhiri C, Morel JB, Vernhettes S, Casacuberta JM, Lucas H, Grandbastien MA. The promoter of the tobacco Tnt1 retrotransposon is induced by wounding and by abiotic stress. Plant Mol Biol. 1997;33:257–266. doi: 10.1023/a:1005727132202. [DOI] [PubMed] [Google Scholar]
- Ogra Y, Suzuki KT. Nuclear trafficking of metallothionein: possible mechanisms and current knowledge. Cell Mol Biol. 2000;46:357–365. [PubMed] [Google Scholar]
- Pontier D, Gan S, Amasino RM, Roby D, Lam E. Markers for hypersensitive response and senescence show distinct patterns of expression. Plant Mol Biol. 1999;39:1243–1255. doi: 10.1023/a:1006133311402. [DOI] [PubMed] [Google Scholar]
- Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D. Activation of hsr203, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant-Microbe Interact. 1998;6:544–554. doi: 10.1094/MPMI.1998.11.6.544. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritisch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanz A, Moreno JI, Castresana C. PIOX, a new pathogen-induced oxygenase with homology to animal cyclooxygenase. Plant Cell. 1998;10:1523–1537. doi: 10.1105/tpc.10.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Response of plant antioxidant defense genes to environmental stress. Adv Genet. 1990;28:1–41. doi: 10.1016/s0065-2660(08)60522-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan C, Posewitz MC, George GN, Winge DR. Characterization of copper chaperone Cox17 of Saccharomyces cerevisiae. Biochemistry. 1998;37:7572–7757. doi: 10.1021/bi980418y. [DOI] [PubMed] [Google Scholar]
- Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science. 1992;257:85–88. doi: 10.1126/science.1621099. [DOI] [PubMed] [Google Scholar]
- Whalen MC, Innes RW, Bent AF, Staskawicz BJ. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Chen Z. Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol. 1999;120:217–225. doi: 10.1104/pp.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Chen Z. Harpin-induced hypersensitive cell death is associated with altered mitochondrial functions in tobacco cells. Mol Plant-Microbe Interact. 2000;13:183–190. doi: 10.1094/MPMI.2000.13.2.183. [DOI] [PubMed] [Google Scholar]
- Ye B, Maret W, Valle BL. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc Natl Acad Sci USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Goldsbrough PB. Structure, organization and expression of the metallothionein gene family in Arabidopsis. Mol Gen Genet. 1994;248:318–328. doi: 10.1007/BF02191599. [DOI] [PubMed] [Google Scholar]