Abstract
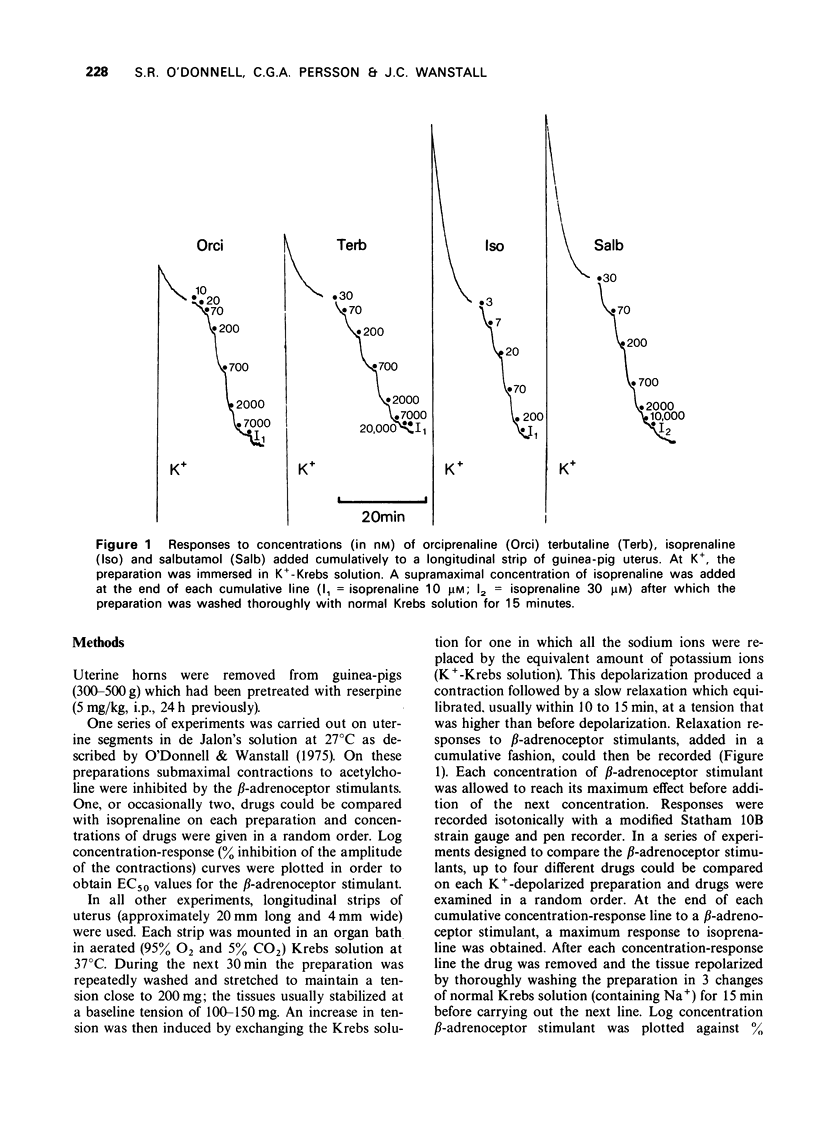
1 A comparison of six β-adrenoceptor stimulants has been carried out on in vitro preparations of guinea-pig uterus which were depolarized in K+-Krebs solution. Results have also been obtained on uterine preparations in which contractions to acetylcholine were inhibited. The establishment of the conditions for the K+-depolarized preparations are described.
2 There was no significant difference between potency values (mean neg log EC50 values) for any of the drugs on the two types of uterine preparation i.e. the preparations had the same sensitivity to the drugs.
3 There was a less than two-fold difference between the relative potency values for the β-adrenoceptor stimulants on the two types of uterine preparation. The relative potency values (isoprenaline = 100) on the K+-depolarized preparation were fenoterol 74.1, salbutamol 15.1, rimiterol 13.5, terbutaline 8.2 and orciprenaline 5.6.
4 The relative potency values obtained on uterine preparations were less than three-fold different from those previously found for guinea-pig trachea (after inhibition of extraneuronal uptake).
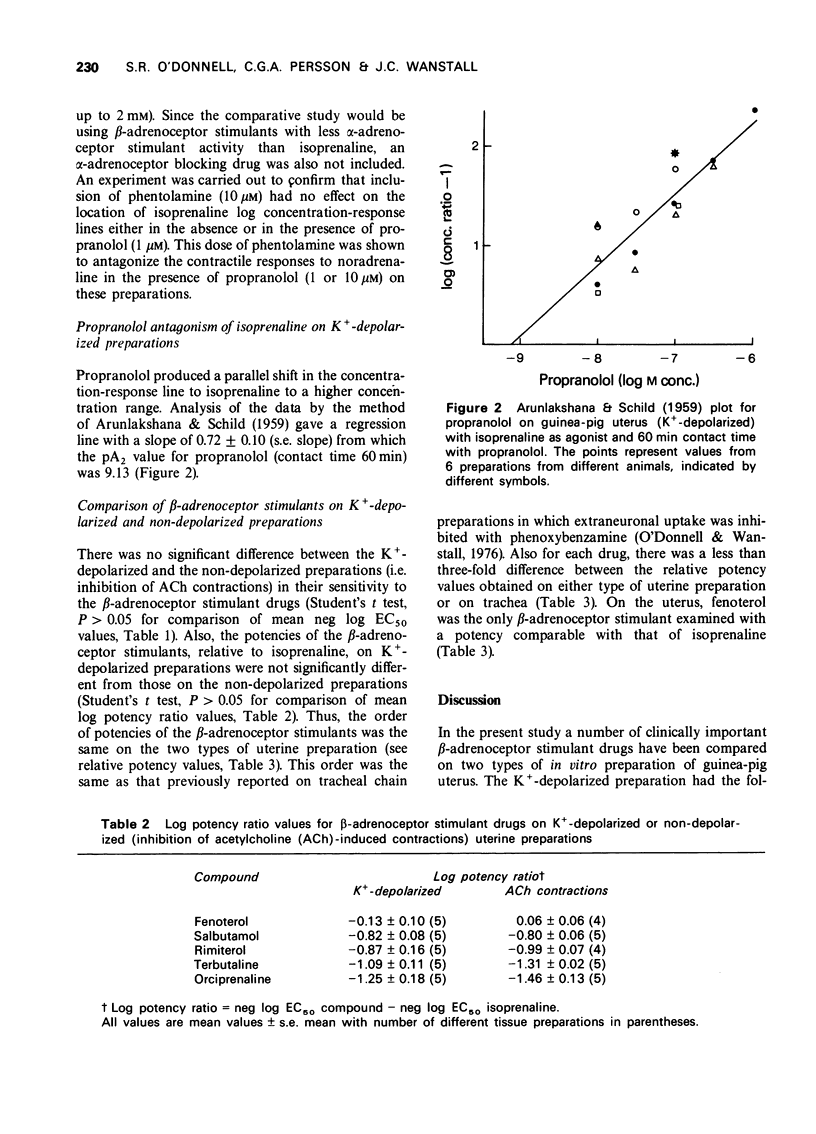
5 The pA2 value for propranolol on the K+-depolarized uterine preparations was 9.13.
6 It is concluded that the K+-depolarized guinea-pig uterine preparation can be used for quantitative studies on β-adrenoceptor stimulant drugs. It lacks spontaneous activity, drugs can be added cumulatively and several drugs can be compared on a single preparation. In addition, the results obtained support the classification of the β-adrenoceptors in guinea-pig uterus and trachea in the same sub-group (β2).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Aziz A., Ghazal A., Daabees T. The effect of mestranol and lynoestrenol on the sensitivity of the isolated rat uterus to the inhibitory action of sympathomimetic agents. J Reprod Fertil. 1974 Oct;40(2):463–466. doi: 10.1530/jrf.0.0400463. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Ingemarsson I., Persson C. G. Relaxing effects of beta-receptor stimulators in isolated, gravid human myometrium. Life Sci. 1973 Aug 16;13(4):335–344. doi: 10.1016/0024-3205(73)90225-7. [DOI] [PubMed] [Google Scholar]
- Blinks J. R. Evaluation of the cardiac effects of several beta adrenergic blocking agents. Ann N Y Acad Sci. 1967 Feb 10;139(3):673–685. doi: 10.1111/j.1749-6632.1967.tb41237.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. J., Ikoku C. The adrenergic receptors in the guinea pig uterus. Can J Physiol Pharmacol. 1966 May;44(3):491–493. doi: 10.1139/y66-058. [DOI] [PubMed] [Google Scholar]
- EDMAN K. A., SCHILD H. O. CALCIUM AND THE STIMULANT AND INHIBITORY EFFECTS OF ADRENALINE IN DEPOLARIZED SMOOTH MUSCLE. J Physiol. 1963 Nov;169:404–411. doi: 10.1113/jphysiol.1963.sp007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN K. A., SCHILD H. O. The need for calcium in the contractile responses induced by acetylcholine and potassium in the rat uterus. J Physiol. 1962 May;161:424–441. doi: 10.1113/jphysiol.1962.sp006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. B., Levy G. P. Differentiation of beta-adrenoreceptors by the use of blocking agents. J Pharm Pharmacol. 1970 Feb;22(2):145–146. doi: 10.1111/j.2042-7158.1970.tb08414.x. [DOI] [PubMed] [Google Scholar]
- Gibson A., Pollock D. The effects of drugs on the sensitivity of the rat anococcygeus muscle to agonists. Br J Pharmacol. 1973 Nov;49(3):506–513. doi: 10.1111/j.1476-5381.1973.tb17261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Towart R. Uptake kinetics and ion requirements for extraneuronal uptake of noradrenaline by arterial smooth muscle and collagen. Br J Pharmacol. 1973 Mar;47(3):556–567. doi: 10.1111/j.1476-5381.1973.tb08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth M., Schnieden H. Letter: Salbutamol and inhibition of uterine contractions. J Pharm Pharmacol. 1973 Dec;25(12):996–998. doi: 10.1111/j.2042-7158.1973.tb09992.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. Neuronal uptake processes for amines and amino acids. Adv Biochem Psychopharmacol. 1970;2:109–132. [PubMed] [Google Scholar]
- Kulkarni P. S., Wakade A. R., Kirpekar S. M. Sympathetic innervation of guinea pig uterus and ovary. Am J Physiol. 1976 May;230(5):1400–1405. doi: 10.1152/ajplegacy.1976.230.5.1400. [DOI] [PubMed] [Google Scholar]
- Lands A. M., Luduena F. P., Buzzo H. J. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967 Nov 1;6(21):2241–2249. doi: 10.1016/0024-3205(67)90031-8. [DOI] [PubMed] [Google Scholar]
- Liggins G. C., Vaughan G. S. Intravenous infusion of salbutamol in the management of premature labour. J Obstet Gynaecol Br Commonw. 1973 Jan;80(1):29–32. doi: 10.1111/j.1471-0528.1973.tb02126.x. [DOI] [PubMed] [Google Scholar]
- Miller J. W. Adrenergic receptors in the myometrium. Ann N Y Acad Sci. 1967 Feb 10;139(3):788–798. doi: 10.1111/j.1749-6632.1967.tb41247.x. [DOI] [PubMed] [Google Scholar]
- Nesheim B. I. Comparison of alpha- and beta-receptor stimulation in the circular and longitudinal muscle of the oestrogen and progesterone dominated rabbit uterus. Acta Pharmacol Toxicol (Copenh) 1974 Apr;34(4):295–304. doi: 10.1111/j.1600-0773.1974.tb03526.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R. An examination of some -adrenoreceptor stimulants for selectivity using the isolated trachea and atria of the guinea pig. Eur J Pharmacol. 1972 Sep;19(3):371–379. doi: 10.1016/0014-2999(72)90104-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Hexoprenaline: beta-adrenoreceptor selectivity in isolated tissues from the guinea-pig. Clin Exp Pharmacol Physiol. 1975 Nov-Dec;2(6):541–547. doi: 10.1111/j.1440-1681.1975.tb01859.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Potency and selectivity in vitro of compounds related to isoprenaline and orciprenaline on beta-adrenoceptors in the guinea-pig. Br J Pharmacol. 1974 Nov;52(3):407–417. doi: 10.1111/j.1476-5381.1974.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. Some aspects of the trachea-heart selectivity of rimiterol in vitro and in vivo in guinea-pigs. Arch Int Pharmacodyn Ther. 1977 Apr;226(2):214–223. [PubMed] [Google Scholar]
- O'Donnell S. R., Wanstall J. C. The contribution of extraneuronal uptake to the trachea-blood vessel selectivity of beta-adrenoceptor stimulants in vitro in guinea-pigs. Br J Pharmacol. 1976 Jul;57(3):369–373. doi: 10.1111/j.1476-5381.1976.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H. O. The action of isoprenaline in the depolarized rat uterus. Br J Pharmacol Chemother. 1967 Nov;31(3):578–592. doi: 10.1111/j.1476-5381.1967.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman M. A., Levy B. Selective beta adrenergic receptor blockade in the rat. J Pharmacol Exp Ther. 1972 Aug;182(2):256–263. [PubMed] [Google Scholar]


