Abstract
N-Acylethanolamines (NAEs) are endogenous lipids in plants produced from the phospholipid precursor, N-acylphosphatidylethanolamine, by phospholipase D (PLD). Here, we show that seven types of plant NAEs differing in acyl chain length and degree of unsaturation were potent inhibitors of the well-characterized, plant-specific isoform of PLD—PLDα. It is notable that PLDα, unlike other PLD isoforms, has been shown not to catalyze the formation of NAEs from N-acylphosphatidylethanolamine. In general, inhibition of PLDα activity by NAEs increased with decreasing acyl chain length and decreasing degree of unsaturation, such that N-lauroylethanolamine and N-myristoylethanolamine were most potent with IC50s at submicromolar concentrations for the recombinant castor bean (Ricinus communis) PLDα expressed in Escherichia coli and for partially purified cabbage (Brassica oleracea) PLDα. NAEs did not inhibit PLD from Streptomyces chromofuscus, and exhibited only moderate, mixed effects for two other recombinant plant PLD isoforms. Consistent with the inhibitory biochemical effects on PLDα in vitro, N-lauroylethanolamine, but not lauric acid, selectively inhibited abscisic acid-induced closure of stomata in epidermal peels of tobacco (Nicotiana tabacum cv Xanthi) and Commelina communis at low micromolar concentrations. Together, these results provide a new class of biochemical inhibitors to assist in the evaluation of PLDα physiological function(s), and they suggest a novel, lipid mediator role for endogenously produced NAEs in plant cells.
Hydrolysis of membrane phospholipids by phospholipase D2 (PLD, EC 3.1.4.4) activity in plants has been known for decades (Hanahan and Chaikoff, 1947); however, its precise physiological roles in plants are only beginning to be understood (for review, see Chapman et al., 1998; Munnik et al., 1998; Wang, 2001b). Recent evidence at the molecular level indicates that there are at least four functional isoforms of PLD in higher plants, designated α, β, γ, and δ, and their biochemical properties differ substantially (Wang, 2001a). PLDα catalyzes the well-characterized transphosphatidylation activity, which requires millimolar concentrations of Ca2+ for optimal activity. At least two isoforms (β and γ) appear to be optimally activated by micromolar concentrations of Ca2+ and binding of inositol-containing phospholipids (Pappan et al., 1997), and these exhibit phospholipid substrate selectivity that differ markedly from that of PLDα (Pappan et al., 1998). The recently described PLDδ activity is membrane associated and activated by free oleic acid (Wang and Wang, 2001).
Evidence for the physiological function of PLDα points to a role in the degradation/reorganization of subcellular membranes, as well as a role in signal transduction (for review, see Chapman et al., 1998). This membrane degradation is manifested at the cellular level by loss of compartmentation leading to cell death, such as in phytohormone-initiated, PLD-mediated senescence (Thompson, 1988; Fan et al., 1997). The unregulated activity of PLDα in plant cells, then, potentially would lead to membrane damage and loss of cellular function, and cells likely have mechanisms in place to regulate PLDα activity. In addition, a signal transduction role for the PLDα isoform has been implicated from studies in several plant systems in which PLDα mediates, in part, the cellular responses to abscisic acid (ABA; Fan et al., 1997; Ritchie and Gilroy, 1998; Jacob et al., 1999; Frank et al., 2000; Sang et al., 2001).
Recent evidence in tobacco (Nicotiana tabacum) cells indicated that a novel class of lipids, N-acylethanolamines (NAEs), was released from the membrane phospholipid, N-acylphosphatidylethanolamine (NAPE), in a signal-mediated fashion (Chapman et al., 1998; Tripathy et al., 1999). A PLD-type activity was identified that hydrolyzed NAPE to NAE in vitro (Chapman et al., 1998), and a subsequent biochemical survey of PLD catalytic properties of recombinant isoforms (Pappan et al., 1998) suggested that the activity in tobacco microsomes likely was attributed to the β or γ isoforms. Moreover, although PLDα hydrolyzed virtually all other phospholipids, it did not catalyze the formation of NAEs from NAPE. Ethanolamine-containing lipids appear to be particularly important in modulating the activity of PLDs. High levels of phosphatidylethanolamine (80 mol%) are important in general for optimal PLD activity in vitro (Wang, 2001a). Furthermore, lysophosphatidyl-ethanolamine (LPE) was recently shown to be inhibitory toward cabbage (Brassica oleracea) PLDα (Ryu et al., 1997). These data, as well as the varied and potent biological activities ascribed to NAEs in animal systems (Di Marzo, 1998), prompted us to hypothesize that plant NAEs might act as lipid mediators to modulate PLDα activity.
Here, we report that seven NAE molecular species inhibited PLDα activity, and those types of NAEs recently identified in elicitor-treated plant cells (Chapman et al., 1998; Tripathy et al., 1999), N-lauroylethanolamine (NAE 12:0) and N-myristoylethanolamine (NAE 14:0), were the most potent, with IC50 estimates in the nanomolar range. Treatment of epidermal cell layers of tobacco and Commelina communis with NAE 12:0 abrogated the ABA-induced closure of stomatal guard cells, a process mediated by PLDα (Jacob et al., 1999; Sang et al., 2001). Together, our results suggest a novel lipid-mediator role for NAEs in higher plants as a potential endogenous inhibitor of PLDα, and they suggest that products of PLDβ or γ (NAEs) can regulate the activity of PLDα in plant cells. This may represent a mechanism for protecting cell membranes from unregulated PLDα-mediated phospholipid degradation, and for attenuating ABA signaling pathways.
RESULTS
NAE and PLDα Activity
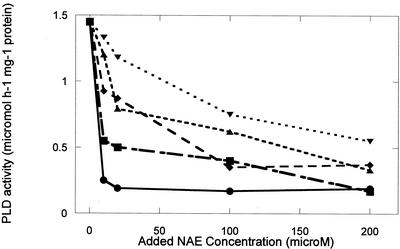
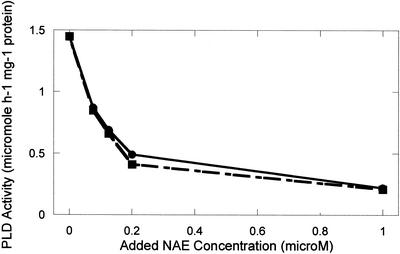
NAE types identified previously in various plant species (Chapman et al., 1998, 1999) were synthesized from ethanolamine and the respective acyl chlorides, and were 95% to 99% pure as judged by thin-layer chromatography (TLC) and gas chromatography-mass spectrometry (Chapman et al., 1999). All NAE types inhibited the activity of castor bean (Ricinus communis) PLDα expressed in Escherichia coli cells (Figs. 1 and 2). All NAEs were effective inhibitors at high concentrations (200 μm), similar to the concentrations reported for LPE (Ryu et al., 1997). In general, the long-chain, unsaturated NAEs demonstrated less inhibitory effects on castor bean PLDα than did saturated or shorter chain types (Fig. 1). In the presence of 50 to 200 μm NAE 12:0 or NAE 14:0, the castor bean PLDα was completely inactive (not shown). As a consequence, submicromolar to low micromolar concentrations of these NAEs were tested for their inhibitory effects on recombinant castor bean PLDα (Fig. 2). The inhibitory effects of NAE 12:0 and NAE 14:0 on PLD activity were similar and were evident at submicromolar concentrations.
Figure 1.
NAE inhibition of recombinant castor bean PLDα expressed in E. coli. Addition of various NAE types to PLD assays inhibited the hydrolytic activity of PLD measured toward phosphatidylcholine (PC) substrate. Protein (20 μg, E. coli lysate) was added to initiate the reaction. NAE types were included at 10 to 200 μm and included the following: NAE 18:3 (▾), NAE 18:2 (▴), NAE 18:1 (♦), NAE 18:0 (▪), and NAE16:0 (●). Data points are the averages of duplicate samples with the range generally less than 12%. Results plotted are from a single experiment and are representative of replicate experimental results.
Figure 2.
NAE inhibition of recombinant castor bean PLDα expressed in E. coli. Addition of various NAE types to PLD assays inhibited the hydrolytic activity of PLD measured toward PC substrate. Protein (20 μg, E. coli lysate) was added to initiate the reaction. NAE types were included at 0.1 to 1.0 μm, and included NAE 14:0 (●) and NAE 12:0 (▪). Data points are the averages of duplicate samples with the range generally less than 10%. Results plotted are from a single experiment and are representative of replicate experimental results.
Table I summarizes the IC50 values for all of the NAEs tested with recombinant castor bean PLDα. In general, the concentration range of inhibition was dependent on NAE chain length and degree of unsaturation and varied through several orders of magnitude, with medium-chain, saturated NAEs being the most potent (IC50 values in the nanomolar range), and long-chain, polyunsaturated NAEs being the least potent (IC50 values in the micromolar range). For example, the inhibitor concentration of NAEs that reduced the maximal PLD activity by 50% ranged from approximately 0.15 μm for NAE 12:0 to approximately 80 μm for NAE 18:3, which accounts for a 500-fold difference in inhibition by the different NAE types. Together, these results clearly demonstrate that NAEs (especially NAE 12:0 and NAE 14:0) are potent inhibitors of plant PLDα in vitro.
Table I.
IC50 values of various NAEs for the inhibition of recombinant castor PLDα expressed in E. coli
| N-Acylethanolamine Type | IC50 Values |
|---|---|
| Acyl Chain | μm |
| 12:0 | 0.15 |
| 14:0 | 0.15 |
| 16:0 | 5.00 |
| 18:0 | 10.00 |
| 18:1 | 40.00 |
| 18:2 | 30.00 |
| 18:3 | 80.00 |
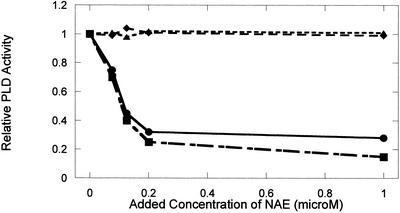
To evaluate the extent of NAE inhibition on PLD from different species, we examined the effects of NAE on partially purified cabbage PLDα and a commercial preparation of Streptomyces chromofuscus PLD. The cabbage PLDα, like the castor bean PLDα, does not catalyze the hydrolysis of NAPE in vitro (see Pappan et al., 1998), whereas the S. chromofuscus enzyme does (Schmid et al., 1986), yet both are optimally active toward many phospholipid classes at mm amounts of Ca2+. Figure 3 shows that NAE 12:0 and NAE 14:0 at submicromolar to low micromolar amounts were potent inhibitors of cabbage PLDα in a manner similar to the castor bean enzyme, but these NAEs had no effect on the activity of S. chromofuscus PLD. In separate control experiments, exogenous myristic acid up to 10 μm did not influence the activity of the cabbage or the castor bean PLDα (data not shown). Other workers have demonstrated that ethanolamine itself has no inhibitory effect on PLD (Ryu et al., 1997), suggesting that the inhibitory effect of NAE is conferred by a structural specificity of the entire molecule, and may be an inherent property of plant PLDα isoforms.
Figure 3.
NAE inhibition of cabbage PLD activity, but not of S. chromofuscus PLD activity. Adding increasing amounts of NAE 12:0 or NAE 14:0 to cabbage (Type IV; Sigma, St. Louis) PLD assays (● and ▪, respectively) reduced relative activity in a concentration-dependent manner. Adding increasing amounts of NAE 12:0 or NAE 14:0 to S. chromofuscus PLD assays (♦ and ▴, respectively) had no effect on enzyme activity. Relative PLD hydrolytic activity from a representative set of experiments is plotted for comparative purposes (points are the average of duplicate samples with a range less than 8%). Specific activity toward PC substrate of the cabbage PLD in the absence of NAE was 15 μmol min−1 mg−1 protein, whereas specific activity of the S. chromofuscus toward PC substrate was 19 μmol min−1 mg−1 protein. These evident inhibitory trends were similar in replicate experiments.
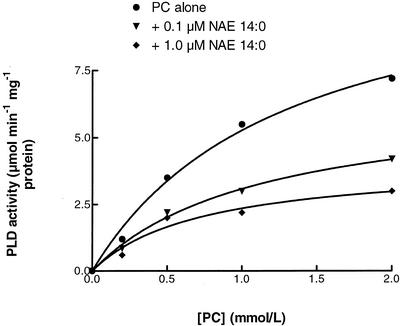
Substrate concentration experiments with increasing amounts of PC in the presence of NAE 14:0 supported conclusions that NAE inhibits the activity of PLDα (Fig. 4). NAE 14:0, in a concentration-dependent manner, inhibited cabbage PLD activity at all substrate (PC) concentrations examined. Although kinetics of membrane-associated enzymes like PLD are complex and should be interpreted with caution (Carmen et al., 1995), inhibition of PLDα by NAE is not likely to be competitive because inhibition is not reversed at high substrate concentrations. The data indicate that the apparent Vmax of PLD with PC substrate was reduced by a factor of three in the presence of 1 μm NAE 14:0. The apparent Km of PLD toward PC was decreased somewhat by adding NAE 14:0, suggesting that NAE increases the affinity of PLD for PC substrate (or perhaps more accurately, affinity for substrate-containing liposomes). It is possible that NAE promotes a membrane surface-associated, inactive state of the enzyme, but the precise mechanism by which NAE acts to inhibit PLD activity must await detailed kinetic analyses and structural information for PLD enzymes.
Figure 4.
Initial velocity measurements of cabbage PLD made at increasing substrate (PC) concentrations without (▾) or with 0.1 μm NAE 14:0 (♦), or with 1.0 μm NAE 14:0 (▵). Data points are averages of triplicate values with sd (less than 10%) omitted for clarity. Lines represent the data fit to the Michaelis-Menton equation with software (GraphPad Prism; GraphPad Software, San Diego). Kinetic parameters estimated by curve fitting were as follows: Apparent Vmax values of 11.96, 6.49, or 4.06 μm min−1 mg−1 protein were estimated with PC alone, with 0.1 μm NAE 14:0, or with 1.0 μm NAE 14:0, respectively, whereas Km values of 1.27, 1.10, or 0.72 μm were estimated with PC alone, with 0.1 μm NAE 14:0, or with 1.0 μm NAE 14:0, respectively. Absolute values of kinetic parameters varied in replicate experiments, but trends were identical; i.e. addition of NAE showed concentration-dependent reductions in apparent Vmax and Km for PLD toward its PC substrate.
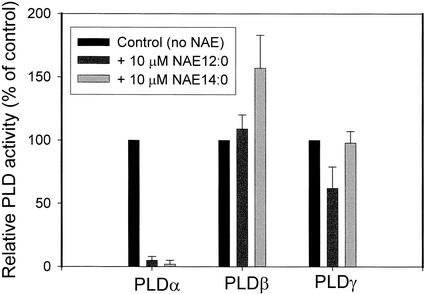
Two other plant PLDs, Arabidopsis PLDβ and γ, were evaluated for their influence by NAE (Fig. 5). Both of these enzymes produce NAE from the phospholipid precursor NAPE in vitro. NAE 12:0 did not influence the transmethylation of PC by PLDβ, whereas NAE 12:0 (at 10 μm) inhibited PLDγ by about 50%, albeit not as potently as PLDα. NAE 14:0 appeared to stimulate somewhat the activity of PLDβ, whereas it had no significant effect on PLDγ activity.
Figure 5.
Comparison of NAE effects on recombinant castor bean PLDα, recombinant Arabidopsis PLDβ, and recombinant Arabidopsis PLDγ expressed in E. coli. Assays were conducted as described in “Materials and Methods.” Relative PLD enzyme activity is plotted for comparative purposes in the presence of 10 μm NAE 12:0 or NAE 14:0. Bars represent the mean and sd of three independent experiments. Control activity is the activity toward PC substrate alone; for PLDα, it was the rate of PC hydrolysis (1.3 μmol h−1 mg−1 protein), and for PLDβ/γ, it was rate of PC transphosphatidylation (β, 85 nmol h−1 mg−1 protein; γ, 72 nmol h−1 mg−1 protein).
NAE inhibition of PLDα in vitro raised the possibility that NAE could act as a lipid mediator to regulate PLDα activity in vivo. We predicted that exogenously supplied NAE would delay or prevent ABA-induced closure of stomatal guard cells, a process clearly shown by transgenic approaches to involve PLDα (Sang et al., 2001) in vivo. Stomatal apertures, measured at the midpoint between two adjacent guard cells, were significantly greater in epidermal peels of tobacco leaves treated with NAE (1 or 10 μm) and ABA (10 μm) compared with those treated with ABA alone, after 30 min (P < 0.0001) and 60 min (P < 0.0001; Table II). Effects were more pronounced at higher concentrations of NAE. Results were generally similar for measurements made with C. communis guard cells under the same conditions (Table III). The inhibitory effect seemed specific to NAE 12:0 because lauric acid (a 12-carbon free fatty acid) did not prevent the ABA-induced stomatal closure (P > 0.4; Table IV). This was consistent with what was observed in vitro where free fatty acid had no effect on PLDα activity. Hence, NAE 12:0, in two plant species, appeared to inhibit ABA-induced stomatal closure, consistent with inhibition at the biochemical level of PLDα.
Table II.
Diameters of stomatal pores (including walls of guard cells), measured at their midpoints, in epidermal peels of tobacco leaves untreated (control) or treated with 0.02 mM ABA alone (ABA), 0.02 mm ABA + 0.001 mm NAE12:0 (ABA + 1 NAE), or 0.02 mm ABA + 0.01 mm NAE12:0 (ABA + 10 NAE) for 10, 30, and 60 min
| Treatment | Mean Pore Diameter in Micrometers (se)
|
||
|---|---|---|---|
| 10 min | 30 min | 60 min | |
| Control (0.067% [v/v] DMSO dimethyl sulfoxide) | 7.84 (0.16), n = 71 | 7.91 (0.22), n = 53 | 8.28 (0.19), n = 56 |
| ABA | 7.79 (0.14), n = 52 | 6.74 (0.22), n = 54 | 6.18 (0.14), n = 62 |
| ABA + 1 NAE | 8.23 (0.24), n = 47 | 8.39 (0.26), n = 61 | 8.60 (0.24), n = 59 |
| ABA + 10 NAE | 8.74 (0.32), n = 41 | 8.79 (0.37), n = 45 | 9.23 (0.24), n = 31 |
Stomates were induced to open by incubation of epidermal peels floated on 5 mm MES NaOH (pH 6.1), 22 mm KCl, and 1 mm CaCl2 for 2 h under white light (125 μm m−2 s−1) prior to treatments (see “Materials and Methods”). Measurements were made with between 30 and 70 guard cell pairs for each treatment at each time point. Data represent mean and se and from a single representative experiment. Replicate experiments showed identical trends. Comparisons by t test: Control versus ABA (30 and 60 min), P < 0.0001; ABA versus ABA + 1 NAE (30 and 60 min), P < 0.0001; ABA versus ABA + 10 NAE (30 and 60 min), P < 0.0001.
Table III.
Diameters of stomatal pores (including walls of guard cells), measured at their midpoints, in epidermal peels of C. communis leaves untreated (control) or treated with 0.02 mm ABA alone (ABA), 0.02 mm ABA + 0.001 mm NAE12:0 (ABA + 1 NAE), or 0.02 mm ABA + 0.01 mm NAE12:0 (ABA + 10 NAE) for 10, 30, and 60 min
| Treatment | Mean Pore Diameter in Micrometers (se)
|
||
|---|---|---|---|
| 10 min | 30 min | 60 min | |
| Control (0.067% [v/v] DMSO) | 13.02 (0.46), n = 21 | 13.65 (0.44), n = 21 | 13.74 (0.37), n = 24 |
| ABA | 10.43 (0.38), n = 27 | 8.82 (0.39), n = 23 | 7.18 (0.31), n = 31 |
| ABA + 1 NAE | 10.31 (0.31), n = 27 | 10.20 (0.35), n = 28 | 9.66 (0.32), n = 33 |
| ABA + 10 NAE | 11.10 (0.29), n = 26 | 11.18 (0.18), n = 18 | 11.14 (0.33), n = 25 |
Stomates were induced to open by incubation of epidermal peels floated on 5 mm MES NaOH (pH 6.1), 22 mm KCl, and 1 mm CaCl2 for 2 h under white light (125 μm m−2 s−1) prior to treatments (see “Materials and Methods”). Measurements were made with between 20 and 30 guard cell pairs for each treatment at each time point. Data represent mean and se from a single representative experiment. Replicate experiments showed identical trends. Comparisons by t test: Control versus ABA (30 and 60 min), P < 0.0001; ABA versus ABA + 1 NAE (60 min), P < 0.0001; ABA versus ABA + 10 NAE (30 and 60 min), P < 0.0001.
Table IV.
Diameters of stomatal pores (including walls of guard cells), measured at their midpoints, in epidermal peels of tobacco leaves untreated (control) or treated with 0.02 mm ABA alone (ABA), 0.02 mm ABA + 0.01 mm NAE12:0 (ABA + NAE), or 0.02 mm ABA + 0.01 mm lauric acid (ABA + free fatty acid [FFA]) for 20 and 40 min
| Treatment | Mean Pore Diameter in Micrometers (se)
|
|
|---|---|---|
| 20 min | 40 min | |
| Control (0.067% [v/v] DMSO) | 9.20 (0.17), n = 97 | 8.99 (0.17), n = 102 |
| ABA | 8.40 (0.18), n = 99 | 8.07 (0.17), n = 104 |
| ABA + NAE | 9.30 (0.17), n = 95 | 9.10 (0.16), n = 112 |
| ABA + FFA | 8.53 (0.20), n = 97 | 8.18 (0.16), n = 115 |
Stomates were induced to open by incubation of epidermal peels floated on 5 mM MES NaOH (pH 6.1), 22 mm KCl, and 1 mm CaCl2 for 2 h under white light (125 μm m−2 s−1) prior to treatments (see “Materials and Methods”). Measurements were made with between 90 and 115 guard cell pairs for each treatment. At time zero, average diameters of stomatal pores were 8.99 ± 0.17 μm. After incubation for 2 h in the dark, these stomates measured 7.00 ± 0.12 μm. Data represent mean and se from a single representative experiment. Replicate experiments showed identical trends. Comparisons by t test: Control versus ABA, P < 0.001; ABA versus ABA + NAE, P < 0.001; ABA versus ABA + FFA, P > 0.4.
DISCUSSION
Previous studies where PLD isoforms were expressed in E. coli to examine the activity toward NAPE indicated a key difference of the PLDβ/γ isoforms compared with PLDα (Pappan et al., 1998). Only PLDβ and γ isoforms were able to catalyze the formation of NAE from NAPE in vitro, whereas PLDα was inactive toward this substrate. NAEs were formed in tobacco cell suspensions, and a PLD activity was identified in microsomes that catalyzed the formation of NAE from NAPE in vitro (Chapman et al., 1998). As a consequence, we proposed that activation of PLDβ or γ, but not α, was responsible for the metabolic release of NAEs from tobacco cell suspensions. These NAEs were identified structurally as NAE 12:0 and NAE 14:0, and NAE formation was subsequently shown to occur in leaves of intact tobacco plants (Tripathy et al., 1999). Inhibition of PLDα by endogenous metabolites of PLDβ (or γ) may provide new insights into the complex regulation of lipid signaling in plants by this diverse and growing class of phospholipases.
Several inhibitors of mammalian PLD have been identified. These inhibitors include fodrin (Lukowski et al., 1996), synaptojanin (Kim et al., 1996), and clathrin assembly protein (Lee et al., 1997), as well as several lipids including ceramide (Venable et al., 1996), alkylphosphate esters (Dittrich et al., 1996), and lysophosphatidyl-Ser (Kawabe et al., 1998). An intriguing observation indicated that oleate-dependent PLD from rat brain was inhibited by several acidic phospholipids, of which polyphosphatidylinositol-bisphosphate (PIP2) was the most effective inhibitor (Kanfer et al., 1996). By contrast, the PIP2-stimulated PLD was inhibited by oleate (Hammond et al., 1995). This unique interaction in which an activator for one PLD isoform is an inhibitor of another gives an example of possible PLD regulation and “crosstalk” between different PLD isozymes in eukaryotic cells. The hydrolysis of NAPE by PLDβ and γ to form NAE and its inhibition of PLDα may be a similar form of regulation between the different PLD isozymes that is distinctive to plant cells because animal systems do not appear to have a PLDα ortholog (Wang, 2001b).
LPE was shown recently to inhibit plant PLDα and to have a profound effect on the physiological symptoms associated with postharvest senescence of flowers and fruits (Ryu et al., 1997). It is interesting that there are two key differences between LPE and NAE inhibition of PLDα. First, LPE inhibition of PLD (like akylphosphate ester inhibition of mammalian PLD; Dittrich et al., 1996) increased with increasing chain length and unsaturation, opposite to NAE (Figs. 1 and 2; Table I). Although the conditions that favor LPE formation in plants remain to be elucidated, and the amounts and types of endogenous LPE in plant tissues are uncertain, the similar down-regulation of PLDα by these structurally diverse ethanolamine-containing lipids is of interest. LPE and NAE are derived from different phospholipid precursors and are produced by different phospholipase classes, which implies that plant cells may possess two alternative mechanisms for accomplishing the same purpose of down-regulating PLDα activity in vivo. The second notable difference is in the concentration range for inhibition. LPE 18:1 was the most potent inhibitor of cabbage PLDα, and this was in the range of 10 to 200 μm (Ryu et al., 1997). NAE 12:0 and NAE 14:0 were the most potent inhibitors of castor bean and cabbage PLDα activity, and this was in the range of 0.1 to 1 μm (Figs. 2 and 3), two orders of magnitude lower than LPE and within the concentration range of NAEs measured in vivo in plant tissues (for summary, see Chapman, 2000).
PLD has been implicated by others in the signal transduction pathway of guard cells in response to ABA (Jacob et al., 1999; Sang et al., 2001). Recent transgenic approaches with plants devoid of PLDα activity clearly demonstrated that abrogation of PLDα activity delayed the ABA-induced closure of stomata and resulted in profound physiological effects in plants exposed to drought stress (Sang et al., 2001). Our results are consistent with these findings and they indicate that selective biochemical inhibition of PLDα by NAEs results in an interference with ABA-induced stomatal closure (Tables II–IV). In addition, our results continue to point to a general role for PLDα in ABA signaling as has been inferred from studies with a number of plants systems (Fan et al., 1997; Ryu et al., 1997; Ritchie and Gilroy, 1998; El Maarouf, 1999; Jacob et al., 1999; Frank et al., 2000). Membrane-permeable NAEs should provide a new biochemical tool for the dissection of PLDα physiological function(s) in plants. Moreover, the results presented here suggest that PLDα may be at least one molecular target for endogenously produced NAEs in higher plants.
MATERIALS AND METHODS
Chemicals
Dioleoyl-[214C-oleoyl]glycero-3-phosphorylcholine (57 mCi mmol−1) was purchased from NEN Life Sciences (Boston). Soybean PC, PIP2, phosphatidylethanolamine(dioleoyl), acyl chlorides, ampicillin, abscisic acid (±, cis/trans), lauric acid, phenylmethylsulfonyl fluoride, cabbage (Brassica oleracea) PLD Type V, Streptomyces chromofuscus PLD, bovine serum albumin, Coomassie Brilliant Blue, and isopropyl β-d-thiogalactoside were from Sigma Chemical. All other reagents were purchased from Fisher Scientific (Pittsburgh) unless otherwise specified.
Synthesis of NAEs
NAEs were synthesized by the addition of 25 mg of acyl chloride in 2.5 mL of dichloromethane to 2.5 mL of ethanolamine according to Devane et al. (1992). The reaction was allowed to proceed for 15 min at room temperature with gentle swirling. The reaction was stopped by the addition of 10 mL of ultrapure water. The organic layer was collected and washed two additional times with 10 mL of ultrapure water. Samples were dried under a stream of N2, resuspended in methanol as stock solutions, purified by preparative TLC as necessary, and stored at −20°C. NAE yield and purity were determined by gas chromatography-mass spectrometry (Chapman et al., 1999).
Expression of PLD in Escherichia coli
The recombinant castor bean (Ricinus communis) PLDα and Arabidopsis PLDβ and γ in pBluescript (SK−) were provided by Dr. X. Wang (Kansas State University, Manhattan). PLDs were expressed in E. coli (JM109) cells and were assayed in cell-free lysates essentially as described by Pappan et al. (1997). In brief, PLDs were induced by the addition of 2 mm isopropyl β-d-thiogalactoside to E. coli cultures (25 mL), which were then grown overnight at 30°C. Cells were harvested by centrifugation and were washed with 50 mm Tris-HCl, pH 8, 150 mm NaCl, 0.25 mm phenylmethylsulfonyl fluoride, and 2 mm EDTA. Cells were ruptured by sonication, and lysates were centrifuged at 10,000g for 5 min. Supernatants were assayed for PLD activity and protein content.
PLD Activity Assays and NAE Inhibition Studies
PLD isoforms were assayed under two different sets of conditions previously determined to yield optimal activity for the respective recombinant proteins and to distinguish between the biochemical requirements for the PLDs (Pappan et al., 1998; Wang, 2001a). PLDα activity was measured in high calcium, PIP2-independent conditions, whereas PLDβ and γ were assayed in the presence of micromolar calcium, and in the presence of PIP2. For castor bean and cabbage PLDα (and for Streptomyces chromofuscus PLD), hydrolysis of radiolabeled PC was used to assess enzyme activity. For PLDβ and γ isoforms, transphosphatidylation of radiolabeled PC in the presence of 1% (v/v) ethanol was used to estimate the relative PLD activity of these isoforms. Enzyme reactions for PLDα were in 100 mm MES (pH 6.5), 25 mm CaCl2, 0.5 mm SDS, and 0.4 mm lipid vesicles (soybean PC plus 0.02 μCi dioleoyl [sn2-oleoyl [1-14C] PC). Enzyme reactions for PLDβ and γ were in 100 mm MES (pH 7.0), 0.05 mm CaCl2, 80 mm KCl, and 0.4 mm lipid vesicles (16 nmol 14C-PC same as above, 112 nmol dioleoylPE, and 6 nmol PIP2) in a final volume of 0.15 mL. The various NAE types were introduced into the reaction with other lipids, or from stock solutions in 67% (v/v) DMSO that were diluted to the appropriate final concentration (see below). Controls with appropriate final amounts of DMSO did not differ appreciably in activity from those without DMSO (final DMSO concentration was generally less than 0.1%, v/v). PLD reactions were initiated by the addition of enzyme and were stopped with boiling isopropanol. Lipids were extracted into CHCl3, and radioactivity was quantified by radiometric scanning of lipid classes separated on TLC plates and/or by liquid-scintillation counting as described previously (Pappan et al., 1998). Protein content was estimated according to Bradford (1976) using bovine serum albumin as a standard.
Measurements of Stomatal Pore Diameter
Epidermal cell layers were peeled with forceps from the lower epidermis of leaves of tobacco (Nicotiana tabacum cv Xanthi) and of Commelina communis. Tobacco plants were greenhouse grown (16-h daylength, supplemented with high-intensity sodium lamps when warranted), and leaves (fourth emerged leaf, not fully expanded) were harvested from 6- to 10-week-old plants. C. communis plants were purchased from a local garden center and acclimated for 2 weeks to the same greenhouse conditions as tobacco prior to harvest of stems with newly emerged leaves. Plant material was harvested and placed in distilled water for 1 h under white light (125 μmol m−2 s−1) in a growth chamber. Epidermal layers were peeled from turgid leaves and were floated on 0.5 mL of 5 mm MES-KOH (pH 6.1), 22 mm KCl, and 1 mm CaCl2 for 1 to 2 h under the same light conditions (Sang et al., 2001).
Epidermal layers with uniformly opened stomata were transferred randomly to the above buffer alone (including 0.067% [v/v] DMSO as a control), buffer plus ABA (0.02 mm, ± cis/trans), or buffer plus ABA (0.02 mm) and NAE 12:0 (0.001 or 0.01 mm). In some experiments, lauric acid (a 12:0 free fatty acid) at 0.01 mm was used to compare with NAE 12:0. Lipids were dissolved as 10 mm stock solutions in 67% (v/v) DMSO (warmed to 45°C prior to each experiment), and were diluted to appropriate final concentrations just before each experiment. Likewise, ABA was dissolved as a 10 mm stock solution in 10% (v/v) DMSO, and was diluted to the appropriate final concentration just before each experiment.
Two to three peels (approximately 0.5–1 cm2 each) were routinely floated on 0.5 mL of experimental solution on large glass cover slips in petri dishes under light conditions above. Images were taken at appropriate time intervals of random locations over each preparation (7–15 independent views for each time point) using an inverted microscope (Diaphot TMD, 20× objective lens, 0.4 numerical aperture; Nikon, Melville, NY) fitted with a CCD camera (model C2400 75i; Hamamatsu, Hamamatsu City, Japan). Stomatal diameters, including guard cell walls, were calculated after the experiments from calibrated digital images using morphometric imaging software (Metamorph 4.6r3; Universal Imaging, West Chester, PA). For tobacco, about 10 stomata could be visualized in each view, and for C. communis, about three stomata were visible in each view. This approach allowed for rapid, efficient evaluation of large numbers of stomata in each treatment at each time point with epidermal preparations harvested and treated under identical conditions. Subsequent statistical comparisons (unpaired t test) of mean pore diameter were performed with software (SigmaPlot version 6.0; SPSS, Chicago or Kaleidograph version 3.5; Synergy Software, Reading, PA). For some experiments, epidermal peels were placed in the dark for the duration of the experiment as an additional control to verify that guard cells were responding appropriately.
ACKNOWLEDGMENTS
We thank Dr. Elison Blancaflor (Samuel R. Noble Foundation) for advice regarding microscopic evaluation of stomata, and the Samuel R. Noble Foundation for use of greenhouse and microscopy facilities. We also thank Dr. Xuemin Wang (Kansas State University) for recombinant PLDs and advice regarding protein expression and characterization. Ms. Jane Hodson (University of North Texas) assisted with statistical analyses of stomatal data, and Mr. Rhidaya Shrestha (University of North Texas) provided assistance with graphics.
Footnotes
This work was supported by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program (agreement no. 99–35304–8002).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001974.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carmen GM, Deems RA, Dennis EA. Lipid signaling enzymes and surface dilution kinetics. J Biol Chem. 1995;270:18711–18714. doi: 10.1074/jbc.270.32.18711. [DOI] [PubMed] [Google Scholar]
- Chapman KD. Emerging physiological roles for N-acylphosphatidylethanolamine metabolism in plants: signal transduction and membrane protection. Chem Phys Lipids. 2000;108:221–230. doi: 10.1016/s0009-3084(00)00198-5. [DOI] [PubMed] [Google Scholar]
- Chapman KD, Tripathy S, Venables BJ, Desouza A. N-Acylethanolamines: formation and molecular composition of a new class of plant lipids. Plant Physiol. 1998;116:1163–1168. doi: 10.1104/pp.116.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD, Venables BJ, Blair R, Jr, Bettinger C. N-Acylethanolamines in seeds: quantification of molecular species and their degradation upon imbibition. Plant Physiol. 1999;120:1157–1164. doi: 10.1104/pp.120.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoids and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiological relevance. Biochim Biophys Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Dittrich N, Nossner G, Kutscher B, Ulbrich-Hofmann R. Alkylphosphate esters as inhibitors of phospholipase D. J Enzyme Inhib. 1996;11:67–75. doi: 10.3109/14756369609038223. [DOI] [PubMed] [Google Scholar]
- El Maarouf H, Zuily-Fodil Y, Gareil M, d'Arcy-Lameta A, Pham-Thi AT. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Mol Biol. 1999;39:1257–1265. doi: 10.1023/a:1006165919928. [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase Dα retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell. 1997;9:2183–2196. doi: 10.1105/tpc.9.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D. Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell. 2000;12:111–124. doi: 10.1105/tpc.12.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S, Alshuller Y, Sung T. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- Hanahan DJ, Chaikoff IL. A new phospholipid splitting enzyme specific for an ester linkage between the nitrogenous base and the phosphoric acid group. J Biol Chem. 1947;169:699–703. [PubMed] [Google Scholar]
- Jacob T, Ritchie SM, Assman SM, Gilroy S. Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1999;96:12192–12197. doi: 10.1073/pnas.96.21.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer JN, McCartney DG, Singh IN, Freysz L. Acidic phospholipids inhibit the phospholipase D activity of rat brain neuronal nuclei. FEBS Lett. 1996;383:6–8. doi: 10.1016/0014-5793(96)00205-0. [DOI] [PubMed] [Google Scholar]
- Kawabe K, Kodaki T, Katayama K, Okamura S, Mori M, Yamashita S. Identification of lipid inhibitor of mammalian phospholipase D. J Biochem. 1998;123:870–875. doi: 10.1093/oxfordjournals.jbchem.a022018. [DOI] [PubMed] [Google Scholar]
- Kim JH, Suh YG, Lee TG, Kim Y, Bae SS, Kim MJ, Lambeth JD, Suh PG, Ryu SH. Inhibition of phospholipase D by a protein factor from bovine brain cytosol: partial purification and characterization of the inhibition mechanism. J Biol Chem. 1996;271:25213–25219. doi: 10.1074/jbc.271.41.25213. [DOI] [PubMed] [Google Scholar]
- Lee C, Kang HS, Chung JK, Sekiya F, Kim JR, Jan JS, Kim SR, Bae YS, Morris AJ, Rhee SG. Inhibition of phospholipase D by clathrin assembly protein 3 (AP3) J Biol Chem. 1997;272:15986–15992. doi: 10.1074/jbc.272.25.15986. [DOI] [PubMed] [Google Scholar]
- Lukowski S, Lecomte M, Mira J. Inhibition of phospholipase D activity by fodrin. J Biol Chem. 1996;271:36–43. doi: 10.1074/jbc.271.39.24164. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine R, Musgrave A. Phospholipid signaling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Pappan K, Austin-Brown S, Chapman K, Wang X. Substrate selectivities and lipid modulation of plant phospholipase Dα, -β, and -γ. Arch Biochem Biophys. 1998;353:131–140. doi: 10.1006/abbi.1998.0640. [DOI] [PubMed] [Google Scholar]
- Pappan K, Zheng S, Wang X. Identification and characterization of a novel plant phospholipase D that requires polyphosphoinositides and submicromolar calcium for activity in Arabidopsis. J Biol Chem. 1997;272:7048–7052. doi: 10.1074/jbc.272.11.7048. [DOI] [PubMed] [Google Scholar]
- Ritchie SM, Gilroy S. Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA. 1998;95:2697–2702. doi: 10.1073/pnas.95.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Bjorn K, Ozgen M, Palta J. Inhibition of phospholipase D by lysophosphatidylethanolamine, a lipid-derived senescence retardant. Proc Natl Acad Sci USA. 1997;94:12717–12721. doi: 10.1073/pnas.94.23.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Zheng S, Li W, Huang B, Wang X. Regulation of plant water loss by manipulating the expression of phospholipase Dα. Plant J. 2001;28:1–11. doi: 10.1046/j.1365-313x.2001.01138.x. [DOI] [PubMed] [Google Scholar]
- Schmid P, Natarajan V, Weis BK, Schmid HHO. Hydrolysis of N-acylated glycerophospholipids by phospholipase A2 and D: a method of identification and analysis. Chem Phys Lipids. 1986;41:195–207. doi: 10.1016/0009-3084(86)90022-8. [DOI] [PubMed] [Google Scholar]
- Thompson JE. The molecular basis for membrane deterioration during senescence. In: Nooden LD, Leopold AC, editors. Senescence and Aging in Plants. New York: Academic Press; 1988. pp. 51–81. [Google Scholar]
- Tripathy S, Venables BJ, Chapman K. N-Acylethanolamines in signal transduction of elicitor perception: attenuation of alkalinization response and activation of defense gene expression. Plant Physiol. 1999;121:1299–1308. doi: 10.1104/pp.121.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable M, Bielawska A, Obeid L. Ceramide inhibits phospholipase D in a cell-free system. J Biol Chem. 1996;271:24800–24805. doi: 10.1074/jbc.271.40.24800. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang X. A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 2001;127:1102–1112. [PMC free article] [PubMed] [Google Scholar]
- Wang X. Multiple forms of phospholipase D in plants: the gene family, catalytic and regulatory properties, and cellular functions. Prog Lipid Res. 2001a;39:109–149. doi: 10.1016/s0163-7827(00)00002-3. [DOI] [PubMed] [Google Scholar]
- Wang X. Plant phospholipases. Annu Rev Plant Physiol Plant Mol Biol. 2001b;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]