Abstract
Interactions between photosynthesis, mitochondrial respiration (mitorespiration), and chlororespiration have been investigated in the green alga Chlamydomonas reinhardtii using flash illumination and a bare platinum electrode. Depending on the physiological status of algae, flash illumination was found to induce either a fast (t1/2 ≈ 300 ms) or slow (t1/2 ≈ 3 s) transient inhibition of oxygen uptake. Based on the effects of the mitorespiratory inhibitors myxothiazol and salicyl hydroxamic acid (SHAM), and of propyl gallate, an inhibitor of the chlororespiratory oxidase, we conclude that the fast transient is due to the flash-induced inhibition of chlororespiration and that the slow transient is due to the flash-induced inhibition of mitorespiration. By measuring blue-green fluorescence changes, related to the redox status of the pyridine nucleotide pool, and chlorophyll fluorescence, related to the redox status of plastoquinones (PQs) in C. reinhardtii wild type and in a photosystem I-deficient mutant, we show that interactions between photosynthesis and chlororespiration are favored when PQ and pyridine nucleotide pools are reduced, whereas interactions between photosynthesis and mitorespiration are favored at more oxidized states. We conclude that the plastid oxidase, similar to the mitochondrial alternative oxidase, becomes significantly engaged when the PQ pool becomes highly reduced, and thereby prevents its over-reduction.
Photosynthesis and respiration, the two major bioenergetic processes of living organisms, coexist within plant cells. Although the photosynthetic electron transport chain (ETC) is clearly restricted to chloroplasts, a respiratory ETC, originally thought to be solely located in mitochondria, has been suggested to be also present in chloroplasts (Bennoun, 1982; Peltier et al., 1987). This chloroplast-based respiration, which has been called chlororespiration to differentiate it from mitorespiration (Bennoun, 1982), probably has its origins in the cyanobacterial endosymbiotic ancestor of chloroplasts (Scherer, 1990). The concept of chlororespiration was initially proposed to account for the effects of respiratory inhibitors, and particularly of inhibitors of terminal oxidases, on photosynthesis in unicellular green algae (Bennoun, 1982). It was reported that cyanide, CO, or salicyl hydroxamic acid (SHAM) increased the redox level of the plastoquinone (PQ) pool, as measured by chlorophyll (Chl) fluorescence induction curves. In addition, flash illumination of algae was found to induce the inhibition of a respiratory process (Peltier et al., 1987). The insensitivity of the flash-induced O2 signal to both antimycin A and SHAM, inhibitors of the mitochondrial ETC, and the requirement for PS I led to the conclusion that chlororespiration, rather than mitorespiration, was inhibited by flash excitation of PS I (Peltier et al., 1987). However, reduction of the PQ pool or inhibitions of O2 uptake could also be explained by an inhibition of mitorespiration coupled to interactions between chloroplasts and mitochondria (Gans and Rebeillé, 1990; Bennoun, 1994; Hoefnagel et al., 1998). Mitorespiration and photosynthesis have been shown to interact through ATP, reducing power and metabolite exchange between chloroplasts and mitochondria (Hoefnagel et al., 1998). Due to the difficulty in differentiating the effects of inhibitors upon mitorespiration or chlororespiration in intact cells, the existence of chlororespiration has been called into question (Bennoun, 1994; Hoefnagel et al., 1998).
The concept of chlororespiration has received some support from the discovery of molecular components likely involved in this process. First, an NADH dehydrogenase complex showing homologies with bacterial complex I has been found in higher plant chloroplasts (Guedeney et al., 1996; Burrows et al., 1998; Sazanov et al., 1998; Horvath et al., 2000; Shikanai and Endo, 2000). Recently, a terminal oxidase involved in carotenoid biosynthesis has been discovered in higher plant chloroplasts (Carol et al., 1999; Wu et al., 1999; Carol and Kuntz, 2001). Based on immunoblotting experiments and the effects of inhibitors, it was proposed that a homolog of this oxidase is located in the thylakoid membrane of Chlamydomonas reinhardtii (Cournac et al., 2000b).
The presence in plant cells of two respiratory ETCs and of one photosynthetic ETC raises the question of how these bioenergetic pathways interact physiologically. To answer this question, unambiguous characterization of these interactions needs to be obtained. The identification of propyl gallate as a potent inhibitor of the chlororespiratory oxidase (Cournac et al., 2000a, 2000b; Josse et al., 2000) has provided a new tool to investigate chlororespiration in vivo. In the present paper, we use a fast bare platinum O2 electrode and flash illumination to kinetically resolve in vivo interactions between photosynthesis, chlororespiration, and mitorespiration. We show that, depending on experimental conditions, flash illumination can induce either a transitory inhibition of chlororespiration (fast transient: t1/2 ≈ 300 ms) or a transitory inhibition of mitorespiration (slow transient: t1/2 ≈3 s). By monitoring in vivo the redox status of PQs using Chl fluorescence measurements, and the redox status of pyridine nucleotide by measuring blue-green fluorescence, we show that cellular redox conditions regulate the interactions between photosynthesis, chlororespiration, and mitorespiration.
RESULTS
Depending on Experimental Conditions, Flash Illumination Can Induce Inhibition of Chlororespiration or Mitorespiration
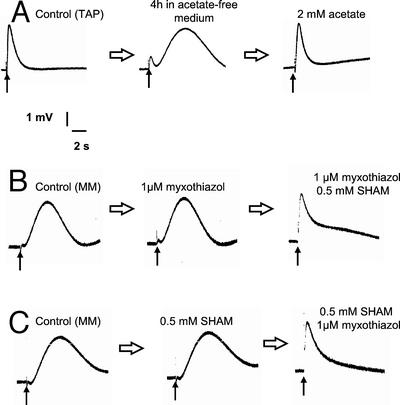
Single flash illumination of dark-adapted algae has been shown previously to induce transient perturbation of respiratory O2 uptake measured using a bare platinum electrode (Peltier et al., 1987). This technique allows a time-resolved characterization of interactions of the photosynthetic ETC with respiratory O2 exchange. We used this technique with the aim to discriminate interactions between photosynthetic ETC and mitorespiration or chlororespiration, the former being expected to be slower because it involves communications between different cellular compartments. When wild-type (WT) C. reinhardtii cells grown on a Tris-acetate phosphate (TAP) medium were resuspended in a minimal medium and deposited at the surface of a bare platinum O2 electrode, a flash-induced O2 signal was observed in response to a short (2-μs) saturating single-turnover flash. This O2 signal consists of a transient increase in O2 concentration (t1/2 rise ≈ 300 ms) and has been reported previously to result from the transitory inhibition of chlororespiration by the flash-induced activity of PS I (Peltier et al., 1987; Cournac et al., 2000b). We monitored how these amperometric signals are altered by variations in mitochondrial activity, which can be modulated either by varying the acetate supply, or by using inhibitors. We observed that after a few hours of starvation in the absence of acetate, the chlororespiratory signal progressively disappeared and was replaced by a different O2 rise with much slower dynamics (t1/2 rise ≈ 3 s; Fig. 1A). Addition of 2 mm acetate restored the initial signal (Fig. 1A). When algae were grown on a minimal medium, a slow O2 transient was observed in response to a single flash illumination. In these conditions, addition of 2 mm acetate transformed the slow O2 transient into a fast transient within a few minutes (data not shown). The fast O2 transient was inhibited by 1 mm propyl gallate (Fig. 2, A and B), an inhibitor of the chloroplast oxidase involved in chlororespiration, thus confirming previous studies concluding that it is due to chlororespiration (Cournac et al., 2000b). In contrast, the slow O2 transient was insensitive to propyl gallate (Fig. 2, C and D), therefore suggesting that it is not due to the transitory inhibition of chlororespiration by PS I. We then tested the effect of mitochondrial inhibitors on these signals. It was shown previously (Peltier et al., 1987, 1995) and confirmed in our hands (not shown) that the fast O2 transient is insensitive to inhibition of mitochondrial activity. When added separately, myxothiazol or SHAM, inhibitors of the mitochondrial cytochrome oxidase and alternative oxidase (AOX) pathways, respectively, did not affect the slow O2 transient. However, when these two inhibitors were present together, thus inhibiting mitorespiration, the slow O2 transient disappeared and was replaced by a fast transient (Fig. 1, B and C). Because, like mitorespiration, the slow O2 transient is insensitive to the separate addition of myxothiazol and SHAM, but is inhibited when both compounds are added together, we conclude that it is likely due to the transitory inhibition of mitorespiration.
Figure 1.
Flash-induced variations in O2 concentration measured in dark-adapted C. reinhardtii cells using a bare platinum electrode. Before each experiment, the algal sample was let in the dark until equilibration of the O2 signal. In these conditions, O2 consumption by the cells and the electrode matches O2 dissolution from the surrounding atmosphere. A single flash (2-μs duration) illumination was applied when indicated by the arrow (↑). In these conditions, the resulting transient increase in O2 concentration corresponded to the transitory inhibition of O2 uptake (see Peltier et al., 1987). A, TAP-grown cells resuspended in minimal medium; the flash-induced O2 transient was recorded after equilibration in the dark (Control), after 4 h of starvation in an acetate-free medium, and after addition of 2 mm acetate. B, Cells grown in minimal medium; the flash-induced O2 transient was recorded after equilibration in the dark. Then, myxothiazol (1 μm) and SHAM (0.5 mm) were added sequentially; the flash-induced transient was recorded after addition of each inhibitor and equilibration in the dark. C, Same as B, but inhibitors were added in the reverse order.
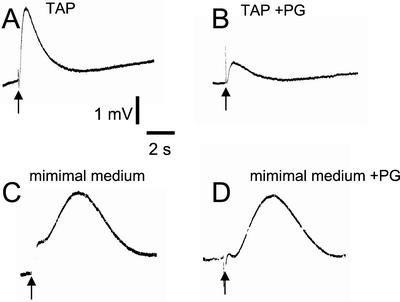
Figure 2.
Effect of propyl gallate addition on fast and slow flash-induced variations in O2 concentration observed in C. reinhardtii cells. Experimental conditions are the same as in Figure 1. A, Fast flash-induced O2 transient cells grown in TAP medium were harvested and resuspended in minimal medium and the flash-induced O2 transient was recorded immediately after equilibration in the dark. B, Same as A after addition of 1 mm propyl gallate. C, Slow flash-induced O2 transient-cells grown in minimal medium. D, Same as C after addition of 1 mm propyl gallate.
Effect of Inhibitors of Mitorespiration and Chlororespiration on O2 Uptake Rates
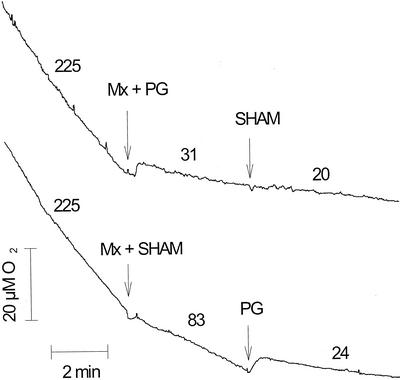
To determine O2 exchange rates involved in mitorespiration and chlororespiration in the dark, we then studied the effects of myxothiazol, SHAM, and propyl gallate on respiratory O2 uptake rates measured in the dark using a Clarke O2 electrode (Fig. 3). Previous studies have shown that the plastid terminal oxidase (PTOX) involved in chlororespiration is much more sensitive to propyl gallate than to SHAM (Cournac et al., 2000a, 2000b), whereas the mitochondrial AOX is sensitive to both inhibitors (Siedow and Girvin, 1980; Berthold, 1998). When added alone, myxothiazol, SHAM, and propyl gallate did not significantly inhibit the dark O2 uptake rate (not shown), indicating that the different oxidative pathways can somehow compensate each other. However, when both myxothiazol and SHAM were added, the O2 uptake rate was inhibited by about 60% due to the inhibition of mitorespiration. Subsequent addition of propyl gallate further inhibited O2 uptake (Fig. 3). When myxothiazol and propyl gallate were first added in combination, respiration was inhibited by about 80%. In these conditions, subsequent addition of SHAM did not induce any significant further inhibition (Fig. 3). Note that the difference in O2 uptake rates reported in the figure (31 versus 20 nmol min−1 mg Chl−1) is most likely overestimated because the O2 uptake rate progressively decreased during the period after propyl gallate addition. No significant effect on the O2 uptake rate was observed after SHAM addition. Based on these experiments, it can be concluded that a propyl gallate-sensitive and SHAM-insensitive O2 uptake, which likely represents chlororespiration, exists in C. reinhardtii cells in the dark. Its rate can be estimated to about 60 nmol min−1 mg−1 Chl, at least when mitorespiration is inhibited.
Figure 3.
Effects of myxothiazol, SHAM, and propyl gallate additions on dark O2 uptake rates measured in WT C. reinhardtii cells using a Clarke O2 electrode. Cells grown in a TAP medium were centrifuged and resuspended in a minimal medium. Algal concentration in the measuring chamber of the electrode was 21 μg Chl mL−1. Numbers indicate O2 uptake rates in nmol min−1 mg Chl−1, averaged over the corresponding period, excluding the first 2 min after addition of inhibitors.
Changes in Redox States of Pyridine Nucleotides and PQs Monitored by in Vivo Measurements of Blue-Green and Red Fluorescence
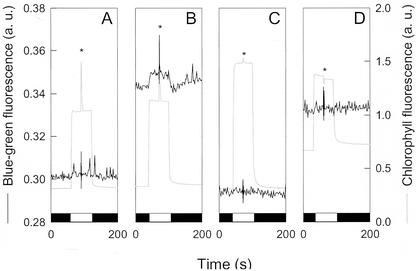
To understand the interactions occurring between mitorespiration, chlororespiration, and photosynthesis, we performed simultaneous in vivo measurements of Chl and blue-green fluorescence. Chl fluorescence is related to the redox level of the PS II acceptor (QA) and reflects the redox status of the PQ pool. Variations in blue-green fluorescence have been reported to be related to changes in the reduction status of NAD(P) (Cerovic et al., 1993; Latouche et al., 2000). In WT C. reinhardtii cells, a dark-to-light transition induced a strong and fast increase in blue-green fluorescence (Fig. 4A); this is due to the reduction of the pyridine nucleotide pool by the photosynthetic ETC and PS I because it does not occur without PS I (Fig. 4C). After turning the light off, the blue-green fluorescence level experienced a sharp transitory decrease. Inhibition of mitochondrial activity by simultaneous addition of myxothiazol and SHAM resulted in an increase of both red and blue-green fluorescence levels in the dark (Fig. 4B). This increase in the dark Chl fluorescence (F0) indicates a reduction of the PQ pool. The increase in blue-green fluorescence in the dark likely indicates a reduction of the pyridine nucleotide pool, but the extent of this reduction is difficult to evaluate from this experiment because some of the fluorescence increase is due to intrinsic fluorescence of the inhibitors for (see “Materials and Methods”). Light-induced variations in blue-green fluorescence were affected by inhibition of mitorespiration, the sharp decrease in blue-green fluorescence observed after a flash or after the period of actinic illumination being suppressed. Photosynthetic activity, estimated using a saturating light pulse by the Chl fluorescence ratio ΔF/Fm (Genty et al., 1989), was strongly inhibited in these conditions, likely due to a fully reduced PQ pool.
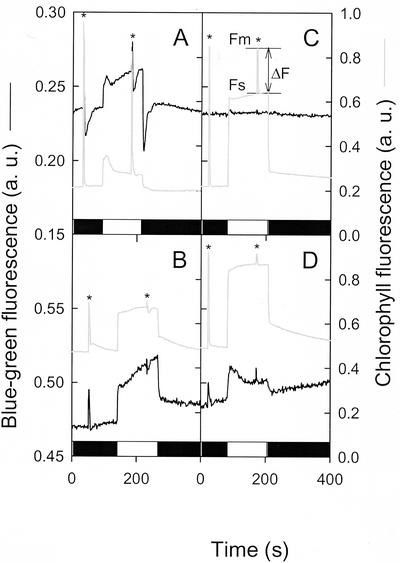
Figure 4.
Measurements of red (Chl) and blue-green [NAD(P)H] fluorescence during dark-light-dark transitions in WT C. reinhardtii cells and in a PS I-deficient mutant (psaAΔ). Cells were deposited on a glass fiber filter at a density of 5 μg Chl cm−2. Black boxes represent dark periods and light boxes represent actinic light periods (24 μmol photons m−2 s−1). Light-saturating pulses (1 s) were applied when indicated by an asterisk. A, WT; B, WT treated with myxothiazol (4 μm) and SHAM (0.8 mm); C, PS I-deficient mutant psaAΔ. Chl fluorescence values used for determination of the photosynthesis quantum yield (ΔF/Fm) are indicated on the Chl fluorescence trace in this graph. D, PS I-deficient mutant psaAΔ treated with myxothiazol (4 μm) and SHAM (0.8 mm).
To study the redox interactions occurring between chlororespiration and mitorespiration, we analyzed a C. reinhardtii mutant devoid of PS I. In such mutants, the contribution of PS I to light-induced blue-green fluorescence changes is absent and fine modifications in the redox state of the pyridine nucleotide pool (for instance, because interactions between chlororespiration and mitorespiration) can be studied. Limited PS II activity involving reoxidation of the PQ pool by a chlororespiratory oxidase has been reported to occur in the absence of PS I (Peltier and Thibault, 1988; Cournac et al., 2000b). During a dark-to-light transition, no significant change in blue-green fluorescence was observed (Fig. 4C). In the light, the Chl fluorescence level strongly increased, indicating a higher reduction state of the PQ pool. The PS II-dependent electron flow rate, as measured by ΔF/Fm, was much lower than in WT but significantly greater than zero (Fig. 4C). When mitochondrial activity was inhibited by simultaneous addition of myxothiazol and SHAM, both blue-green and red fluorescence levels increased, as observed in the WT. The PS II-dependent electron flow (ΔF/Fm) was strongly inhibited in response to the inhibition of mitorespiration, as previously reported (Peltier and Thibault, 1988; Cournac et al., 2000b). Note that inhibition of PS II activity by mitochondrial inhibitors correlated tightly with initial rates of respiration, related to the residual load of acetate (data not shown). In these conditions, a light-induced increase in the blue-green fluorescence was clearly visible (Fig. 4D). Such an increase cannot be due to the reduction of NADP+ to NADPH by PS I because mutant strains used in this experiment are devoid of PS I (Redding et al., 1999). It could be explained, however, if one posits that cellular NAD(P)H pools can be re-oxidized via chlororespiration through the PQ pool. Upon illumination, PS II would compete with chlororespiration for the PQ pool and, thus, would inhibit net oxidation of NAD(P)H.
To test this hypothesis, we studied the effect of propyl gallate, an inhibitor of the chlororespiratory oxidase, on blue-green fluorescence changes (Fig. 5). For this purpose, we used a different experimental device that allowed the separation of variations of the blue-green fluorescence signal due to changes in the redox state of the pyridine nucleotide pool from those due to the intrinsic fluorescence of inhibitors (see “Materials and Methods”). Note that, in this experiment, the effect of mitochondrial inhibitors on basal Chl fluorescence and PS II activity (Fig. 5B) was less pronounced that on Figure 4, due to a longer period in an acetate-free medium before measurement. Despite this, we still observed a significant increase in the blue-green fluorescence related to NAD(P)H in the dark as well as a light-induced transient as in Figure 4 (Fig. 5B). In the presence of propyl gallate, the stationary Chl fluorescence level measured under actinic light was higher, due to an almost complete inhibition of the PS II-dependent electron flow, as shown by the strong decrease in ΔF/Fm (Fig. 5C). On the other hand, propyl gallate had no effect on blue-green fluorescence levels (Fig. 5C). However, when added to algae previously treated by myxothiazol and SHAM, propyl gallate strongly increased Chl fluorescence levels (Fig. 5D), thus indicating that the PQ pool became highly reduced. This indicates that the dark redox state of PQs is controlled by two phenomena: reduction by stromal pools, whose redox state depends on metabolic and mitochondrial activities, and oxidation by a propyl gallate-sensitive oxidase. The light-induced increase in blue-green fluorescence observed in Figure 5B disappeared in the presence of propyl gallate (Fig. 5D), thus showing that it required the presence of an active chlororespiratory process, as hypothesized above.
Figure 5.
Measurements of red (Chl) and blue-green [NAD(P)H] fluorescence during dark-light-dark transitions in PS I-deficient C. reinhardtii mutants (psaAΔ). TAP-grown cells were centrifuged and resuspended in minimal medium and placed in a stirred quartz cuvette. A, Control; B, myxothiazol (4 μm) and SHAM (0.8 mm); C, propyl gallate (1 mm); D, myxothiazol, SHAM, and propyl gallate. Black boxes represent dark periods and light boxes represent actinic light periods (24 μmol photons m−2 s−1). Light saturating pulses (1 s) were applied when indicated by an asterisk.
DISCUSSION
Based on its insensitivity to mitorespiration inhibitors (myxothiazol and SHAM) and to its sensitivity to the chloroplast oxidase inhibitor propyl gallate, the fast (t1/2 rise ≈ 300 ms) O2 transient observed in response to flash illumination is attributed to a transient inhibition of chlororespiration. This confirms previous interpretations (Peltier et al., 1987, 1995; Cournac et al., 2000b). In the absence of respiratory inhibitors, this chlororespiratory signal was observed when algae were supplied with acetate. In the absence of acetate or when carbohydrate reserves were exhausted, flash illumination induced a transitory inhibition of O2 uptake, resulting in a much slower O2 transient (t1/2 rise ≈ 3 s). This slow transient was assigned to an inhibition of mitorespiration and not chlororespiration because it was insensitive to propyl gallate, but was suppressed and replaced by a fast transient when mitorespiration was inhibited. Therefore, we posit the existence within plant cells of two types of redox interactions between ETCs: (a) interactions between photosynthetic and chlororespiratory ETCs, and (b) interactions between photosynthetic and mitorespiration ETCs. Both types of interactions can be kinetically resolved in vivo, photosynthesis/mitorespiration interactions, which require the involvement of metabolic interactions between chloroplasts and mitochondria, developing more slowly than photosynthesis/chlororespiration interactions, which are restricted to thylakoid membranes. Measurements of Chl and blue-green fluorescence show that chlororespiration is significantly engaged and interacts with PS II activity when pyridine nucleotide and PQ pools are reduced. Then, the switch from one type of interaction to the other would be determined by changes in the cellular redox status. At relatively oxidized cellular redox status, photosynthesis/mitorespiration interactions are favored, whereas photosynthesis/chlororespiration interactions are favored when cellular pools are more reduced (i.e. in the presence of acetate or mitochondrial inhibitors).
Flash-induced inhibition of O2 uptake is interpreted as an oxidation of PQs mediated by PS I. At the same time, flash-induced PS II activity should stimulate O2 uptake through a reduction of PQs. The analysis of flash-induced O2 transients in WT and PS I-deficient mutants of C. reinhardtii showed that both stimulation by PS II and inhibition by PS I occur in WT (Ravenel and Peltier, 1992). Because stimulation by PS II is slower (t1/2 ≈ 1 s) than inhibition by PS I (t1/2 ≈ 300 ms), the resulting transient appears as an inhibition. The location of chlororespiratory and photosynthetic electron carriers within thylakoid membranes could explain such a difference. In higher plant chloroplasts, both the Ndh complex (Berger et al., 1993; Sazanov et al., 1996; Horvath et al., 2000) and PTOX (Joët et al., 2002) have been located in stroma lamellae, i.e. in the vicinity of PS I and cyt b6f. On the other side, PS II reaction centers are exclusively located in grana. Plastoquinol diffusion between grana (where PS II is mainly located) and stroma lamellae has been shown to be a slow process operating in the time scale of seconds (Joliot et al., 1992), which may account for the slower stimulation of O2 uptake by PS II.
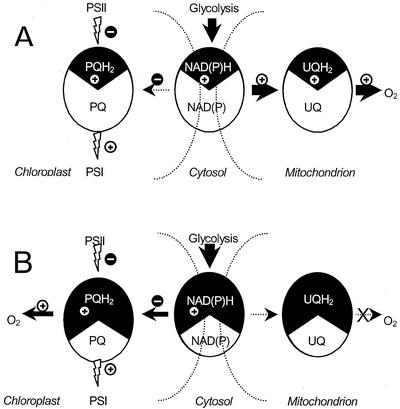
PTOX, recently shown to be involved in chlororespiration (Cournac et al., 2000b), shows sequence homologies with AOX, the plant mitochondrial AOX (Carol et al., 1999; Wu et al., 1999; Carol and Kuntz, 2001). Interestingly enough, AOX has been suggested to function as an “energy overflow,” only becoming active when the cytochrome pathway is saturated with electrons (Vanlerberghe and McIntosh, 1997). Similarly, chlororespiration was found to be active when cellular redox carriers are reduced. Therefore, we suggest that PTOX, like AOX, would only become active when PQs are sufficiently reduced. These conditions can be created by reducing the cytosolic and mitochondrial electron carriers either with the presence of acetate or by inhibition of mitorespiration (see Fig. 6). Therefore, we propose the following train of events to explain the flash-induced O2 transients. In the case of photosynthesis/mitorespiration interactions (when cellular electron carriers are relatively oxidized), PTOX would not be active. Rereduction of PQs would be achieved by an NAD(P)H-PQ oxidoreductase, thereby diverting electrons from the mitochondrial ETC (Fig. 6A), probably via metabolic shuttles such as the OAA/malate shuttle. In the case of photosynthesis/chlororespiration interactions (fast O2 transient), the PQ redox level would be high enough to engage PTOX in plastoquinol oxidation. In these conditions, rereduction of P700+ by plastoquinol (via cyt b6f and plastocyanin) would reroute some electrons from the chlororespiratory chain toward PS I, thereby explaining the transient inhibition of chlororespiration (Fig. 6B).
Figure 6.
Schematic representations of electron transfer pathways in dark-adapted C. reinhardtii: effect of a single turnover flash. Different electron carriers are figured: the PQ pool of stroma lamellae, the mitochondrial ubiquinone pool, and the pyridine nucleotide pool, the latter being present in the three cellular compartments (chloroplast, mitochondria, and cytosol) and supposed to be in redox equilibrium thanks to metabolic shuttles. Arrows indicate electron transfer pathways, width being representative of the electron flow rate. Flash illumination generates charge separations. At the donor side of PS I, a positive charge (⊕) is created and interacts with the PQ pool of stroma lamellae. At the PS II acceptor side, a negative charge () is created. Because PS II is located in grana, this charge interacts slowly with the PQ pool of stroma lamellae. Two situations are depicted. A, When mitochondrial respiration is active (in the absence of acetate), electron carriers are relatively oxidized, and chlororespiration is not significantly engaged. The positive charge generated by PS I is transferred to the PQ pool of stroma lamellae and is compensated by an electron transfer from the NAD(P)H pool, resulting in a transitory decrease in mitochondrial respiration. B, When mitochondrial respiration is inhibited, electron carriers are relatively reduced, and chlororespiration is active. In this situation, the positive charge generated by PS I is transferred to the PQ pool of stroma lamellae and directly results in a transitory decrease in chlororespiration. This decrease develops before the positive charge is compensated by electron transfer from the NAD(P)H pool or from PS II.
Interactions of photosynthesis with either chlororespiration or mitorespiration likely involve an NAD(P)H-PQ oxidoreductase activity. In higher plant chloroplast, an Ndh complex homologous to the bacterial complex I has been identified. This complex has been shown to be functional and to be involved in the non-photochemical reduction of PQs (Burrows et al., 1998; Kofer et al., 1998; Shikanai et al., 1998; Horvath et al., 2000). In the case of the green alga C. reinhardtii, such an Ndh complex is most likely absent (Peltier and Cournac, 2002). From the effect of respiratory inhibitors on the PS II-independent H2 production, which relies on donation of electrons to the PQ pool, it was concluded that an NAD(P)H-PQ oxidoreductase with properties different from a complex I-type enzyme could be involved in this process (Cournac et al., 1998). Note that, from inhibitor studies and from the analysis of tobacco (Nicotiana tabacum) ndh mutants, such a pathway has also been described in higher plant chloroplasts (Corneille et al., 1998; Cournac et al., 1998; Yamane et al., 2000). Whatever the nature of the enzyme implied, reduction of PQs by NAD(P)H appears efficient enough to compete with reduction by PS II in PS I-deficient mutants when mitochondria are inhibited. This is also likely the case in WT, and could explain part of the sensitivity of photosynthesis to mitochondrial inhibitors. More generally, this could be a mechanism controlling PS II activity when stromal pools are reduced.
The existence of chlororespiration has become controversial during the last decade (Bennoun, 1982, 1998; Peltier et al., 1987, 1995; Peltier and Schmidt, 1991; Bennoun, 1994), some of the initial results being explained by the existence of redox interactions between chloroplasts and mitochondria. We have shown in the present study that interactions between photosynthesis and chlororespiration, as well as interactions between photosynthesis and mitorespiration, do, in fact, occur within plant cells and that they are controlled by cellular redox conditions.
MATERIALS AND METHODS
Algal Material
Chlamydomonas reinhardtii cells were grown either on a TAP medium or on a minimal medium (Harris, 1989). Algal cultures were maintained at room temperature under continuous agitation and low illumination (about 25 μmol photons m−2 s−1 for WT strains grown on minimal medium and about 1 μmol photons m−2 s−1 for strains grown on TAP medium). The WT strain used in this work was isolated as an mt+ segregant of a cross between two strains isogenic to the 137c strain (Harris, 1989). PS I-deficient mutants were made by deletions of psaA in this strain as previously described (Fischer et al., 1996; Redding et al., 1999; Cournac et al., 2000b).
O2 Exchange Measurements
Algae were harvested in exponential phase by low-speed centrifugation (600g) and resuspended in a HEPES-KOH buffer (35 mm, pH 7.2). One milliliter of the algal suspension was placed at 25°C in the reaction chamber of a Clark-type O2 electrode (Hansatech, King's Lynn, UK).
Flash-Induced O2 Exchange Measurements
Cells were harvested during exponential growth by low-speed centrifugation (600g) and resuspended in a 50 mm Tris buffer (pH 7.2) containing 0.1 m KCl to provide a sufficient conductivity for the amperometric measurements. Flash-induced O2 exchange measurements were performed using a bare platinum electrode system as described by Schmid and Thibault (1979). The cells were allowed to settle on the electrode for about 30 min before measurements were made. O2 was flushed at the surface of the sample to maintain a sufficient O2 concentration at the algal level (Peltier et al., 1987). The electrode system was covered by a conic reflector in which an aperture for a xenon flash (2-μs duration, model FX 201, PerkinElmer Life Sciences, Boston) was adapted to provide flash illumination. The O2 signal was recorded on the screen of an oscilloscope (Tektronix, Wilsonville, OR).
Fluorescence Measurements
Cells were harvested during exponential growth by low-speed centrifugation (600g) and resuspended in a 35 mm HEPES buffer (pH 7.2). Fluorescence measurements were performed in a front-face configuration on a new version of the pulsed fluorimeter described elsewhere (Cerovic et al., 1993), simultaneously recording blue-green (pyridine nucleotide) and red (Chl) fluorescence. A high-power xenon flash lamp (L4633, Hamamatsu, Massy, France) was used as a pulsed excitation light source (1-μs duration). Excitation light pulses were passed through a 340-nm interference filter (transmittance = 33%, bandwidth = 10 nm, 03FIU008, Melles Griot, Magny les hameaux, France). The blue-green fluorescence was measured with a photomultiplier-based detector (photomultiplier R5600U-01, Hamamatsu) insensitive to continuous light, protected by a UV-blocking filter (KV408, Schott, Clichy, France) and a blue glass filter (CS 4–96, Corning, ARIES, Chatillon, France). The red fluorescence was measured with a photodiode detector protected by a UV-blocking filter (KV408) and a 682-nm interference filter (transmittance = 85%, bandwidth = 22 nm, 682DF22 EM XF47, Omega, Brattleboro, VT). The actinic light was provided by an array of red light emitting diodes (HLMP-8150, Hewlett-Packard, Les Ulis, France).
In the experiment shown in Figure 4, algae were deposited onto a glass microfiber filter (AP40, Millipore, Saint-Quentin-Yvelines, France). A 20-mm-diameter disc was cut in the filter and placed in a thermoregulated (25°C) sample holder. Inhibitor treatments were realized in the dark by depositing at the surface of the sample 0.5 mL of resuspension buffer containing the desired inhibitor concentration; after 5 min, the excess solution was sipped and the algae were kept in the dark until measurements were performed. With this protocol, variations in the basal level of blue-green fluorescence in response to the addition of inhibitors could not be corrected from the background fluorescence of inhibitors (some of them emitting in the blue-green region), so that only light-induced variations must be considered. In the experiment shown in Figure 5, standard quartz cells (1-cm optical path), containing algae resuspended in a HEPES buffer solution (40 mm, pH = 7.2), were used in a thermoregulated (25°C) sample holder. The latter configuration, although less favorable in terms of signal/noise ratio, allowed us to determine the part of variations in fluorescence due to changes in the redox state of pyridine nucleotides induced by inhibitor treatments, by correcting fluorescence signals from the intrinsic fluorescence of inhibitors. Two effects were taken in consideration: the screening of excitation due to the absorption of UV by inhibitors (corrected using the red fluorescence decrease observed immediately after the addition of inhibitors) and intrinsic blue-green fluorescence of inhibitors (estimated from the rise of blue-green fluorescence immediately after addition).
ACKNOWLEDGMENTS
We thank Drs. Thierry Joët and David Stern for communication of unpublished data, and Drs. Bernard Genty, Michel Havaux, and Jérôme Lavergne for helpful discussions and comments. We also acknowledge the skillful technical assistance of Patrick Carrier, Bernard Dimon, and Jacqueline Massimino.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.001636.
LITERATURE CITED
- Bennoun P. Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA. 1982;79:4352–4356. doi: 10.1073/pnas.79.14.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P. Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochim Biophys Acta. 1994;1186:59–66. [Google Scholar]
- Bennoun P. Chlororespiration, sixteen years later. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 675–683. [Google Scholar]
- Berger S, Ellersiek U, Westhoff P, Steinmuller K. Studies on the expression of NDH-H, a subunit of the NAD(P) H-plastoquinone oxidoreductase of higher plant chloroplasts. Planta. 1993;190:25–31. [Google Scholar]
- Berthold DA. Isolation of mutants of the Arabidopsis thalianaalternative oxidase (ubiquinol:oxygen oxidoreductase) resistant to salicylhydroxamic acid. Biochim Biophys Acta. 1998;1364:73–83. doi: 10.1016/s0005-2728(98)00015-2. [DOI] [PubMed] [Google Scholar]
- Burrows PA, Sazanov LA, Svab Z, Maliga P, Nixon PJ. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndhgenes. EMBO J. 1998;17:868–876. doi: 10.1093/emboj/17.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Kuntz M. A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci. 2001;6:31–36. doi: 10.1016/s1360-1385(00)01811-2. [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. Mutations in the Arabidopsis gene immutanscause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell. 1999;11:57–68. doi: 10.1105/tpc.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerovic ZG, Bergher M, Goulas Y, Tosti S, Moya I. Simultaneous measurement of changes in red and blue fluorescence in illuminated chloroplasts and leaf pieces: the contribution of NADPH to the blue fluorescence signal. Photosynth Res. 1993;36:193–204. doi: 10.1007/BF00033038. [DOI] [PubMed] [Google Scholar]
- Corneille S, Cournac L, Guedeney G, Havaux M, Peltier G. Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts: characterization of a NAD(P) H-plastoquinone oxidoreductase activity. Biochim Biophys Acta. 1998;1363:59–69. doi: 10.1016/s0005-2728(97)00074-1. [DOI] [PubMed] [Google Scholar]
- Cournac L, Guedeney G, Joët T, Rumeau D, Latouche G, Cerovic Z, Redding K, Horvath E, Medgyesy P, Peltier G. Non-photochemical reduction of intersystem electron carriers in chloroplasts of higher plants and algae. In: Garab G, editor. Photosynthesis: Mechanism and Effects. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1877–1882. [Google Scholar]
- Cournac L, Josse EM, Joet T, Rumeau D, Redding K, Kuntz M, Peltier G. Flexibility in photosynthetic electron transport: a newly identified chloroplast oxidase involved in chlororespiration. Philos Trans R Soc Lond Ser B Biol Sci. 2000a;355:1447–1453. doi: 10.1098/rstb.2000.0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G. Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem. 2000b;275:17256–17262. doi: 10.1074/jbc.M908732199. [DOI] [PubMed] [Google Scholar]
- Fischer N, Stampacchia O, Redding K, Rochaix JD. Selectable marker recycling in the chloroplast. Mol Gen Genet. 1996;251:373–380. doi: 10.1007/BF02172529. [DOI] [PubMed] [Google Scholar]
- Gans P, Rebeillé F. Control in the dark of the plastoquinone redox state by mitochondrial activity in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;1015:150–155. [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Guedeney G, Corneille S, Cuine S, Peltier G. Evidence for an association of ndh B, ndh Jgene products and ferredoxin-NADP-reductase as components of a chloroplastic NAD(P) H dehydrogenase complex. FEBS Lett. 1996;378:277–280. doi: 10.1016/0014-5793(95)01473-x. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas sourcebook. A comprehensive guide to biology and laboratory use. San Diego: Academic Press; 1989. [DOI] [PubMed] [Google Scholar]
- Hoefnagel MHN, Atkin OK, Wiskich JT. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim Biophys Acta. 1998;1366:235–255. [Google Scholar]
- Horvath EM, Peter SO, Joet T, Rumeau D, Cournac L, Horvath GV, Kavanagh TA, Schafer C, Peltier G, Medgyesy P. Targeted inactivation of the plastid ndhBgene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol. 2000;123:1337–1349. doi: 10.1104/pp.123.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T, Genty B, Josse E-M, Kuntz M, Cournac L, Peltier G (2002) Involvement of a plastid terminal oxidase in plastoquinine oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem (in press) [DOI] [PubMed]
- Joliot P, Lavergne J, Beal D. Plastoquinone compartmentation in chloroplasts: I. Evidence for domains with different rates of photo-reduction. Biochim Biophys Acta. 1992;1101:1–12. [Google Scholar]
- Josse EM, Simkin AJ, Gaffe J, Laboure AM, Kuntz M, Carol P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000;123:1427–1436. doi: 10.1104/pp.123.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofer W, Koop HU, Wanner G, Steinmüller K. Mutagenesis of the genes encoding subunits A, C, H, I, J and K of the plastid NAD(P) H-plastoquinone-oxidoreductase in tobacco by polyethylene glycol-mediated plastome transformation. Mol Gen Genet. 1998;258:166–173. doi: 10.1007/s004380050719. [DOI] [PubMed] [Google Scholar]
- Latouche G, Cerovic ZG, Montagnini F, Moya I. Light-induced changes of NADPH fluorescence in isolated chloroplasts: a spectral and fluorescence lifetime study. Biochim Biophys Acta Bioenerg. 2000;1460:311–329. doi: 10.1016/s0005-2728(00)00198-5. [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L. Chlororespiration. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:523–550. doi: 10.1146/annurev.arplant.53.100301.135242. [DOI] [PubMed] [Google Scholar]
- Peltier G, Havaux M, Ravenel J. Chlororespiration in unicellular green algae. Studies with mitochondrial respiration deficient mutants. In: Mathis P, editor. Photosynthesis: From Light to Biosphere. II. The Netherlands: Kluwer Academic Publishers; 1995. pp. 887–890. [Google Scholar]
- Peltier G, Ravenel J, Verméglio A. Inhibition of a respiratory activity by short saturating flashes in Chlamydomonas: evidence for a chlororespiration. Biochim Biophys Acta. 1987;893:83–90. [Google Scholar]
- Peltier G, Schmidt GW. Chlororespiration: an adaptation to nitrogen deficiency in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1991;88:4791–4795. doi: 10.1073/pnas.88.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier G, Thibault P. Oxygen exchange studies in Chlamydomonas mutants deficient in photosynthetic electron transport: evidence for a photosystem II-dependent oxygen uptake in vivo. Biochim Biophys Acta. 1988;936:319–324. [Google Scholar]
- Ravenel J, Peltier G. Stimulation of the chlororespiratory electron flow by photosystem II activity in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1992;1101:57–63. [Google Scholar]
- Redding K, Cournac L, Vassiliev IR, Golbeck JH, Peltier G, Rochaix JD. Photosystem I is indispensable for photoautotrophic growth, CO2 fixation, and H2 photoproduction in Chlamydomonas reinhardtii. J Biol Chem. 1999;274:10466–10473. doi: 10.1074/jbc.274.15.10466. [DOI] [PubMed] [Google Scholar]
- Sazanov LA, Burrows P, Nixon PJ. Detection and characterization of a complex I-like NADH-specific dehydrogenase from pea thylakoids. Biochem Soc Trans. 1996;24:739–743. doi: 10.1042/bst0240739. [DOI] [PubMed] [Google Scholar]
- Sazanov LA, Burrows PA, Nixon PJ. The plastid ndhgenes code for an NADH-specific dehydrogenase: isolation of a complex I analogue from pea thylakoid membranes. Proc Natl Acad Sci USA. 1998;95:1319–1324. doi: 10.1073/pnas.95.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S. Do photosynthetic and respiratory electron transport chains share redox proteins. Trends Biochem Sci. 1990;15:458–462. doi: 10.1016/0968-0004(90)90296-n. [DOI] [PubMed] [Google Scholar]
- Schmid GH, Thibault P. Characterization of a light-induced oxygen uptake in tobacco protoplasts. Z Naturforsch. 1979;34c:570–575. [Google Scholar]
- Shikanai T, Endo T. Physiological function of a respiratory complex, NAD(P) H dehydrogenase in chloroplasts: dissection by chloroplast reverse genetics. Plant Biotechnol. 2000;17:79–86. [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A. Directed disruption of the tobacco ndhBgene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA. 1998;95:9705–9709. doi: 10.1073/pnas.95.16.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN, Girvin ME. Alternative respiratory pathway: its role in seed respiration and its inhibition by propyl gallate. Plant Physiol. 1980;65:669–674. doi: 10.1104/pp.65.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Wu DY, Wright DA, Wetzel C, Voytas DF, Rodermel S. The immutans variegation locus of Arabidopsisdefines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell. 1999;11:43–55. doi: 10.1105/tpc.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane Y, Shikanai T, Kashino Y, Koike H, Satoh K. Reduction of Q(A) in the dark: another cause of fluorescence Fo increases by high temperatures in higher plants. Photosynth Res. 2000;63:23–34. doi: 10.1023/A:1006350706802. [DOI] [PubMed] [Google Scholar]