Abstract
Raising the level of extracellular ATP to mm concentrations similar to those found inside cells can block gravitropism of Arabidopsis roots. When plants are grown in Murashige and Skoog medium supplied with 1 mm ATP, their roots grow horizontally instead of growing straight down. Medium with 2 mm ATP induces root curling, and 3 mm ATP stimulates lateral root growth. When plants are transferred to medium containing exogenous ATP, the gravity response is reduced or in some cases completely blocked by ATP. Equivalent concentrations of ADP or inorganic phosphate have slight but usually statistically insignificant effects, suggesting the specificity of ATP in these responses. The ATP effects may be attributable to the disturbance of auxin distribution in roots by exogenously applied ATP, because extracellular ATP can alter the pattern of auxin-induced gene expression in DR5-β-glucuronidase transgenic plants and increase the response sensitivity of plant roots to exogenously added auxin. The presence of extracellular ATP also decreases basipetal auxin transport in a dose-dependent fashion in both maize (Zea mays) and Arabidopsis roots and increases the retention of [3H]indole-3-acetic acid in root tips of maize. Taken together, these results suggest that the inhibitory effects of extracellular ATP on auxin distribution may happen at the level of auxin export. The potential role of the trans-plasma membrane ATP gradient in auxin export and plant root gravitropism is discussed.
In animal cells, extracellular ATP is a regulatory molecule. It binds to purinergic receptors and triggers signaling cascades that lead to diverse responses (Ralevic and Burnstock, 1998). Ectoapyrases, which are NTP/NDP-hydrolyzing enzymes anchored in the plasma membrane with their active site facing toward the extracellular matrix, play the important role of quenching the signaling effects of extracellular ATP (Komoszynski and Wojtczak, 1996), which may transiently reach concentrations as high as millimolar in the extracellular space around stressed or injured cells (Di Virgilio, 1995). The recent results of Lew and Dearnaley (2000), showing that extracellular ATP can induce membrane potential changes in Arabidopsis root hairs, suggest the possibility that extracellular ATP may serve a regulatory role in plants as well.
In addition to the signaling role of extracellular ATP, another role was suggested recently by Thomas et al. (2000). Their data indicated that plants and yeast might use the steep ATP gradient that exists across the plasma membrane in a symport process to help power the efflux of various compounds across the multidrug resistance transporter called P-glycoprotein, or PGP1. PGP1 is an integral membrane protein in the family of ATP-binding cassette (ABC) proteins, many of which are involved in the export of diverse chemicals, especially xenobiotics, from cells (Gros and Hanna, 1996). Thomas et al. (2000) reported that transgenic overexpression of PGP1 in yeast and in Arabidopsis makes these organisms more resistant to the toxic effects of cycloheximide. They observed that transgenic up-regulation of a pea (Pisum sativum) ectoapyrase enzyme in yeast and Arabidopsis similarly enhanced xenobiotic resistance in these organisms. They found a consistent relationship between the ability of an organism to degrade extracellular ATP and its ability to acquire toxin resistance. They also found that PGP1 and ectoapyrase can each increase the resistance of Arabidopsis to toxic levels of cytokinin, implying that the transport of this naturally occurring substance could be influenced by the activity of these proteins. They interpreted these and other supporting data to mean that the hydrolysis of extracellular ATP by ectoapyrases may play a critical role in the mechanism of PGP1-mediated xenobiotic resistance.
The results of Lew and Dearnaley (2000) and Thomas et al. (2000) raise the question of whether plants ever encounter significant levels of ATP in their extracellular matrix. Dissolved (extracellular) ATP accumulates in the sea to concentrations exceeding 100 ng L−1 (Azam and Hodson, 1977), and in soil to concentrations exceeding 40 nm (Thomas et al., 2000). The processes accounting for this extracellular ATP are most likely cell injury and autolysis, secretion, and export of ATP through plasma membrane transporters (Di Virgilio, 1995). The multidrug resistance transporter PGP1 has been implicated in the export of ATP from animal cells (Abraham et al., 1993) and from Arabidopsis and yeast cells (Thomas et al., 2000).
By whatever means ATP escapes to the outside of cells, its concentration in the bulk extracellular medium is typically at least a million-fold less than it is inside cells. However, this concentration difference would not be a static one, and could be relatively small at sites where ATP is first released to the ECM. Manipulating the steepness of the transmembrane ATP gradient by up- or down-regulating ectophosphatase activity can correspondingly up- or down-regulate the effectiveness of PGP1 in transporting substances out of cells (Thomas et al., 2000).
Given the presence of ATP in the ECM of plants, and the well-established role of extracellular ATP in animal signaling pathways, we have started an investigation into the possible role of extracellular ATP in plant growth and development. In our initial studies, we found that adding ATP to the growth medium could inhibit root gravitropism and stimulate lateral root growth in Arabidopsis seedlings. The similarity between root growth phenotypes induced by extracellular ATP and those characteristics of mutants deficient in auxin transport made us suspect that the extracellular ATP effects could be related to auxin transport. Further encouraging this idea is the fact that PGP1-like genes are required for auxin transport (Noh et al., 2001), and extracellular ATP influences the transport effectiveness of PGP1. Here, we describe experiments designed to test this idea and discuss the results, which are consistent with the interpretation that transmembrane ATP gradients can influence the efficiency of auxin export from cells.
RESULTS
Exogenous ATP Inhibits Root Gravitropism and Induces Lateral Roots
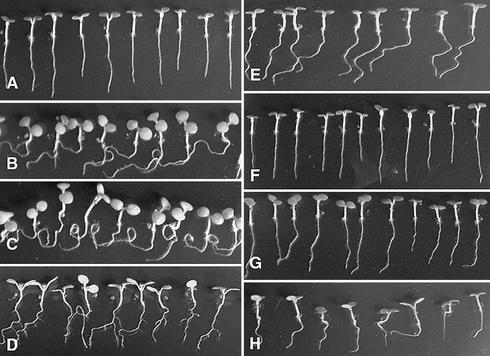
Exogenously added ATP inhibits the gravitropism of the roots of Arabidopsis seedlings grown on Murashige and Skoog (MS) medium. Under these conditions, roots on control medium grow downward with a relatively straight growth (Fig. 1A). When the medium is supplied with 1 mm ATP, the roots grow horizontally instead of growing straight down (Fig. 1B). This effect is primarily attributable to a decreased rate of gravitropism, because with a longer incubation time, the roots bend down more, reaching a 90° bend within another week (data not shown). A medium concentration of 2 mm ATP induces root curling in most plants (Fig. 1C). A minority of these plants show the more horizontal root growth characteristic of growth in 1 mm ATP, and these also grow more downward with increased time in the medium. Media containing 3 mm ATP stimulate lateral root growth (Fig. 1D). Because Suc-free MS medium was used, wild-type plants grown in MS medium with no added ATP had no lateral roots even after several weeks. Under the conditions used, the medium ATP is relatively stable: After 1 week, 75% of the ATP originally added remains in the plate, as judged by a luciferase assay (Thomas et al., 2000).
Figure 1.
Exogenous ATP induces root curling and growth of lateral roots. Shown are 1-week-old Arabidopsis plants (ecotype Wassilewskija) grown on a plate containing MS only (A), or MS +: 1 mm ATP (B), 2 mm ATP (C), 3 mm ATP (D), 2 mm ADP (E), 2 mm AMP (F), 2 mm ITP (G), and 2 mm ADP plus 2 mm phosphate, pH 4.1 (H). For these experiments, the pH of the ATP was not adjusted before adding it into the media.
To test for effects by related compounds, the growth effects of ADP, AMP, and ITP were examined. In medium containing 2 mm ADP, roots were somewhat wavy and exhibited some sideways growth, but no curling roots were observed (Fig. 1E). The roots of plants grown in 2 mm AMP and 2 mm ITP, grew in a direction and form that was similar to those of plants grown in MS medium only (Fig. 1, F and G).
The pH of MS medium is brought down to 4.8 by 1 mm ATP, to 4.2 by 2 mm ATP, and to 3.8 by 3 mm ATP. To test whether the ATP effects could be attributed to the altered pH values of the medium, plants were grown in MS medium with the pH adjusted to 4.1. In the absence of ATP, with the medium at pH 4.1, no root curling was observed (data not shown), which indicated that the root-curling phenotype observed in 2 mm ATP was not caused by pH. To test whether the ATP effects could be attributed to phosphate release, plants were grown in MS with 2 mm ADP plus 2 mm phosphate, pH 4.1. The results (Fig. 1H) showed a very similar growth pattern to roots on ADP alone. The growth angle of some roots deviated from the vertical, and some exhibited slight waviness, but none of the roots had the curling growth form characteristically seen of roots grown in 2 mm ATP (Fig. 1C). Taken together, these results support the conclusion that the root-curling phenotypes of plants grown in 2 mm ATP should be attributed to the effects of ATP.
We also sowed the seeds in MS medium with pH-adjusted ATP. Plant roots grew horizontally at 4 mm ATP and started curling at 5 mm ATP; i.e. at a higher ATP concentration than needed to see the similar phenotypes shown in Figure 1. In this experiment, the ADP control exhibited the same slightly curvy and weakly gravitropic response of roots as shown for the ADP control in Figure 1 (data not shown).
To test whether the ATP effects could be attributed to some chemical product arising from the interaction of ATP with components of the complex MS medium, plants were grown in a MES-buffered media without the MS salts or the B5 vitamins, ± ATP. Without MS, 1 mm ATP still induced horizontal growth, and higher concentrations of ATP still induced growth inhibition (data not shown).
ATP Inhibits Root Gravitropism of Plants Transferred onto ATP-Containing Medium
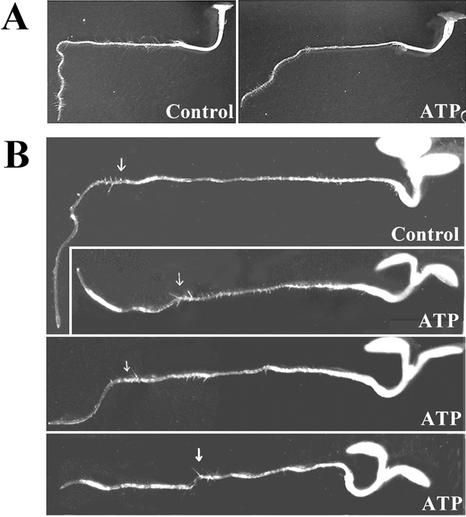
Under continuous light, the roots of plants growing on control MS medium are completely reoriented with a 90° angle by 48 h after being placed in a horizontal position (Fig. 2A). Roots of plants transferred to medium containing 5 mm ATP and turned horizontally bent at a lesser angle, had an unusual bending pattern, or did not bend at all (Fig. 2B) in the same 48-h time period. Less than 10% of the roots of plants transferred to 5 mm ATP curved straight down like those on MS-only medium, and the rest showed a somewhat reduced angle of bending. The roots of plants transferred to medium containing 5 mm ADP had similar gravitropic bending after 48 h as those grown on MS medium only.
Figure 2.
Exogenous ATP inhibits root bending. A, Gravity response of the roots of seedlings grown on media containing MS only for 6 d, transferred to a new plate containing MS only (left) or MS + 5 mm ATP (right), and then turned 90° and grown for 2 more d. B, Bending pattern of the roots of plants grown horizontally 2 d after transfer to: a medium containing MS only or a medium containing MS + 5 mm ATP. Arrows show the start position of root tip after the transfer.
When plants are reoriented to the horizontal under the light, tropic bending is a combination of both gravitropism and phototropism. To separate the gravity response of roots from their phototropic bending, we also tested the effect of extracellular ATP on root gravitropic bending after 6 and 24 h in darkness. As shown in Table I, extracellular ATP can inhibit root gravitropic bending in darkness in a dose-dependant manner. Although results from a representative experiment are reported in Table I, 5 mm ATP significantly reduced gravity response in five separate experiments, whereas 3 mm ATP was statistically significant in three of five experiments. In addition, the magnitude of the effect of ATP was even greater at 6 h after gravity stimulation (data not shown). The effects of ATP and ADP on root growth were not statistically significant, and roots grown in 3 mm ATP showed a faster growth rate but a smaller bending angle. Therefore, the reduction in gravitropic bending in the presence of ATP is not likely attributable to an inhibition in growth rate.
Table I.
Gravity response of Arabidopsis roots in the presence of ATP 24 h after reorientation
| Growth | Curvature | P Value | |
|---|---|---|---|
| mm | degrees | ||
| Control | 3.60 ± 0.33 | 63.5 ± 5.8 | |
| 1 mm ATP | 4.10 ± 0.35 | 56.3 ± 3.9 | 0.32 |
| 3 mm ATP | 3.93 ± 0.37 | 40.0 ± 4.6 | 0.0054 |
| 5 mm ATP | 3.05 ± 0.24 | 34.9 ± 4.6 | 0.0011 |
| 5 mm ADP | 3.36 ± 0.20 | 47.1 ± 5.5 | 0.058 |
Each value is the average and se of 10 separate plants and the P values for gravity response were determined by Student's t test. For all samples, the growth differences had P > 0.15.
The effect of ADP on root gravitropic bending was also examined (Table I). Although 5 mm ADP treatments showed reduced root bending, the reduction was not statistically significant in this experiment or four additional experiments. This experiment was done in Suc-containing medium to support more root growth, but we also tested the effects of extracellular ATP on root bending in Suc-free MS medium, and the results were similar (data not shown).
ATP Alters Auxin Distribution as Estimated Indirectly in DR5-β-Glucuronidase (-GUS) Plants
DR5 is a synthetic auxin-responsive promoter element. Arabidopsis plants transformed with the DR5-GUS construct were used to provide an indirect measurement of endogenous auxin distribution in roots, as described by Ulmasov et al. (1997), and to study the effect of exogenous ATP on the expression of DR5-GUS, as revealed by the resulting staining pattern.
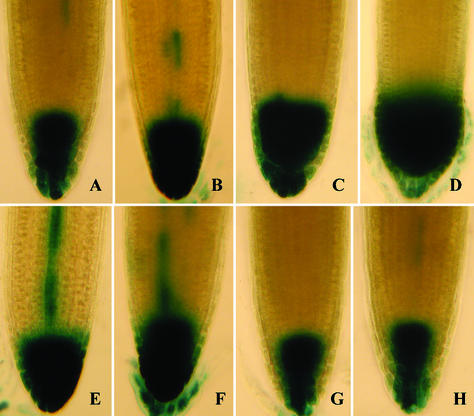
When N-1-naphthylphthalamic acid (NPA) concentration was increased in the medium, GUS staining in DR5-GUS plants was expanded from the center area of the root tip to the whole region of root tip (Fig. 3, A–D), a result similar to that seen by Sabatini et al. (1999) and Casimiro et al. (2001). Most of the GUS staining in DR5-GUS plants grown on medium with 2 mm ATP was similar to that in the roots of plants grown in 1 μm NPA (Fig. 3E) and may represent the accumulation of pools of auxin transported to the cap through the root steele. In the roots of plants grown in 2 mm ITP and AMP, the GUS-staining patterns in the root tip were almost indistinguishable from the control (Fig. 3, G and H). GUS staining in root tips of plants grown in 2 mm ADP were also expanded when compared with control and were similar to those plant grown in 0.1 μm NPA (Fig. 3F). Similar staining patterns were observed in three separate trials.
Figure 3.
Exogenous ATP induces accumulation of GUS stain in the root tips of DR5-GUS seedlings. GUS-stained root tips of DR5-GUS seedlings grown 6 d in NPA at a concentration of: 0 μm (A), 0.1 μm (B), 1 μm (C), and 10 μm (D); or in 2 mm ATP (E), 2 mm ADP (F), 2 mm ITP (G), and 2 mm AMP (H). The pH of ATP was not pre-adjusted.
ATP Increases the Sensitivity of Roots to Exogenous Auxins
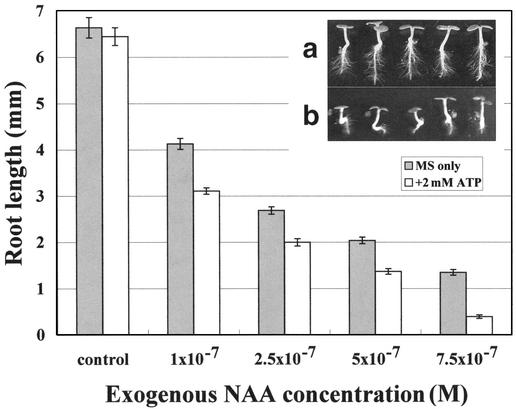
If root tips retain more auxin in the presence of exogenous ATP, it seemed possible that it would take less exogenous auxin to elicit characteristic root responses to auxin when exogenous ATP was present. Although 2 mm ATP could induce root curling, it did not induce any significant root length difference when compared with roots that grow in MS only (Fig. 4). α-Naphthalene acetic acid (NAA) increasingly inhibited root growth as its concentration in the medium was increased from 10−7 m to 7.5×10−7 m, and the addition of 2 mm ATP resulted in a statistically significant further inhibition of the root length (P < 0.001) at all NAA concentrations and a shift of the NAA inhibition curve to the right (Fig. 4).
Figure 4.
Exogenous ATP increases the sensitivity of roots to growth inhibition by exogenous auxin. Wassilewskija seeds were grown in MS medium with different concentrations of NAA in the presence (or absence) of 2 mm ATP. After 6 d, the seedlings were taken out, and the root length was measured. Each data point represents the average ± se (n > 60). The pH of the ATP stock was not pre-adjusted. a, Plants grown in 2.5 × 10−7 m NAA alone; b, plants grown in 2.5 × 10−7 m NAA with 2 mm ATP.
We also tested the effect of exogenous ATP on the sensitivity of root growth to exogenous 2,4-dichlorophenoxyacetic acid (2,4-D) and indole-3-acetic acid (IAA). The results were similar to those observed with NAA. In the presence of 2.5 × 10−7 m 2,4-D, the length of roots grown in medium with added 2 mm ATP was 58.6% of those grown in the same medium without added ATP (P < 0.001). The root lengths of plants grown in 10−7 m IAA plus ATP elongated 36.4% less than the plants grown in 10−7 m IAA (P ∼ 0.05). It should be noted that the effect of IAA was evident when the plants were grown in the dark to prevent IAA breakdown, whereas the other auxin treatments were performed with light-grown plants. Taken together, our data shows that exogenous ATP can increase the sensitivity of roots to exogenous auxins.
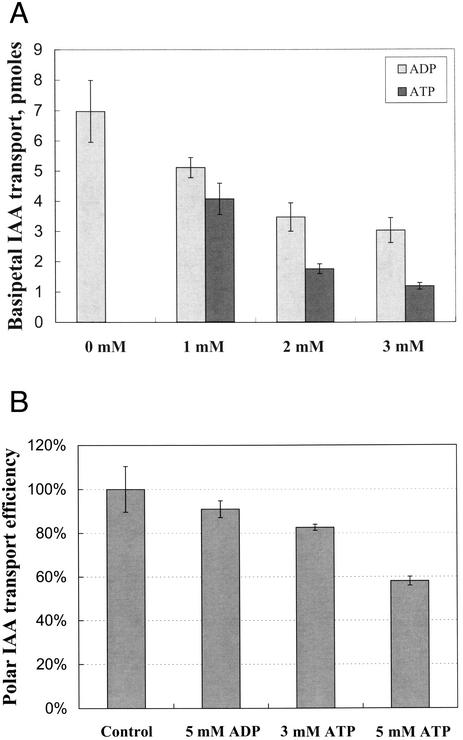
ATP Inhibits Basipetal Auxin Transport in a Dose-Dependent Fashion
The similarity of the GUS-staining pattern of DR5-GUS plants grown in the presence of ATP and of NPA implied that ATP might also work to block auxin transport. To test this idea, the effect of exogenous ATP on basipetal auxin transport in Arabidopsis roots was examined, because basipetal auxin transport has been specifically linked to the control of gravitropic bending (Rashotte et al., 2000). As the concentration of ATP was elevated, there was a dose-dependent decrease in basipetal auxin transport (Fig. 5A). All concentrations of exogenous ATP applied resulted in statistically significant reductions in transport (P < 0.006). Control tests indicated that the inhibitory effects of extracellular ATP on auxin basipetal transport in Arabidopsis roots were also found when the pH of the medium was lower (data not shown). The effect of ADP on basipetal transport was also examined and found to lead to reductions in basipetal auxin transport but of a lower magnitude. The reductions in basipetal transport by ADP are not significant at 1 mm, but are significant at 2 and 3 mm (P < 0.05). In a single experiment, 5 mm AMP had no effect on auxin transport.
Figure 5.
Exogenous ATP inhibits basipetal auxin transport in Arabidopsis and maize roots. A, Basipetal auxin transport in Arabidopsis roots was allowed to precede in darkness for 5 h, at which time the [3H]IAA in a 5-mm root section 1 mm back from the tip was quantified by scintillation counting. Values represent average ± se. B, Basipetal auxin transport in maize roots was allowed to proceed in darkness for 4 h, at which time the [3H]IAA amount in the receiver agar block was quantified by scintillation counting. The experiment was repeated at least four times. The data represent the average ± se (n > 16).
Exogenous ATP can also block basipetal auxin transport in maize (Zea mays) roots (Fig. 5B). If we consider the radioactivity in the control receiver bock (containing MS only) as 100% transport efficiency, the efficiency of auxin polar transport into receiver blocks containing 5 mm ATP and ADP was 58% ± 2.04% and 91.7% ± 3.82% (Fig. 5B). These data represent the average of at least 16 individual measurements. The levels of transport measured at 3 and 5 mm ATP are significantly different from that measured in the absence of ATP (P < 0.001), and the level at 5 mm ATP is also significantly different from that measured at 5 mm ADP (P < 0.001)
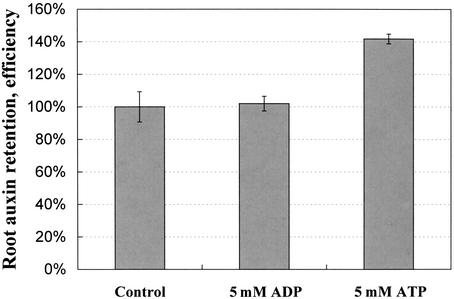
ATP Increases Auxin Retention
Auxin transport is regulated at both its import and export steps. To test whether exogenous ATP inhibits auxin influx or efflux, corn root tips were excised and incubated with MS plus [3H]IAA with or without ATP or ADP. After the tips were washed, the radioactivity remaining in them was measured. If we consider the tritiated auxin content of control root tips as 100%, the amount of [3H]IAA in roots treated with 5 mm ATP and 5 mm ADP were 141.7% ± 3% and 102% ± 4.5%, respectively (Fig. 6).
Figure 6.
Exogenous ATP promotes retention of auxin in corn roots. Corn (yellow dent) root tips (3 mm) were excised and incubated in MS liquid medium (no agar) plus 10−7 m [3H]IAA, with or without ATP or ADP. After the root tips were washed with MS medium, the radioactivity in roots was measured by scintillation counting. The level of [3H]IAA retained in roots treated with 5 mm ATP is significantly different from that in roots treated with 5 mm ADP and in untreated roots. Values represent average ± se (n = 18; P < 0.001).
DISCUSSION
Several experiments were performed to test the specificity of the ATP effect on root growth, gravity response and auxin transport. The effects of extracellular ATP on growth and gravitropism are dose-dependent and are not mimicked by ITP, by AMP, or by inorganic phosphate, thus they cannot be attributed to nonspecific effects of nucleoside triphosphates or to the phosphate release that accompanies ATP degradation. ADP has slight effects on root growth and gravity response, and these were not statistically significant at the lower concentrations where ATP was active. The effect of ADP on auxin transport was insignificant in maize. Although its effect in Arabidopsis was statistically significant, it was of a lower magnitude than the ATP effect. The tests with ADP suggest that some ATP-induced responses can be mimicked weakly by ADP.
Because ATP is an effective chelator and ADP a slightly less effective chelator, an increase in the external ATP or ADP concentrations would also result in lowering the concentration of divalent cations in the wall. However, as described in the “Results,” ATP is more effective in inhibiting gravitropism at pH 4.1 than at pH 5.7, but its effectiveness as a chelator is lower at lower pH values. External ATP is also more effective at inhibiting auxin transport at lower pH values (data not shown). Thus it is unlikely that the effects of exogenous ATP on gravitropism and auxin transport could be attributable to its chemical properties as a chelator. Likewise, because the effects of ATP were observed with or without MS salts or B5 vitamins in the growth medium, it is unlikely that they are attributable to some chemical product arising out of the interaction of ATP with components of the MS medium.
Our findings that extracellular ATP can inhibit root gravitropism is plausibly linked to our data showing that extracellular ATP can also decrease the amount of basipetal auxin transport through roots, stimulate auxin accumulation in root tips, and increase the sensitivity of root responses to exogenous auxin. Taken together these results imply that the mechanism by which extracellular ATP inhibits root gravitropism is by inhibition of auxin transport. This inhibition could occur at the step of auxin import or auxin export. If extracellular ATP alters auxin transport by blocking influx, then less tritiated auxin should be retained in corn roots that are incubated with 5 mm ATP and fed the labeled hormone. However, the results of this experiment showed that 40% more tritiated auxin is retained in corn roots that are incubated with ATP. Of course, this greater retention could be attributable to greater auxin influx into roots, but it seems unlikely that a more rapid uptake of auxin into roots would result in a decreased rate of polar auxin transport. So, taken together, the results strongly suggest that extracellular ATP inhibits auxin transport and that it does so by blocking auxin efflux, not influx.
The exact mechanism by which auxin is transported out of cells is not yet fully understood (for review, see Muday and DeLong, 2001). The prevailing opinion is that it moves out of cells via carriers driven by a chemiosmotic mechanism dependent on the transmembrane pH gradient. The role of PIN proteins in this export in Arabidopsis is clearly essential, but whether these membrane proteins are themselves the transporter has not been demonstrated (Palme and Gälweiler, 1999). Similarly MDR-like proteins also play a critical role in auxin transport out of cells (Noh et al., 2001), but what this specific role is remains to be determined. External ATP could influence the transport activity of either of these putative transport proteins.
If, indeed, the transport of auxin out of cells is electrochemically favored, then it would not directly need the potential energy of an ATP gradient to leave cells. However, as clearly discussed by Martinoia et al. (2002), proteins in the ABC family of transporters, like PGP1, control a wide variety of transport processes that could influence auxin efflux indirectly, including the transport of ions and Glu complexes, and plants altered in their expression of ABC transporters have phenotypes that indicate they have disturbed phytohormone balance. Palme and Gälweiler (1999) and Friml and Palme (2002) emphasize that auxin efflux is regulated by a complex cellular machinery involving many different metabolic processes. Thus, a fundamental disturbance of the transport efficiency of MDR-like transporters could be expected to result in the accumulation of a variety of substances that could affect auxin efflux. To give just one example, a slow-down in the rate of efflux of ATP from cells through MDR (Thomas et al., 2000) could upset the cytosolic ATP/ADP balance and thus alter the balance of kinase-phosphatase activities that so dramatically affect auxin efflux and gravity responses in Arabidopsis roots (Rashotte et al., 2001).
Our data are thus consistent with a model in which an increase in the extracellular [ATP] would depress the transport activity of one or several MDR-like ABC transporters whose cargo indirectly affects auxin transport. If we were to interpret our results in accord with this model, we would predict that significant inhibition of MDR-transporter activity is achieved in Arabidopsis roots only when the concentration of extracellular ATP reaches levels close to those typically found in the cytoplasm (1 mm or higher), because these are the same concentrations that we report here as being the most effective at inhibiting auxin transport and disrupting normal gravitropic responses.
In vivo, one might not expect the concentration of extracellular ATP to approach the mm level except in special microenvironments, such as in regions surrounding cell injury or where there is high exocytotic activity. However, the steepness of the transmembrane ATP gradient could be altered not only by increased extracellular [ATP], but also by changes in the local concentration of cytoplasmic ATP. In this regard, it would be useful to test whether the settling of starch-filled and ATP-producing chloroplasts in shoots after gravistimulation increases the local concentration of ATP near the bottom of those cells, and whether this helps to account for the increase in transport of auxin toward the lower end of horizontally placed shoots.
Similarly, one could increase the steepness of the transmembrane ATP gradient, and, according to the model, increase the rate of auxin transport, by decreasing the extracellular [ATP]. Ectoapyrase can metabolize extracellular ATP very efficiently (Komoszynski and Wojtczak, 1996). Apyrase promoter-GUS staining showed that Arabidopsis apyrases are expressed very highly in root tips and are most concentrated in the gravity-sensing columella cells (Y. Sun and S.J. Roux, unpublished data). Methods to assess the changing intracellular and extracellular concentrations of ATP in vivo, such as those described by Kennedy et al. (1999), will be needed to test whether changes in these concentrations are actually correlated dynamically with changes in auxin transport.
An alternative explanation for the results described is that extracellular ATP is functioning as a signal in plant gravitropism. In plants, extracellular ATP can stimulate generative nuclear division in pollen tubes of Easter lily (Lilium longiflorum; Kamizyo and Tanaka, 1982) and depolarize the membrane potential of root hairs (Lew and Dearnaley, 2000), both of which could be signaling phenomena. In animals, the extracellular ATP signal is transduced into responses by different purinoceptors, some of which are G-protein linked and some of which can sense not only the extracellular ATP signal, but also extracellular NTP, ADP, or AMP (Ralevic and Burnstock, 1998). A search through the Arabidopsis genome database does not reveal any gene products with sequences obviously similar to any of the described animal purinoreceptors, but these receptors are not well conserved even among animals, so more sophisticated structure-based searches would be needed to learn whether there were any potential purinoceptor candidates present in plants.
If external ATP were functioning primarily as a receptor-activating agonist in the root responses described here, its receptor might be responsive to much lower levels of ATP than the millimolar amounts used. Because of the activity of phosphatases and apyrases in the plant cell wall, it may be that millimolar concentrations of applied ATP would be needed to achieve micromolar concentrations at the external face of the plasma membrane. Preliminary experiments indicate that 0.1 mm ATP-γ-S, a poorly hydrolyzable analog of ATP, is as effective as 2 mm ATP in inhibiting root gravitropism and, like 2 mm ATP, does not inhibit root growth at this concentration (data not shown). However, ATP-γ-S would be expected to be a competitive inhibitor of ectoapyrase activity, which, in turn, would lead to higher [ATP] in the wall, so these results are equally consistent with both the agonist model and the ATP gradient model.
The mechanistic basis for the extracellular ATP effects observed remains unresolved. Because such high (millimolar) concentrations of ATP were needed to induce the responses observed, the likelihood of indirect effects, attributable to some reaction that is not physiologically relevant must be considered, and additional control experiments beyond the ones described here will be needed to test this possibility. As a step in this direction, a screen of T-DNA insertional mutants has yielded some lines that are insensitive to 2 mm ATP and others that are hypersensitive to 1 mm ATP, as judged by the root gravitropic responses of these mutants (W. Tang and S.J. Roux, unpublished data). The fact that the phenotypic responses of roots to extracellular ATP can be manipulated genetically makes it likely that cloning and characterizing the genes that affect these responses will allow a rigorous test of the relationship of transmembrane ATP gradients to auxin efflux and plant gravity sensing.
MATERIALS AND METHODS
All Arabidopsis seeds (ecotype Wassilewskija) were surface sterilized by soaking them in 20% (v/v) bleach for 20 min and then washing them extensively with sterilized water. Transgenic Arabidopsis (DR5-GUS) seeds were kindly provided by Dr. Tom Guilfoyle (University of Missouri). Arabidopsis plants were grown under continuous light at 22°C on MS medium (Sigma-Aldrich, St. Louis; 4.3 g L−1], 1× B5 vitamins, 0.5 g L−1 MES, and 1.5% [w/v] agarose [Invitrogen, Carlsbad, CA], pH 5.7). To make media containing ATP or ADP, MS medium was heated in a microwave oven until it was completely dissolved, dispensed into 10-mL sterilized plastic tubes, and placed into a water bath at 45°C. A stock solution (1 m) of ATP or ADP (sodium salt, Sigma-Aldrich) was added to the MS medium and mixed by vortexing just before the plates were poured. The pH of ATP and ADP stocks was adjusted to 5.7 before use, unless indicated otherwise. In one set of experiments, Arabidopsis seeds were grown in a medium that did not contain MS (0.5 g L−1 MES and 1.5% [w/v] agarose, pH 5.7) with or without ATP for 1 week.
Effect of Extracellular ATP on Root Bending in Arabidopsis
Wild-type Arabidopsis seeds were germinated on MS medium containing 1.5% (w/v) Suc for 6 d under continuous light. Seedlings were then transferred to a new MS agar plate containing 1.5% (w/v) Suc and the indicated concentration of ATP or ADP. The plates were turned 90°, and the seedlings grew in their new horizontal orientation for 24 h in darkness. For automatic video digitizer analysis of root gravitropism, root bending angle and growth were recorded at user specified time intervals. Roots that reoriented completely to the new gravity vector resulting in vertical growth were considered to have a bending angle of 90°.
Measurement of ATP Concentration in MS Plates
To measure the ATP concentration in the media, agar block samples were taken from fresh-made and one-week-old plates, each of which contained 10 mL of medium. Agar blocks were weighed, and 2 volumes (100 mg ∼ 100 μL) of extraction buffer (50 mm Tris-HCl, pH 7.8) was added. Agar blocks were then ground in centrifuge tubes and centrifuged at maximum speed for 15 min. Supernatants were taken and diluted 1,000 times with the same extraction buffer, and the ATP concentration was measured with the Sigma-Aldrich ATP bioluminescent assay kit according to the manufacturer's instructions.
GUS Staining as an Indirect Measurement of Auxin Distribution in DR5-GUS Plants
Seeds of DR5-GUS plants were grown in MS medium in the presence of 2 mm ATP, ADP, ITP, or AMP (pH not adjusted) or different concentration of NPA for 1 week and then stained for GUS activity. GUS staining was performed according to Lehman et al. (1996). Arabidopsis seedlings were vacuum fixed in 100 mm sodium phosphate and 1% (v/v) formaldehyde, pH 7.0, for 20 min and then rinsed 5 times in 100 mm sodium phosphate, pH 7.0. The GUS-staining solution (1 mm 5-bromo-4-chloro-3-indolyl-β-d GlcUA [X-Gluc, Sigma-Aldrich], 100 mm sodium phosphate, 10 mm EDTA, 0.5 mm K4Fe(CN)6, 0.5 mm K3Fe(CN)6, and 1% [v/v] Triton X-100, pH 7.0) was then added later, and staining was allowed to proceed overnight.
Measuring Effects of Extracellular ATP on the Inhibition of Root Growth by Different Auxins
Arabidopsis seeds were grown under light on media containing MS plus 2 mm ATP (pH not adjusted), ± NAA or 2,4-D. After 6 d, the seedlings were taken out, and their root length was measured from a digital image using the computer program Image J. For the tests with IAA, the plants were grown in the light on MS medium for 5 d. Their root length was measured, and then the plants were transferred to a new MS plate plus 2 mm ATP (pH 5.7) ± IAA, and continued to grow in darkness for 4 d before their root length was measured again.
Basipetal Auxin Transport in Arabidopsis Roots
Basipetal auxin transport was measured in 7-d-old vertically grown seedlings as reported previously (Rashotte et al., 2001), with the following modifications. Seedlings were germinated on MS plates for 7 d. Plants were either transferred to control plates or to plates containing a range of ATP concentrations between 1 and 5 mm, as indicated, immediately before addition of radioactivity. The pH of the ATP-containing plates was not adjusted in this experiment. Mixtures containing 1% (w/v) agar and 100 nm [3H]IAA were prepared in 3-mL scintillation vials. A narrow-stem transfer pipette was carefully inserted into the hardened agar mixture such that a 1-mm-diameter cylinder of agar was removed. The cylinder containing [3H]IAA agar was applied such that the agar just touched the root tip of the seedlings. Plates remained vertically oriented in the dark, to avoid IAA degradation. After 5 h, the apical 1 mm in contact with the agar line was discarded, and the amount of [3H]IAA transport into a 5-mm segment back from the apical tip was determined. Each root segment was placed into 2.5 mL of scintillation fluid, and the amount of radioactivity within each sample was determined using a scintillation counter (LS6500, Beckman Coulter, Fullerton, CA) for 2 min.
Basipetal Auxin Transport in Maize (Zea mays) Roots
Auxin polar transport was performed according to Wilkins and Scott (1968). In brief, corn seeds were germinated in darkness at 25°C for 4 d. The primary toot tip (1 mm) was cut and discarded. The following 6-mm segment was then excised for use. Twenty segments were placed vertically with their basal ends down on a receiver block of 1.5% (w/v) agar containing MS only or MS with ATP or ADP. A donor block of 1.5% (w/v) agar with 1 μm [3H]IAA was put on the apical (root tip) ends of the segments. Polar transport of the labeled auxin was allowed to proceed in darkness for 4 h. The receiver block was then removed, soaked in 5 mL of scintillation solution for 16 h, and then assayed for its radioactivity by scintillation counting.
Auxin Accumulation in Maize Root Tips
The root tips (3 mm) of 4-d-old etiolated maize seedlings were excised and weighed in centrifuge tubes. 3-[5(n)-3H]IAA (1 μm; Amersham Pharmacia Biotech) in MS solution (no agar) with or without ATP or ADP was added in a volume of 200 μL, incubated in darkness for 4 h, and then washed in the same incubation solution without [3H]IAA. Roots were then soaked in 5 mL of scintillation solution for 16 h, and their radioactivity was measured by a scintillation counter. The counts per minute measured was divided by the weight of the roots (milligrams) to normalize the results.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENT
The authors thank Dr. Tom Guilfoyle (Department of Biochemistry, University of Missouri) for providing the DR5-GUS seeds.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAG2–1347) and by the National Science Foundation (grant no. IBN–0080363 to S.J.R.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013672.
LITERATURE CITED
- Abraham E, Prat A, Gerweck L, Seneveratine T, Arceci R, Kramer R, Guidotti G, Cantiello H. The multidrug resistance (MDR1) gene product functions as an ATP channel. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam F, Hodson R. Dissolved ATP in the sea and its utilization by marine bacteria. Nature. 1977;267:696–697. doi: 10.1038/267696a0. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- Friml J, Palme K. Polar auxin transport: old questions and new concepts? Plant Mol Biol. 2002;49:273–284. [PubMed] [Google Scholar]
- Gros P, Hanna M. The P-glycoprotein family and multidrug resistance: an overview. Handbook Biolog Physics. 1996;7:137–163. [Google Scholar]
- Kamizyo A, Tanaka N. Studies on the generative nuclear division III: effect of exogenous ATP on the generative nuclear division in Lilium longiflorum. Cytologia. 1982;47:195–205. [Google Scholar]
- Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. Glucose generates sub-plasma membrane ATP microdomains in single Islet B cells. J Biol Chem. 1999;274:13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- Komoszynski M, Wojtczak A. Apyrase: function and relationship to ATPase. Biochim Biophys Acta. 1996;1310:233–241. doi: 10.1016/0167-4889(95)00135-2. [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyls. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Lew RR, Dearnaley JDW. Extracellular nucleotide effects on electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci. 2000;153:1–6. [Google Scholar]
- Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolukisaoglu U, Müller-Röber B, Schulz B. Multifunctionality of plant ABC transporters-more than just detoxifiers. Planta. 2002;214:345–355. doi: 10.1007/s004250100661. [DOI] [PubMed] [Google Scholar]
- Muday GK, DeLong A. Polar auxin transport: controlling where and how much. Trends Plant Sci. 2001;6:535–542. doi: 10.1016/s1360-1385(01)02101-x. [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell. 2001;13:2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K, Gälweiler L. PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol. 1999;2:375–381. doi: 10.1016/s1369-5266(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte A, Brady S, Reed R, Ante S, Muday G. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;122:481–490. doi: 10.1104/pp.122.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey B, Leyser HMO, Bechtold N, Weisbeek D et al. An auxin dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Thomas C, Rajagopal A, Windsor B, Dudler R, Lloyd A, Roux SJ. A role for ectophosphatase in xenobiotic resistance. Plant Cell. 2000;12:519–533. doi: 10.1105/tpc.12.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins MB, Scott TK. Auxin transport in roots. Nature. 1968;219:1388–1389. doi: 10.1038/2191388a0. [DOI] [PubMed] [Google Scholar]