Abstract
We report on the molecular cloning of the Phytophthora megasperma H20 (PmH20) glycoprotein shown previously as an inducer of the hypersensitive response, of localized acquired resistance and of systemic acquired resistance in tobacco (Nicotiana tabacum), and of the PmH20 α- and β-megaspermin, two elicitins of class I-A and I-B, respectively. The structure of the glycoprotein shows a signal peptide of 20 amino acids followed by the typical elicitin 98-amino acid-long domain and a 77-amino acid-long C-terminal domain carrying an O-glycosylated moiety. The molecular mass deduced from the translated cDNA sequence is 14,920 and 18,676 D as determined by mass spectrometry. This structure together with multiple sequence alignments and phylogenetic analyses indicate that the glycoprotein belongs to class III elicitins. It is the first class III elicitin protein characterized, which we named γ-megaspermin. We compared the biological activity of the three PmH20 elicitins when applied to tobacco cv Samsun NN plants. Although α- and γ-megaspermin were similarly active, β-megaspermin was the most active in inducing the hypersensitive response and localized acquired resistance, which was assessed by measuring the levels of acidic and basic pathogenesis-related proteins and of the antioxidant phytoalexin scopoletin. The three elicitins induced similar levels of systemic acquired resistance measured as the expression of acidic PR proteins and is increased resistance to challenge tobacco mosaic virus infection.
Elicitins are a family of structurally related proteins that are secreted by Phytophthora and Pythium spp. (Kamoun et al., 1997; Ponchet et al., 1999) and that are able to induce the hypersensitive response (HR) in Nicotiana and Brassica spp. (Ricci et al., 1989; Kamoun et al., 1993). The primary structure of elicitins has been determined after sequencing of purified proteins and/or after sequencing of cloned genes and cDNAs. All known elicitins share a conserved elicitin domain from amino acids 1 to 98. Five different classes have been defined based on the primary structure. Class I-A and I-B enclose 10-kD elicitins that display only the elicitin domain and thus are 98-amino acid-long proteins. Some have an acidic pI, are called α-elicitins, and belong to class I-A. Some have a basic pI, are called β-elicitins, and belong to class I-B. Class II contains highly acidic elicitins, which possess a short hydrophilic C-terminal tail (five to six amino acids long). Class III encloses elicitins with a long (65–101) amino acid C-terminal domain rich in Ser, Thr, Ala, and Pro, an amino acid composition and distribution that suggests potential O-glycosylation sites (Kamoun et al., 1997). Elicitins from Pythium spp. have been either classified into a distinct group called the Pythium spp. group (Kamoun et al., 1997) or as a subgroup of class I (Ponchet et al., 1999). Although several class I-A and I-B elicitins have been purified to homogeneity and investigated for their biological activity, there are no reports on the isolation and biological activities of class II and class III elicitin proteins.
Biological activity of elicitins has been most studied on tobacco (Nicotiana tabacum) plants and tobacco cell cultures. Elicitins are usually applied through the vascular system, either by application to the stem of decapitated plants or to the petiole of detached leaves. This mode of treatment leads to the systemic movement of elicitins, with α- and β-elicitins being equally well translocated (Devergne et al., 1992; Zanetti et al., 1992). This property explains elicitin capacity to induce distal HR and systemic acquired resistance (SAR) against fungal phytopathogens (Kamoun et al., 1993; Bonnet et al., 1996; Picard et al., 2000). The elicitin-induced HR is correlated with features of programmed cell death, production of ethylene, and expression of typical defense responses such as phytoalexins and PR proteins (Milat et al., 1991b; Keller et al., 1996b; Levine et al., 1996). When applied to tobacco cell cultures, elicitins induce rapid protein phosphorylation, Ca2+ influx, extracellular and transient H2O2 production, alkylinization of the extracellular medium, acidification of the cytosol, lipid peroxidation, gene expression, disruption of microtubular cytoskeleton, and cell wall modifications (Blein et al., 1991; Milat et al., 1991a; Viard et al., 1994; Suty et al., 1995; Tavernier et al., 1995; Pugin et al., 1997; Simon-Plas et al., 1997; Dorey et al., 1999; Kieffer et al., 2000; Sasabe et al., 2000; Binet et al., 2001).
Although α- and β-elicitins interact with the same receptor, with the same affinity (Bourque et al., 1998), β-isoforms were shown to be 50- to 100-fold more active to induce distal HR than α-isoforms when applied to decapitated tobacco plants or to the petiole of detached leaves (Ricci et al., 1989; Nespoulos et al., 1992; Kamoun et al., 1993). However, both isoforms are similarly active to induce local HR when directly infiltrated into leaf mesophyll (Kamoun et al., 1993). It was claimed that the latter mode of elicitin application does not lead to SAR activation (Ponchet et al., 1999).
We have screened previously from the culture filtrate of Phytophthora megasperma H20 (PmH20), a pathogen of Douglas fir, for proteinaceous factors inducing the HR on tobacco leaves. We have isolated a glycoprotein and an α- and β-elicitin, termed α- and β-megaspermin (Baillieul et al., 1994, 1995). The glycoprotein showed an apparent molecular mass of 32 kD as determined after SDS-PAGE. The three elicitors share common epitopes as antibodies directed against α-megaspermin interact with the glycoprotein and β-megaspermin (Baillieul et al., 1996). Infiltrated into tobacco leaves, the glycoprotein induces localized acquired resistance (LAR) and SAR. LAR is characterized by the strong activation of a large range of defense responses in the vicinity of the glycoprotein infiltrated site, including acidic and basic PR protein expression (Dorey et al., 1997; Cordelier et al., 2003) and accumulation of the antioxidant phytoalexin scopoletin (Costet et al., 2002b), and by a high level of resistance to challenge tobacco mosaic virus (TMV) infection (Cordelier et al., 2003). The glycoprotein-induced SAR is characterized by the systemic expression of SAR molecular markers, as acidic PR proteins, representing a subset of markers induced during LAR, and by enhanced resistance against TMV infection (Cordelier et al., 2003). LAR provides a higher level of defense responses and of resistance than SAR.
Here, we report on the molecular cloning of the glycoprotein, and of α- and β-megaspermin. Sequence analysis revealed that the glycoprotein is a class III elicitin. The PmH20 glycoprotein is, thus, the first class III elicitin protein to be isolated and characterized. It was termed γ-megaspermin. We compared some biological activities of the three PmH20 elicitins such as the induction of local and distal HR and the ability to induce LAR and SAR after infiltration into tobacco leaves.
RESULTS
Protein Microsequencing
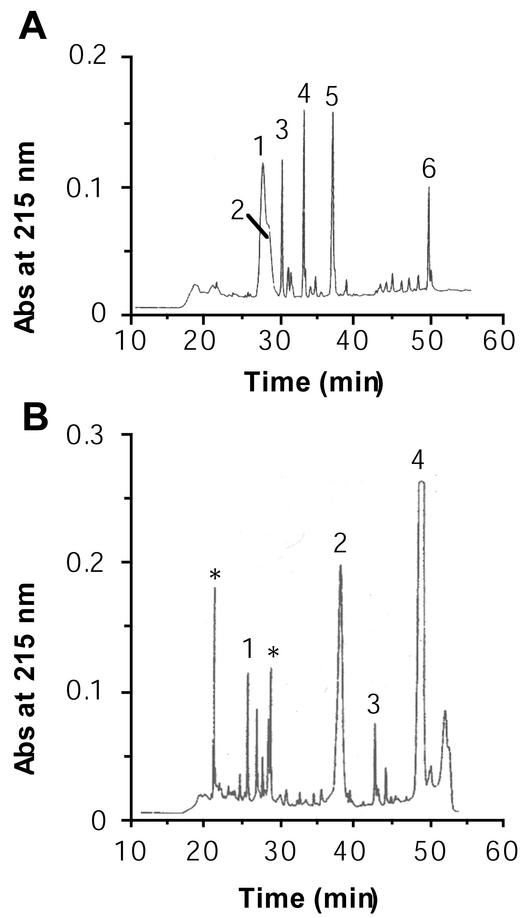
The glycoprotein, α- and β-megaspermin were S-carboxymethylated before N-terminal sequencing and protease digestion. Figure 1 shows the elution profiles after reversed-phase chromatography of the peptides obtained after digestion of the glycoprotein with trypsin or protease V8. The major numbered peptides have been sequenced, and their amino acid sequences are listed in Table I. The trypsic profile shows a broad peak with a shoulder (Fig. 1, peaks 1 and 2). The amino acid sequences of the corresponding peptides T1 and T2 were similar except at three positions where a Hyp was identified in peptide T1 instead of a Pro in peptide T2. In both peptides, three amino acids were not identified (noted * in Table I). A sequence of 113 amino acids was deduced from the different peptides issuing from the glycoprotein (Table I). Analysis of glycoprotein partial sequence revealed an elicitin domain from amino acids 1 to 98 with the six conserved Cys residues at positions 3, 27, 51, 56, 71, and 95. It already suggested that the PmH20 glycoprotein belonged to the elicitin family and thus was renamed γ-megaspermin. A partial amino acid sequence was also deduced for α-megaspermin (Table I) after comparison of the peptide sequences with amino acid sequence of capsicein, an α-elicitin from P. capsici.
Figure 1.
Analysis of protease-digested glycoprotein by reversed-phase HPLC. Peptides were obtained after digestion of reduced and alkylated glycoprotein with trypsin or protease V8. A, HPLC profile of trypsin-digested glycoprotein. B, HPLC profile of protease V8-digested glycoprotein. Numbers indicate the peptides that have been sequenced. Triangles indicate peptides blocked at the N terminus.
Table I.
N-terminal and peptide sequences of the glycoprotein, α - and β -megaspermin
| Glycoprotein | |
| N-T | EACSASEQASAYTSMVGLLQGTALSTCASDSGYNMLYA |
| T1 | SLADNFEPGCTSSHyPA*DAHyPVA**AAHyPS |
| T2 | SLADNFEPGCTSSPA*DAPVA**AAPSP |
| T3 | VDACHELIK |
| T4 | YATSLPTAEQTAAMCK |
| T5 | NVQATNPPDCDLNIPTSGAVMNVK |
| T6 | EACSASEQASAYTSMVGLLQGTALST |
| V8-1 | QTAAMCKVDACHE |
| V8-2 | LIKNVQATNPPDCDLNIPTSGAVMNVKSLADNFEPG*TSS*A*DA*VA |
| V8-3 | VGLLQGTALSTCASDSGYNML |
| V8-4 | EACSASEQASAYTSMVGLLQGTALSTCASDSGYNMLYATSLPTA |
| Ps | EACSASEQASAYTSMVGLLQGTALSTCASDSGYNMLYATSLPTAEQTAAMCKVDACHELIKNVQATNPPDCDLNIPTSGAVM NVKSLADNFEPGCTSS(P/HyP)A*DA(P/HyP)VA**AA(P/HyP)SP |
| α-Megaspermin | |
| N-T | TTCTTTQQTAAYVALVSILSDASFNQCATDSGYSMLTATS |
| T1 | LMCASTACNSMIAK |
| T2 | IITLNAPDCELTVPTSGLVLNVYSYA |
| T3 | TTCTTTQQTAAYVALVSILSDASFNQCATDSGYSMLTATSLPTTDQY |
| Ps | TTCTTTQQTAAYVALYSILSDASFNQCATDSGYSMLTATSLPTTDQYKLMCASTACNSMIAKIITLNAPDCELTVPTSGLVLNVYSYA |
| β-Megaspermin | |
| N-T | TACTATQQTAAYKTLVSILSDASFNKCSTDSGYSMLTAKALPTNAQYKLMCAST |
The glycoprotein was digested with trypsin (T) or V8 protease (V8), and α -megaspermin with trypsin. The nos. refer to the peptides in Figure 1. N-T, N-terminal sequencing of the protein. *, Lack of amino acid detection. HyP, Hydroxy-Pro; (P/hyP), Pro or HyP. A partial primary structure (Ps) was deduced from peptide alignment for the glycoprotein and after comparison with capsicein amino acid sequence for α -megapsermin.
Molecular Cloning of α-, β-, and γ-Megaspermin Full-Length cDNAs
A PCR-based strategy was developed to obtain full-length cDNA clones of α-, β-, and γ-megaspermin. The sequence of the different primers used is listed in Table II. The first step was to generate and to clone elicitin-specific PCR products. This was achieved using degenerated primers for β- and γ-megaspermin, designed according to the known amino acid sequence. Only a single clone showed a translated product identical to γ-megaspermin from amino acids 11 to 75, whereas several β-megaspermin clones were obtained. We used nondegenerated primers for α-megaspermin because there is a strong conservation between the nucleotide sequence of α-elicitins. Several α-megaspermin clones were obtained. From these different partial sequences, gene-specific primers were synthesized to perform 5′- and 3′-RACE reactions. Full-length clones for α-, β-, and γ-megaspermin were obtained by performing PCR using specific primers located in the 5′- and 3′-untranslated regions determined after 5′- and 3′-RACE cloning.
Table II.
Primers used to clone cDNA encoding α -, β -, and γ -megaspermin
| Step | Primer 5′-3′ |
|---|---|
| α-Megaspermin | |
| 1 | (F)ATGAACTTCCGCGCTCTGTTCGCCGCCAC |
| (R)GTACGAGTACACGTTGAGCACC | |
| 2: 5′-RACE | GTACGAGTACACGTTGAGCACC |
| 3: 3′-RACE | TCGCCAAGATCATTACGC |
| 4 | (F)CACCACCACCACCCACT |
| (R)AGCTACTCGTCTTGTCGAAAC | |
| β-Megaspermin | |
| 1 | (F)ACIGCITGYACIGCIACICARCARAC |
| (R)GTITTRCAIGCIGTISWIGCRCACAT | |
| 2: 3′-RACE | ACAAGTGCTCTACGGATTCGGGCTACTC |
| 3: 5′-RACE | AGAAGCCGTTCGCGTACGAGTACACGTTGA |
| 4 | (F)GGCTACCCACCACCATCAAGATG |
| (R)TCACCATTCTGGAAGCGGAAC | |
| γ-Megaspermin (glycoprotein) | |
| 1 | (F)GARGCITGYWSIGCIWSIGARCARGCIWSIGCITAYAC |
| (R)TAYAAITYYAGYGTYAGICCICCYAA | |
| 2: 3′-RACE | CCTACACGTCCATGGTCGGTCTTCTG |
| 3: 5′-RACE | GTTGGTAGCCTGCACGTTCTTGATAAGCTC |
| 4 | (F)GCACCACACTCCAGACTCCC |
| (R)AGCAAGCCACTGGCCAGGCAC | |
(F), Forward; (R), reverse.
Sequence Analysis of α-, β-, and γ-Megaspermin cDNAs
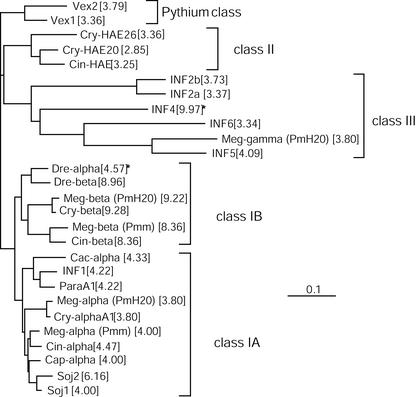
Several full-length cDNA clones of α-, β-, and γ-megaspermin have been sequenced. For each protein, we found at least one cDNA encoding a polypeptide showing 100% identity with the amino acid sequence determined after peptide sequencing (Fig. 2). The γ-megaspermin cDNA sequence encodes a 174-amino acid protein with a signal peptide of 20 amino acids. The calculated molecular mass and pI of the mature protein are 14,920 D and 3.80, respectively. Mass spectrometry indicated a 18,676-D protein as well as dimers, trimers, and tetramers. To further investigate the relationship between γ-megaspermin and elicitins, we aligned the sequences (Fig. 3) and analyzed the phylogeny of the elicitin family by the neighbor joining method (Fig. 4). Like all sequenced elicitins, γ-megaspermin lacks tryptophane and is rich in Ala (24%), Thr (14%), and Ser (9%) residues. There is 44% amino acid identity between the elicitin domain of γ-megaspermin and that of α- or β-megaspermin, and 80% identity between α- and β-megaspermin. The difference in pI between α- and β-type elicitins is attributable to an increased number of basic amino acids for the β-type, whereas acid amino acids remain constant, between 2 and 5. For instance, α- and β-megaspermin have five and four acidic amino acids and three and seven basic amino acids, respectively. Acidic class III elicitins have an increased content in acidic amino acids, 11 for γ-megaspermin, 18 for INF6, 14 for INF5, 14 for INF2b, and 16 for INF2a, and the basic amino acid content remains low, between three and five. The glycoprotein γ-megaspermin is closely related to INF5, a class III 164-amino acid elicitin from P. infestans, showing 94% identity in the elicitin domain and 52% identity in the C-terminal domain. Analysis of the phylogeny also revealed that α- and β-megaspermin belong to class I-A and class I-B, respectively. The elicitins showing the highest amino acid sequence identity to α- and β-megaspermin are α- and β-cryptogein from P. cryptogea and not α- and β-megaspermin from P. megasperma f.sp. megasperma (Pmm). PmH20 α-megaspermin disclose one and seven different amino acids compared with α-cryptogein and Pmm α-megaspermin, respectively. PmH20 β-megaspermin shows two and 14 different amino acids compared with β-cryptogein and Pmm β-megaspermin, respectively. Signal peptide sequence analysis showed that class I-A elicitins have 100% identical signal peptides, as well as class I-B, which are different from class I-A (Table III). Signal peptides of PmH20 α- and β-megaspermin are identical to class I-A and class I-B signal peptides, respectively. Signal peptides from class II and III elicitins are highly conserved but not identical within a class. It is noteworthy that the signal peptide of PmH20 γ-megaspermin is most closely related to that of INF5.
Figure 2.
cDNA coding sequences and translation products of α-, β-, and γ-megaspermin. The peptide signal sequence is underlined.
Figure 3.
Multiple sequence alignments of elicitins. Alignment of 27 elicitin sequences from PmH20 (Meg-α or α-megaspermin [AJ493606], Meg-β or β-megaspermin [AJ493607], and Meg-γ or γ-megaspermin [AJ493608]), Phytophthora sojae (Soj1 [CAA07710] and Soj2 [CAA07711]), Phytophthora capsici (Cap-α [P15571]), Pmm (Meg-α [AAB27563] and Meg-β [AAB27564]), P. cinnamomi (Cin-α [CAB38323], Cin-β [CAB38321], and Cin-HAE [CAB38322]), Phytophthora cryptogea (Cry-α1 [Z34462], Cry-β [Z34459], Cry-HAE20 [CAA84225], and Cry-HAE26 [CAA84226]), Phytophthora infestans (INF1 [U50844], INF2a [AF004951], INF2b [AF004952], INF4 [AF419841], INF5 [AF419842], and INF6 [AF419843]), Phytophthora parasitica (ParaA1 [AAB29433]), Phytophthora cactorum (Cac-α [2009394A]), Phytophthora drechsleri (Dre-α [P35696], Dre-β [P35697]), and Pythium vexans (Vex1 [AAB34416] and Vex2 [AAB34417]). Bank accession number for each protein is indicated between square brackets.
Figure 4.
Phylogeny of the elicitin family from Phytophthora spp. and Pythium vexans. The phylogenetic tree was constructed by the neighbor-joining method based on the mature protein sequences shown in Figure 3. The length of the branches reflects weighted amino acid substitutions and the scale bar represents 10% weighted sequence divergence. Vex1 and Vex2 were used as out groups. The calculated pI for each protein is indicated between square brackets. The five classes representing main clusters of the tree are indicated. The stars indicate proteins that may belong to another class, because two of the criteria defining an elicitin class are the pI and the occurrence of a C-terminal tail after the 98-amino acid-long elicitin domain. Thus, Dre-α would belong to class I-A and Inf4 to class I-B although Inf4 disclose a signal peptide sequence of class III elicitin (see Table III).
Table III.
Peptide signal sequences of the different classes of elicitins
| Class I-A | |
| α-Meg(PmH20) | MNFRALFAATVAALVGSTSA |
| α-Cry | MNFRALFAATVAALVGSTSA |
| α-Cin | MNFRALFAATVAALVGSTSA |
| Para A1 | MNFRALFAATVAALVGSTSA |
| Inf1 | MNFRALFAATVAALVGSTSA |
| Class I-B | |
| β-Meg(PmH20) | MNFTALLAAVAAALVGSANA |
| β-Cry | MNFTALLAAVAAALVGSANA |
| β-Cin | MNFTALLAAVAAALVGSANA |
| Class II | |
| Cin-HAE | MNFATLLAATAAALVGSVSA |
| Cry-HAE20 | MQFTALFAATAVALVGSVSA |
| Cry-HAE26 | MQFTALLAATAAALVGSVSA |
| Class III | |
| Inf2A | MNTKTFLAIAAAAFVGFAAA |
| Inf2B | MNTKTFLAISAAAVVGFAAA |
| γ-Meg(PmH20) | MNTYFAVAAAALAFVASVNG |
| Inf5 | MNTYIAVAAAALAVIASVNG |
| Inf4 | MNFVALIAVTVAVLVGSTNA |
| Inf6 | MNTYFVLASAVAALAGSADA |
Compared Biological Activity of α-, β-, and γ-Megaspermin in Planta
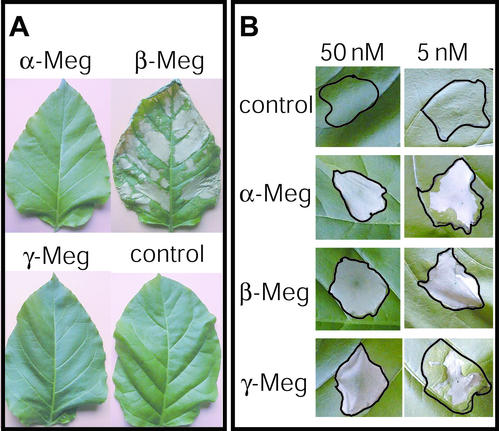
We first compared the induction of distal and local HR induced by α-, β-, and γ-megaspermin in tobacco. We conducted petiole dip assays using 1 ml of 100 nm of each protein. β-Megaspermin induced a strong distal necrosis, whereas α- and γ-megaspermin treated leaves remained without necrotic symptoms (Fig. 5A). Infiltration into tobacco leaves of 50 nm of each protein induced necrosis limited to the infiltration site (Fig. 5B), i.e. there was no distal necrosis in this test. Differences in HR induction became visible when the concentration of the infiltrated elicitins was lowered. For instance, 5 nm α- or γ-megaspermin triggered a partial necrosis, and 5 nm β-megaspermin caused necrosis of the whole infiltrated tissue (Fig. 5B).
Figure 5.
Distal and local induction of hypersensitive necrotic lesions by α-, β-, and γ-megaspermin. A, Petiole dip assay performed with 1 mL of 100 nm elicitin solution or water as a control. B, Infiltration assay performed with 50 or 5 nm elicitin solution or water as a control. Leaves were photographed 4 d after elicitor application.
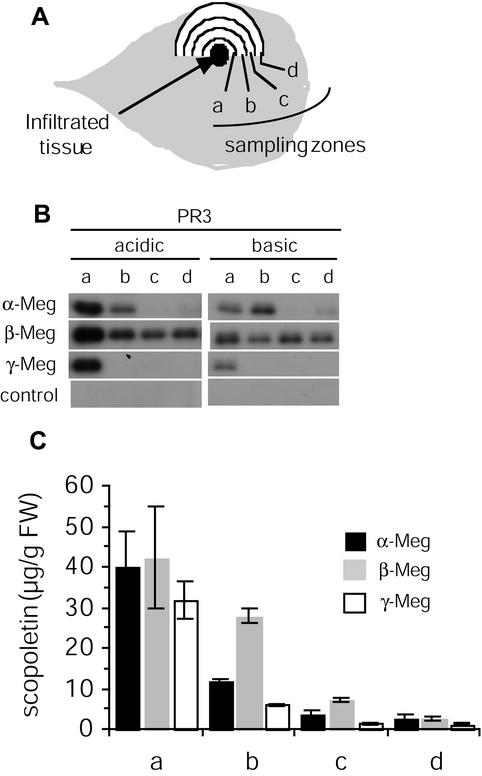
In the infiltration assay, we next compared the ability of the three proteins to induce acidic and basic PR3 protein expression and scopoletin accumulation in tissues beyond the zone of elicitor application, i.e. tissues exhibiting LAR. Tissues infiltrated with 50 nm elicitin were sampled as shown in Figure 6A. The three elicitins appeared similarly active in inducing strong acidic and basic PR3 proteins and scopoletin accumulation in zone “a”, which is adjacent to the infiltration site (Fig. 6, B and C). Then an elicitin-dependent decrease in PR and scopoletin levels occurred. Low acidic and basic PR3 accumulation was found in zones b, c, and d after β-megaspermin treatment and only in zone b after α-megaspermin treatment, and no PR was found in b, c and d after γ-megaspermin application. The rate of scopoletin decrease was highest with γ-megaspermin and lowest with β-megaspermin. Scopoletin levels decreased until they reached similar low levels in zone “d” after treatment with either elicitin.
Figure 6.
Acidic and basic PR3 protein expression and scopoletin accumulation in tissue exhibiting LAR after treatment with α-, β-, or γ-megaspermin. Tobacco leaves were infiltrated with 50 nm α-, β-, or γ-megaspermin or water. Tissues in the vicinity of the infiltrated tissues were collected 24 h after treatments and analyzed. A, Diagram showing the sampling zones. Each zone is 5 mm wide. B, Acidic and basic PR3 protein immunodetection after western blotting. C, Total (free + conjugated forms) scopoletin accumulation quantified by HPLC. No scopoletin was detected in control plants. Results have been expressed as the mean and sd calculated from two independent experiments.
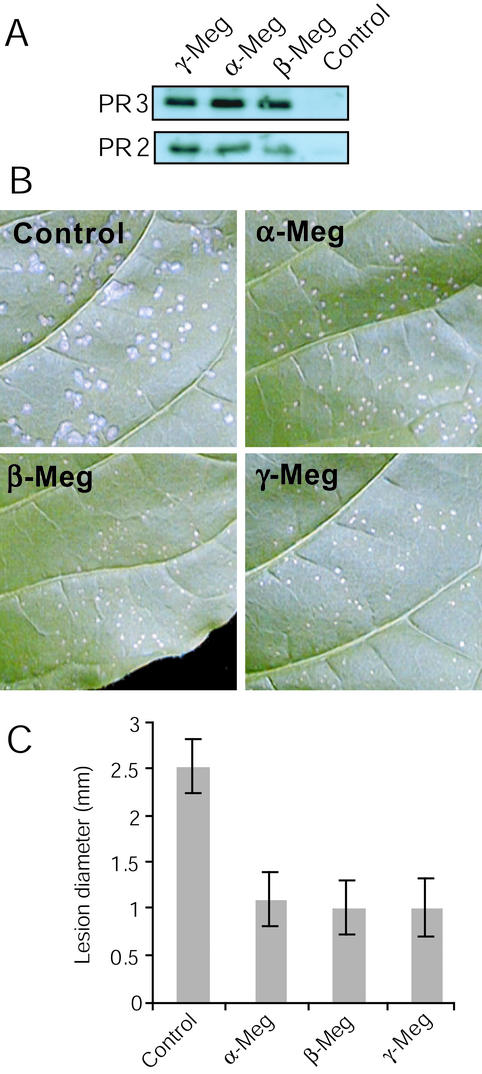
Because we have previously shown that the glycoprotein γ-megaspermin was able to induce SAR when infiltrated into tobacco leaves (Cordelier et al., 2003) and because it was claimed that the latter method of elicitin application does not lead to SAR activation (Ponchet et al., 1999), we investigated whether α- and β-megaspermin would induce SAR. Tobacco plants were infiltrated with 50 nm of elicitins (one elicitin per plant, six–eight infiltration sites per leaf, and three leaves per plant), and tissues from the systemic noninfiltrated leaves were collected 7 d after treatment to probe for acidic PR2 and PR3. Figure 7A shows a similar PR2 and PR3 accumulation in the systemic leaf of plants treated with α- or β- or γ-megaspermin, indicating a similar ability to induce SAR at the molecular level. We also analyzed SAR induction after treatment with 50 nm β-cryptogein, a type I-B elicitin from P. cryptogea, and found similar levels of PR2 and PR3 expression (data not shown). SAR expression was also analyzed for increased resistance to challenge TMV infection. Three leaves per plants were infiltrated at 6 to 8 spots with either elicitin at 50 nm. Six days later, the systemic nontreated leaves were inoculated with TMV, and lesions were observed after a further 6-d period. We also included a 50 nm β-cryptogein treatment (data not shown). Figure 7B shows a clear reduction in TMV lesion size in leaves treated with α-, β-, or γ-megaspermin compared with control. Measuring lesion diameter (Fig. 7C), we found no significant difference in the reduction in lesion size after either treatment, and the reduction rate was 60%. The β-cryptogein treatment resulted in the similar decrease in lesion size. Together with the acidic PR2 and PR3 expression analysis, this result suggested that α-, β-, and γ-megaspermin infiltrated at 50 nm into tobacco leaves induced a similar level of SAR.
Figure 7.
SAR expression in plants infiltrated with α-, β-, or γ-megaspermin. Three leaves per tobacco plant were infiltrated at six to eight spots with 50 nm α-, β-, or γ-megaspermin or water. Seven days after treatments, tissues from the upper noninfiltrated systemic leaf were either collected to probe for acidic PR2 and PR3 proteins by western blotting (A) or challenge inoculated with TMV and inoculated leaves were photographed after a further 6 d period (B), and lesion diameter were measured and expressed as the mean and sd calculated from a minimum of 100 lesions (C).
DISCUSSION
We have described the molecular cloning of full-length cDNAs encoding the PmH20 glycoprotein, inducing the HR, LAR, and SAR in tobacco, as well as the PmH20 α- and β-megaspermin. The comparison of the glycoprotein deduced amino acid sequence with that of a large set of elicitins, including the PmH20 α- and β-megaspermin, indicates that the glycoprotein belongs to the class III elicitin family. A glycoprotein of similar Mr was partially purified from P. capsici (Nespoulous et al., 1999). Its N-terminal amino acid sequence shows homology to the PmH20 glycoprotein. No homology to class III elicitins was reported for the P. capsici glycoprotein and no biological activity toward plants was tested. The PmH20 glycoprotein, renamed γ-megaspermin, is thus the first class III elicitin protein to be purified to homogeneity, studied for its biological activity in tobacco, and for which the complete amino acid and cDNA sequences have been characterized.
Besides the typical elicitin domain from amino acids 1 to 98 of the mature protein, with the six conserved Cys residues, γ-megaspermin discloses also unusual features for elicitins. Class I-A and I-B elicitins are holoproteins (Ponchet et al., 1999). The C-terminal domain of γ-megaspermin, from amino acids 99 to 154, is rich in Ser, Thr, Ala, and Pro, an amino acid composition and distribution that suggests potential O-glycosylation sites (Wilson et al., 1991). We have previously shown that γ-megaspermin carries an oligosaccharide moiety (Baillieul et al., 1995). Sequence analysis using the NetNGlyc 1.0, NetOGlyc (Hansen et al., 1997), and DictyOGlyc 1.1 (Gupta et al., 1999) prediction programs from the Centre for Biological Sequence Analysis indicates no N-glycosylation consensus sequences but several potential O-glycosylation sites located in the C-terminal domain. We have no experimental demonstration for the precise site(s) of O-glycosylation. Thr-121, -127, and -128 are potential sites because they were not identified after sequencing trypsic peptides T1 and T2 and peptide V8–2 and because they were suggested as potential sites running the NetOGlyc prediction program. The difference between the calculated molecular mass of the mature protein obtained after translation of the cDNA coding sequence, 14,920 D, and the experimental molecular mass obtained after mass spectrometry analysis, 18,676 D, suggests, considering one glycosylation site, 23 sugar residues, N-acetylhexosamine (mass of 203 D), and hexose (mass of 162 D) assuming the loss of one molecule of water in the formation of O-glycosylated bonds. Peptide sequencing revealed also the occurrence of γ-megaspermin with hydroxylated Pro residues in the C-terminal domain and γ-megaspermin without such posttranslational modifications.
On the basis of purification rates from 1 L of culture medium, the most abundant elicitin produced by PmH20 is β-megaspermin for which several tens of milligrams of the protein can be obtained. The two other elicitins appear similarly abundant because several milligrams of each protein was purified. This report further extends at the protein level previous findings showing the occurrence in the same Phytophthora spp. isolate of mRNA encoding elicitins from different classes (Kamoun et al., 1997). Furthermore, we have shown previously that γ-megaspermin homologs are secreted by such various Phytophthora spp. as P. cryptogea, P. cinnamomi, P. capsici, and P. parasitica (Baillieul et al., 1996). It suggests that production by Phytophthora spp. of elicitins from different classes is a rule rather than an exception. We did not obtain evidence, during protein purification or during cDNA cloning, of the production by PmH20 of class II elicitins.
It was reported that the partially purified 32-kD glycoprotein from P. capsici, showing homology in the N-terminal sequence with our PmH20 γ-megaspermin, displays phospholipase A2 activity, whereas other elicitins are devoid of such activity (Nespoulous et al., 1999). We tested different purified fractions of γ-megaspermin as well as α- and β-megaspermin and could not detect any phospholipase A2 activity. So far, it is not known whether γ-megaspermin displays an enzymatic activity.
The availability of three PmH20 elicitin proteins from three different classes allowed us to compare their elicitor activity in tobacco. Although α- and γ-megaspermin appeared similarly active in the different tests, β-megaspermin was shown to be the most active. β-megaspermin, but not α- and γ-megaspermin, caused distal necrosis. Such differences have been reported already for α- and β-elicitins (Kamoun et al., 1993; Bourque et al., 1998) and were shown to be attributable to elicitin diffusion through the vascular system (Devergne et al., 1992; Zanetti et al., 1992). Different pI between elicitins could explain their differential biological activities. β-Megaspermin has a calculated pI of 9.22, whereas that of α- and γ-megaspermin is 3.8. An acidic electric point would restrict elicitin diffusion at the acidic physiological pH in the negatively charged cell wall, resulting in lower amounts of acidic elicitins interacting with plasma membrane binding sites. The restriction would be even enhanced for γ-megaspermin because of the O-glycosylated C-terminal domain, because O-glycosidic side chains have been hypothesized to anchor proteins in cell walls (Kapteyn et al., 1999). Data using radiolabeled γ-megaspermin suggest that the protein would not migrate through the vascular system when applied to the petiole of a detached leaf, whereas α- and β-megaspermin do. The results with γ-megaspermin need, however, to be confirmed.
Elicitins behave differently when infiltrated into the leaf mesophyll. The three megaspermins showed to be similarly active, at 50 nm, in inducing the HR, which remained restricted to the infiltration site. A similar observation was reported previously comparing α- and β-type elicitins infiltrated at 100 nm into tobacco leaves (Kamoun et al., 1993). Differences in HR induction become visible when the concentration of the infiltrated PmH20 elicitins is lowered: for instance, whereas 5 nm α- or γ-megaspermin can trigger a partial necrosis, 5 nm β-megaspermin still causes necrosis of the whole infiltrated tissue. As for the distal necrosis activity, pI differences may explain such discrepancies between acidic and basic elicitins infiltrated at very low doses.
Expression of defense responses during LAR, i.e. in tobacco leaf tissues adjacent to tissues infiltrated with a HR dose of elicitin (50 nm), was shown for γ-megaspermin (Dorey et al., 1997) and cryptogein (Keller et al., 1996a). Here, we compared such expression after infiltration with the three PmH20 elicitins, representative of three elicitin classes. The treatment with either elicitin leads to the similar accumulation of PR proteins and of scopoletin in the tissues most proximal to the HR lesion, i.e. the 5-mm-wide tissues in contact with the necrotic lesion. Analyzing more distal tissues, β-megaspermin triggered a stronger response because PR protein and scopoletin accumulation was detected in distal tissues compared with treatments with α- or γ-megaspermin. This observation is puzzling. We have previously shown that in the infiltration assay, no radiolabeled γ-megaspermin (Dorey et al., 1997) or α-megaspermin (S. Kauffman, unpublished data) could be detected beyond the infiltration site. Radiolabeled β-cryptogein (50 nm), which shows only two amino acid changes compared with β-megaspermin, was also shown to strictly restrict to the infiltrated site (Keller et al., 1996a). Thus, the biochemical responses induced by either elicitin in the vicinity of the infiltration site, and characterizing LAR, result from the diffusion of a plant signal(s). We have previously shown that neither salicylic acid nor reactive oxygen intermediates would act as diffusible signals liberated by γ-megaspermin-treated tissues undergoing the HR (Dorey et al., 1997, 1998; Costet et al., 1999, 2002a; Cordelier et al., 2003). Data based on genetic and pharmacological approaches suggest that ethylene liberated by the HR cells would act as a LAR-diffusible signal regulating only a subset of defense responses during γ-megaspermin induced LAR (Cordelier et al., 2003). An explanation for the difference in LAR intensity induced by the three elicitins could be a different ethylene production without noticeable differences in cell death establishment. The higher intensity in cell death for β-megaspermin compared with α- and γ-megaspermin after infiltration low elicitin dose, i.e. 5 nm, support this hypothesis.
Tobacco plants infiltrated with either PmH20 elicitin or with β-cryptogein develop SAR. When infiltrated at a concentration that induces the HR with the same kinetics and intensity, i.e. 50 nm, typical SAR molecular markers, acidic PR2 and PR3 proteins, are similarly induced in the systemic nontreated leaves, and a similar reduction in TMV lesion size is observed on the SAR leaves. Because elicitins remain localized in the infiltration assay, SAR induction results from the systemic diffusion of a plant SAR signal(s). SAR has also been shown to be induced after the application of class I-A and I-B elicitins to decapitated tobacco plants, but it is a consequence of the systemic movement of the elicitins (Bonnet et al., 1996; Keller et al., 1996a). The infiltration assay, thus, more closely mimics an incompatible interaction, as in such interaction the pathogen remains localized at its site of penetration and pathogen-induced SAR results from the systemic diffusion of a plant signal (Sticher et al., 1997).
INF1, a class I-A elicitin from P. infestans, functions as an avirulence factor inducing the HR: INF1-deficient P. infestans strains induce disease lesions in Nicotiana benthamiana (Kamoun et al., 1998). Cryptogein high-affinity binding sites with receptor properties occur on tobacco plasma membrane preparations (Wendehenne et al., 1995). Thus, such receptor would function as a resistance gene in the gene-for-gene model, which is thought to be sufficient to explain resistance of Nicotiana spp. to Phytophthora spp. (Kamoun et al., 1999). Class I-A and I-B elicitins interact with the same tobacco receptor, with the same affinity (Bourque et al., 1998). Preliminary in vivo competition experiments indicate that 100 nm γ-megaspermin can inhibit the oxidative burst induced by a saturating 25 nm concentration of β-megaspermin applied to tobacco cell suspensions. It suggests that the class III elicitin γ-megaspermin would also interact with the same receptor of class I-A and I-B elicitins.
The function of the C-terminal glycosylated domain following the elicitin domain of γ-megaspermin remains to be elucidated. If class III elicitins interact with the same receptor interacting with class I-A and I-B elicitins, then the occurrence of this C-terminal domain seems not hinder the binding to the receptor. Class I-A and I-B elicitins function as sterols carriers, and β-elicitins are much more efficient than α-elicitins (Mikes et al., 1997, 1998). Experimental evidence suggests that the formation of an elicitin-sterol complex is a prerequisite for binding to the receptor and subsequent elicitor activity (Osman et al., 2001). To further investigate the latter proposed mode of action of elicitins, analysis of the sterol-carrying activity of γ-megaspermin would provide new insight in elicitin activity, particularly whether the C-terminal domain would enhance or reduce sterol-carrying activity and/or the affinity to the putative receptor, or has no effect.
In conclusion, elicitins are a remarkable model to study the perception by plants of fungal avirulent proteins and to unravel the triggered transduction pathways leading to resistance, which involves the HR and the production of host diffusible signals inducing LAR and SAR. Elicitins induce the HR, LAR, and SAR when applied in nanomolar concentrations. They are relatively easy to purify to homogeneity and in high amounts. They occur as different structural classes. Recent examples of unraveled mechanisms are the involvement of nitrate efflux as an essential component in elicitin-induced HR (Wendehenne et al., 2002), the involvement of reactive oxygen intermediates as negative regulators of LAR rather than as diffusible signals (Costet et al., 2002a), and the effects of cytosolic free calcium in response to elicitins (Lecourieux et al., 2002). Molecular characterization of the receptor of elicitins should be the next challenge to obtain an integrated view of elicitin mode of action.
MATERIALS AND METHODS
Enzymatic Cleavage and Microsequencing
The three elicitins were isolated from the culture medium of Phytophthora megasperma H20 (PmH20) and purified to homogeneity as described (Baillieul et al., 1995). Before enzymatic digestion and N-terminal sequencing, the proteins were reduced with dithiothreitol and S-carboxymethylated with iodoacetamide (Stone et al., 1989). The S-carboxymethylated elicitins were digested with sequencing grade trypsin or protease V8 (Roche Diagnostics, Mannheim, Germany). Thirty nanomoles of elicitins was digested with 1 μg of protease. Trypsin digestion was performed according to Stone et al. (1989). For treatment with protease V8, the glycoprotein was dissolved in 100 mm ammonium carbonate buffer and 0.4 m urea, pH 7.8. Incubation was overnight at 25°C. The peptide mixture was loaded onto a C18 reversed-phased column (Waters, St. Quentin-Yvelines, France) equilibrated with 0.1% (v/v) trifluoroacetic acid. The elution was performed with a linear 0% to 70% (v/v) gradient of acetonitrile in 0.1% (v/v) aqueous trifluoroacetic acid. Microsequencing of peptides was carried out by the Edman method using a sequencer (model 473A, Applied Biosystems, Foster City, CA).
cDNA Cloning
Total RNA was extracted from PmH20 mycelium using Trizol according to manufacturer (Invitrogen, Carlsbad, CA). Reverse transcript were obtained from 2 μg of total RNA as described (Sambrook and Russel, 2001). Specific cDNAs were amplified by reverse transcriptase-PCR. After electrophoresis on a 1% (w/v) agarose gel and subsequent purification of cDNAs (Qiaquick purification kit, Qiagen USA, Valencia, CA), amplified products of appropriate length were cloned into pDrive vector according to manufacturer (Qiagen PCR cloning kit) and sequenced. 5′- and 3′-RACE reactions were conducted according to manufacturer (Smart RACE cDNA amplification kit, BD Biosciences Clontech, Palo Alto, CA).
Plants and Treatments
Tobacco (Nicotiana tabacum cv Samsun NN) plants were grown in a greenhouse and were placed 2 to 3 d before treatment in a growth room at 22°C ± 1°C with a photoperiod of 18 h. For plant infiltration treatments and for the petiole dip assay, the concentrated elicitin solution (at least 40 μm) was diluted into water to reach the desired concentration. Infiltrations were made with a syringe into the mesophyll of fully developed leaves. About 100 μL of solution was applied to infiltrate leaf areas of 3 to 4 cm2. The petiole dip assay was conducted on freshly cut tobacco leaves. Leaf petioles were dipped into a 1.5-mL microtube containing 1 mL of a 100 nm elicitin solution or water. One milliliter of the solution was usually taken up after about 2 h. Then leaves were transferred to small beakers containing water and kept in a growth room at 22°C ± 1°C.
PR Protein and Scopoletin Analysis
PR protein detection was performed on protein extracts made from 80 to 150 mg fresh weight tissue. Samples were ground in 2.5 volumes of MES buffer, pH 6, containing 14 mm β-mercaptoethanol and charcoal. The crude extract was clarified by centrifugation and used for PR protein immunodetection. Protein extracts corresponding to 4 mg fresh weight were loaded onto a 12.5% (w/v) resolving polyacrylamide gel. Electrophoresis was performed in Tris-Gly buffer (19.2 mm Tris and 2.5 mm Gly), pH 8.8, under a constant voltage of 100 V using the MiniProtean gel apparatus (Bio-Rad, Hercules, CA). After electrophoresis, proteins were transferred onto an Immobilon-P membrane (Millipore, Bedford, MA) for 1 h under a constant voltage of 8.5 V cm−1 in the electrophoresis Tris-Gly buffer containing 20% (w/v) methanol. After transfer, the membrane was soaked for 1 h at room temperature in the milk-PBS buffer containing 8 g L−1 NaCl, 0.2 g L−1 KCl, 1.15 g L−1 Na2HPO4, 0.21 g KH2PO4, 0.1% (v/v) Tween 20, and 5% (w/v) defatted powdered milk. The membrane was then soaked overnight at 4°C in the milk-PBS buffer containing the primary rabbit antibodies directed against the target PR protein (dilution 1/10,000). After four washes in the milk-PBS buffer, the membrane was soaked 2 h at room temperature in the same buffer containing the secondary antibodies (goat anti-rabbit antibodies, dilution 1/10,000) conjugated with alkaline phosphatase. After a first wash with the milk-PBS buffer, four additional washes were made in the PBS buffer exempt of milk and Tween 20. Immunodetection was performed with the immun-star chemiluminescent kit of Bio-Rad.
For scopoletin analysis, 50 to 100 mg of fresh leaf tissues was ground in 2.5 volumes of 90% (v/v) methanol containing 100 ng of 4-methylumbelliferone used as an internal standard to calculate the recovery rate for each sample. The cellular debris were pelleted by centrifugation, and the collected supernatant was left at −20°C for 1 h to flocculate chlorophylle, which was eliminated by centrifugation. The supernatant was diluted 10 times in the HPLC buffer containing 30 mm NaH2PO4 and 5% (v/v) acetonitrile, pH 3. Fifty and 150 μL of each sample were injected onto a C18 Nova Pak column (Waters). Elution was performed using a 0% to 30% (w/v) acetonitrile gradient in 30 mm NaH2PO4, pH 3, in 20 min at 1 mL min−1. Eluted compounds were detected by fluorescence using the Waters 470 scanning fluorescence detector calibrated for scopoletin detection (λex = 290 nm, λem = 402), and by UV absorption using the Waters 996 photodiode array detector set to perform every second a spectrum from 200 to 500 nm. Identification of the compounds was based on cochromatography with authentic standards.
ACKNOWLEDGMENTS
We thank Pierrette Geoffroy and Monique Leret (Institut de Biologie Moléculaire des Plantes-Centre National de la Recherche Scientifique) for the HPLC analysis of protease digested α- and γ-megaspermin and for peptide sequencing, respectively, and Dr. Michel Jacquinot (the Institute for Structural Biology, Grenoble, France) for the mass spectrometry analysis of γ-megaspermin.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.012658.
LITERATURE CITED
- Baillieul F, Fritig B, Kauffmann S. Occurrence among Phytophthoraspecies of a glycoprotein eliciting a hypersensitive response in tobacco and its relationships with elicitins. Mol Plant-Microbe Interact. 1996;9:214–216. [Google Scholar]
- Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S. A new elicitor of the hypersensitive response in tobacco: A fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995;8:551–560. doi: 10.1046/j.1365-313x.1995.8040551.x. [DOI] [PubMed] [Google Scholar]
- Baillieul F, Saindrenan P, Fritig B, Kauffmann S. A model system for the dissection of the hypersensitive response: A fungal glycoprotein elicits a HR in tobacco. In: Daniels MJ, Downie JA, Osbourn AE, editors. Advances in Molecular Genetics of Plant-Microbe Interactions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 323–326. [Google Scholar]
- Binet M, Humbert C, Lecourieux D, Vantard M, Pugin A. Disruption of microtubular cytoskeleton induced by cryptogein, an elicitor of hypersensitive response in tobacco cells. Plant Physiol. 2001;125:564–572. doi: 10.1104/pp.125.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blein J-P, Millat M-L, Ricci P. Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophthora cryptogea. Plant Physiol. 1991;95:486–491. doi: 10.1104/pp.95.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet P, Bourdon E, Ponchet M, Blein J-P, Ricci P. Acquired resistance triggered by elicitins in tobacco and other plants. Eur J Plant Pathol. 1996;102:181–192. [Google Scholar]
- Bourque S, Ponchet M, Binet MN, Ricci P, Pugin A, Lebrun-Garcia A. Comparison of binding properties and early biological effects of elicitins in tobacco cells. Plant Physiol. 1998;118:1317–1326. doi: 10.1104/pp.118.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordelier S, de Ruffray P, Fritig B, Kauffmann S. Biological and molecular comparison between localized and systemic acquired resistance induced in tobacco by a Phytophthora megaspermaglycoprotein elicitin. Plant Mol Biol. 2003;51:109–118. doi: 10.1023/a:1020722102871. [DOI] [PubMed] [Google Scholar]
- Costet L, Dorey S, Fritig B, Kauffmann S. A pharmacological approach to test the diffusible signal activity of reactive oxygen intermediates in elicitor-treated tobacco leaves. Plant Cell Physiol. 2002a;43:91–98. doi: 10.1093/pcp/pcf012. [DOI] [PubMed] [Google Scholar]
- Costet C, Fritig B, Kauffmann S. Scopoletin expression in elicitor-treated and tobacco mosaic virus infected tobacco plants. Physiol Plant. 2002b;115:228–235. doi: 10.1034/j.1399-3054.2002.1150208.x. [DOI] [PubMed] [Google Scholar]
- Costet L, Cordelier S, Dorey S, Baillieul F, Fritig B, Kauffmann S. Relationship between localized acquired resistance (LAR) and the hypersensitive response (HR): HR is necessary for LAR to occur, and salicylic acid is not sufficient to trigger LAR. Mol Plant-Microbe Interact. 1999;12:655–662. [Google Scholar]
- Devergne JC, Bonnet P, Panabières F, Blein JP, Ricci P. Migration of the fungal protein cryptogein within tobacco plants. Plant Physiol. 1992;99:843–847. doi: 10.1104/pp.99.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey S, Baillieul F, Pierrel MA, Saindrenan P, Fritig B, Kauffmann S. Spatial and temporal induction of cell death, defense genes, and accumulation of salicylic acid in tobacco leaves reacting hypersensitively to a fungal glycoprotein elicitor. Mol Plant-Microbe Interact. 1997;10:646–655. [Google Scholar]
- Dorey S, Baillieul F, Saindrenan P, Fritig B, Kauffmann S. Tobacco class I and II catalases are differentially expressed during elicitor-induced hypersensitive cell death and localized acquired resistance. Mol Plant-Microbe Interact. 1998;11:1102–1109. [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S. H2O2from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation or scopoletin consumption in elicitin-treated cultured tobacco cells. Plant Physiol. 1999;121:163–172. doi: 10.1104/pp.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Jung E, Gooley AA, Williams KL, Brunak S, Hansen J. Scanning the available Dictyostelium discoideum proteome for O-linked GlcNAc glycosylation sites using neural networks. Glycobiology. 1999;9:1009–1022. doi: 10.1093/glycob/9.10.1009. [DOI] [PubMed] [Google Scholar]
- Hansen JE, Lund O, Rapacki K, Brunak S. O-GLYCBASE version 2.0: a revised database of O-glycosylated proteins. Nucleic Acids Res. 1997;25:278–282. doi: 10.1093/nar/25.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S, Hraber P, Sobral B, Nuss D, Govers F. Initial assessment of gene diversity for the oomycete pathogen Phytophthora infestansbased on expressed sequences. Fungal Genet Biol. 1999;28:94–106. doi: 10.1006/fgbi.1999.1166. [DOI] [PubMed] [Google Scholar]
- Kamoun S, Lindquist H, Govers F. A novel class of elicitin-like genes from Phytophthora infestans. Mol Plant-Microbe Interact. 1997;10:1028–1030. doi: 10.1094/MPMI.1997.10.8.1028. [DOI] [PubMed] [Google Scholar]
- Kamoun S, Van West P, Vleeshouwers VGAA, de Groot KE, Govers F. Resistance of Nicotiana benthamiana to Phytphthora infestansis mediated by the recognition of the elicitor protein INF1. Plant Cell. 1998;10:1413–1425. doi: 10.1105/tpc.10.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun S, Young M, Glascock CB, Tyler BM. Extracellular protein elicitors from Phytophthora: host-specificity and induction of resistance to bacterial and fungal phytopathogens. Mol Plant-Microbe Interact. 1993;6:15–25. [Google Scholar]
- Kapteyn JC, Van Egmond P, Sievi E, Van Den Ende H, Makarow M, Klis FM. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and beta 1,6-glucan-deficient mutants. Mol Microbiol. 1999;31:1835–1844. doi: 10.1046/j.1365-2958.1999.01320.x. [DOI] [PubMed] [Google Scholar]
- Keller H, Blein JP, Bonnet P, Ricci P. Physiological and molecular characteristics of elicitin-induced systemic acquired resistance in tobacco. Plant Physiol. 1996a;110:365–376. doi: 10.1104/pp.110.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H, Bonnet P, Galiana E, Pruvot L, Friedrich L, Ryals J, Ricci P. Salicylic acid mediates elicitin-induced systemic acquired resistance, but not necrosis in tobacco. Mol Plant-Microbe Interact. 1996b;9:696–703. [Google Scholar]
- Kieffer F, Lherminier J, Simon-Plas F, Nicole M, Paynot M, Elmayan T, Blein JP. The fungal elicitor cryptogein induces cell wall modifications on tobacco cell suspension. J Exp Bot. 2000;51:1799–1811. doi: 10.1093/jexbot/51.352.1799. [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A. Analysis and effects of cytosolic free calcium elevations in response to elicitors in Nicotiana plumbaginifoliacells. Plant Cell. 2002;14:2627–2641. doi: 10.1105/tpc.005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Mikes V, Milat ML, Ponchet M, Panabieres F, Ricci P, Blein JP. Elicitins, proteinaceous elicitors of plant defense, are a new class of sterol carrier proteins. Biochem Biophys Res Commun. 1998;245:133–139. doi: 10.1006/bbrc.1998.8341. [DOI] [PubMed] [Google Scholar]
- Mikes V, Milat ML, Ponchet M, Ricci P, Blein JP. The fungal elicitor cryptogein is a sterol carrier protein. FEBS Lett. 1997;416:190–192. doi: 10.1016/s0014-5793(97)01193-9. [DOI] [PubMed] [Google Scholar]
- Milat ML, Ducruet JM, Ricci P, Marty F, Blein JP. Physiological and structural changes in tobacco leaves treated with cryptogein, a proteinaceous elicitor from Phytophthora cryptogea. Phytopathology. 1991a;81:1364–1368. [Google Scholar]
- Milat ML, Ricci P, Blein JP. Capsidiol and ethylene production by tobacco cells in response to cryptogein, en elicitor from Phytophthora cryptogea. Phytochemistry. 1991b;30:2171–2173. [Google Scholar]
- Nespoulous C, Gaudemer O, Huet JC, Pernollet JC. Characterization of elicitin-like phospholipases isolated from Phytophthora capsiciculture filtrate. FEBS Lett. 1999;452:400–406. doi: 10.1016/s0014-5793(99)00654-7. [DOI] [PubMed] [Google Scholar]
- Nespoulos C, Huet J-C, Pernollet J-C. Structure-function relationships of a and β elicitins, signal proteins involved in the plant-Phytophthorainteraction. Planta. 1992;186:551–557. doi: 10.1007/BF00198035. [DOI] [PubMed] [Google Scholar]
- Osman H, Vauthrin S, Mikes V, Milat ML, Panabieres F, Marais A, Brunie S, Maume B, Ponchet M, Blein JP. Mediation of elicitin activity on tobacco is assumed by elicitin-sterol complexes. Mol Biol Cell. 2001;12:2825–2834. doi: 10.1091/mbc.12.9.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard K, Ponchet M, Blein JP, Rey P, Tirilly Y, Benhamou N. Oligandrin: A proteinaceous molecule produced by the mycoparasite Pythium oligandrum induces resistance to Phytophthora parasiticainfection in tomato plants. Plant Physiol. 2000;124:379–395. doi: 10.1104/pp.124.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchet M, Panabieres F, Milat ML, Mikes V, Montillet JL, Suty L, Triantaphylides C, Tirilly Y, Blein JP. Are elicitins cryptograms in plant-Oomycete communications? Cell Mol Life Sci. 1999;56:1020–1047. doi: 10.1007/s000180050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugin A, Franchisse J-M, Tavernier E, Bligny R, Gout E, Douce R, Guern J. Early events induced by the elicitor cryptogein in tobacco cells: involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell. 1997;9:2077–2091. doi: 10.1105/tpc.9.11.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci P, Bonnet P, Huet J-C, Sallantin M, Beauvais-Cante F, Bruneteau M, Billard V, Michel G, Pernollet J-C. Structure and activity of proteins from pathogenic fungi Phytophthoraeliciting necrosis and acquired resistance in tobacco. Eur J Biochem. 1989;183:555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sasabe M, Takeuchi K, Kamoun S, Ichinose Y, Govers F, Toyoda K, Shiraishi T, Yamada T. Independent pathways leading to apoptotic cell death, oxidative burst and defense gene expression in response to elicitin in tobacco cell suspension culture. Eur J Biochem. 2000;267:5005–5013. doi: 10.1046/j.1432-1327.2000.01553.x. [DOI] [PubMed] [Google Scholar]
- Simon-Plas F, Rustérucci C, Milat M-L, Humbert C, Montillet J-L, Blein JP. Active oxygen species production in tobacco cells elicited by cryptogein. Plant Cell Environ. 1997;20:1573–1579. [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux J-P. Systemic acquired resistance. Annu Rev Phytopathol. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Stone KL, LoPresti MB, Crawford JM, DeAngelis R, Williams KR. Enzymatic digestion of proteins and HPLC peptide isolation. In: Matsudaira PT, editor. A Practical Guide to Protein and Peptide Purification for Microsequencing, San Diego: Academic Press; 1989. pp. 31–47. [Google Scholar]
- Suty L, Blein JP, Ricci P, Pugin A. Early changes in gene expression in tobacco cells elicited with cryptogein. Mol Plant-Microbe Interact. 1995;8:644–651. [Google Scholar]
- Tavernier E, Wendehenne J-P, Blein J-P, Pugin A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of a hypersensitive reaction in tobacco cells. Plant Physiol. 1995;109:1025–1031. doi: 10.1104/pp.109.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Blein JP. Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Binet MN, Blein JP, Ricci P, Pugin A. Evidence for specific, high-affinity binding sites for a proteinaceous elicitor in tobacco plasma membrane. FEBS Lett. 1995;374:203–207. doi: 10.1016/0014-5793(95)01108-q. [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Lamotte O, Frachisse J-M, Barbier-Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell. 2002;14:1937–1951. doi: 10.1105/tpc.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IBH, Gavel Y, von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991;275:529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti A, Beauvais F, Huet J-C, Pernollet J-C. Movement of elicitins, necrosis-inducing proteins secreted by Phytophthorasp., in tobacco. Planta. 1992;187:163–170. doi: 10.1007/BF00201933. [DOI] [PubMed] [Google Scholar]