Abstract
We examined the learning and memory of neurotrophin-4 (NT4)−/− mice by using fear conditioning. In both cue and context conditioning, we found significant deficits in the NT4 mutants at 2 and 24 h after training but not at 30 min. Hippocampal slices from the mutant mice showed normal basal synaptic transmission, short-term plasticity, and decremental long-term potentiation (LTP) at the Schaffer collateral-CA1 synapses. These findings, together with the normal short-term memory, suggest that the hippocampal development of NT4−/− mice is largely unaffected. However, consistent with the long-term memory defects, the long-lasting LTP at the same synapses was attenuated significantly in the mutant mice. Our results suggest that NT4 plays a physiological role essential for hippocampus- and amygdala-dependent long-term memory and hippocampal long-lasting LTP and that NT4 may be useful in the therapy of acquired disorders of learning and memory.
In mammals, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), and neurotrophin-4 (NT4) are a family of structurally related neurotrophic factors (1–8). Neurotrophins exert their functions through two types of receptors, the trks and p75. The trks are a family of receptor tyrosine kinases (9–11), and trkB is the main receptor for BDNF and NT4 (5, 6, 12–14). All four neurotrophins are ligands for p75, a neurotrophin receptor that does not have kinase activity and belongs to the tumor necrosis factor receptor super family (15, 16).
There is increasing evidence that suggests that neurotrophins play a functional role in regulating synaptic plasticity (17–20). It was first found that BDNF and NT3 can cause a rapid, but reversible, increase in the frequency of miniature excitatory postsynaptic currents at neuromuscular synapses in culture (21). Since then, various studies have shown that exogenous neurotrophins facilitate synaptic function in nerve-muscle synapses (22, 23), cultured hippocampal neurons (24–26), and hippocampal slices (27, 28). Lack of BDNF severely impairs decremental long-term potentiation (D-LTP; refs. 29 and 30) and fully compromises the long-lasting LTP (L-LTP; ref. 31) in the hippocampus. Similar results were obtained from experiments with anti-trkB antibody or trkB-IgG to block trkB function (28, 31, 32). A recent study showed that NT3+/− mice are deficient in short-term plasticity but not LTP at lateral perforant path-dentate granule cell synapses (33). Strong evidence comes from the studies of Patterson et al. (30) and Korte et al. (34). They showed that recombinant BDNF or virus-mediated BDNF expression can quickly rescue impaired LTP in BDNF−/− hippocampal slices (30, 34). These experiments argue that neurotrophins play a direct and acute role in synaptic plasticity.
Studies focused on adult animals found that intracerebroventricular infusion of NGF, NT3, and NT4 can improve age-related declines in spatial memory (35, 36). Cerebral cortex injection of anti-NGF antibody led to learning and memory impairment (37). Intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus (38). These experiments are consistent with the in vitro studies and suggest that neurotrophins play a functional role in synaptic plasticity. It has been difficult to assess the function of neurotrophins genetically in the adult animal, because targeted gene disruption of NGF, BDNF, NT3, and trks leads to postnatal lethality (10, 39, 40). However, mice carrying a targeted disruption in a single copy of these genes are viable. It was found recently that BDNF+/− mice have a modest deficit in spatial learning but not memory retention (41). Interestingly, BDNF+/− and BDNF−/− mice have very similar LTP phenotypes (29, 30). However, BDNF+/− mice do not have detectable neuronal loss, whereas BDNF−/− mice have severe peripheral nervous system deficits (42, 43). It was also found that NGF+/− mice display significant deficits in memory acquisition and retention as well as basal forebrain cholinergic neuron reduction; a long infusion of NGF into the lateral ventricle of these mice abolished the deficits in spatial memory but not the neuronal loss (44). These studies suggest that the memory and LTP phenotypes in neurotrophin mutant mice may be independent from neuronal loss.
NT4 is the last mammalian neurotrophin identified, and it is expressed in many regions of the brain (5, 6). NT4 enhances glutamatergic synaptic transmission in cultured hippocampal neurons (24). Overexpression of NT4 in the myocytes of a Xenopus nerve-muscle cultures causes a higher level of spontaneous synaptic activity and enhances evoked synaptic transmission (23). However, it is not known whether NT4 plays a role in learning and memory or hippocampal LTP formation. Targeted deletion of the NT4 gene in mice results in a homozygous viable mouse strain that does not show any overt phenotype except neuronal reductions in two peripheral ganglia, nodose and geniculate ganglia, where visceral and facial sensory neurons are located (45, 46). Because NT4−/− mice are the only viable strain of neurotrophin knockouts and are without overt morphological and physiological phenotypes, these animals are particularly suitable for studies of the physiological functions of neurotrophins in adult animals. In the present study, we have explored the role of NT4 in memory and hippocampal LTP formation. We demonstrate that NT4−/− mice have deficits in long-term memory and hippocampal L-LTP but are normal in short term-memory and hippocampal D-LTP.
Materials and Methods
Mice.
Both wild-type (wt) and NT4−/− mice used in the experiments were 129/svJ inbred mice. NT4−/− mice were identified by Southern blot analysis of tail DNA samples. All experiments were done with 5- to 8-month-old male mice that were kept in a 12-h:12-h light:dark cycle. Tests were always conducted during the light phase of the cycle. The mouse facility at University of California, Los Angeles, is fully accredited by the American Association for the Accreditation of Laboratory Animal Care. Mice were maintained in accordance with the Animal Welfare Act and the Department of Health and Human Services guide.
Nociception Tests.
A 1-s continuous electrical shock of increasing intensity was given to mice in the conditioning chamber (described in the following section). An interval of 10 s was given between shocks. The sequence of currents used in the test was as follows: 0.05 mA, 0.1 mA, 0.15 mA, 0.2 mA, 0.25 mA, 0.3 mA, 0.35 mA, 0.4 mA, 0.45 mA, and 0.5 mA. The levels of currents that elicit flinch, jump/run, and vocalization for each mouse were recorded. The tests were done in a blind fashion.
Fear Conditioning.
The mice were first put through a training trial in which each animal was placed in a conditioning chamber (context) for 2 min before the onset of a discrete tone (the conditioned stimulus or CS). The tone lasted for 30 s, and at the end of the sound, a 2-s continuous electrical foot shock (0.75 mA) was given to the mouse. The mouse stayed in the chamber for another 30 s after the shock before it was sent back to its home cage. Freezing frequency was recorded at 5-s intervals for the whole training session. The conditioning chamber is connected to a master shocker (Germini Shocker, San Diego Instruments). Each group consisted of eight NT4−/− and eight wt age-matched male mice.
For contextual conditioning, mice were tested at 30 min, 2 h, and 24 h after the training trial. Each mouse was placed in the conditioning chamber where it had been trained and was analyzed by consecutively measuring the freezing response over a 5-min period. Complete immobility (except for respiratory movement) over a 5-s interval was scored as “freezing.”
For cue conditioning, mice were tested at 30 min, 2 h, and 24 h after the training trial. Each mouse was placed in a different chamber for 3 min and then exposed to a CS identical to the one to which it had been exposed in the training trial. The CS lasted for 3 min. The freezing responses over the entire 6 min were measured and recorded. All investigations were done in a blinded fashion.
Electrophysiology.
Transverse hippocampal slices 500-μm-thick were prepared from adult male NT4−/− mice or age-matched wt mice (5–8 months old). Slices were kept in oxygenated artificial cerebrospinal fluid (ACSF) in a holding chamber for at least 1 h and then transferred to a submerged chamber for recording while being perfused continuously with warm ACSF (30 ± 1°C) at a flow rate of 2–3 ml/min. The composition of the ACSF was (in mM): NaCl, 120; NaHCO3, 25; KCl, 3.3; NaH2PO4, 1.23; CaCl2, 1.8; MgSO4, 1.2; and d-glucose, 10 (pH 7.4). Schaffer collateral fibers were stimulated by constant stimulus pulses (0.1 ms; 30–400 μA) delivered through a sharpened monopolar tungsten electrode placed in the stratum radiatum layer of area CA1. Evoked field excitatory postsynaptic potentials (fEPSPs) were recorded extracellularly from the same layer with a glass electrode filled with 2 M NaCl. At the beginning of each experiment, a synaptic input–output curve was generated by plotting fEPSP slopes vs. their corresponding presynaptic fiber volley sizes, elicited at different stimulus intensities. Slices that showed maximal fEPSP sizes <3 mV were rejected. Test pulses that evoked 40–50% of the maximal fEPSP slope were then applied once per minute for baseline collection. In some experiments, paired pulses were applied at various interpulse intervals with test pulse intensity, and resulting paired-pulse facilitation (PPF) was calculated as percentages of increase of the initial slope of the second fEPSP compared with that of the first fEPSP.
A single high-frequency stimulus train (100 Hz; 1 s) was applied at the test pulse intensity to induce the D-LTP, whereas the L-LTP was elicited by four consecutive 100-Hz trains with 5-min intertrain intervals. The test pulses, as used for baseline collection, were resumed immediately after the tetanization to monitor the changes in fEPSP. The amount of LTP was expressed as percentages of increase in the slope of fEPSPs relative to the baseline.
Data Analysis.
For electrophysiological data, recorded signals were digitized and stored on computer disks for off-line analysis. The group data were expressed as means ± SEM and subjected to a one-way ANOVA to test for overall statistical significance. Comparison between groups was made by Student's t test. Statistical significance was defined as P < 0.05.
Results
Significant Memory Retention Deficits of NT4−/− Mice in Fear Conditioning.
To examine the role of NT4 in learning and memory, we focused on a fear-conditioning test (47, 48). Our NT4−/− and wt control mice do not perform well in the water maze test, because they are inbred 129/SvJ mice; it is known that 129/SvJ mice perform poorly in the water maze test but are highly suitable for the fear-conditioning test (49). Fear conditioning is based on the ability of normal animals to learn to fear a previously neutral stimulus, the CS, because of its temporal association with an aversive stimulus, such as a foot shock. When exposed to the CS, conditioned animals tend to refrain from all but respiratory movement, a response known as freezing. We used two types of CS: an audible tone for cue-dependent fear conditioning and an environmental context for context-dependent conditioning (50, 51). Quantitation is provided by the percentage of time spent freezing. Cued conditioning depends on the amygdala, whereas contextual conditioning requires correct functioning of both hippocampus and amygdala (48, 51). By using fear conditioning, robust learning can be accomplished in a 3-min trial.
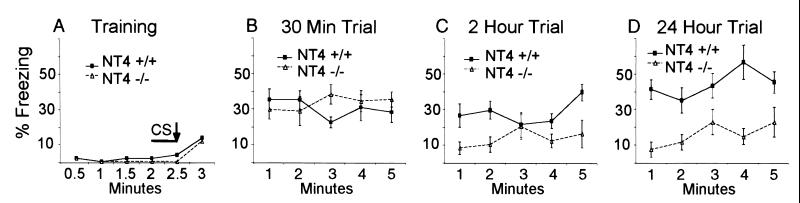
Fig. 1A shows the results of a typical training trial. There was no significant difference in freezing between the mutant and wt mice after the shock (P = 0.31), and both groups showed significantly increased freezing time during the training (P < 0.001). For the contextual conditioning experiment, we divided mice into three groups and tested the animals at 30 min, 2 h, and 24 h after a single training trial. The freezing levels of NT4−/− and wt mice were indistinguishable 30 min after training (Fig. 1B; P = 0.12). Both groups displayed freezing for about 30% of the 5-min testing period. These data show that NT4−/− mice can be conditioned contextually and that the memory is retained for at least 30 min. However, there were significant differences between NT4−/− and wt mice at 2 and 24 h after training (Fig. 1 C and D). At 2 h after training, the average level of freezing of wt mice was 29%, and that of the NT4−/−mice was 14% (P < 0.0001). An even stronger difference was detected 24 h after training, with wt showing 44% freezing and NT−/− showing 15% (P < 0.0001). These data indicate that NT4−/− mice can learn and remember for a short period after contextual conditioning, but between 30 min and 2 h, they loose the memory acquired during the training.
Figure 1.
Contextual conditioning with a single training trial. (A) The freezing of one group of NT4−/− (n = 8) and wt (n = 8) mice during training. The solid line indicates the duration of the tone (CS). The arrow indicates the 2-s foot shock. ANOVA showed no significant difference between NT4−/− and wt mice (P = 0.31). The freezing responses of all other groups of mice for both context and cue conditioning tests are very similar to what is shown in A. (B–D) Three separate groups of NT4−/− and wt mice were tested for contextual conditioning at 30 min (B), 2 h (C), and 24 h (D) after training. ANOVA showed highly statistically significant differences between NT4−/− and wt mice at 2 and 24 h (P < 0.0001) but no significant difference at 30 min (P = 0.12).
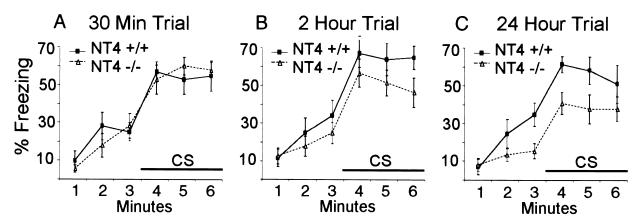
In the cued conditioning test, another three groups of NT4−/− and wt mice were placed in a different chamber at 30 min, 2 h, and 24 h after a single training trial. After 3 min, the same audible tone (CS) previously paired with the foot shock during training was given, and the freezing time was recorded (Fig. 2 A–C). Again, there was no significant difference between mutant and wt mice 30 min after the training trial (Fig. 2A), with both sets of animals showing significantly increased freezing caused by the CS (P < 0.001 for both). At 2 h after training (Fig. 2B), there was no difference between mutant and wt mice before CS (P = 0.31) and a significant difference during CS (P = 0.027). At 24 h after training (Fig. 2C), these differences were more pronounced (P = 0.021 before CS; P = 0.004 during CS). Consistent with the contextual conditioning data, these results again show that the NT4−/− mice have a deficit in long-term but not short-term memory.
Figure 2.
Cue conditioning with a single training trial. (A–C) Three groups of NT4−/− and wt mice were tested for cued conditioning at 30 min (A), 2 h (B), and 24 h (C) after training. Similar to contextual conditioning, ANOVA showed statistically significant differences between NT4−/− and wt mice at 2 h (P = 0.027) and 24 h (P = 0.004) but no significant difference at 30 min (P = 0.42).
Sensitivity to Foot Shock Is Normal in NT4−/− Mice.
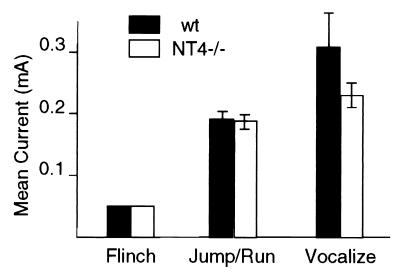
An important control is to examine whether NT4−/− mice have altered nociceptive reaction to shock, because changes in pain sensitivity can affect conditioning (52). For both NT4−/− and wt mice, we determined the threshold current to elicit the three progressive reactions to increasing electrical foot shock: flinch, jump/run, and vocalization. Fig. 3 shows that there were no differences between NT4−/− and wt mice in current levels required to elicit flinch and jump/run. The level of current required to cause vocal responses from NT4−/− mice was lower than that of wt mice and tended toward but did not reach statistical significance (P = 0.051). These data indicate that pain sensitivity and the physical responses to pain, including motor performance and vocalization, are not impaired in the NT4 knockout mice and cannot account for the abnormal performance in fear conditioning. Taken together, our behavioral analyses of NT4−/− mice indicate that NT4 is required for long-term memory retention involving hippocampus and amygdala.
Figure 3.
Sensitivity to foot shock in NT4−/− and wt type mice. The sensitivity to foot shock was determined by assessing the amount of current required to elicit three stereotypical responses in NT4−/− and wt mice. Eight mice of each genotype were tested, and there was no difference between NT4−/− and wt type mice (P > 0.25). The level of current required to cause vocal responses from NT4−/− mice was lower than that of wt mice but did not reach statistical significance (P = 0.051).
Normal Basal Synaptic Transmission and Short-Term Plasticity in NT4−/− Mice.
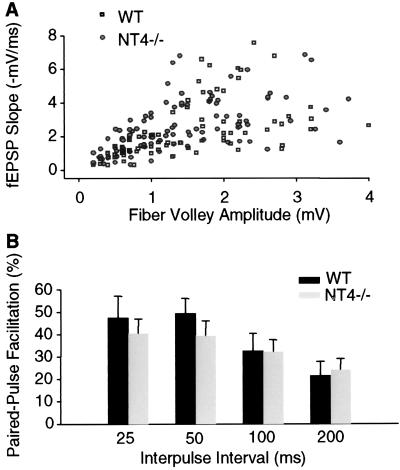
To study the function of NT4 further, electrophysiological analysis was performed in hippocampal slices. We first examined basal excitatory transmission in area CA1 of the hippocampus by using field potential recordings. No marked difference in the input–output coupling during Schaffer collateral stimulation was observed between slices from mutant and wt mice (Fig. 4A). The ratio of fEPSP slopes to presynaptic fiber volley sizes, determined at 50% maximum slope level, was 1.90 ± 0.26 ms−1 in NT4−/− mice (n = 13 slices; eight mice), which was not significantly different from that in wt (1.67 ± 0.24 ms−1; n = 9 slices; eight mice; P = 0.38).
Figure 4.
Basal synaptic transmission and PPF are normal in area CA1 of hippocampal slices from NT4−/− mice. (A) The scatter plot of fEPSP slopes vs. fiber volley amplitudes during Schaffer collateral stimulation. No marked difference was shown in the distribution of values obtained from mutants (n = 13 slices; eight mice) and wt mice (n = 9 slices; eight mice). (B) PPF was calculated as percentage of increase of the second fEPSP slope from the first one. No significant difference was detected between the mutants and wt mice for the facilitation measured at indicated interpulse intervals (n = 9 slices; six mice for each group; P > 0.30 for all comparisons).
We next examined PPF, a short-term synaptic plasticity caused by presynaptic enhancement of neurotransmission (ref. 53; Fig. 4B). At interpulse intervals of 25, 50, 100, and 200 ms, the PPF of fEPSP slopes in NT4−/− mice was 40 ± 6%, 39 ± 7%, 32 ± 5%, and 24 ± 5%, respectively (n = 9 slices; six mice). These results were not significantly different from the PPF in wt mice: 47 ± 10%, 49 ± 7%, 33 ± 8%, and 22 ± 6% at corresponding interpulse intervals (n = 9 slices; six mice; P > 0.30 for all comparisons). Thus, neither basal synaptic function nor short-term synaptic plasticity seemed significantly perturbed at the Schaffer collateral-CA1 synapses of NT4 mutant mice.
Impaired Hippocampal L-LTP but Not D-LTP in NT4−/− Mice.
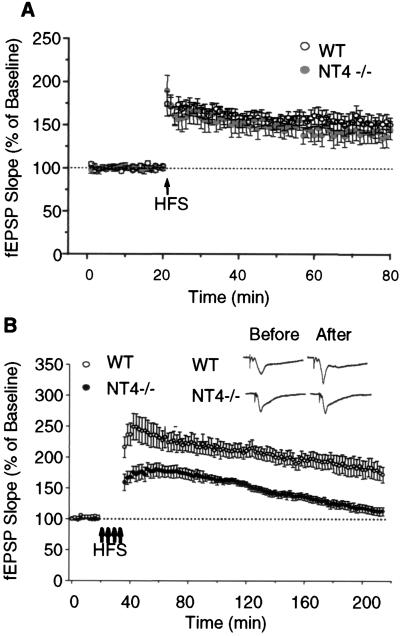
The role of NT4 in long-term synaptic plasticity was investigated by using two different high-frequency stimulation paradigms. First, a single high-frequency stimulation (100 Hz; 1 s) was applied to induce the D-LTP at CA1 synapses. As shown in Fig. 5A, the D-LTP of knockouts was not significantly different from that of the wt mice. The potentiation immediately, 30 min, and 60 min posttetanus was 174 ± 14%, 152 ± 7%, and 150 ± 8% of the baseline for wt (n = 12 slices; 10 mice) and 189 ± 18%, 150 ± 9%, and 144 ± 11% of the baseline for mutants (n = 11 slices; 8 mice), respectively (P > 0.05 for all comparisons).
Figure 5.
NT4 mutation attenuates the L-LTP but not D-LTP in area CA1. (A) D-LTP induced by single high-frequency stimulation (HFS; 100 Hz; 1 s). No significant difference was observed between the wt (n = 12 slices; 10 mice) and mutant mice (n = 11 slices; 8 mice) at all time points tested. (B) L-LTP induced by four stimulus trains (100 Hz; 1 s) at 5-min intervals. Note the wt mice (n = 8 slices; six mice) showed robust and lasting LTP over a period of 3 h. In contrast, the mutant mice (n = 7 slices; seven mice) displayed a continuously decaying potentiation after receiving the same tetanization (P < 0.05 for all time points tested). (Insets) Sample fEPSP traces recorded from area CA1 of wt and mutant slices before and 3 h after four stimulus trains. (Bars = 2 mV and 10 ms.)
Another stimulation paradigm, consisting of four consecutive high-frequency trains (100 Hz; 1 s) with 5-min intertrain intervals, was applied to induce L-LTP at CA1 synapses. As demonstrated in Fig. 5B, the wt mice (n = 8 slices; six mice) showed robust and lasting LTP over a period of 3 h: 232 ± 17%, 218 ± 14%, 190 ± 10%, and 172 ± 12% at 30, 60, 120, and 180 min posttetanus, respectively. In contrast, the mutant mice (n = 7 slices; seven mice) displayed a reduced and continuously decaying potentiation after receiving the same tetanization. The corresponding LTP values for mutant mice were 176 ± 8%, 167 ± 4%, 136 ± 6%, and 112 ± 7%, significantly lower than those for the wt (P < 0.05 for all comparisons). These results show that NT4 mutation selectively impairs L-LTP but not D-LTP in area CA1. The observation that D-LTP induced by one stimulus train is not significantly altered in NT4−/− mice, whereas the early component of L-LTP is reduced, supports the notion that L-LTP recruits signaling pathways distinct from those for D-LTP immediately after tetanization (54) and suggests that NT4 signaling is essential for the expression of normal L-LTP.
Discussion
Using fear conditioning, we demonstrate herein that lack of endogenous NT4 severely hampers the hippocampus- and amygdala-dependent long-term memory (2 h and 24 h) but does not affect the learning and short-term memory (30 min) in inbred 129/SvJ mice. Our electrophysiological analysis shows that, in the hippocampal CA1 region of adult NT4−/− mice, basal synaptic transmission, PPF, and D-LTP are normal, but the L-LTP is impaired significantly. These data suggest that NT4 plays an essential role in the formation of long-term memory and underlying long-term synaptic plasticity of adult brain. A critical aspect of our data is that NT4 deficiency selectively impairs long-term memory and L-LTP in the hippocampus of adult mice, suggesting that the observed deficits are likely to be functional rather than developmental. In addition, our control experiments show that the behavioral deficits of NT4−/− mice are not due to motor, sensory, motivational, or attention abnormalities.
NT4 Deficiency Disrupts Memory Consolidation in Hippocampus and Amygdala.
Amygdala and hippocampus have been shown to be the key brain regions underlying fear conditioning (51, 55). Although high concentrations of neurotrophins (56) are present in amygdala, their function has not been addressed previously. The present study demonstrates deficits in the long-term memory for both contextual and auditory fear conditioning in NT4 mutants, indicating that NT4 deficiency could disrupt memory consolidation in both hippocampus and amygdala. Thus, our data provide evidence for involvement of the neurotrophin in amygdala-dependent memory.
It is possible that endogenous NT4 is critically involved in activity-dependent, associative long-term synaptic plasticity in fear-conditioning circuits. If the associative plasticity occurs in brain structures afferent to the amygdala such as the hippocampus, attenuation of hippocampal L-LTP, which weakens the hippocampal output to the amygdala, could be attributed to the deficits in contextual fear conditioning seen in NT4 mutants. The concurrent deficits in both contextual and auditory fear conditioning in NT4−/− mice suggest that the synaptic plasticity within the amygdala is likely to be altered by NT4 deficiency as well. Considerable progress has been made in recent years with regards to the relationship between fear conditioning and long-term synaptic plasticity in the amygdala. It has been shown that LTP can be induced in the basolateral amygdala in vivo by high-frequency stimulation of excitatory afferent from hippocampus (57). LTP induction in the MG-LA (lateral nucleus of amygdala) pathway enhances auditory conditioning-evoked responses (58), and reciprocally, fear conditioning induces LTP-like increases in synaptic transmission in this pathway (59). It is therefore plausible that failure of induction or maintenance of L-LTP-like changes at amygdala synapses contributes to the impairment of long-term memory for fear conditioning in NT4 knockouts. Further studies will be needed to test this possibility.
Do Different Neurotrophins Play Different Roles in Hippocampus-Dependent Learning and Memory?
It is well documented that the different neurotrophins have specific roles in the survival of various types of neurons (39, 40), differentiation of the pyramidal neurons in the visual cortex (60, 61), and ocular dominance column formation (62). Little is known about whether different neurotrophins play different roles in learning and memory formation. Behavioral analyses have been reported on only two neurotrophin heterozygous mutant strains, NGF+/− and BDNF+/− mice (41, 44), and a neurotrophin receptor mutant strain, p75−/− mice (63). All three strains of mice have significant deficits of learning in the water maze test. In addition to learning, p75−/− mice have deficits in inhibitory avoidance and motor activity, and NGF+/− mice display significant deficits in memory retention. The significant neuronal loss in the central nervous system of p75−/− and NGF+/− mice made it difficult to assess the precise role of these two molecules in learning and memory. In contrast to p75 and NGF+/− mice and similar to BDNF+/− mice, NT4−/− mice do not have detectable central nervous system neuronal reduction (45, 46). We show herein that NT4−/− mice are, to our knowledge, the only neurotrophin mutant mice that have normal learning and short-term memory in the fear-conditioning test. Moreover, opposite of the effect of BDNF in memory retention, NT4 affects the retention of long-term memory. Our data suggest that the function of NT4 may be complementary to BDNF in learning and memory formation. It would be interesting to study mice that have hippocampal-specific gene knockout of trkB, the receptor for both BDNF and NT4. If trkB is responsible for both BDNF and NT4 signaling, such knockout mice would have deficits in both learning and memory retention. During the review of this paper, Minichiello and colleagues (64) produced such mice. They showed that inactivation of trkB in the forebrain of mice hampers space learning. The effect of trkB on memory retention remains to be determined.
Previous electrophysiological studies regarding the effect of neurotrophins in the hippocampus have focused on BDNF and NT3. As mentioned in the introduction, a reduced dose of NT3 only selectively impairs short-term plasticity in the dentate gyrus (33), whereas deficiency of BDNF results in severely diminished D-LTP and L-LTP in the CA1 and CA3 regions (29–31). We show herein that the deficits in synaptic function of NT4−/− mice differ from those previously observed in BDNF−/− mice in several aspects, although they both are the ligands of trkB. First, at the Schaffer collateral-CA1 synapses, the basal synaptic transmission and short-term synaptic plasticity measured by PPF remain normal in NT4 mutants, whereas BDNF mutants display reduced basal transmission and PPF (30). Second, the NT4 mutation selectively impairs the L-LTP induced by multiple high-frequency trains but has little effect on the D-LTP induced by a single train. In contrast, both D-LTP and L-LTP are hampered in BDNF mutants (29–31). Thus, the function of endogenous NT4 at hippocampal synapses seems to be more selective, focusing on the long-lasting form of LTP. These data are consistent with our behavioral analysis and suggest that different neurotrophins may play different roles in hippocampus-dependent learning and memory.
Possible Underlying Mechanisms for Synaptic Plasticity Deficits in NT4 Mutants.
Our findings raise an interesting question as to how NT4 and BDNF, both of which bind and activate trkB receptors, could have differential effects in the hippocampus. One explanation is that BDNF may have a more prominent influence than does NT4 on formation and refinement of synapses in the hippocampus, such that loss of BDNF causes more extensive deficits in synaptic function. Alternatively, NT4 and BDNF interact differentially with trkB to activate distinct signaling pathways and therefore affect different aspects of synaptic function. In fact, the differential activation of trkB by BDNF and NT4 has been demonstrated recently in mice carrying point mutations in trkB that cause loss of NT4 signaling without major effects on BDNF responses (65). It is possible that different neurotrophins, NT4, BDNF, and NT3, each have their own specific action in modulating synaptic function. These differential actions may work in tandem to maintain normal synaptic transmission and plasticity in adult hippocampus and may contribute to hippocampus-dependent learning and memory processes.
It is well established that hippocampal L-LTP and long-term memory share a common requirement for gene transcription and protein synthesis (66, 67). The correlative impairment of both L-LTP and long-term memory in NT4 mutant mice suggests that loss of NT4/trkB signaling may compromise the synthesis of new proteins that are crucial for long-term information storage. Indeed, the deficits in L-LTP of NT4−/− mice are similar to what is observed with protein synthesis inhibitor-treated hippocampal slices (66, 68).
Finally, our studies suggest that drugs that act as NT4 mimetics may be attractive candidates for the therapy of acquired disorders of learning and memory caused by abnormal neuronal function, especially if there are defects in consolidation of short-term into long-term memories.
Acknowledgments
This work is supported by National Institutes of Health Grants DA08571 and DA05010 (to C.-W.X.), as well as grants from UCLA Alzheimer's Disease Center (to C.-W.X.), UCLA School of Medicine (to D.S.), the Kolliner Foundation (to X.L.), and UCLA Center for Aging (to X.L.).
Abbreviations
- LTP
long-term potentiation
- D-LTP
decremental LTP
- L-LTP
long-lasting LTP
- wt
wild-type
- CS
conditioned stimulus
- fEPSP
field excitatory postsynaptic potential
- PPF
paired-pulse facilitation
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140204597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140204597
References
- 1.Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y A. Nature (London) 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 2.Hohn A, Leibrock J, Bailey K, Barde Y A. Nature (London) 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- 3.Jones K R, Reichardt L F. Proc Natl Acad Sci USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisonpierre P C, Belluscio L, Squinto S, Ip N Y, Furth M E, Lindsay R M, Yancopoulos G D. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- 5.Berkemeier L R, Winslow J W, Kaplan D R, Nikolics K, Goeddel D V, Rosenthal A. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- 6.Ip N Y, Ibaaanez C F, Nye S H, McClain J, Jones P F, Gies D R, Belluscio L, Le Beau M M, Espinosa R d, Squinto S P, et al. Proc Natl Acad Sci USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibaanez C F. J Neurobiol. 1994;25:1349–1361. doi: 10.1002/neu.480251104. [DOI] [PubMed] [Google Scholar]
- 8.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 9.Barbacid M. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 10.Barbacid M. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 11.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Nanduri V, Jing S A, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones K R, Reichardt L F, Barbacid M. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soppet D, Escandon E, Maragos J, Middlemas D S, Reid S W, Blair J, Burton L E, Stanton B R, Kaplan D R, Hunter T, et al. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 14.Squinto S P, Stitt T N, Aldrich T H, Davis S, Bianco S M, Radziejewski C, Glass D J, Masiakowski P, Furth M E, Valenzuela D M, et al. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- 15.Chao M V, Bothwell M A, Ross A H, Koprowski H, Lanahan A A, Buck C R, Sehgal A. Science. 1986;232:518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- 16.Chao M V. J Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- 17.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 18.Lu B, Figurov A. Rev Neurosci. 1997;8:1–12. doi: 10.1515/revneuro.1997.8.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Fitzsimonds R M, Poo M M. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 20.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 21.Lohof A M, Ip N Y, Poo M M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 22.Stoop R, Poo M M. Prog Brain Res. 1996;109:359–364. doi: 10.1016/s0079-6123(08)62118-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang X H, Poo M M. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- 24.Lessmann V, Gottmann K, Heumann R. NeuroReport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 25.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y X, Zhang Y, Lester H A, Schuman E M, Davidson N. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 28.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 29.Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 31.Korte M, Kang H, Bonhoeffer T, Schuman E. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 32.Kang H, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 33.Kokaia M, Asztely F, Olofsdotter K, Sindreu C B, Kullmann D M, Lindvall O. J Neurosci. 1998;18:8730–8739. doi: 10.1523/JNEUROSCI.18-21-08730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer W, Bjeorklund A, Chen K, Gage F H. J Neurosci. 1991;11:1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer W, Sirevaag A, Wiegand S J, Lindsay R M, Bjeorklund A. Proc Natl Acad Sci USA. 1994;91:8607–8611. doi: 10.1073/pnas.91.18.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez H, Miranda M I, Bermúdez-Rattoni F. J Neurosci. 1997;17:3796–3803. doi: 10.1523/JNEUROSCI.17-10-03796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messaoudi E, Bârdsen K, Srebro B, Bramham C R. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- 39.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 40.Conover J C, Yancopoulos G D. Rev Neurosci. 1997;8:13–27. doi: 10.1515/revneuro.1997.8.1.13. [DOI] [PubMed] [Google Scholar]
- 41.Linnarsson S, Bjeorklund A, Ernfors P. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 42.Ernfors P, Lee K F, Jaenisch R. Nature (London) 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 43.Jones K R, Farianas I, Backus C, Reichardt L F. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen K S, Nishimura M C, Armanini M P, Crowley C, Spencer S D, Phillips H S. J Neurosci. 1997;17:7288–7296. doi: 10.1523/JNEUROSCI.17-19-07288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conover J C, Erickson J T, Katz D M, Bianchi L M, Poueymirou W T, McClain J, Pan L, Helgren M, Ip N Y, Boland P, et al. Nature (London) 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Ernfors P, Wu H, Jaenisch R. Nature (London) 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- 47.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 48.Holland P C, Bouton M E. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 49.Crawley J N, Belknap J K, Collins A, Crabbe J C, Frankel W, Henderson N, Hitzemann R J, Maxson S C, Miner L L, Silva A J, et al. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 50.Kim J J, Fanselow M S. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 51.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 52.Fanselow M S, Bolles R C. J Comp Physiol Psychol. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 53.Katz B, Miledi R. J Physiol (Paris) 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abel T, Nguyen P V, Barad M, Deuel T A, Kandel E R, Bourtchouladze R. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 55.LeDoux J E, Cicchetti P, Xagoraris A, Romanski L M. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conner J M, Lauterborn J C, Yan Q, Gall C M, Varon S. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maren S, Fanselow M S. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogan M T, LeDoux J E. Neuron. 1995;15:127–136. doi: 10.1016/0896-6273(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 59.Rogan M T, Steaubli U V, LeDoux J E. Nature (London) 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 60.McAllister A K, Lo D C, Katz L C. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 61.McAllister A K, Katz L C, Lo D C. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 62.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 63.Peterson D A, Dickinson-Anson H A, Leppert J T, Lee K F, Gage F H. J Comp Neurol. 1999;404:1–20. doi: 10.1002/(sici)1096-9861(19990201)404:1<1::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 64.Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V, Rossi-Arnaud C, Lipp H P, Bonhoeffer T, Klein R. Neuron. 1999;24:401–414. doi: 10.1016/s0896-6273(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 65.Minichiello L, Casagranda F, Tatche R S, Stucky C L, Postigo A, Lewin G R, Davies A M, Klein R. Neuron. 1998;21:335–345. doi: 10.1016/s0896-6273(00)80543-7. [DOI] [PubMed] [Google Scholar]
- 66.Frey U, Krug M, Reymann K G, Matthies H. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen P V, Abel T, Kandel E R. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 68.Otani S, Marshall C J, Tate W P, Goddard G V, Abraham W C. Neuroscience. 1989;28:519–526. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]