Abstract
The effect of cadmium (Cd) on high-affinity sulfate transport of maize (Zea mays) roots was studied and related to the changes in the levels of sulfate and nonprotein thiols during Cd-induced phytochelatin (PC) biosynthesis. Ten micromolar CdCl2 in the nutrient solution induced a 100% increase in sulfate uptake by roots. This was not observed either for potassium or phosphate uptake, suggesting a specific effect of Cd2+ on sulfate transport. The higher sulfate uptake was not dependent on a change in the proton motive force that energizes it. In fact, in Cd-treated plants, the transmembrane electric potential difference of root cortical cells was only slightly more negative than in the controls, the external pH did not change, and the activity of the plasma membrane H+-ATPase did not increase. Kinetics analysis showed that in the range of the high-affinity sulfate transport systems, 10 to 250 μm, Cd exposure did not influence the Km value (about 20 μm), whereas it doubled the Vmax value with respect to the control. Northern-blot analysis showed that Cd-induced sulfate uptake was related to a higher level of mRNA encoding for a putative high-affinity sulfate transporter in roots. Cd-induced sulfate uptake was associated to both a decrease in the contents of sulfate and glutathione and synthesis of a large amount of PCs. These results suggest that Cd-induced sulfate uptake depends on a pretranslational regulation of the high-affinity sulfate transporter gene and that this response is necessary for sustaining the higher sulfur demand during PC biosynthesis.
The use of higher plants in phytoextraction of heavy metals from polluted soil is not only based on their ability to take up, translocate, and accumulate the metals, but also on mechanisms able to alleviate their toxic effects (Salt et al., 1998). Knowledge of these mechanisms is crucial in the basic understanding of the tolerance to improve plants of biotechnological interest (Zenk, 1996).
Cd exposure in higher plants rapidly induces the synthesis of phytochelatins (PCs), a class of heavy-metal-binding peptides with the general structure (γ-Glu-Cys)n-Gly (Steffens, 1990; Rauser, 1995). PCs are synthesized nontranslationally from reduced glutathione (GSH) in a transpeptidation reaction catalyzed by the enzyme PC synthase. PCs form complexes with Cd2+, reducing the activity of the metal in the cytosol (Cobbett, 2000). Cd-PC complexes are then compartmentalized into the vacuole, probably by means of an ATP-binding cassette-type transporter localized in the tonoplast (Salt and Rauser, 1995). The crucial role of PCs in plant Cd2+ detoxification pathway was supported by the isolation of two mutants of Arabidopsis, cad1 and cad2, which are deficient in PC and GSH biosynthesis, respectively, and are consequently more sensitive to Cd (Howden et al., 1995a, 1995b; Cobbett et al., 1998).
PC biosynthesis is closely dependent on sulfur metabolism (Cobbett, 2000; Leustek et al., 2000). In fact, Cd exposure induces the activity of enzymes involved in the sulfate reductive assimilation pathway and GSH biosynthesis. Nussbaum and co-workers (1988) showed that Cd2+ accumulation into maize (Zea mays) seedlings was related to an increase in the activity of both ATP-sulfurylase and adenosine 5′-phosphosulfate reductase, the first two enzymes in the sulfate assimilation pathway. Other works report on the induction of enzyme activities involved in GSH biosynthesis, such as γ-glutamyl-Cys (γEC) synthetase in maize and glutathione synthetase in pea (Pisum sativum), indicating a cellular response to a transient GSH depletion during PC biosynthesis (Rüegsegger et al., 1990; Rüegsegger and Brunold, 1992). The molecular basis of this response has recently been outlined in studies conducted on Indian mustard (Brassica juncea), where Cd2+ accumulation induces a coordinate transcriptional regulation of genes involved in sulfate assimilation (Heiss et al., 1999; Lee and Leustek, 1999) and GSH biosynthesis (Schäfer et al., 1998).
The general alteration of the sulfur metabolic pathways induced by Cd2+ is a possible consequence of an increase in the GSH demand driven by PC biosynthesis. In other words, exposure to Cd would induce an “additional sink” increasing the need for thiol compounds by cells (Tukendorf and Rauser, 1990; Heiss et al., 1999).
Considering these aspects, it seems particularly interesting to draw attention toward the effects of Cd2+ on transport systems involved in sulfate uptake from the soil solution and in its translocation to the shoot. Several high-affinity sulfate transporters and low-affinity sulfate transporters have been cloned and characterized with respect to their kinetic profiles and regulation, enlarging our knowledge on the control of the sulfur fluxes in higher plants (Leustek and Saito, 1999; Saito, 2000; Smith et al., 2000). These transporters may have specialized functions, because they differ in affinity for sulfate and in spatial expression.
Molecular studies conducted on Indian mustard have shown that the general activation of sulfur metabolism induced by Cd2+ is associated with a down-regulation of a low-affinity sulfate transporter, which is probably expressed in the central cylinder of the roots and, thus, likely involved in sulfate translocation (Heiss et al., 1999). The authors suggest that this effect could be related to the need for maintaining high-sulfate levels in the roots, to sustain a high-sulfur assimilation rate during PC biosynthesis. The role of other root sulfate transporters during Cd2+ stress still needs investigation.
In the present work we report on the effects of Cd2+ on sulfate uptake focusing the analysis on the high-affinity sulfate transporter of maize roots.
RESULTS
Plant Growth and Cd Accumulation
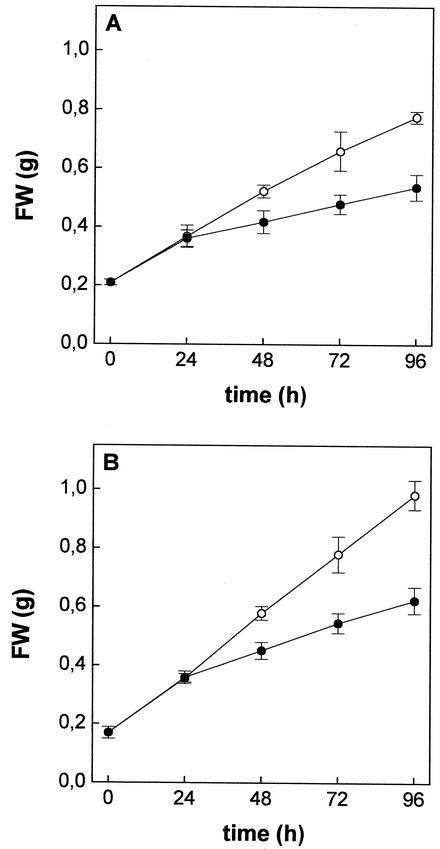
Maize plants grown in the presence of 10 μm Cd2+ showed significant growth reduction of both roots (Fig. 1A) and shoots (Fig. 1B). In the controls, fresh weight of both roots and shoots increased linearly over a 96-h period; the growth rates were 0.14 and 0.20 g fresh weight d−1 for roots and shoots, respectively. Cd did not affect the growth of either roots or shoots over the first 24 h; thereafter, the fresh weights increased linearly with the time, though with lower rates than in the controls: 0.06 and 0.09 g fresh weight d−1 for roots and shoots, respectively. Moreover, during the first 48 h of Cd exposure, the relative water content of both roots and shoots did not significantly change compared with the control (data not shown). At the end of the experimental period, the leaves of Cd-treated plants showed symptoms of chlorosis and necrotic areas began to be evident at their tips. Roots did not show any apparent damage.
Figure 1.
Effects of Cd2+ exposure on growth of roots (A) and shoots (B) of maize plants. Plants were grown for 96 h in a complete nutrient solution supplemented (●) or not (○) with 10 μm CdCl2. Plants were harvested at different times, blotted with paper towels, and weighed. Data points and error bars are means and se of two experiments run in quadruplicate (n = 8).
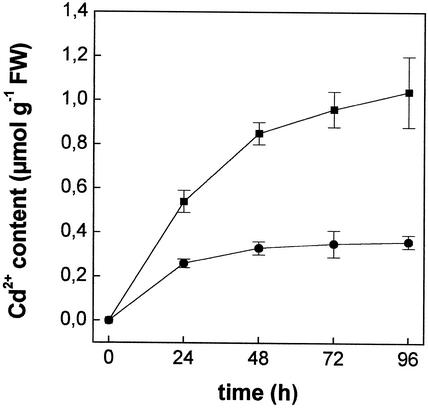
Figure 2 shows the time course of Cd2+ accumulation over a 96-h period. Cd was linearly accumulated in roots up to 48 h; thereafter, the concentration of the metal kept increasing, though at lower rates. In shoots, Cd2+ concentration increased greatly in the first 24 h, although to a lesser extent than in roots, and then it remained constant after 48 h. The concentration of Cd2+ in roots was about 2-fold higher than in shoots during the exposure period.
Figure 2.
Time course of Cd2+ accumulation in roots (▪) and shoots (●) of maize plants grown in a complete nutrient solution supplemented with 10 μm CdCl2. Plants were harvested at different times, and their Cd2+ contents were measured, after complete mineralization, by atomic absorption spectrophotometry. Data points and error bars are means and se of two experiment run in triplicate (n = 6).
Effect of Cd on Thiols and Sulfate Concentration in Roots
Cd accumulation in roots was accompanied by a progressive increase in total nonprotein thiols (NPT; Table I); after 48 h of Cd2+ exposure, NPT levels were more than 4-fold higher than those of the controls. Analysis of thiols showed this increase as primarily attributable to the synthesis of PCs, which accounted for about 87% of NPT (expressed as GSH equivalents) after 48 h of treatment (Table I). Exposure to Cd led to a substantial increase in Cys and γEC levels in roots, whereas it induced a significant decrease in GSH concentration (Table I).
Table I.
Effects of Cd2+ exposure on the concentrations of NPT, Cys, γEC, GSH, and PCs in maize roots
| Thiol Concentrations
|
|||||
|---|---|---|---|---|---|
| 0 h
|
24 h
|
48 h
|
|||
| Control | Control | Cd | Control | Cd | |
| nmol GSH equivalent g−1 fresh wt | |||||
| NPT | 231.3 ± 10.3 | 222.2 ± 10.1 | 464.5 ± 17.4 | 199.0 ± 7.2 | 1,159.3 ± 50.2 |
| Cys | 9.3 ± 0.3 | 8.9 ± 0.2 | 12.0 ± 0.6 | 8.1 ± 0.5 | 15.6 ± 0.6 |
| γEC | 3.6 ± 0.2 | 4.1 ± 0.3 | 12.6 ± 0.4 | 4.2 ± 0.3 | 28.7 ± 1.2 |
| GSH | 198.4 ± 7.0 | 194.3 ± 4.3 | 112.3 ± 7.9 | 188.2 ± 11.4 | 105.3 ± 6.6 |
| PCs | – | – | 327.6 ± 19.1 | – | 1,009.7 ± 50.6 |
Plants were grown for 24 or 48 h in a complete nutrient solution supplemented or not with 10 μm CdCl2. At different times, roots were excised from shoots, grounded in liquid N2, and an HCl extract was analyzed for thiol content. Values are means ± se of three experiments run in triplicate (n = 9).
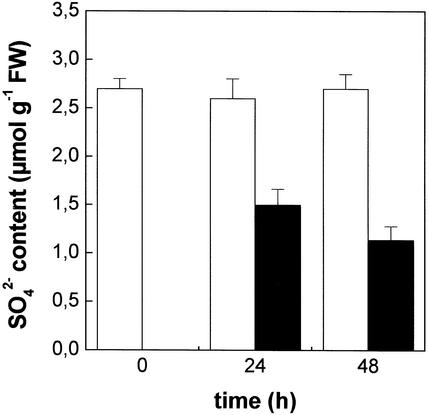
Not only did Cd increase the NPT level, but it also affected the sulfate content of the roots (Fig. 3). In fact, in roots of Cd-treated plants, the level of sulfate was lower than in the controls (−42% and −58%, after 24 and 48 h, respectively).
Figure 3.
Effect of Cd2+ exposure on the sulfate content of maize roots. Plants were grown for 24 or 48 h in a complete nutrient solution supplemented (black bars) or not (white bars) with 10 μm CdCl2. At different times, roots were excised from shoots, rinsed with distilled water, and homogenized. The sulfate content was measured turbidimetrically. Bars and error bars are means and se of two experiments run in quadruplicate (n = 8).
Effects of Cd on Sulfate, Phosphate (Pi), and K+ Uptake
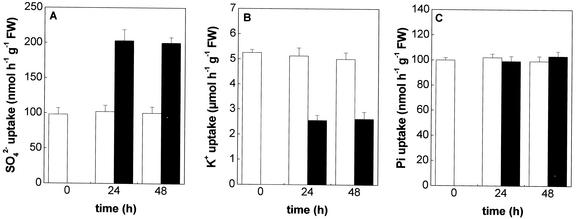
Sulfate uptake by roots was assessed in short-term experiments (10 min) using 35SO42− as a tracer. The rate of sulfate uptake, measured at 200 μm external SO42− concentration, was found to be twice that in roots of plants grown with 10 μm Cd2+ in the nutrient solution than in the control. Such an effect, detected after 24 h of Cd exposure, was maintained up to 48 h (Fig. 4A). No changes on sulfate influx into the roots were detectable when untreated plants were supplied with Cd at the moment of sulfate uptake measurement or after 1 h of preincubation with the metal (data not shown).
Figure 4.
Effects of Cd2+ exposure on SO42− (A), K+ (B), and Pi (C) uptake in maize roots. Plants were grown for 24 or 48 h in a complete nutrient solution supplemented (black bars) or not (white bars) with 10 μm CdCl2. The influxes were evaluated by measuring the rates of 35SO42−, 86Rb+, and 32Pi absorption into roots of intact plants over a 10-min pulse. The incubation solutions contained 200 μm SO42−, 240 μm K+, and 40 μm Pi. Bars and error bars are means and se of three experiments in run quadruplicate (n = 12).
Cd exposure significantly reduced (about −50% as compared with the control) K+ uptake by roots (Fig. 4B), whereas it did not affect Pi uptake (Fig. 4C), when measured at 240 and 40 μm external concentration, respectively.
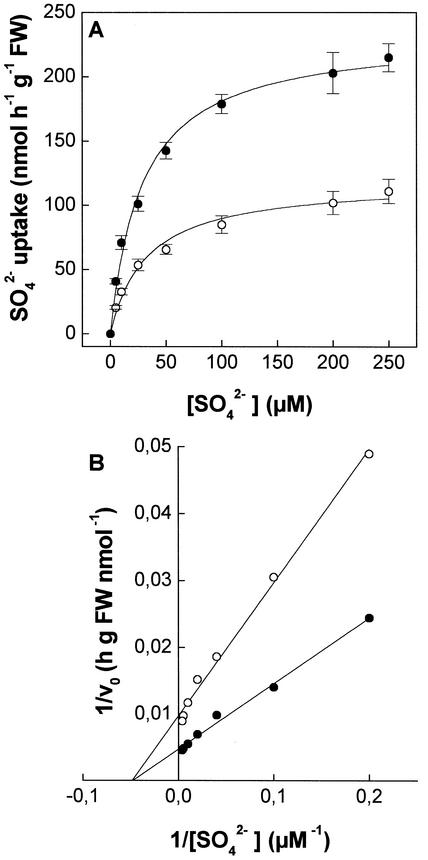
In Figure 5A, the influxes of sulfate into Cd-treated (24 h) and control roots are reported as the function of sulfate external concentration in the range of 10 to 250 μm. Influx isotherms can be described by a simple Michaelis-Menten function. Least squares fitting (Fig. 5B) revealed that the two kinetics were similar in Km (20.3 ± 5.0 and 20.5 ± 3.1 μm for control and Cd-treated plants, respectively), but different in Vmax values (102.4 ± 5.2 and 208.1 ± 6.9 nmol h−1 g−1 fresh weight, for control and Cd-treated plants, respectively).
Figure 5.
Sulfate influx isotherms in maize roots (A) and relative Lineweaver-Burk plot (B). Plants were grown for 24 h in a complete nutrient solution supplemented (●) or not (○) with 10 μm CdCl2. The influxes were evaluated by measuring the rates of 35SO42− absorption into roots of intact plants over a 10-min pulse. Sulfate concentrations ranged from 10 to 250 μm. Data points and error bars are means and se of three experiments in quadruplicate (n = 12).
Transmembrane Electric Potential Difference and in Vitro Plasma Membrane H+-ATPase Activity
To investigate the effects of Cd2+ exposure on proton motive force, which energizes secondary active transports, the transmembrane electric potential difference (Em) of root cortical cells and the in vitro activity of the plasma-membrane H+-ATPase were measured. Em values were slightly more negative (about 10 mV) in Cd-treated than in control roots (Table II). These differences were detected after 24 h of exposure to Cd2+ and were maintained for at least 48 h. The H+-ATPase activity was studied in plasma membrane vesicles obtained by roots of both Cd-treated and control plants. As shown in Table II, the exposure to Cd did not significantly affect the basal plasma-membrane H+-ATPase activity of roots, even after a 48-h treatment.
Table II.
Effect of Cd2+ exposure on Em values and on in vitro plasma membrane H+-ATPase activity of maize root cells
| Time |
Em
|
H+-ATPase Activity
|
||
|---|---|---|---|---|
| Control | Cd | Control | Cd | |
| h | mV | μmol ADP released h−1 mg−1 protein | ||
| 0 | −96 ± 2 | – | – | – |
| 24 | −96 ± 3 | −105 ± 1 | 16.2 ± 0.5 | 18.4 ± 1.1 |
| 48 | −94 ± 2 | −104 ± 3 | 15.4 ± 0.7 | 17.0 ± 0.3 |
Plants were grown for 24 or 48 h in a complete nutrient solution supplemented or not with 10 μm CdCl2. Em values were measured in roots of intact plants perfused with the complete nutrient solution supplemented or not with 10 μm CdCl2. The values are the means ± se of three experiments (n > 20). ATPase activity was measured as ADP release in plasma membrane vesicles from control or Cd-exposed plants. The values are the means ± se of three experiments in run triplicate (n = 9).
cDNA Cloning and Northern-Blot Analysis
A partial cDNA clone encoding a putative high-affinity sulfate transporter was amplified by reverse transcriptase-PCR technique, using the total RNA extracted from sulfur-starved roots as the source of template. Amplification primers, designed on conserved sequences of different high-affinity sulfate transporter clones (see “Materials and Methods”), yielded a single PCR product of the expected size. Sequence analysis restored a cDNA fragment (HAST) of 928 bp with a predicted translation product very similar to a wide range of higher plant high-affinity sulfate transporters. In particular, protein sequence showed high identity with maize ZmST-701 (99%), barley (Hordeum vulgare) HVST1 (83%), and Triticum tauschii TTST2 (82%). The sequence has been registered at the National Center for Biotechnology Information (NCBI/GenBank accession no. AY059461).
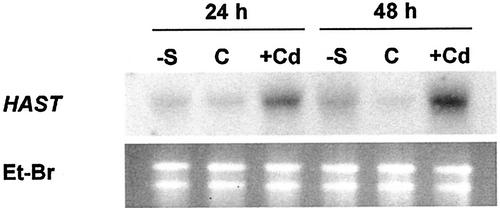
Northern-blot analysis was carried out on total RNA extracted from roots of maize plants grown for 24 or 48 h in the complete nutrient solution, supplemented or not with 10 μm Cd2+, or in the minus-sulfate solution. Analysis was performed using HAST cDNA as a probe. Results showed higher HAST transcript levels in roots of both Cd-treated and sulfur-starved plants than in the controls (Fig. 6).
Figure 6.
Northern-blot analysis of HAST expression in maize roots. Total RNA was extracted from roots of sulfur-starved (−S), control (C), and Cd-exposed (Cd) plants. Thirty micrograms of total RNA was loaded onto each lane. Blots were hybridized with 32P-labeled HAST probe. Ribosomal RNAs were stained on the gel with ethidium bromide (Et-BR) and used to check loading.
DISCUSSION
Maize plants accumulated Cd in roots and translocated it to shoots. However, the amount of Cd into the shoot during 96 h of exposure accounted for only about 30% of the total Cd2+ in the whole plant, according to the high Cd retention capability reported for maize roots (Florijn and Van Beusichem, 1993; Rauser and Meuwly, 1995). Hence, a strong need for Cd2+ detoxification into the root cells was created.
The main strategy for Cd2+ detoxification in plant cells is based on chelation by PCs and subsequent compartmentalization of the Cd-PCs complex (Clemens, 2001). The PC biosynthesis induced by Cd2+ enhances the cell requirement for thiol compounds and can lead to a transient depletion of the GSH pools (Scheller et al., 1987; Steffens, 1990). In Indian mustard, the need for maintaining a high rate of PC biosynthesis and adequate GSH levels may be met by a coordinate transcriptional regulation of genes involved in sulfate assimilation and GSH biosynthesis (Schäfer et al., 1998; Heiss et al., 1999; Lee and Leustek, 1999). Moreover, this response has also been hypothesized to be a possible mechanism modulating PC biosynthesis (Cobbett, 2000), as supported by the observation that overexpression of genes encoding either γEC synthetase or glutathione synthetase enhanced PC biosynthesis after Cd exposure (Yong et al., 1999; Zhu et al., 1999). The results reported here show a general activation of sulfur metabolism in roots of plants grown in the presence of Cd2+, as the dramatic increase in the levels of NPT indicates (Table I). This response, consistent with the Cd-induced thiol biosynthesis, observed by Rüegsegger and Brunold (1992), is attributable to increases in both Cys and γEC concentration and to a large production of PCs (Table I). The negative influence of Cd2+ on GSH level in roots could be explained as a depletion effect due to PC biosynthesis (Scheller et al., 1987; Steffens, 1990; Rüegsegger and Brunold, 1992).
The rate of sulfate uptake by roots of plants grown in the presence of Cd2+ for 24 or 48 h was twice than that of the control (Fig. 4A). This behavior was detected in the complete nutrient solution at 200 μm SO42− external concentration. No effect on sulfate uptake was observed after short-time treatment (10 min-1 h) when Cd2+ was supplied to control plants (data not shown), suggesting that the above-described increase was not due to a direct action of Cd on the transport systems involved in sulfate uptake.
Molecular (Smith et al., 1995, 2000) and kinetic (Hawkesford et al., 1993; Smith et al., 1995, 1997) studies indicate that sulfate uptake is energetically coupled to the H+-electrochemical gradient (ΔμH+) across the plasma membrane, through H+-SO42− symport mechanisms. Changes in ΔμH+ may influence the activity of H+-symporters (Hawkesford et al., 1993; Chrispeels et al., 1999). Roots of Cd-treated plants did not modify, compared with the controls, the pH of the medium during sulfate uptake experiments (data not shown). This suggests that Cd2+ did not change the net H+ efflux from roots, according to the very similar activities of H+-ATPase measured in vitro in plasma membrane vesicles from Cd-treated or control plants (Table II). Furthermore, an enhancement of the chemical component of ΔμH+ by Cd2+ can also be excluded from the results obtained by Fodor et al. (1995), which showed a direct inhibitory effect of Cd2+ on H+-ATPase activity measured in plasma membrane vesicles purified by wheat and sunflower roots. An actual effect of Cd2+ on the electric component of ΔμH+ can be excluded because the values of Em in root cells were only slightly more negative in Cd-treated than in control plants (Table II). Similar to sulfate, K+ and Pi transports are dependent on H+ electrochemical gradient across the plasma membrane (Ullrich-Eberius et al., 1984; Thibaud et al., 1988; Sacchi and Cocucci, 1992; Daram et al., 1998), but unlike sulfate, their uptake was inhibited or unaffected, respectively, by Cd treatment (Fig. 4, B and C). The lower K+ uptake justifies the slightly more negative values of Em in root cells of Cd-treated plants.
These results suggest that sulfate uptake increase is a specific effect of Cd accumulation and does not depend on an enhancement in the proton motive force across the plasma membrane.
In Cd-treated (24 h) and control roots, influx isotherms, obtained in a wide range of sulfate external concentration (10–250 μm), approximated a single Michaelis-Menten kinetic (Fig. 5A). The values of apparent Km were similar to those of other high-affinity sulfate transport systems in intact plants (Lee, 1982; Clarkson et al., 1983; Honda et al., 1998), suggesting that sulfate uptake was mediated by a high-affinity transport system in the observed range of concentrations. The affinity of the transport system for sulfate does not seem to change after Cd treatment (Fig. 5B), as shown by the Km values, which were 20.3 ± 5.0 and 20.5 ± 3.1 μm for control and Cd-treated plants, respectively. This finding suggests that similar sulfate transport systems are involved in mediating sulfate uptake in Cd-treated and control roots. However, the Vmax value in Cd-treated plants was found to be twice that in the controls (Fig. 5B), suggesting the hypothesis that sulfate uptake increase was a consequence of a higher expression of high-affinity sulfate transport systems of the roots. To minimize possible artifacts attributable to Cd2+ toxicity, kinetic experiments were performed only on plants exposed to Cd for 24 h, when a general action of the metal was not apparent (Fig. 1). For lengthier treatments, whereas Cd led to a progressive chlorosis and apical necrosis in leaves, it did not cause any evident damage to the root system, which is different from that reported by Nussbaum et al. (1988) in the same species, though at higher Cd2+ concentrations.
To study the effect of Cd2+ on the expression of sulfate transporters, we cloned a partial cDNA sequence encoding a putative high-affinity sulfate transporter, expressed in sulfur-starved maize roots. Sequence analysis showed high homologies among this fragment and other sequences of maize and several other species, confirming the identity of HAST as a putative high-affinity sulfate transporter.
Northern-blot analysis showed that Cd2+ exposure, as well as sulfur starvation, increased the levels of HAST mRNA (Fig. 6). The time course of this response closely correlated with the observed enhancement in sulfate uptake. This relationship suggests that the increase in sulfate uptake induced by Cd2+, was mainly attributable to a pretranslational regulation of the gene encoding the high-affinity sulfate transporter of the roots. However, the existence of an additional mechanism of allosteric regulation of the carrier cannot be ruled out because of the cytoplasmic pool of sulfate. In fact, Cd accumulation in roots induced a depletion of the cell sulfate content (Fig. 3). Such an effect, which may seem in contrast with the higher sulfate uptake, could be explained as a consequence of the Cd-induced activation of the sulfate-reductive assimilation pathway (Nussbaum et al., 1988; Heiss et al., 1999).
The higher sulfate influx in Cd-treated roots could reflect an adaptive response that contributes to support the sulfate demand during PC biosynthesis. Coordinate transcriptional regulation of the genes encoding the high-affinity sulfate transporter and other enzymes involved in sulfate assimilation has been described in roots of sulfur-starved maize plants, and it is thought to be a cellular response to transient GSH depletion (Bolchi et al., 1999). Likewise, the exposure of plants to Cd2+, in the presence of an adequate sulfate supply, results in a higher expression of the high-affinity sulfate transporter, probably as a consequence of a decrease in either cell GSH or sulfate levels. Moreover, the rapid effect of Cd on sulfur nutritional status of the plants (Table I) would account for the differences observed in the timing of HAST expression in Cd-treated and sulfur-starved plants (Fig. 6). Different from sulfate withdrawal, Cd exposure rapidly induces additional sinks of thiol compounds, which in turn drive a higher demand for sulfate uptake.
In conclusion, the results of the present work show that maize roots respond to Cd2+ exposure increasing the sulfate uptake activity mainly by up-regulating a gene encoding for a HAST of the roots. Such a response could represent the first step of an adaptive process required to ensure an adequate supply of sulfur compounds during Cd-induced PC synthesis and may be interpreted as a classical demand-driven regulation of ion transport systems.
MATERIALS AND METHODS
Seed Germination and Plant Growth Conditions
Maize (Zea mays L. cv Dekalb DK 300) caryopses were sown on filter paper saturated with distilled water and incubated at 26°C in the dark. Three days later, seedlings selected for uniform growth were transplanted into 5-L tanks (18 seedlings per tank) containing an aerated complete nutrient solution [200 μm KNO3, 200 μm Ca(NO3)2, 40 μm KH2PO4, 200 μm MgSO4, 25 μm Fe-tartrate, 30 μm H3BO4, 5 μm MnCl2, 1 μm CuCl2, 1 μm ZnCl2, and 0.1 μm (NH4)6Mo7O24, pH 6.50] and kept in a growth chamber at 26°C and 80% relative humidity during the 14-h light period and at 22°C and 70% relative humidity during the 10-h dark period. Three days after transplanting, the complete nutrient solution was supplemented with 10 μm CdCl2 or substituted with a minus sulfate solution, where MgSO4 was replaced by an equimolar amount of MgCl2. Hydroponic solutions were renewed daily to minimize nutrient depletion.
Determination of Cd
Plants were harvested and roots were washed for 10 min in ice-cold 5 mm CaCl2 solution to displace extracellular Cd (Rauser, 1987). Roots were excised and gently blotted with paper towels. Cd content was measured, after complete mineralization in a mixture of nitric, sulfuric, and perchloric acid (5:1:1, v/v), by atomic absorption spectrophotometry (SpectrAA-20, Varian, Palo Alto, CA).
Determination of Thiols
Roots were ground in liquid nitrogen, extracted in 1:2 (w/v) ice-cold 0.1 mm HCl and 1 mm Na2EDTA, and the homogenates were centrifuged 15 min at 15,000g and 4°C. The supernatants were collected and immediately subjected to thiol analysis.
For total NPTs, 200 μL of supernatant was mixed to 1.8 mL of 0.6 mm 5,5′-dithiobis-(2-nitrobenzoic acid) and 250 mm K-Pi buffer, pH 8.00. NPT content was measured spectrophotometrically by reading the A412.
Cys, γEC, and total glutathione contents were measured, after reduction with dithiothreitol (DTT) and derivatization with monobromobimane, by reverse phase HPLC and fluorescence detection as described by Schupp and Rennenberg (1988).
Total PC concentration was estimated by subtracting Cys, γEC, and GSH from NPT (Schäfer et al., 1997). All results were expressed as nanomoles of GSH equivalent per gram fresh weight.
Determination of Sulfate
Roots were rinsed three times in distilled water and blotted with paper towels. Sulfate was extracted by homogenizing the samples in 1:10 (w/v) ice-cold 0.1 n HNO3. After heating at 80°C for 40 min, the extracts were filtered and the sulfate contents were then determined according to the turbidimetric method described by Tabatabai and Bremner (1970).
Sulfate, K+, and Pi Influxes
Sulfate influxes into the roots were measured by determining the rates of 35S uptake over a 10-min pulse in incubation solutions labeled with the radiotracer. Three plants were placed onto 400 mL of a fresh complete nutrient solution, containing different MgSO4 concentrations (10–250 μm), supplemented or not with 10 μm CdCl2, aerated, and thermoregulated at 26°C. Radioactive pulses were started by adding [35S]H2SO4 to the uptake solutions. Specific activities varied from 4.7 KBq μmol−1 (250 μm) to 118.0 KBq μmol−1 (10 μm). At the end of the incubation period, roots were excised from shoots, rinsed twice for 1 min in 400 mL of a corresponding nonradioactive solution at 4°C, blotted with paper towels, and then heated for 20 min at 80°C in 0.1 n HNO3 (10 mL g−1 fresh weight).
K and Pi influxes were measured, in the same conditions, in the complete nutrient solution, containing 86RbCl (14.0 KBq μmol−1) and [32P]H3PO4 (29.5 KBq μmol−1) as radiotracers in the presence or in the absence of 10 μm CdCl2. K and Pi concentrations were 240 and 40 μm, respectively.
Radioactivity was detected on aliquots of the supernatants by liquid scintillation counting in a Beckman LS 6000SC (Beckman Coulter, Inc., Fullerton, CA).
Measurement of Transmembrane Electric Potential Difference in Root Cells
Transmembrane electric potential differences were measured using a high-impedance electrometer amplifier (K5–700, World Precision Instruments, New Haven, CT) and microelectrodes pulled from single-barreled borosilicate glass tubings (World Precision Instruments, New Haven, CT) filled with 3 m KCl (adjusted to pH 2.00 to reduce tip potential). Electrode resistance varied between 10 and 15 MΩ.
In brief, one maize plant was transferred into a 300-mL plexiglas vessel connected with a horizontal chamber, on the bottom of which three roots were fixed and impaled for Em recording. The whole system was perfused at 300 mL h−1 with an aerated and thermoregulated (26°C) complete nutrient solution, supplemented or not with 10 μm CdCl2. Electrodes were inserted, perpendicularly to the main axis of the roots, into the cortical cells.
Plasma-Membrane Vesicle Isolation and H+-ATPase Assay
Plasma-membrane vesicles were purified essentially as described by Palmgren et al. (1990). In brief, 30 g of maize roots were homogenized in a mortar with 120 mL of ice-cold 330 mm Suc, 50 mm MOPS-BisTris propane (pH 7.50), 5 mm EDTA, 1 mm DTT, 0.5 mm phenylmethylsulfonyl fluoride, and 1% (w/v) bovine serum albumin. The homogenate was filtered and centrifuged for 15 min at 13,000g and for 30 min at 100,000g. The microsomal fraction was processed at 4°C using the three-step batch procedure described by Larsson et al. (1987), with a two-phase system consisting of 6.5% (w/w) dextran T500, 6.5% (w/w) polyethyleneglycol 3350, 330 mm sorbitol, 5 mm K-Pi (pH 7.80), 5 mm KCl, 1 mm DTT, and 0.1 mm EDTA.
Plasma-membrane H+-ATPase activity was measured (50–100 μg of membrane proteins) after the oxidation of NADH coupled to the hydrolysis of ATP, as described by Palmgren et al. (1990). Oxidation of NADH was followed at 340 nm with a V-550 spectrophotometer (Jasco, Tokyo).
Membrane protein assay was carried out according to Bradford (1976), using γ-globulin as a standard.
Isolation of a HAST cDNA Partial Clone
A partial cDNA clone encoding a putative HAST of maize roots, was isolated by reverse transcriptase-PCR technique. Total RNA was extracted (see below) from roots of maize plants grown for 3 d in the minus sulfate solution and first-strand cDNA was prepared with Moloney murine leukemia virus-reverse transcriptase. One-tenth of undiluted cDNA was used for PCR. Amplification primers (sense, 5′-CAGGCTAGGGTTTATCATAG-3′; antisense, 5′-GTTCTYGGCCKTGTYACTTG-3′) were designed on highly conserved regions of barley (Hordeum vulgare) HVST1 (NCBI/GenBank accession no. X96431; nucleotide position 677–696 on the plus strand; 1,596–1,615 on the minus strand), maize ZmST1–701 (NCBI/GenBank accession no. AF016306; nucleotide position 151–170 on the plus strand), Stylosanthes hamata SHST1 (NCBI/GenBank accession no. X82255; nucleotide position 1,600–1,619 on the minus strand), and Triticum tauschii TTST2 (NCBI/GenBank accession no. AJ238245; nucleotide position 3,444–3,463 on the minus strand) sequences. DNA amplification reactions were conducted with the AmpliTaq Gold Polymerase (PerkinElmer Applied Biosystems, Foster City, CA) under the following conditions: 2 min of initial denaturation at 95°C, followed by 40 cycles of 45-s denaturation at 94°C, 45-s annealing at 52°C, and 2-min extension at 72°C. Amplification product was cloned into a EcoRV site of pBluescript II KS vector (Stratagene, La Jolla, CA). The partial cDNA clone was sequenced, and its identity was verified by comparative analysis in the protein sequence database at the NCBI.
Total RNA Extraction and Northern-Blot Analysis
Total RNA was extracted from roots of Cd-exposed, sulfur-starved, and control plants using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Thirty micrograms of total RNA per lane was separated by electrophoresis at 5 V cm−1 in 1.3% (w/v) agarose gel containing 6% (v/v) formaldehyde, transferred to Hybond N+ nylon membrane (Amersham-Pharmacia Biotech, Uppsala) by capillary blotting in 20× SSC, and then fixed by UV cross-linking. The blot was hybridized with 32P[dCTP]-labeled HAST probe synthesized with Multiprime DNA labeling system kit (Amersham-Pharmacia Biotech). Prehybridization and hybridization were conducted according to the nylon membrane manufacturer's instructions. Membrane was washed for 10 min with 2× SSC in 0.1% (w/v) SDS at room temperature, for 10 min with 1× SSC in 0.1% (w/v) SDS at 65°C for 20 min, and then for 10 min with 0.1× SSC in 0.1% (w/v) SDS at 65°C.
ACKNOWLEDGMENTS
We thank Dr. Silvia Morgutti and Marco Resmini for the precious contributions given during the writing of this paper.
Footnotes
This work was supported by the National Research Council of Italy (Progetto Finalizzato Biotecnologie, Subproject 2, Line 2.1. and CNR-Agenzia 2000, code CNRG00857B).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002659.
LITERATURE CITED
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by l-cysteine. Plant Mol Biol. 1999;39:527–537. doi: 10.1023/a:1006148815106. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Crawford NM, Schroeder JI. Proteins for transport of water and mineral nutrients across the membranes of plant cells. Plant Cell. 1999;11:661–676. doi: 10.1105/tpc.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT, Smith FW, Vanden Berg PJ. Regulation of sulphate transport in tropical legume, Macroptilum atropurpureum, cv. Siratro J Exp Bot. 1983;34:1463–1483. [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Daram P, Brunner S, Persson BL, Amerhein N, Bucher M. Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta. 1998;206:225–233. doi: 10.1007/s004250050394. [DOI] [PubMed] [Google Scholar]
- Florijn PJ, Van Beusichem ML. Uptake and distribution of cadmium in maize inbred lines. Plant Soil. 1993;150:25–32. [Google Scholar]
- Fodor E, Szabó-Nagy A, Erdei L. The effect of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J Plant Physiol. 1995;147:87–92. [Google Scholar]
- Hawkesford M, Davidian JC, Grignon C. Sulphate/proton cotransport in plasma-membrane vesicles isolated from roots of Brassica napus L.: increased transport in membranes isolated from sulphur-starved plants. Planta. 1993;202:918–921. [Google Scholar]
- Heiss S, Schäfer HJ, Haag-Kerwer A, Rausch T. Cloning sulfur assimilation genes of Brassica juncea L.: cadmium differentially affects the expression of a putative low-affinity sulfate transport and isoforms of ATP sulfurylase and APS reductase. Plant Mol Biol. 1999;39:847–857. doi: 10.1023/a:1006169717355. [DOI] [PubMed] [Google Scholar]
- Honda C, Fujiwara T, Chino M. Sulfate uptake in Arabidopsis thaliana. J Plant Nutr. 1998;21:601–614. [Google Scholar]
- Howden R, Anderson CR, Goldsbrough PB, Cobbett CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 1995a;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Anderson CR, Cobbett CS. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol. 1995b;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Widdell S, Kjelbom P. Preparation of high purity plasmamembrane. Methods Enzymol. 1987;148:568–588. [Google Scholar]
- Lee RB. Selectivity and kinetics of ion uptake by barley plants following nutrient deficiency. Ann Bot. 1982;50:429–449. [Google Scholar]
- Lee S, Leustek T. The effect of cadmium on sulfate assimilation enzymes in Brassica juncea. Plant Sci. 1999;141:201–207. [Google Scholar]
- Leustek T, Martin MN, Bick J-N, Davies JP. Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:141–165. doi: 10.1146/annurev.arplant.51.1.141. [DOI] [PubMed] [Google Scholar]
- Leustek T, Saito K. Sulfate transport and assimilation in plants. Plant Physiol. 1999;120:637–643. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum S, Schmutz D, Brunold C. Regulation of assimilatory sulfate reduction by cadmium in Zea mays L. Plant Physiol. 1988;88:1407–1410. doi: 10.1104/pp.88.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Askerlund P, Fredrikson K, Widdel S, Sommarin M, Larson C. Sealed inside-out and right-side-out plasma membrane vesicles. Plant Physiol. 1990;92:871–880. doi: 10.1104/pp.92.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Compartmental efflux analysis and removal of extracellular cadmium from roots. Plant Physiol. 1987;85:62–65. doi: 10.1104/pp.85.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins and related peptides. Plant Physiol. 1995;109:1141–1149. doi: 10.1104/pp.109.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE, Meuwly P. Retention of cadmium in roots of maize seedlings. Plant Physiol. 1995;109:195–202. doi: 10.1104/pp.109.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegsegger A, Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegsegger A, Schmutz D, Brunold C. Regulation of glutathione synthesis by cadmium in Pisum sativum L. Plant Physiol. 1990;93:1579–1584. doi: 10.1104/pp.93.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi GA, Cocucci M. Effects of deuterium oxide on growth, proton extrusion, potassium influx, and in vitro plasma membrane activities in maize root segments. Plant Physiol. 1992;100:1962–1967. doi: 10.1104/pp.100.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Regulation of sulfate transport and synthesis of sulfur-containing amino acids. Curr Opin Plant Biol. 2000;3:188–195. [PubMed] [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatin across the tonoplast of oat roots. Plant Physiol. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Schäfer HJ, Greiner S, Rausch T, Haag-Kerwer A. In seedlings of heavy metal accumulator Brassica juncea Cu2+ differentially affect transcript amounts for γ-glutamylcysteine synthetase (γ-ECS) and metallothionein (MT2) FEBS Lett. 1997;404:216–220. doi: 10.1016/s0014-5793(97)00132-4. [DOI] [PubMed] [Google Scholar]
- Schäfer HJ, Haag-Kerwer A, Rausch T. cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy metal accumulator Brassica juncea L: evidence for Cd induction of a putative mitochondrial γ-glutamylcysteine synthetase isoform. Plant Mol Biol. 1998;37:87–97. doi: 10.1023/a:1005929022061. [DOI] [PubMed] [Google Scholar]
- Scheller HV, Huang B, Hatch E, Goldsbrough PB. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987;85:1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione content of spruce needles (Picea abies L.) Plant Sci. 1988;57:113–117. [Google Scholar]
- Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT. Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci USA. 1995;92:9373–9377. doi: 10.1073/pnas.92.20.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher A, Warrilow AGS. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Smith FW, Rae AL, Hawkesford MJ. Molecular mechanisms of phosphate and sulphate transport in plants. Biochim Biophys Acta. 2000;1465:236–245. doi: 10.1016/s0005-2736(00)00141-3. [DOI] [PubMed] [Google Scholar]
- Steffens JC. The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:553–575. [Google Scholar]
- Tabatabai MA, Bremner JM. A simple turbidimetric method of determining total sulfur in plant material. Agron J. 1970;62:805–806. [Google Scholar]
- Thibaud JB, Davidian JC, Sentenac H, Soler A, Grignon C. H+ cotransports in corn roots as related to the surface pH shift induced by active H+ excretion. Plant Physiol. 1988;88:1469–1473. doi: 10.1104/pp.88.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukendorf A, Rauser WE. Changes in glutathione and phytochelatins in roots of maize seedlings exposed to cadmium. Plant Sci. 1990;70:155–166. [Google Scholar]
- Ullrich-Eberius CI, Novacky AJ, van Bel AJE. Phosphate uptake in Lemna gibba G.: energetics and kinetics. Planta. 1984;161:46–52. doi: 10.1007/BF00951459. [DOI] [PubMed] [Google Scholar]
- Yong LZ, Pilon-Smits EAH, Tarun AS, Weber SU, Juanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999;119:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenk MH. Heavy-metal detoxification in higher plants: a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999;119:73–79. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]