Abstract
Transgenic tobacco (Nicotiana tabacum) with altered levels of mitochondrial alternative oxidase (AOX) were used to examine the potential role of this electron transport chain protein in resistance to tobacco mosaic virus. We examined the effect of AOX expression on the salicylic acid-induced resistance in susceptible plants and the resistance responses of plants harboring the N-gene. A lack of AOX did not compromise the ability of salicylic acid treatment to heighten the resistance of susceptible plants. In plants with the N-gene, a lack of AOX did not compromise the ability of the hypersensitive response to restrict the virus or the ability of the plant to develop systemic acquired resistance. Overexpression of AOX did not heighten the resistance of susceptible plants, but did result in smaller hypersensitive response lesions, suggesting a link between mitochondrial function and this programmed cell death event. We conclude that AOX is not a critical component of the previously characterized salicylhydroxamic acid-sensitive pathway important in viral resistance.
Mitochondria play a central role in energy and carbon metabolism of eukaryotic cells, being the site of the tricarboxylic acid cycle and oxidative phosphorylation pathways (Siedow and Day, 2000). As well, mitochondria have other important functions such as taking an active role in programmed cell death (PCD) pathways of animals (Green and Reed, 1998) and possibly plants (Jones, 2000; Lam et al., 2001).
In plants, the electron transport chain supporting oxidative phosphorylation branches at ubiquinone (Siedow and Day, 2000; Vanlerberghe and Ordog, 2002). Electrons can flow from ubiquinone through the usual cytochrome (cyt) pathway or to alternative oxidase (AOX). Electron flow from ubiquinone to AOX bypasses two of three sites of energy conservation supporting oxidative phosphorylation, thereby reducing the energy efficiency of respiration. Salicylhydroxamic acid (SHAM) is an artificial chemical inhibitor of AOX activity and has been used in AOX studies in intact tissues and isolated mitochondria (Schonbaum et al., 1971; Siedow and Day, 2000).
An effective means to induce AOX gene expression in tobacco (Nicotiana tabacum) and other plant species is by artificial chemical inhibition of the cyt pathway by compounds such as CN or antimycin A (Vanlerberghe and McIntosh, 1994; Wagner and Wagner, 1997). Expression is also induced in tobacco and other species by treatment with salicylic acid (SA; Rhoads and McIntosh, 1993). In fact, one of the first roles identified for SA in plants was to induce AOX expression in thermogenic flowers (Raskin et al., 1987). The mechanism of action of SA in this regard is unknown.
Tobacco mosaic virus (TMV) infection of tobacco plants harboring the N resistance gene results in plant PCD at the site of infection, restricting the virus to the immediate region (Whitham et al., 1994; Dawson, 1999). This induction of PCD at a site of pathogen infection is known as the hypersensitive response (HR) and is commonly associated with pathogen resistance (Heath, 2000). Another induced defense response often accompanying the HR is the development of systemic acquired resistance (SAR). SAR is induced several days following pathogen infection and is a long-lasting heightened resistance to a broad spectrum of pathogens throughout the plant (Gaffney et al., 1993). For example, in N-gene tobacco plants displaying SAR, TMV infection results in a reduced number and size of HR lesions (Ross, 1961). TMV infection of susceptible (nn genotype) tobacco results in accumulation and systemic movement of virus, leading to the mosaic symptoms and stunted growth typical of the diseased state (Dawson, 1999).
SA is a key component of plant signal transduction pathway(s) involved in defense responses against bacterial, fungal, and viral pathogens (Dempsey et al., 1999; Alvarez, 2000). For example, SA levels increase 20-fold in TMV-inoculated leaves of N-gene tobacco, preceding the activation of defense responses (Malamy et al., 1990). As well, pretreatment of TMV-susceptible tobacco plants with SA delays TMV accumulation and the onset of disease symptoms (White, 1979; Chivasa et al., 1997). Transgenic tobacco plants expressing the nahG gene (encoding a bacterial SA-metabolizing enzyme) are unable to accumulate SA. Such plants fail to develop SAR (Gaffney et al., 1993), and the HR is compromised in its ability to restrict the virus (Mur et al., 1997). Although the importance of SA in plant resistance responses to numerous pathogens is well established, the mechanism of action of SA, in particular resistance phenomenon, remains a subject of much debate (Dempsey et al., 1999; Alvarez, 2000).
In recent years, a series of studies have indicated that tobacco resistance to TMV occurs via a SHAM-sensitive pathway (Chivasa et al., 1997; Chivasa and Carr, 1998; Naylor et al., 1998; Ji and Ding, 2001). The SA-induced resistance of susceptible (nn genotype) plants and the resistance responses of N-gene plants are compromised by SHAM (Chivasa et al., 1997). Furthermore, pretreatment of plants with cyt pathway inhibitors strongly induces AOX gene expression and this treatment is able to heighten the resistance of nn genotype plants (similar to that achieved by SA treatment) and substitute for SA (in nahG plants) in the N-gene-mediated resistance responses (Chivasa and Carr, 1998). In addition, effects of the respiratory inhibitors on viral resistance are again compromised by SHAM. Based on these pharmacological and correlative data, it has been hypothesized that a role for SA accumulation in viral resistance is to induce AOX and that resistance responses are dependent upon AOX activity and hence are antagonized by SHAM (outlined in Murphy et al., 1999). Also, resistance responses to bacterial and fungal pathogens were unaffected by SHAM, suggesting that the SHAM-sensitive pathway is viral specific (Murphy et al., 1999).
Here, we use transgenic tobacco plants with altered levels of AOX protein to evaluate whether this component of mitochondrial electron transport is critical in viral resistance responses.
RESULTS
Experiments with Susceptible Plants
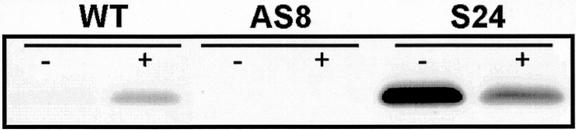
Susceptible (nn genotype) plants were used to examine the effect of AOX expression on TMV accumulation and SA-induced resistance. When plants were grown hydroponically and supplied with 2 mm SA in their growth medium, there was a dramatic increase in the level of leaf mitochondrial AOX (Fig. 1). In an alternate manner, transgenic plants with constitutive expression of a sense AOX transgene (S24) maintained very high levels of AOX in the presence or absence of SA. In transgenic plants harboring an antisense AOX transgene (AS8), AOX protein was never detected, indicating effective inhibition of AOX expression, even under strong inducing (+SA) conditions (Fig. 1).
Figure 1.
The level of leaf mitochondrial AOX protein in wild-type and transgenic (AS8 and S24) plants. AS8 plants contain an antisense AOX transgene that results in a silencing of leaf AOX expression, whereas S24 plants contain a sense AOX transgene that results in constitutive high levels of leaf AOX protein. Plants were either left untreated (−) or were treated for 12 h with 2 mm SA in their hydroponic medium prior to mitochondrial isolation. Mitochondrial protein (100 μg) was separated by SDS-PAGE, transferred to nitrocellulose, and probed with a monoclonal antibody against AOX. Typical results are shown.
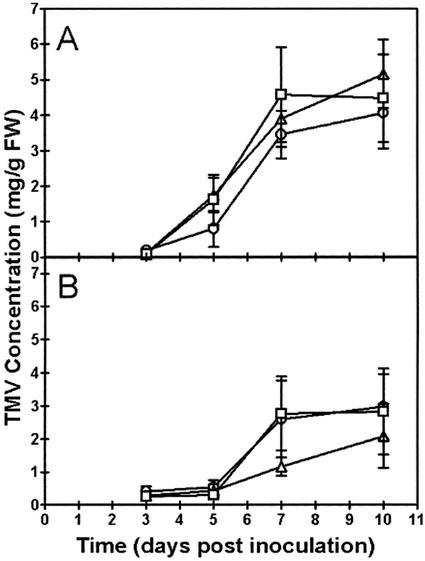
We compared the accumulation of TMV coat protein in wild-type and transgenic plants that were untreated or treated with SA. Treatment with SA involved daily changes of the hydroponic medium supplemented with 0.1 mm SA, whereas untreated plants received daily changes of the same medium without SA. The SA treatment was provided for 7 d prior to TMV inoculation and throughout the experimental period following inoculation. In untreated wild-type plants, no TMV coat protein (in leaves 2 and 3 above the inoculated leaf) was detected at 3 d postinoculation (DPI), but significant amounts were present in most cases by 5 DPI (Fig. 2A). Rapid accumulation of coat protein then occurred between 5 and 7 DPI, with little further accumulation by 10 DPI. This pattern of accumulation correlated well with typical visible symptoms of TMV disease in the plant. The earliest visible symptom (vein clearing at the base of third leaf above inoculated leaf) became apparent between 4 and 5 DPI. By 10 DPI, symptoms were more obvious and included leaf curling, chlorophyll loss (mosaic discoloration), stunting of growth, and leaf burn (necrosis). There was no significant difference (one-way analysis of variance [ANOVA]) between wild-type, AS8, and S24 plants in the extent of coat protein accumulation at any of the time points examined (Fig. 2A). As well, all three plant lines showed similar visible disease symptoms at all stages of the experiment.
Figure 2.
The level of TMV coat protein in leaves of wild-type and transgenic plants. A, Plants were grown in the absence of SA. B, The hydroponic medium of plants was supplemented with 0.1 mm SA for 7 d prior to TMV inoculation and throughout the postinoculation period. Results for wild-type (□), AS8 (▵), and S24 (○) plants are shown. TMV coat protein in leaves 2 and 3 above the inoculated leaf was determined using an ELISA assay. Data represent the mean ± se from eight independent experiments (n = 8). In each experiment, data for each time point were obtained from a separate plant. Hence, the complete data set is based upon a total of 192 individual plants. The ELISA assay indicated that mock-inoculated plants measured at 10 DPI had no TMV coat protein.
When wild-type plants were treated with SA, there was a heightened resistance against TMV as indicated by a delay or absence of visible symptoms of disease and accumulation of coat protein during the experimental period (Fig. 2B). In this case, no plants showed significant accumulation of TMV coat protein or any visible symptoms of disease at 5 DPI. The level of TMV coat protein at 7 and 10 DPI was highly variable (Fig. 2B) because in five of the eight plants at 7 DPI and in five of the eight plants at 10 DPI, no coat protein (or visible disease symptoms) was observed.
When AS8 and S24 plants were treated with SA, none of the plants accumulated significant levels of TMV coat protein or displayed visible disease symptoms at 5 DPI. Again, the level of TMV coat protein at 7 and 10 DPI was highly variable (Fig. 2B) due to a complete lack of coat protein in some plants. At 7 DPI, four of the eight AS8 plants and four of the eight S24 plants lacked any coat protein. At 10 DPI, three of the eight AS8 plants and four of the eight S24 plants lacked any coat protein. Again, lack of coat protein in individual plants correlated well with a lack of visible disease symptoms. In summary, for the SA-treated plants, we found no significant difference (one-way ANOVA) between wild-type, AS8, and S24 plants in the extent of coat protein accumulation at any of the time points examined (Fig. 2B). Mock-inoculated plants (±SA) never accumulated coat protein or showed disease symptoms.
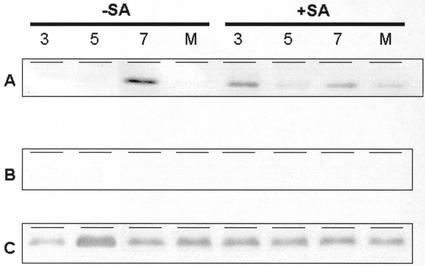
For the above experiments, we also examined the level of AOX protein in leaves 2 and 3 above the inoculated leaf. As expected, S24 plants maintained high levels of AOX protein at each time point, with or without SA treatment (Fig. 3C). Also, no AOX protein was ever detected in AS8 leaves (Fig. 3B). In the wild type at 3 DPI (prior to any significant accumulation of TMV coat protein; Fig. 2), we consistently found that the level of AOX protein was high in SA-treated plants and very low in untreated plants (Fig. 3A). In a similar manner, the level of AOX in mock-inoculated SA-treated wild-type plants (at 10 DPI) was high, whereas the level in mock-inoculated untreated plants remained low (Fig. 3A). These results indicate that the SA treatments used in these experiments maintained high levels of leaf AOX in wild-type plants throughout the experimental period. It is interesting that we found that significant amounts of AOX protein accumulated by 7 DPI in untreated (−SA) plants. This accumulation correlated with severe symptoms of TMV disease (such as extensive necrosis) in the leaves by this time point.
Figure 3.
The level of leaf mitochondrial AOX protein in wild-type (A), AS8 (B), and S24 (C) plants grown in hydroponic culture. The (+SA) plants were grown with 0.1 mm SA in their hydroponic medium for 7 d prior to TMV inoculation and throughout the postinoculation period. Numbers refer to DPI, whereas the M refers to mock-inoculated plants, in which case mitochondria were isolated at 10 DPI. Mitochondria were isolated from leaves 2 and 3 above the inoculated leaf, and mitochondrial protein (40 μg) was separated by SDS-PAGE, transferred to nitrocellulose, and probed with a monoclonal antibody against AOX. Typical results are shown.
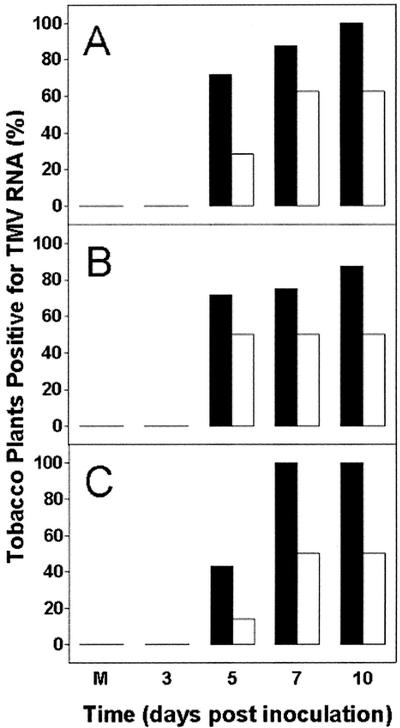
For the above experiments, we also used northern analysis to score for the presence or absence of TMV coat protein RNA (in leaves 2 and 3 above the inoculated leaf) as an independent measure of the degree of TMV accumulation. Figure 4 summarizes the northern results of all experiments, showing the percentage of plants that scored positive for TMV coat protein RNA at each time point. In untreated (−SA) wild-type plants, over 70% of plants were positive for viral RNA at 5 DPI, whereas only 29% of SA-treated plants tested positive at this time point (Fig. 4A). A similar delay by SA treatment was evident in AS8 and S24 plants (Fig. 4, B and C). For all three plant lines, almost all untreated (−SA) plants were positive for viral RNA by 7 and 10 DPI. In an alternate manner, in SA-treated plants of each line, only about one-half of all plants contained detectable viral RNA at these time points. In all cases, a lack of detectable viral RNA in particular plants correlated well with an absence of TMV coat protein or visible symptoms of disease in these same plants (see above). In summary, the northern results verified that SA treatment was able to delay or prevent TMV accumulation over the experimental period and that all three plant lines acted similarly. Note that no bands were ever detected in mock-inoculated plants (at 10 DPI) or in TMV-inoculated plants (±SA) at 3 DPI.
Figure 4.
Summary of northern results establishing the presence or absence of TMV coat protein RNA in leaf tissue from wild-type (A), AS8 (B), and S24 (C) plants. Data represent the percentage of plants with detectable TMV coat protein RNA. Plants were grown in the absence (black bars) or presence (white bars) of SA and were inoculated with TMV, as explained in the legend to Figure 3. Total RNA was isolated from leaves 2 and 3 above the inoculated leaf, and 5 μg of RNA was separated on agarose gels containing formaldehyde and used for northern analysis with a radiolabeled DNA probe recognizing TMV coat protein RNA. This allowed plants to be readily scored for the presence or absence of TMV coat protein RNA. The M refers to a plant that was mock inoculated, in which case RNA was isolated at 10 DPI. Each bar represents data obtained from eight separate plants, each from a separate experiment.
Experiments with Resistant Plants
Plants harboring the N-gene were used to examine the effect of AOX expression on resistance responses (HR and SAR). Initial experiments indicated that the resistant plants grown in growth chambers maintained the same profiles of AOX expression as seen in hydroponically grown susceptible plants. That is, AS8 plants lacked detectable AOX protein, wild-type plants displayed low levels of AOX protein, and S24 plants displayed high levels of AOX protein (data not shown).
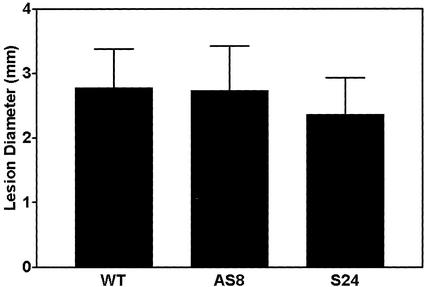
Wild-type, AS8, and S24 plants each generated well-defined, circular HR lesions within approximately 3 d after TMV inoculation. As well, we saw no difference between plant lines in the total number of lesions produced (data not shown). However, we did find that S24 lesions were significantly smaller (one-way ANOVA) than wild-type or AS8 lesions by about 15% (Fig. 5). This result was observed in all five experiments. In an alternate manner, wild-type and AS8 plants had almost identical mean lesion diameters (differing by only 1.5%). After measuring lesions, plants were left in growth chambers for an additional 1 to 3 weeks. In this case, we never observed the appearance of satellite lesions or lesions in upper uninoculated leaves, suggesting that in each plant line the original HR had effectively localized the virus.
Figure 5.
Diameter of HR lesions on TMV-inoculated leaves of wild-type and transgenic (AS8 and S24) tobacco plants containing the N-gene. Lesion diameters were determined at 7 DPI. Data are from five independent experiments. In each experiment, six plants each of wild type, AS8, and S24 were used (each with a single inoculated leaf). Approximately 50 lesions per leaf were measured. Data are the mean ± sd (wild type, n = 1,773; AS8, n = 1,467; S24, n = 1,640).
Some component of the N-gene-mediated HR is temperature sensitive (Whitham et al., 1994). Plants grown above 28°C and inoculated with TMV do not mount an HR, but instead allow the virus to accumulate and spread systemically. If such systemic infected plants are then shifted to a permissive temperature, there is extensive and synchronized lesion formation in the virus-infected tissues. We grew plants at 30°C and inoculated one leaf with TMV. Plants were then kept at 30°C for an additional 7 to 14 d, over which time the plants (all three lines) developed typical disease symptoms similar to that seen in susceptible plants. Plants were then shifted to 20°C and were monitored closely to evaluate characteristics of the death response in tissues with accumulated virus. In each experiment, lesion development became visible at 9 h following transfer to the permissive temperature. Cell death occurred first along the veins and following the leading edge of chlorotic mosaic areas. Between 9 and 12 h, the initial areas of death expanded, particularly in leaves showing disease symptoms. Plants were monitored for approximately 36 h, but necrotic regions did not expand further than what was observed by 12 h. In each experiment, the amount of time elapsed prior to the initial signs of necrosis, the progression of death between 9 and 12 h, the lesion morphology, and the overall extent of necrosis did not noticeably differ between the three plant lines.
In a related experiment, plants grown at 22°C were inoculated with TMV to induce HR lesions. These plants were subsequently moved to 30°C for 7 d before being moved back to the permissive temperature. After this final temperature transfer, we never saw the development of additional lesions on wild-type, AS8, or S24 plants. This experiment further illustrates that the original HR response was, in all cases, effective in restricting the virus.
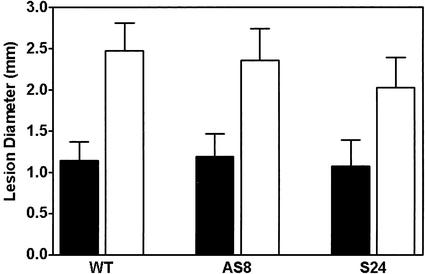
An experiment outlined in the legend to Figure 6 was used to evaluate the ability of each plant line to induce SAR. In all three plant lines, significantly smaller lesions formed on plants that had been previously inoculated with TMV compared with plants that had been previously only mock inoculated (Fig. 6). This indicates that each plant line had developed a heightened resistance to TMV in response to the initial viral inoculation, i.e. a classical SAR response. As well, far fewer lesions were seen after the second inoculation in plants (of each plant line) previously exposed to virus (data not shown).
Figure 6.
Diameter of HR lesions in wild-type and transgenic (AS8 and S24) tobacco plants displaying SAR. In these experiments, plants were initially inoculated with TMV (black bars) or they were mock inoculated (white bars). Seven days after this initial inoculation, an upper leaf (two leaves above the initial inoculated leaf) was inoculated and lesion diameters on this upper leaf were determined after 7 d. Data are the mean ± sd (black bars, wild type, n = 22; AS8, n = 38; S24, n = 14; white bars, wild type, n = 150; AS8, n = 147; S24; n = 169).
DISCUSSION
SA plays a key role in plant resistance responses to pathogens (see Introduction). SA is also known to induce AOX gene expression (Raskin et al., 1987; Rhoads and McIntosh, 1993), and increases in AOX expression have been noted in the response of different plants to viral and other pathogen attacks (Chivasa et al., 1997; Lennon et al., 1997; Chivasa and Carr, 1998; Lacomme and Roby, 1999; Simons et al., 1999). Furthermore, several studies indicate that viral resistance (SA-induced resistance in susceptible plants and N-gene-mediated resistance responses) is antagonized by SHAM, a well-known inhibitor of AOX activity (Chivasa et al., 1997; Chivasa and Carr, 1998; Naylor et al., 1998). Hence, it has been suggested that an important role for SA in plant viral resistance is to induce AOX and that resistance responses are dependent upon AOX activity and hence compromised by SHAM (Murphy et al., 1999). Nonetheless, another study found that whereas the level of AOX protein was increased in local and systemic tobacco tissues following TMV infection, no increase in AOX activity could be detected using the oxygen isotope discrimination technique (Lennon et al., 1997). It should also be noted that whereas the SHAM-sensitive pathway is viral specific (Murphy et al., 1999), the induction of AOX is not. AOX induction occurs in response to other pathogen infections as well (Lacomme and Roby, 1999; Simons et al., 1999) and hence may be a general consequence of pathogen infection rather than a specific resistance response to virus.
Here, we used transgenic plants with altered levels of AOX protein to critically assess the potential role of AOX in plant viral resistance. In susceptible plants, a lack of AOX in AS8 did not compromise the ability of SA to delay symptom development or to reduce accumulation of TMV protein and RNA in upper uninoculated leaves (Figs. 2 and 4 and text). In N-gene plants, a lack of AOX did not compromise effective restriction of the virus by the HR (Fig. 5 and text), and it did not compromise the effective establishment of SAR (Fig. 6).
If the ability of SA treatment to increase the viral resistance of susceptible plants is primarily due to its ability to increase AOX activity (as previous experiments with SHAM suggest), then one might expect that constitutive overexpression of AOX might substitute for SA treatment. We found this not to be the case. Untreated (−SA) S24 plants were found to develop disease symptoms and to accumulate TMV in upper uninoculated leaves at a similar rate to the wild-type and AS8 plants (Figs. 2 and 4 and text).
The key evidence in previous experiments suggesting an involvement of AOX in viral resistance were experiments using SHAM to inhibit AOX activity (Chivasa et al., 1997; Chivasa and Carr, 1998; Naylor et al., 1998). In past plant respiration studies, SHAM has been primarily used in short-term (minutes) assays to estimate the activity or capacity for AOX respiration. However, the usefulness of SHAM in the long-term (days) assays done to evaluate AOX involvement in viral resistance will be severely hampered by the nonspecific effects of this inhibitor. Effects of SHAM on several other plant processes beside AOX activity have often been described (Rich et al., 1978; Moller and Berczi, 1986; Pressig and Kuc, 1987; Moller et al., 1988; Singh et al., 1992; Kawano et al., 1998). Furthermore, several of these other SHAM targets such as peroxidases, lipoxygenases, and plasmalemma NAD(P)H oxidase activities (Moller and Berczi, 1986; Pressig and Kuc, 1987; Kawano et al., 1998) have themselves been implicated in plant resistance to pathogens. A further complication is that SHAM treatment likely generates small amounts of SA in plant tissue (due to contamination of SHAM with SA or breakdown of SHAM to SA in the tissue). One apparent result of this is induction of PR-1 protein by SHAM treatment (Chivasa et al., 1997; Chivasa and Carr, 1998). Other complications of the pharmacological approach were that many experiments required a long incubation (up to 9 d) of isolated leaf discs, the need to inoculate leaf discs with TMV, and the use of high concentrations of SA with leaf discs (Chivasa et al., 1997; Chivasa and Carr, 1998). In our hands, these experimental approaches were problematic and not pursued further.
Another important experiment previously used to establish a role for AOX in viral resistance involved treatment of plants with the cyt pathway inhibitors CN and antimycin A (Chivasa and Carr, 1998). These AOX-inducing treatments were able to substitute for SA in resistance responses, and this effect was again compromised by SHAM. The inhibitor approach may be problematic. First, the inhibitors have other targets beside respiration. CN inhibits many enzymes, including catalase, ascorbate peroxidase, and some superoxide dismutase isozymes (Zollner, 1989), whereas antimycin A is an inhibitor of photosynthetic electron transport (Moss and Bendall, 1984). Second, marked inhibition of energy metabolism is a drastic treatment likely to initiate a multitude of cell stress responses (Yu et al., 2001), some of which could conceivably impact pathogen resistance. Third, in treatments including a cyt pathway inhibitor and SHAM (a key treatment to evaluating the role of AOX), all of mitochondrial electron transport will be disrupted. It is perhaps not surprising that in these conditions, induced (and presumably energy-requiring) plant resistance responses would be compromised.
We found that overexpression of AOX reduced the size of HR lesions (Fig. 5). This effect did not compromise the ability of the HR to restrict the virus. Reduction in lesion size is usually interpreted as a heightened resistance to virus. Nonetheless, the reduction in size (15%) is small in comparison with the reduction of lesion size that is achieved during SAR (Fig. 6). Hence, overexpression of AOX should not be interpreted as substituting for the N-gene-mediated development of SAR. Also, the establishment of SAR reduces the size and number of HR lesions (Ross, 1961), and we saw no significant change in lesion number in S24 plants in an initial inoculation with TMV. Therefore, our hypothesis is that overexpression of AOX in N-gene plants has not significantly heightened viral resistance (just as it did not heighten the resistance of susceptible plants), but rather that overexpression has dampened the progression of PCD at the lesion periphery.
The effects of the addition of exogenous SA have recently been compared between wild-type tobacco suspension cells and antisense cells lacking AOX (Robson and Vanlerberghe, 2002). We find that addition of SA completely abolishes the whole-cell respiration of antisense cells and that this eventually leads to a loss of cyt c from the mitochondrion (abolishing the cyt pathway) and an induction of PCD (as shown by the accumulation of oligonucleosomal fragments of DNA). SA-induced cyt c release and PCD have been recently documented in animal cells (Pique et al., 2000). SA was also shown to dramatically reduce respiration of tobacco cells, although its site of action in respiration was unclear (Xie and Chen, 1999). These results support the notion that SA accumulation may disrupt mitochondrial function during responses to pathogens and that AOX may play a role in modulating entry into a PCD pathway involving the mitochondrion. One can hypothesize that the ability of AOX to modulate this PCD pathway may relate to its ability to dampen the mitochondrial generation of reactive oxygen species by preventing overreduction of electron transport chain components such as ubiquinone (Maxwell et al., 1999; Parsons et al., 1999; Yip and Vanlerberghe, 2001). Such a function might be critical given that reactive oxygen species are widely implicated as signaling molecules in most PCD pathways in plants and animals (Jabs, 1999). Further study of such phenomenon may shed light on the ability of AOX overexpression to slightly reduce lesion size in response to TMV.
In conclusion, we find no evidence that AOX is a critical component of the SHAM-sensitive signal transduction pathway important for plant viral resistance, but we do suggest that AOX may play a role in HR cell death, as previously suggested by expression data (Lacomme and Roby, 1999).
MATERIALS AND METHODS
Plant Material
The transgenic tobacco (Nicotiana tabacum cv Petit Havana SR1) with altered levels of mitochondrial AOX (due to the introduction of sense or antisense AOX gene constructs) has been previously described (Vanlerberghe et al., 1994). This cultivar of tobacco lacks the N-gene and hence is susceptible to TMV disease (Dawson, 1999). Experiments were performed on T2 generation seedlings resistant to kanamycin. To introduce the N-gene, mature pollen from wild-type and T2 transgenic plants was applied to the pistil of tobacco cv Xanthi (with anthers removed). Seed was collected and kanamycin-resistant seedlings were used.
Growth Conditions
Susceptible plants were grown in hydroponics to facilitate treatment of the plants with SA. Plants were grown in one-tenth Hoagland medium (Hoagland and Arnon, 1950), which was changed daily. When indicated, this medium was supplemented with SA. Plants were grown with a 16-h photoperiod at approximately 25°C and at a light intensity of approximately 100 μmol photons m−2 s−1. To inoculate plants, carborundum was applied to the adaxial surface of one panel of one leaf and 10 μg of TMV (in buffer containing 10 mm Tris-HCl and 1 mm EDTA, pH 7.2) was rubbed on the surface. The leaf was then rinsed under running water and the plant was returned to hydroponics. Mock-inoculated plants were treated similarly but without TMV.
Resistant plants were grown in soil in growth chambers (Conviron, Winnipeg, Canada) with a 16-h photoperiod, a light intensity of approximately 200 μmol m−2 s−1, and 50% relative humidity. Unless stated otherwise, temperature was 22°C (light) and 20°C (dark). Watering alternated between tap water and one-tenth Hoagland medium. Plants were inoculated (or mock inoculated) as described above except that 5 μg of TMV was applied to the entire adaxial surface of a fully expanded leaf (sixth or seventh lowest leaf).
Experimental Analyses
Mitochondria were isolated by a mini-prep procedure previously described (Boutry et al., 1984). Reducing SDS-PAGE and immunoblot analysis of protein from isolated mitochondria was performed as before using a monoclonal antibody against AOX (Vanlerberghe et al., 1998). The level of TMV coat protein in leaves 2 and 3 above the inoculated leaf of susceptible plants was determined using an ELISA-based kit (Agdia, Elkhart, IN) according to the manufacturer's instructions. Total RNA was isolated from leaves 2 and 3 above the inoculated leaf of susceptible plants by a mini-prep procedure (Verwoerd et al., 1989). Northern analysis was performed by standard methods using a DNA probe recognizing TMV coat protein RNA. Lesion sizes on resistant plants were carefully measured with a caliper.
ACKNOWLEDGMENTS
We thank Dr. Robin Cameron (University of Toronto) for helpful suggestions throughout this work and for her critical reading of the manuscript. We also thank Alice Cheung (University of Toronto), Nathan Swartsman (University of Toronto at Scarborough), and Duarte Rodriques (University of Toronto at Scarborough) for their contributions to this work, and we thank Dr. Zhixiang Chen (University of Idaho, Moscow) for the gift of a probe recognizing TMV coat protein RNA.
Footnotes
This work was supported by the Natural Sciences and Engineering Research Council of Canada, by the Canada Foundation for Innovation, by the Ontario Research and Development Challenge Fund, and by a Premiers Research Excellence Award of Ontario (all funds to G.C.V.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003855.
LITERATURE CITED
- Alvarez ME. Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol. 2000;44:429–442. doi: 10.1023/a:1026561029533. [DOI] [PubMed] [Google Scholar]
- Boutry M, Faber AM, Charbonnier M, Briquet M. Microanalysis of plant mitochondrial protein synthesis products: detection of variant polypeptides associated with male sterility. Plant Mol Biol. 1984;3:445–452. doi: 10.1007/BF00033392. [DOI] [PubMed] [Google Scholar]
- Chivasa S, Carr JP. Cyanide restores N gene-mediated resistance to tobacco mosaic virus in transgenic tobacco expressing salicylic acid hydroxylase. Plant Cell. 1998;10:1489–1498. doi: 10.1105/tpc.10.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivasa S, Murphy AM, Naylor M, Carr JP. Salicylic acid interferes with tobacco mosaic virus replication via a novel salicylhydroxamic acid-sensitive mechanism. Plant Cell. 1997;9:547–557. doi: 10.1105/tpc.9.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson WO. Tobacco mosaic virus virulence and avirulence. Phil Trans R Soc Lond B. 1999;354:645–651. doi: 10.1098/rstb.1999.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF. Salicylic acid and disease resistance in plants. Crit Rev Plant Sci. 1999;18:547–575. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessman H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Heath MC. Hypersensitive response-related death. Plant Mol Biol. 2000;44:321–334. doi: 10.1023/a:1026592509060. [DOI] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ 347.
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Ji L-H, Ding S-W. The suppressor of transgene RNA silencing encoded by cucumber mosaic virus interferes with salicylic acid-mediated virus resistance. Mol Plant-Microbe Interact. 2001;14:715–724. doi: 10.1094/MPMI.2001.14.6.715. [DOI] [PubMed] [Google Scholar]
- Jones A. Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci. 2000;5:225–230. doi: 10.1016/s1360-1385(00)01605-8. [DOI] [PubMed] [Google Scholar]
- Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: the earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- Lacomme C, Roby D. Identification of new early markers of the hypersensitive response in Arabidopsis thaliana. FEBS Lett. 1999;459:149–153. doi: 10.1016/s0014-5793(99)01233-8. [DOI] [PubMed] [Google Scholar]
- Lam E, Kato N, Lawton N. Programmed cell death, mitochondria and the plant hypersensitive response. Nature. 2001;411:848–853. doi: 10.1038/35081184. [DOI] [PubMed] [Google Scholar]
- Lennon AM, Neuenschwander UH, Ribas-Carbo M, Giles L, Ryals JA, Siedow JN. The effects of salicylic acid and tobacco mosaic virus infection on the alternative oxidase of tobacco. Plant Physiol. 1997;115:783–791. doi: 10.1104/pp.115.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IM, Berczi A. Salicylhydroxamic acid-stimulated NADH oxidation by purified plasmalemma vesicles from wheat roots. Physiol Plant. 1986;68:67–74. [Google Scholar]
- Moller IM, Berczi A, van der Plas LHW, Lambers H. Measurement of the activity and capacity of the alternative pathway in intact plant tissues: identification of problems and possible solutions. Physiol Plant. 1988;72:642–649. [Google Scholar]
- Moss DA, Bendall DS. Cyclic electron transport in chloroplasts: the Q cycle and the site of action of antimycin A. Biochim Biophys Acta. 1984;767:389–395. [Google Scholar]
- Mur LAJ, Bi Y-M, Darby RM, Firek S, Draper J. Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersal during lesion establishment in TMV-infected tobacco. Plant J. 1997;12:1113–1126. doi: 10.1046/j.1365-313x.1997.12051113.x. [DOI] [PubMed] [Google Scholar]
- Murphy AM, Chivasa S, Singh DP, Carr JP. Salicylic acid-induced resistance to viruses and other pathogens: a parting of the ways? Trend Plant Sci. 1999;4:155–160. doi: 10.1016/s1360-1385(99)01390-4. [DOI] [PubMed] [Google Scholar]
- Naylor M, Murphy AM, Berry JO, Carr JP. Salicylic acid can induce resistance to plant virus movement. Mol Plant-Microbe Interact. 1998;11:860–868. [Google Scholar]
- Parsons HL, Yip JYH, Vanlerberghe GC. Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiol. 1999;121:1309–1320. doi: 10.1104/pp.121.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique M, Barragan M, Dalmau M, Bellosillo B, Pons G, Gil J. Aspirin induces apoptosis through mitochondrial cytochrome c release. FEBS Lett. 2000;480:193–196. doi: 10.1016/s0014-5793(00)01922-0. [DOI] [PubMed] [Google Scholar]
- Pressig CL, Kuc JA. Inhibition by salicylhydroxamic acid, BW755C, eicosatetraynoic acid and disulfiram of hypersensitive resistance elicited by arachidonic acid or poly-l-lysine in potato tuber. Plant Physiol. 1987;84:891–894. doi: 10.1104/pp.84.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production in Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Rhoads DM, McIntosh L. Cytochrome and alternative pathway respiration in tobacco: effects of salicylic acid. Plant Physiol. 1993;103:877–883. doi: 10.1104/pp.103.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PR, Wiegand NK, Blum H, Moore AL, Bonner WD. Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978;525:325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Robson CA, Vanlerberghe GC. Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and -independent pathways of programmed cell death. Plant Physiol. 2002;129:1908–1920. doi: 10.1104/pp.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AF. Systemic acquired resistance induced by localized virus infections in plants. Virology. 1961;14:340–358. doi: 10.1016/0042-6822(61)90319-1. [DOI] [PubMed] [Google Scholar]
- Schonbaum GR, Bonner WD, Storey RT, Bahr JT. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971;47:124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN, Day DA. Respiration and photorespiration. In: Buchanan B, Gruissem W, Jones R, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 676–728. [Google Scholar]
- Simons BH, Millenaar FF, Mulder L, Van Loon LC, Lambers H. Enhanced expression and activation of the alternative oxidase during infection of Arabidopsis with Pseudomonas syringae pv tomato. Plant Physiol. 1999;120:529–538. doi: 10.1104/pp.120.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Chen C, Gibbs M. Characterization of an electron transport pathway associated with glucose and fructose respiration in the intact chloroplasts of Chlamydomonas reinhardtii and spinach. Plant Physiol. 1992;100:327–333. doi: 10.1104/pp.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Mitochondrial electron transport regulation of nuclear gene expression: studies with the alternative oxidase gene of tobacco. Plant Physiol. 1994;105:867–874. doi: 10.1104/pp.105.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L, Yip JYH. Molecular localization of a redox-modulated process regulating plant mitochondrial electron transport. Plant Cell. 1998;10:1551–1560. doi: 10.1105/tpc.10.9.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Ordog SH. Alternative oxidase: integrating carbon metabolism and electron transport in plant respiration. In: Foyer CH, Noctor G, editors. Advances in Photosynthesis and Respiration: Photosynthetic Nitrogen Assimilation and Associated Carbon Metabolism. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2002. (in press) [Google Scholar]
- Vanlerberghe GC, Vanlerberghe AE, McIntosh L. Molecular genetic alteration of plant respiration: silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiol. 1994;106:1503–1510. doi: 10.1104/pp.106.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BMM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acid Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AM, Wagner MJ. Changes in mitochondrial respiratory chain components of Petunia cells during culture in the presence of antimycin A. Plant Physiol. 1997;115:617–622. doi: 10.1104/pp.115.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N: similarity to Toll and the interleukin-1 receptor. Cell. 1994;78:1101–1115. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Xie Z, Chen Z. Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells. Plant Physiol. 1999;120:217–225. doi: 10.1104/pp.120.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip JYH, Vanlerberghe GC. Mitochondrial alternative oxidase acts to dampen the generation of active oxygen species during a period of rapid respiration induced to support a high rate of nutrient uptake. Physiol Plant. 2001;112:327–333. doi: 10.1034/j.1399-3054.2001.1120305.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Nickels R, McIntosh L. A genome approach to mitochondrial-nuclear communication in Arabidopsis. Plant Physiol Biochem. 2001;39:345–353. [Google Scholar]
- Zollner H. Handbook of Enzyme Inhibitors. New York: VCH Publishers; 1989. pp. 273–274. [Google Scholar]