Abstract
CLPG1, an endopolygalacturonase (endoPG) gene of Colletotrichum lindemuthianum, was transferred to tobacco (Nicotiana tabacum) leaves by using the Agrobacterium tumefaciens transient delivery system. The following four constructs were prepared: CLPG1, with or without its signal peptide (SP; PG1, PG1ΔSP); CLPG1 with the tobacco expansin1 SP instead of its own SP (Exp::PG1ΔSP); and a mutated version of the latter on two amino acids potentially involved in the catalytic site of CLPG1 (D202N/D203N). Chlorotic and necrotic lesions appeared 5 to 7 d postinfiltration, exclusively in response to CLPG1 fused to the expansin SP. The lesions were correlated to the production of an active enzyme. Necrosis-inducing activity, as well as endoPG activity, were completely abolished by site-directed mutagenesis. Ultrastructural immunocytolocalization experiments indicated that the expansin SP addressed CLPG1 to the cell wall. Staining of parenchyma cells revealed the progressive degradation of pectic material in junction zones and middle lamella as a function of time after infiltration, ultimately leading to cell separation. A 30% decrease in the GalUA content of the cell walls was simultaneously recorded, thereby confirming the hydrolytic effect of CLPG1 on pectic polysaccharides, in planta. The elicitor activity of CLPG1 was further illustrated by the induction of defense responses comprising active oxygen species and β-1,3-glucanase activity, before leaf necrosis. Altogether, the data demonstrate that an appropriate SP and a functional catalytic site are required for the proper expression and elicitor activity of the fungal endoPG CLPG1 in tobacco.
Endopolygalacturonases (endoPGs) are a class of pectinases that participate in the degradation of plant cell walls by catalyzing the hydrolysis of the homogalacturonan domain of pectic polysaccharides, a linear chain of α-1,4-linked galacturonosyl residues. Depending on the source of enzyme, the degradation proceeds either by a strict endo-mode or by an endo-/exo-mode of cleavage (Cook et al., 1999). The released pectic fragments are mostly composed of linear oligogalacturonides (OGAs). The extent of degradation varies according to the level of methylesterification of the α-d,1-4-linked GalUA residues that compose the linear homogalacturonan chain because endoPGs only cleave unesterified rows of GalUA. Degradation can also be controlled by the presence of polygalacturonase-inhibiting proteins (PGIPs), a class of Leu-rich repeat proteins found in the cell walls of many plants.
The OGAs released by cleavage of homogalacturonan were the first oligosaccharins, that is biologically active oligosaccharides (Darvill et al., 1992), that were isolated from commercial pectin and plant cell walls. OGA-induced responses have been reviewed extensively (Côté and Hahn, 1994; Ridley et al., 2001). The likelihood that such fragments elicit defense mechanisms when produced in planta is based on their effects when they are externally supplied to plant cells and tissues. Thus, early responses such as plasma membrane depolarization, ion fluxes, and cytosol acidification, are induced in suspension-cultured cells after treatment with OGAs (Mathieu et al., 1991). It has also been reported that active oxygen species (AOS) are produced very rapidly after OGAs are supplied to plant cells and seedlings (Legendre et al., 1993; Lee et al., 1999; Orozco-Cardenas and Ryan, 1999).
However, there is no direct evidence that endoPGs elicit defense responses via the release of pectic fragments when expressed in planta. For example, recent reports indicate that the catalytic activity of xylanase, another cell wall-degrading enzyme, is not required for its ability to elicit ethylene synthesis (Enkerli et al., 1999; Furman-Matarasso et al., 1999). In a previous work, we showed that the CLPG1 endoPG of Colletotrichum lindemuthianum, a fungal pathogen of French bean (Phaseolus vulgaris), elicits defense responses when supplied to French bean seedlings (Lafitte et al., 1993) and that this effect is mimicked by the pectic fragments recovered upon hydrolysis of the host cell walls (Boudart et al., 1998). To probe the function of this endoPG in planta, the Agrobacterium tumefaciens transient expression system was retained to deliver CLPG1 to plant tissues. Agro-infiltration has proved a powerful tool to study the effects of microbial molecules on plant tissues, most notably fungal (Van der Hoorn et al., 2000), bacterial (Van den Ackerveken et al., 1996; Rathjen et al., 1999), and viral (Bendahmane et al., 2000) avirulence proteins. In the present study, various constructs of CLPG1-cDNA were prepared. Among them, a mutated version of CLPG1 was obtained by site-directed mutagenesis on two Asp residues potentially involved in the catalytic site of the enzyme, according to their alignment with catalytic residues of other microbial endoPGs. The effects resulting in expressing an active endoPG, in planta, on defense responses and cell wall structure are reported.
RESULTS
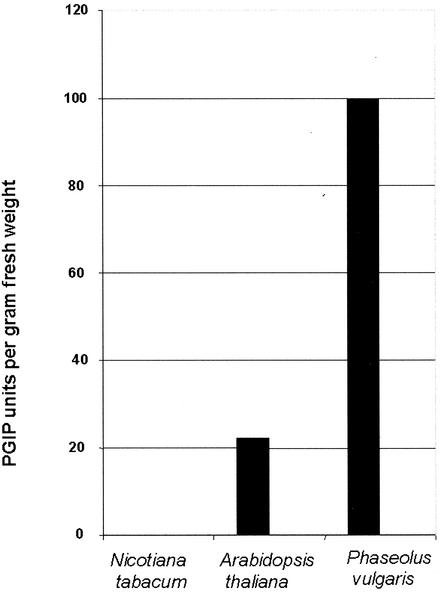
The endoPG-CLPG1 of C. lindemuthianum is a potent elicitor of defense responses in French bean, the host plant of this fungus, and in non-host plants. To assess the effects of its expression in planta, tobacco (Nicotiana tabacum) was retained as a recipient plant because of the feasibility of agro-infiltrating tobacco tissues, and of the lack of polygalacturonase inhibitory activity (PGIP) against CLPG1 in this plant, as preliminary checked according to Lafitte et al. (1984). As shown in Figure 1, the level of PGIP was high in French bean, much lower in Arabidopsis, and undetectable in tobacco.
Figure 1.
Measurement of PGIP activity against CLPG1 in protein extracts from tobacco cv Samsun NN, Arabidopsis (ecotype Columbia), and French bean seedlings. The experiment was repeated two times with the same results.
Agro-Infiltration of Leaf Tissues with CLPG1 Induces Necrosis in Tobacco
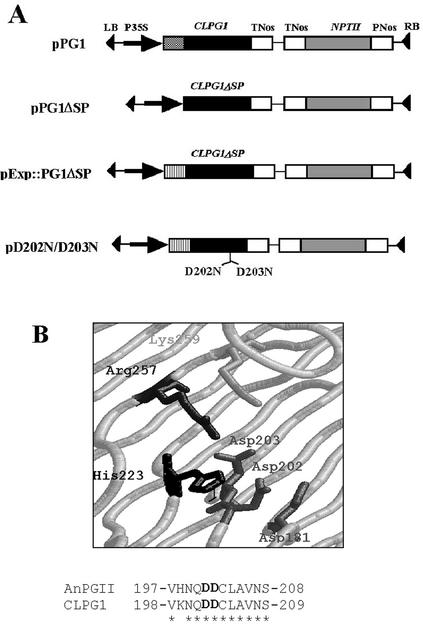
CLPG1 encodes a 363-amino acid proprotein beginning with a 26-amino acid signal peptide (SP) at the N terminus (Centis et al., 1996). To transiently express CLPG1 in tobacco mesophyll cells, the CLPG1 coding sequence was fused, with or without its SP coding sequence, to the cauliflower mosaic virus 35S promoter (constructs pPG1 and pPG1ΔSP; Fig. 2). Two additional constructs in which the SP of the cell wall protein expansin replaced the SP of CLPG1 coding sequence were obtained (pExp::PG1ΔSP; pD202N/D203N; Fig. 2). In the latter, two adjacent aspartic residues that have been shown to be crucial for enzymatic activity of fungal endoPGs (Armand et al., 2000) were mutated to Asn in pExp::PG1ΔSP (Fig. 2).
Figure 2.
A, Structure of T-DNA constructs transferred to plant cells via A. tumefaciens. Theed into the pIPM0 binary vector with (pPG1) or without (pPG1ΔSP) its own SP, or with the SP of the tobacco expansin1 gene (pExp::PG1ΔSP). An additional construct was obtained from pExp::PG1ΔSP by site-directed mutagenesis of Asp-202 and Asp-203 codons putatively involved in the catalytic site of the enzyme, giving rise to two Asn (pD202N/D203N). Each construct was under the control of cauliflower mosaic virus 35S promoter and of the nopaline synthase terminator (tNos). B, Three-dimensional representation of the putative catalytic site of CLPG1, as modelized by analogy with AnPGII endoPG of Aspergillus niger (van Santen et al., 1999) using the SWISS-MODEL software (http://swissmodel.expasy.org/; Guex and Peitsch, 1999). The amino acid sequences of the catalytic site of AnPGII endoPG (upper line) and of CLPG1 (lower line) are represented (amino acid identity shown by asterisks).
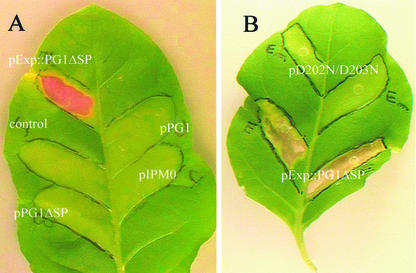
The infiltration of tobacco leaves with agrobacteria carrying the construct pExp::PG1ΔSP resulted in a severe necrosis of the infiltrated area (Fig. 3A). The first symptoms progressively appeared 4 to 5 d postinfiltration (dpi) as slightly discolored zones with few localized necrotic spots. One to 2 d later, necrotic lesions started to spread throughout the entire infiltrated area. In contrast, infiltration with pPG1ΔSP, pPG1, or the empty vector pIPM0 did not result in any symptom (Fig. 3A), even 4 weeks postinfiltration.
Figure 3.
Symptoms induced in tobacco leaves infiltrated with A. tumefaciens carrying: A, pPG1, pPG1ΔSP, pExp::PG1ΔSP, or the empty vector; and B, pExp::PG1ΔSP or pD202N/D203N. Leaves were also infiltrated with infiltration medium (10 mm MgSO4 + 250 μm acetosyringone) as a control. The infiltration area was outlined with a black marker pen. Leaves were photographed 2 weeks postinfiltration, using a CCD-IRIS color video camera. Necrosis was only observed upon A. tumefaciens transformation with pExp::PG1ΔSP.
The necrotic effect of Exp::PG1ΔSP was abolished when D202 and D203 were mutated to N, thereby indicating that these two aspartic residues are essential for CLPG1 effect (Fig. 3B).
EndoPG Activity Is Only Recorded in Tobacco Leaves Agro-Infiltrated with pExp::PG1ΔSP
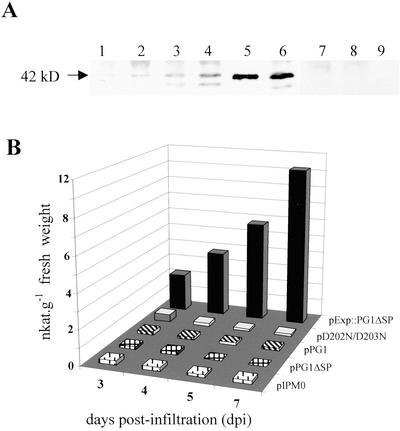
Western-blot experiments were carried out to look for the presence of translation products in tobacco leaves infiltrated with agrobacteria carrying the various constructs (Fig. 4A). A major protein band, with the same apparent molecular mass as the pure CLPG1 (42 kD), was revealed with the antiserum against CLPG1 in leaf extracts agro-infiltrated with pExp::PG1ΔSP. The 42-kD protein was already detectable 3 to 4 dpi. Expression increased as a function of time and was also high in tissues transformed with D202N/D203N. An additional minor band was revealed, whose intensity might correspond to a processed or unglycosylated form of the protein, whereas no protein cross-reacting with the CLPG1 antiserum could be detected in leaf material transformed with pPG1, pPG1ΔSP, or the empty vector pIPM0.
Figure 4.
Time course measurement of endoPG in protein extracts prepared from whole leaves infiltrated with agrobacteria carrying either pPG1, pPG1ΔSP, pExp::PG1ΔSP, or the mutant pD202N/D203N. A, Western-blot analysis of protein extracts of tobacco leaves agro-infiltrated with pExp::PG1ΔSP (lanes 1–4), pD202N/D203N (lane 6), pPG1 (lane 7), pPG1ΔSP (lane 8), or pIPM0 (lane 9). Lanes 1 through 4 corresponded to 3, 4, 5, and 7 dpi, respectively, and lanes 6 through 9 to 7 dpi. Pure CLPG1 (42 kD) was in lane 5. Proteins were separated by SDS-PAGE on a 10% (w/v) polyacrylamide gel and blotted onto a nitrocellulose membrane. Proteins cross-reacting with a CLPG1 polyclonal antiserum were revealed with a secondary goat anti-rabbit antiserum conjugated to alkaline phosphatase. Alkaline phosphatase activity was revealed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. B, endoPG activity was colorimetrically assessed. The experiment was repeated three times with the same time course increase of endoPG activity upon infiltration with pExp::CLPG1ΔSP.
A time course analysis of endoPG activity was simultaneously performed on the same tissues. Figure 4B shows that the enzyme activity was only expressed in the leaves infiltrated with pExp::PG1ΔSP, and increased linearly during the 1st week postinfiltration, where it reaches values as high as 12 nanokatals (nkat) g fresh weight−1. Comparatively, only basal amounts of endoPG activity were measured in leaf tissues agro-infiltrated with pPG1, pPG1ΔSP, the empty vector control, or the mutant pD202N/D203N. Interestingly, the protein produced by this mutant was inactive, as expected from its structure. The above results demonstrate that an appropriate SP and a functional catalytic site are required for the proper expression and activity of CLPG1 in planta.
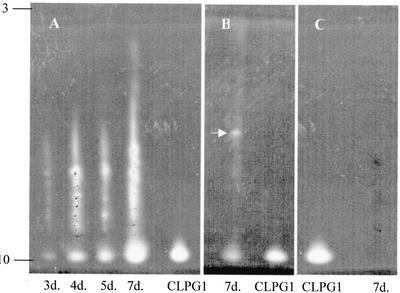
To further characterize the enzyme that was expressed in planta from pExp::PG1ΔSP, a zymogram of endoPG was performed after isoelectric focusing of the proteins extracted from the agro-infiltrated leaves, as a function of time. As shown on Figure 5A, a main spot of increasing intensity, focusing with a similar pI as the pure CLPG1 (pI = 10.1) was observed as early as 3 d post-agro-infiltration. Because of the presence of freeze-dried insoluble material in the samples, most probably polysaccharides, part of the activity was not totally resolved, and appeared as a smear. An additional experiment in which this material was allowed to settle in the bottom of the tube before isoelectric focusing confirmed the presence of an active enzyme of the same pI as CLPG1. It also allowed the detection of an additional endoPG isoform focusing at a neutral or slightly acidic pI value (arrow, Fig. 5B). The fact that this isoform was absent in leaves infiltrated with pD202N/D203N (Fig. 5C) rules out the possibility that it originated from A. tumefaciens.
Figure 5.
Zymogram of endoPG activity in protein extracts recovered from tobacco leaves 3 to 7 dpi with A. tumefaciens carrying pExp::PG1ΔSP (A) and 7 dpi with A. tumefaciens carrying pD202N/D203N (C). endoPG activity was assayed by analytical isoelectric focusing. The active endoPG focused at a pI identical to pure CLPG1 protein (pI = 10.1). B, An additional endoPG isoform focusing at a neutral or slightly acidic pI value (arrow) was also revealed.
CLPG1 Is Localized in the Cell Wall of Tobacco Leaf Tissues Expressing Exp::PG1ΔSP
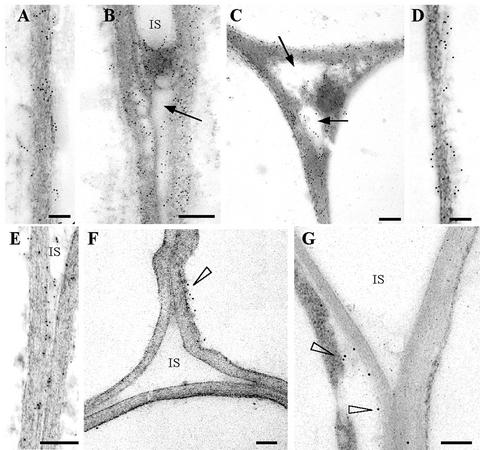
Immunogold labeling of tissues infiltrated with agrobacteria carrying pExp:: PG1ΔSP with an antiserum against CLPG1 showed the presence of numerous gold particles throughout the cell walls and intercellular spaces of the parenchyma cells (Fig. 6, A–D). There was no significant difference in labeling density between 4 (Fig. 6, A and B) and 7 (Fig. 6, C and D) dpi. Gold particles within the cell walls and intercellular spaces were also visible in tissues 4 d post-agro-infiltration with the mutant D202N/D203N (Fig. 6E). Very few gold particles were observed on ultrathin sections in the pIPM0 control, preferentially localized between the cell wall and cytoplasm (Fig. 6F).
Figure 6.
Immunogold labeling of leaf parenchyma cell walls 4 (A, B, and E) and 7 (C and D) d postinfiltration with A. tumefaciens carrying pExp::PG1ΔSP (A–D) or the mutant pD202N/D203N (E). Labeling was achieved with antiserum against CLPG1 and gold-conjugated goat antiserum to rabbit IgG. Gold particles were found within the cell walls (A and B) and in intercellular spaces (C and D) at nearly identical levels at 4 and 7 dpi. Note degradation of pectic material in intercellular spaces (IS; arrows). Gold particles were also observed within the cell walls and intercellular spaces 4 dpi with A. tumefaciens carrying the mutant pD202N/D203N (E), without detectable pectin degradation. A few gold particles were observed in leaves agro-infiltrated with the empty vector (cell wall-cytoplasm interface, arrowhead, F), and in sections treated with the secondary antibody alone (arrowheads, G). Sections were contrasted with uranyl acetate. Scale bar = 0.3 μm.
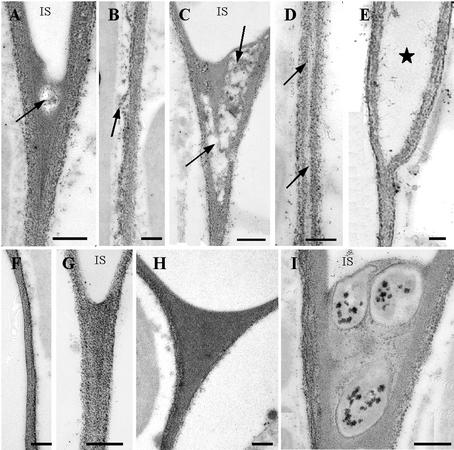
Staining of the parenchyma cell wall polysaccharides with the periodic acid-thiocarbohydrazide-silver proteinate (PATAg) reagent allowed the visualization of the effect of CLPG1 in planta. As shown in Figure 7, A through E, the cell walls were degraded in a progressive manner, beginning as small limited areas at the cell corner 4 dpi (Fig. 7A), and then extending to the whole intercellular space after 7 d (Fig. 7C). Moreover, the region corresponding to the middle lamella between two cells appeared less contrasted (Fig. 7D) as a result of pectic matrix degradation and progressive dissociation of adjacent cells (Fig. 7E). On the contrary, the cell walls of control, non-agro-infiltrated tissues (Fig. 7, F and G), or tissues agro-infiltrated with the mutant D202N/D203N (Fig. 7H) or the empty vector pIPM0 (Fig. 7I), remained evenly PATAg stained with no apparent cell wall degradation, despite the presence of the bacteria in the intercellular spaces (Fig. 7I).
Figure 7.
Electron micrographs of parenchyma cell walls stained with the PATAg reagent for polysaccharides visualization. A through E, Plant expressing Exp::PG1ΔSP: A, 4 dpi, the solubilization of cell wall components was limited to the cell corners (arrow); B, was also visible along the cell walls between two tricellular junctions; C, 7 dpi, cell wall degradation was clearly visible in the cell corner where large areas were cleared out (arrow); and D, in the middle lamella (arrow), ultimately leading to separation of the two walls (E). F and G, Control, non-agro-infiltrated tissues. H, Plants expressing D202N/D203N were heavily stained with no apparent alteration of the walls. I, Three bacteria in the corner of an intercellular space (IS). Note the absence of cell wall degradation in contact to bacteria. Bar = 0.3 μm.
Sugar Content of Cell Walls Is Modified in Tobacco Leaves Expressing Exp::PG1ΔSP
The GalUA content of the cell walls of tobacco leaves infiltrated with pExp::PG1ΔSP was reduced by 31% to 35% at 4 and 7 dpi, respectively (Table I). Simultaneously, a noticeable increase in the neutral sugars Rha, Ara, and to a lesser extent Gal and Xyl was also observed. Such an increase reflects a modification in the ratio between the sugars that compose cell wall polysaccharides because of an important release of GalUA from CLPG1-digested homogalacturonan domains of pectin. The decrease in GalUA did not vary much between 4 and 7 d, an effect that could be associated, at least in part, with the level of immunolocalized CLPG1 observed in infiltrated tissues (Fig. 6, A–E) whose maximum intensity was recorded around 4 dpi.
Table I.
Sugar composition of cell walls (mol % mg CW−1) from tobacco leaves infiltrated with A. tumefaciens carrying pExp::PG1ΔSP or with the infiltration medium (control)
| Sugars
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GalUA
|
Rha
|
Ara
|
Gal
|
Xyl
|
||||||
| 4 d | 7 d | 4 d | 7 d | 4 d | 7 d | 4 d | 7 d | 4 d | 7 d | |
| mol % | ||||||||||
| Control | 35.2 ± 0.1 | 36.9 ± 2.4 | 2.1 ± 0.2 | 2.1 ± 0.2 | 4.1 ± 0.7 | 3.5 ± 1 | 3.7 ± 0.2 | 4.6 ± 1 | 3.7 ± 1.2 | 3.7 ± 1.2 |
| pExp::CLPG1DSP | 24.1 ± 1.4 | 24.3 ± 2.5 | 3.8 ± 0.8 | 4.4 ± 1 | 7.2 ± 0.8 | 8.9 ± 0.6 | 7.2 ± 1.6 | 7.4 ± 0.7 | 5.7 ± 1 | 6.6 ± 1.8 |
Cell walls were extracted 4 and 7 d postinfiltration and hydrolyzed with 2 n trifluorhydric acid according to the procedure described in “Materials and Methods.” Sugar analysis was performed using high-performance anion-exchange chromatography-pulsed-amperometric detection chromatography (Dionex, Sunnyvale, CA).
A 31% to 35% decrease in GalUA content of cell walls was calculated when Exp::PG1ΔSP was expressed at 4 and 7 d postinfiltration, respectively. The total amount of GalUA and neutral sugars per mg cell wall varied from 284 mg and 304 mg in the control, to 259 mg and 293 mg when Exp::PG1ΔSP was expressed, at 4 and 7 d postinfiltration, respectively.
Expression of Exp::PG1ΔSP none in Planta Induces Defense Responses
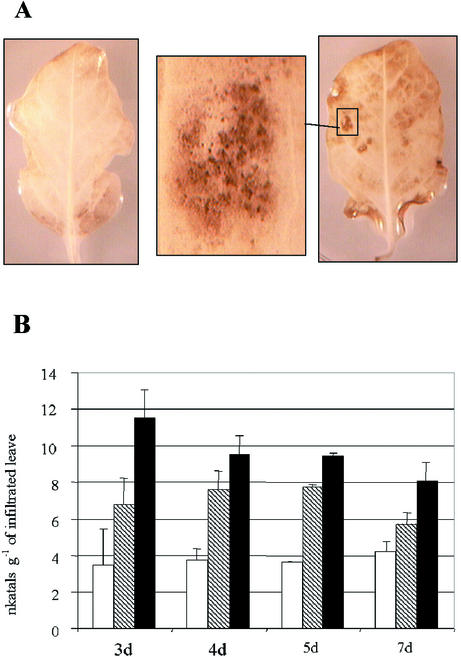
The manganese/diaminobenzidine (DAB) uptake method was used to follow the production of AOS as a function of time after agro-infiltration. The leaves expressing Exp::PG1ΔSP gradually appeared more intensely brown with time than those agro-infiltrated with the empty vector, as shown 5 dpi (Fig. 8A). AOS were clearly distinguishable as dark-brown deposits localized in areas of leaves not showing visible necrosis (Fig. 8A, insert) or surrounding small-sized necrosis at the beginning of the necrotic stage.
Figure 8.
AOS detection and time course measurement of β-1,3-glucanase activity in agro-infiltrated tobacco leaves. A, AOS were detected as dark-brown deposits (right, insert) 5 dpi in leaf tissues expressing Exp::PG1ΔSP using the DAB uptake method. AOS were not detected in leaves agro-infiltrated with the empty vector (left). B, Time course measurement of β-1,3-glucanase activity of tobacco leaves infiltrated with agrobacteria carrying either pExp::PG1ΔSP (black box) or the empty vector (hatched box). Control leaves (white box) were infiltrated with infiltration medium (10 mm MgSO4 + 250 μm acetosyringone). β-1,3-glucanase activity was colorimetrically assessed.
An early induction of β-1,3-glucanase activity was measured in response to the in planta expression of Exp::PG1ΔSP, with a maximum activity 3 dpi (Fig. 8B). Interestingly, significant amounts of β-1,3-glucanase activity were also detected in response to agrobacteria carrying the empty vector pIPM0, as compared with the MgSO4 control. Nevertheless, β-1,3-glucanase activity was comparatively produced in larger amounts when Exp::PG1ΔSP was expressed.
DISCUSSION
The A. tumefaciens transient expression system was used for the delivery and expression of a fungal endoPG in planta. Tobacco plants were retained as the recipient plant material. In contrast to French bean, the host plant of the fungal endoPG, tobacco cv Samsun NN, can be easily transformed and lacks detectable PGIP activity against CLPG1. This allowed to establish the conditions required for the production of an active CLPG1 protein, and to look for the intrinsic effects of this enzyme in absence of PGIP.
The nature of the SP appeared to be crucial for the proper expression of CLPG1. Although all constructs were driven by the 35S promoter to ensure sufficient expression, only those harboring the SP of expansin, a plant cell wall protein, were functional. Neither those containing nor lacking the SP of CLPG1 yielded detectable levels of protein or enzyme activity. The fact that CLPG1-SP allows the secretion of the enzyme in the fungus, but not when transferred to plant leaves, suggests that it is not functional in plants. A similar situation was already reported in Arabidopsis plants transformed with the cutinase cDNA of Fusarium solani (Sieber et al., 2000). In comparison, the SP of expansin proved to be the only one satisfactory, in that it allowed the targeting of CLPG1 to the plant cell wall.
Although both CLPG1 and the mutated version of CLPG1 were expressed to high levels, endoPG activity was only recorded in tissues agro-infiltrated with Exp::CLPG1ΔSP, thereby confirming that the two aspartic residues 202 and 203 are essential for the catalytic activity of the enzyme. This situation offered the possibility to compare the effects of the active and the inactive CLPG1 protein in tobacco tissues. A series of events, beginning with CLPG1 synthesis and activity 2 to 3 dpi (dpi), were recorded. They successively comprise cell wall degradation and β-1,3-glucanase activity induction (3–4 dpi), AOS production and chlorosis (5 dpi), and, finally, leaf necrosis (7 dpi). It was most noticeable that cell wall degradation preceded and was required for the oxidative burst and hypersensitive response-like necrosis because the inactive CLPG1 failed to elicit any response. This work also showed that cell death followed the onset of AOS production and was strictly associated to it.
The possibility that these responses were mediated by an endoPG-PGIP type of interaction was ruled out because of the absence of PGIP against this enzyme in tobacco. Instead, the accumulation of active enzyme and decrease in the GalUA content of the cell wall indicated that OGAs were released from the homogalacturonan domain of pectin. The elicitor effect of OGAs on various plant defense mechanisms (Ridley et al., 2001), notably of CLPG1-released OGAs (Boudart et al., 1998), suggests that they might mediate, at least in part, the responses reported in this work. This would imply that PGIP is not absolutely required for maintaining elicitation by OGAs. However, other mechanisms might also be involved because tobacco cv Samsun NN tissues infiltrated with tobacco cell wall-derived OGAs at 50 and 250 μg mL−1 did not develop necrotic lesions, contrasting with what was observed when CLPG1 was exogenously supplied (G. Boudart, unpublished data) or produced in planta (this work). The appearance of a novel endoPG of plant origin is but one example of additional host responses that might reinforce the effect of CLPG1. In addition, cell separation (Fig. 7E) can profoundly affect the mechanical strength of the cell wall and associated signaling phenomena. An unexpected perspective on the role of pectin in plant development was recently highlighted in transgenic apple (Malus domestica) trees overexpressing an endoPG (Atkinson et al., 2002). The expression of a fungal endoPG in plant tissues reported in this work provides a means to investigate such alterations.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum cv Samsun NN) was grown on vermiculite in a growth chamber at 75% hygrometry, with a photoperiod of 12 h light at 110 μE m−2 s−1 and 25°C, and 12 h dark at 22°C.
Construction of Binary Vectors with CLPG1 Cassettes
Recombinant plasmids were obtained by inserting various constructs of the CLPG1 encoding sequence into IPM0, a plasmid derived from the binary vector pBin19 (Rancé et al., 1998). The construct contained in pPG1 was based on the entire CLPG1 coding sequence that was amplified by PCR with Pyrococcus furiosus DNA polymerase (Promega, Madison, WI) using CLPG1 cDNA cloned in pGEM-T (Promega) as template. A KpnI site was introduced at the 5′ end of the forward primer (GGGGTACCATGGTCTCTTACCTCTTCGTGCTCGGC) and a XbaI site was introduced at the 5′ end of the reverse primer (GCTCTAGACGTAAAGACTCAGCCGCTTAGCAAGCA). The amplified 1.1-kb fragment was digested by KpnI and XbaI and inserted between the 35S promoter and the nopaline synthase terminator of IPM0 previously restricted with the same enzymes.
The construct contained in pPG1ΔSP was prepared by PCR amplification with P. furiosus DNA polymerase of the CLPG1 sequence encoding the mature endoPG. A BamHI site was introduced at the 5′ end of the forward primer (GGATCCAAGAAAGCCAGCTGCACCTTCACCGAT) and the SP6 oligonucleotide was used as the downstream primer. The amplified 1-kb fragment that corresponded to CLPG1 without its SP was cloned into pGEMT. The plasmid was amplified in Escherichia coli, purified, and digested with BamHI and SpeI. The restricted fragment was ligated as above to the pIPM0 binary vector previously digested with BamHI and XbaI.
In pExp::PG1ΔSP, the SP of CLPG1 was replaced by the SP of the tobacco expansin1 gene (Link and Cosgrove, 1998). The double-stranded DNA sequence of expansin1 SP was obtained by annealing the corresponding synthetic sense strand and its antisense complement (Isoprim, Toulouse, France). KpnI and BamHI restriction sites were created at the 5′ and 3′ end of each strand, respectively. The 0.1-kb double-stranded DNA was digested by KpnI and BamHI, ligated in a single step to PG1ΔSP, and inserted into pIPM0 previously restricted with BamHI and SpeI and with KpnI and XbaI, respectively.
A mutated version of the pExp::PG1ΔSP construct in which the amino acids D202 and D203 were mutated to N was prepared by a double PCR strategy according to a previously described protocol (Bowman et al., 1990). In brief, a first PCR was performed on pExp::ΔPG1 using an oligonucleotide primer containing the mutated codons (underlined with modified bases in bold; CAAGAACCAGAACAACTGCCTCGCCGT-3′) and a second primer complementary to a vector sequence downstream the pExp::PG1ΔSP insert (CAGCTATGACCATGATTACGC). After PCR amplification, the template DNA was digested by the DpnI endonuclease. An aliquot of the DpnI-digested PCR products was added to a second PCR reaction containing two primers complementary to sequences upstream (GGGGTACCAAGAAAATGGCCAACATTGGC) and downstream (GCTCTAGAC-GTAAAGACTCAGCCGCTTAGCAAGCA) of the DNA insert, respectively. The amplified product was digested by KpnI and XbaI and ligated to the binary pIPM0 vector previously restricted with the same enzymes giving rise to the plasmid D202N/D203N. The nucleotide sequence of each construct was checked, and the resulting recombinant plasmids were amplified in E. coli XL1 blue (Stratagene, La Jolla, CA) before being transferred into Agrobacterium tumefaciens.
Agro-Infiltration of Plant Tissues
Constructs in pIPM0 binary vector were introduced into A. tumefaciens strain LBA4404 by electroporation. A. tumefaciens cells were inoculated into 4 mL of yeast extract broth supplemented with 50 μg mL−1 kanamycin and 50 μg mL−1 streptomycin and grown at 28°C overnight with shaking (200 rpm). Cultures were diluted 1:100 (v/v) in 50 mL of fresh yeast extract broth plus antibiotics and grown for 2 d at 28°C. Cells were pelleted (15 min, 3,000g at 4°C) and washed twice with cold 10 mm MgSO4, and then resuspended to an OD600 of 1 (2 × 109 colony forming units mL−1) in cold 10 mm MgSO4 supplemented with 250 μm acetosyringone. Young expanding leaves of 6-week-old plants were pressure infiltrated with the bacterial suspension using a sterile 2-mL needleless syringe. The infiltrated area was immediately outlined with a marker pen. For experiments requiring plant extracts, the whole leaf was agro-infiltrated.
EndoPG Activity Assays
Infiltrated leaves were ground in liquid nitrogen with a mortar and pestle. The powder (1 g) was added to 3 mL of cold extraction buffer composed of 100 mm MOPS buffer (pH 6.7) containing 2 m NaCl, 10 mm dithiothreitol, 2 mm phenylmethylsulfonyl fluoride, and protease inhibitors (1% [v/v] standard cocktail, Sigma, St. Louis). The homogenate was let under constant stirring at 4°C for 1 h before being centrifuged at 2,500g for 30 min at 4°C. The proteins of the supernatants were precipitated by addition of solid ammonium sulfate up to 70% saturation, under constant stirring at 4°C overnight. The pellet recovered upon centrifugation at 13,500g for 10 min was resuspended in 250 μL of 1 m NaCl and the solubilized material was exhaustively dialyzed against 2% (v/v) glycerol. The dialyzed extract was then adjusted to 1.5 mL with 2% (v/v) glycerol and used as enzyme source for the measurement of endoPG activity by two different methods.
The colorimetric method allowed to assess endoPG activity by measuring the release of GalUA residues from polygalacturonic acid (PGA) (Sigma). The standard assay (1 mL) contained the enzyme extract (50 μL), 1 mg of PGA in 50 mm Na-acetate buffer pH 5.2 (500 μL), and 450 μL of the same buffer. After incubation for 2 h at 30°C, 2 volumes of absolute ethanol were added to the reaction mixture to precipitate the non-digested PGA. A control, in which the enzyme extract was omitted in the reaction mixture and only added after ethanol, was simultaneously performed. After vortexing, the precipitates were centrifuged at 13,500g for 10 min and the supernatant (200 μL) was analyzed for its uronic acid content at 520 nm by the Blumenkrantz-Asboe procedure (Blumenkrantz and Asboe-Hansen, 1973). EndoPG activity was expressed as nkat per gram fresh weight.
The endoPG activity was also assessed by analytical isoelectric focusing of the above protein extract. One milliliter of dialyzed extract was transferred into an Eppendorf tube (Eppendorf Scientific, Westbury, NY), rapidly frozen in liquid nitrogen, and freeze dried. The dry powder was resuspended in a minimal volume (100 μL) of a cold ampholine carrier solution (pH 3.5–10, 3% [w/v] in water, Pharmacia Biotech, Uppsala). A 15-μL aliquot was applied onto the surface of an Ampholine PAGplate gel (pH 3.5–10, Pharmacia) as small droplets (1–2 μL) width-wise at the cathode side. Isoelectric focusing was performed with a Multiphor apparatus coupled to an EPS 3501 XL power supply (Pharmacia). After focusing, the gel was rinsed with 50 mm Na-acetate buffer (pH 5.2) and incubated for 1 h at 30°C in the presence of PGA (0.2% [w/v] in the acetate buffer). The endoPG activity was then visualized in the gel with ruthenium red staining (0.025% [w/v] in water) according to Lisker and Retig (1974).
PGIP Assay
Crude protein extracts were prepared from tobacco cv Samsun NN, Arabidopsis (ecotype Columbia), and French bean (Phaseolus vulgaris cv P12R) seedlings. Plants were grown under the conditions described above, and were 6, 4, and 2 weeks old, respectively. The seedlings were rapidly frozen in liquid N2 and ground with a mortar and pestle. The powder (1 g) was suspended into 5 mL of cold extraction buffer (50 mm Na-acetate buffer [pH 5.2] and 1 m NaCl) and constantly stirred at 4°C for an hour. After centrifugation of the homogenates at 5,000g for 10 min at 4°C, the supernatants were recovered and exhaustively dialyzed against the same buffer without NaCl. Dialyzed extracts were adjusted to the same volume and assayed for PGIP activity against 0.25 nkat of the CLPG1 endoPG according to Lafitte et al. (1984). PGIP activity was expressed as units per gram fresh weight, one unit corresponding to the amount of PGIP required for reducing CLPG1 activity by 50%.
Western-Blot Analysis
Dialyzates (100 μL) were subjected to gel electrophoresis under denaturating conditions in a 10% (w/v) acrylamide gel. After migration, the proteins were electroblotted onto nitrocellulose transfer membranes by using a semidry apparatus (Bio-Rad Laboratories, Hercules, CA) at a constant current (1 mA cm2). Blots were soaked for 30 min in Tris-buffered saline (TBS) buffer (20 mm Tris-HCl [pH 7.5] and 150 mm NaCl) containing 5% (w/v) nonfat dried milk powder, and then incubated overnight in TBS-T20 (TBS and 0.05% [v/v] Tween 20) containing the CLPG1 antiserum (1:10,000 [v/v] dilution). After washing in TBS-T20, the blots were incubated for 4 h in TBS-T20 containing alkaline phosphatase-conjugated goat anti-rabbit IgE (1:5,000 [v/v] dilution). After washing in TBS-T20, the antigen-antibody complex was visualized using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Promega).
β-1,3-Glucanase Assay
β-1,3-glucanase activity was assessed by measuring the reducing sugars released from 1% (w/v) laminarin (Sigma) by incubation of 5 mg of laminarin with 25 μL of the dialyzed protein extract in 50 mm acetate buffer (pH 5.2) at 50°C for 30 min, according to a previously published procedure (Daugrois et al., 1992). The amount of reducing sugars was estimated by the Somogyi (1952) procedure. The β-1,3-glucanase activity was expressed as nkat per gram fresh weight.
Electron Microscopy and Immunolabeling
Small pieces of tissues were cut off from agro-infiltrated leaves and fixed in 0.5% (v/v) glutaraldehyde and 4.5% (v/v) paraformaldehyde in 50 mm Na-cacodylate buffer (pH 7.2) for 2 h at room temperature. The fixed samples were dehydrated in a graded aqueous ethanol series (20%, 40%, 60%, 75%, 80%, and 100% [v/v], two times for 15 min each step). They were then infiltrated in LR White resin according to the following schedule: 2:1; 1:1; and 1:2 (v/v) ethanol:LR White resin for 3 h each step, followed by 100% LR White overnight, and an additional 24 h with renewed LR White. The infiltrated samples were embedded in gelatin capsules and polymerized for 24 h at 60°C. For transmission electron microscopy, ultrathin sections (80 nm in thickness) were cut with a diamond knife on an UltracutE microtome (Leica, Rueil-Malmaison, France) and collected on gold grids.
For immunolabeling experiments, sections were first incubated in a blocking solution composed of 0.5% (w/v) bovine serum albumin in TBS plus Tween 20 (TBST; 50 mm Tris-HCl [pH 7.5], 150 mm NaCl, and 0.1% [v/v] Tween 20) for 30 min. After being blotted dry, the grids were incubated overnight with the primary rabbit CLPG1 polyclonal antiserum (Hugouvieux et al., 1995), diluted 1:100 (v/v) with TBST. The grids were then washed with TBST before being transferred into a droplet of goat anti-rabbit IgG conjugated with 10 nm of colloidal gold diluted 1:25 (v/v) in TBST for 2 h at room temperature. The sections were washed with TBST and finally with water. After immunolabeling, the sections were stained with 5% (w/v) uranyl acetate for 30 min. Control experiments were performed without primary antibody. Sections were labeled by PATAg for polysaccharide visualization (Thiéry, 1967). They were observed on an H600 electron microscope (Hitachi, Tokyo) at 75 kV.
In Planta Detection of AOS
Generation of 02− was detected in agro-infiltrated leaves using the DAB technique according to Lu and Higgins (1998). Agro-infiltrated leaves were harvested as a function of time after infiltration and vacuum infiltrated with a solution containing 0.2% (w/v) of 3,3′-diaminobenzidine tetrahydrochloride, 20 mm MnCl24H20, and 10 mm sodium azide. The leaves were transferred onto a wet filter paper in a petri dish and left in the dark at room temperature for 1 h. The leaves were boiled in 95% (v/v) ethanol until the chlorophyll content was completely removed, and stored in 95% (v/v) ethanol. Photographs were acquired using a CCD-IRIS color video camera (Sony, Japan).
Sugar Analysis
Agro-infiltrated leaves were ground in liquid N2; the whole recovered powder (about 2 g) was extracted twice in 40 mL of boiling aqueous ethanol (20:80 [v/v]). The insoluble residue was extracted successively with chloroform/methanol (1:1 [v/v]) and acetone. The recovered insoluble cell wall residue was dried at room temperature and then hydrolyzed in 2 n trifluorhydric acid at 120°C for 1 h. The glycosyl residue composition of the hydrolysate was determined by high-performance anion-exchange chromatography-pulsed-amperometric detection (Dionex) according to Boudart et al. (1998).
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011585.
LITERATURE CITED
- Armand S, Wagemaker MJ, Sanchez-Torres P, Kester HC, van Santen Y, Dijkstra BW, Visser J, Benen JA. The active site topology of Aspergillus niger endopolygalacturonase II as studied by site-directed mutagenesis. J Biol Chem. 2000;275:691–696. doi: 10.1074/jbc.275.1.691. [DOI] [PubMed] [Google Scholar]
- Atkinson RG, Schröder R, Hallett IC, Cohen D, MacRae EA. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002;129:122–133. doi: 10.1104/pp.010986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahmane A, Querci M, Kanyuka K, Baulcombe DC. Agrobacterium transient expression system as a tool for the isolation of disease resistance genes: application to the Rx2 locus in potato. Plant J. 2000;21:73–81. doi: 10.1046/j.1365-313x.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. A new method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Boudart G, Lafitte C, Barthe JP, Frasez D, Esquerré-Tugayé MT. Differential elicitation of defense responses by pectic fragments in bean seedlings. Planta. 1998;206:86–94. [Google Scholar]
- Bowman S, Tischfield JA, Stambrook PJ. An efficient and simplified method for producing site-directed mutations by PCR. J Methods Cell Mol Biol. 1990;2:254–260. [Google Scholar]
- Centis S, Dumas B, Fournier J, Marolda M, Esquerré-Tugayé MT. Isolation and sequence analysis of Clpg1, a gene coding for an endopolygalacturonase of the phytopathogenic fungus Colletotrichum lindemuthianum. Gene. 1996;170:125–129. doi: 10.1016/0378-1119(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Cook BJ, Clay RP, Bergmann CW, Albersheim P, Darvill AG. Fungal polygalacturonases exhibit different substrate degradation patterns and differ in their susceptibilities to polygalacturonase-inhibiting proteins. Mol Plant-Microbe Interact. 1999;12:703–711. doi: 10.1094/MPMI.1999.12.8.703. [DOI] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structure and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Darvill A, Augur C, Carlson RW, Cheong J, Eberhard S, Hahn MG, Lo VM, Marfa V, Meyer B, Mohnen D et al. Oligosaccharins: oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology. 1992;2:181–198. doi: 10.1093/glycob/2.3.181. [DOI] [PubMed] [Google Scholar]
- Daugrois JH, Lafitte C, Barthe JP, Faucher C, Touzé A, Esquerré-Tugayé MT. Purification and characterization of two basic β-1,3-glucanases induced in Colletotrichum lindemuthianum-infected bean seedlings. Arch Biochem Biophys. 1992;292:468–474. doi: 10.1016/0003-9861(92)90017-q. [DOI] [PubMed] [Google Scholar]
- Enkerli J, Felix G, Boller T. The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol. 1999;121:391–398. doi: 10.1104/pp.121.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman-Matarasso N, Cohen E, Du Q, Chejanovsky N, Hanania U, Avni A. A point mutation in the ethylene-inducing xylanase elicitor inhibits the β-1–4-endoxylanase activity but not the elicitation activity. Plant Physiol. 1999;121:345–351. doi: 10.1104/pp.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb viewer: an environment for protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hugouvieux V, Centis S, Lafitte C, Esquerré-Tugayé MT. Characterization of Colletotrichum lindemuthianum endopolygalacturonase with molecular probes. C R Acad Sci Paris. 1995;318:113–120. [Google Scholar]
- Lafitte C, Barthe JP, Gansel X, Dechamp-Guillaume G, Faucher C, Mazau D, Esquerré-Tugayé MT. Differential induction by endopolygalacturonase of β-1,3 glucanases in Phaseolus vulgaris isolines susceptible and resistant to Colletotrichum lindemuthianum race β. Mol Plant-Microbe Interact. 1993;6:628–634. [Google Scholar]
- Lafitte C, Barthe JP, Montillet JL, Touzé A. Glycoprotein inhibitors of Colletotrichum lindemuthianum endopolygalacturonase in near isogenic lines of Phaseolus vulgaris resistant and susceptible to anthracnose. Physiol Plant Pathol. 1984;25:39–53. [Google Scholar]
- Lee S, Choi H, Suh SS, Doo IS, Oh KY, Choi E, Schroeder-Taylor AT, Low PS, Lee Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999;121:147–152. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BM, Cosgrove DJ. Acid-growth response and α-expansins in suspension cultures of bright yellow 2 tobacco. Plant Physiol. 1998;118:907–916. doi: 10.1104/pp.118.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker N, Retig N. Detection of polygalacturonase and pectin lyase isoenzymes in polyacrylamide gels. J Chromatogr. 1974;96:245–249. doi: 10.1016/s0021-9673(00)98570-4. [DOI] [PubMed] [Google Scholar]
- Lu H, Higgins VJ. Measurement of active oxygen species generated in planta in response to elicitor AVR9 of Cladosporium fulvum. Physiol Mol Plant Pathol. 1998;52:35–51. [Google Scholar]
- Mathieu Y, Kurdjian A, Xia H, Guern J, Koller A, Spiro M-D, O'Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancé I, Fournier J, Esquerré-Tugayé MT. The incompatible interaction between Phytophthora parasitica var. nicotianae race 0 and tobacco is suppressed in transgenic plants expressing antisense lipoxygenase sequences. Proc Natl Acad Sci USA. 1998;95:6554–6559. doi: 10.1073/pnas.95.11.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen JP, Chang JH, Staskawicz BJ, Michelmore RW. Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J. 1999;18:3232–3240. doi: 10.1093/emboj/18.12.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, Metraux JP, Nawrath C. Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell. 2000;12:721–738. doi: 10.1105/tpc.12.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- Thiéry JP. Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microsc. 1967;6:987–1018. [Google Scholar]
- Van den Ackerveken G, Marois E, Bonas U. Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell. 1996;87:1307–1316. doi: 10.1016/s0092-8674(00)81825-5. [DOI] [PubMed] [Google Scholar]
- Van der Hoorn RAL, Laurent F, Roth R, De Wit JGM. Agroinfiltration is a versatile tool that facilitates comparative analysis of Avr9/Cf-9-induced and Avr4/Cf-4-induced necrosis. Mol Plant-Microbe Interact. 2000;13:439–446. doi: 10.1094/MPMI.2000.13.4.439. [DOI] [PubMed] [Google Scholar]
- van Santen Y, Benen JA, Schroter KH, Kalk KH, Armand S, Visser J, Dijkstra BW. 1. 68-Å crystal structure of endopolygalacturonase II from Aspergillus niger and identification of active site residues by site-directed mutagenesis. J Biol Chem. 1999;274:30474–30480. doi: 10.1074/jbc.274.43.30474. [DOI] [PubMed] [Google Scholar]