Abstract
The vegetative phenotype of the auxin-resistant diageotropica (dgt) mutant of tomato (Lycopersicon esculentum Mill.) includes reduced gravitropic response, shortened internodes, lack of lateral roots, and retarded vascular development. Here, we report that early fruit development is also dramatically altered by the single-gene dgt lesion. Fruit weight, fruit set, and numbers of locules and seeds are reduced in dgt. In addition, time to flowering and time from anthesis to the onset of fruit ripening are increased by the dgt lesion, whereas ripening is normal. The dgt mutation appears to affect only the early stages of fruit development, irrespective of allele or genetic background. Expression of members of the LeACS (1-aminocyclopropane-1-carboxylic acid synthase, a key regulatory enzyme of ethylene biosynthesis) and LeIAA (Aux/IAA, auxin-responsive) gene families were quantified via real-time reverse transcriptase-polymerase chain reaction in both dgt and wild-type fruits, providing the first analysis of Aux/IAA gene expression in fruit. The dgt lesion affects the expression of only certain members of both the LeACS and LeIAA multigene families. Different subsets of LeIAA gene family members are affected by the dgt mutation in fruits and hypocotyls, indicating that the DGT gene product functions in a developmentally specific manner. The differential expression of subsets of LeIAA and LeACS gene family members as well as the alterations in dgt fruit morphology and growth suggest that the early stages of fruit development in tomato are regulated, at least in part, by auxin- and ethylene-mediated gene expression.
The onset of ovary development into fruit (fruit set) and fruit development are usually triggered by signals from pollination and fertilization. Fertilization-independent fruit set can also occur either naturally in parthenocarpic fruits (genetic parthenocarpy) or by induction via exogenous application of auxin or GAs to flowers. Reproductive processes in fleshy fruits have been perhaps best studied in tomato (Lycopersicon esculentum Mill.; Gillaspy et al., 1993; Giovannoni, 2001), and here we apply the availability of an auxin-resistant mutant of tomato to further elucidate the biochemical, genetic, and molecular mechanisms that regulate fruit set and the early stages of fruit development.
Artificial induction via auxin has long been used to study parthenocarpy in tomato (Gustafson, 1937). Application of auxin transport inhibitors that block export of auxins from the ovary also stimulates the development of parthenocarpic fruits (Beyer and Quebedeaux, 1974), an observation that is consistent with reports of higher levels of auxins in ovaries of parthenocarpic tomato fruits (Mapelli et al., 1978; Mapelli and Lombardi, 1982).
Auxins are also involved in cell expansion in fruit tissues. During tomato fruit development, two peaks in auxin content occur (Gillaspy et al., 1993). The first auxin peak occurs 10 d after anthesis, coinciding with the beginning of cell expansion. The second auxin peak appears later and coincides with the final phase of embryo development. In non-parthenocarpic tomato varieties, the number of seeds affects final fruit size (Varga and Bruinsma, 1986). Thus, embryo-synthesized auxin could be the source for the second auxin peak (Hocher et al., 1992). In accordance, in parthenocarpic fruits, this second peak is not detected and fruits are correspondingly smaller (Mapelli et al., 1978).
It is likely that auxin regulation of fruit development involves gene expression. Auxin induces the expression of several gene families, including the SAUR (small auxin up-regulated RNA), GH3, and Aux/IAA genes (Guilfoyle, 1998). The Aux/IAA genes constitute a family of early auxin response genes (Abel and Theologis, 1996) encoding proteins that contain nuclear localization signals and have short half-lives (Abel et al., 1994; Oeller and Theologis, 1995). The ability of Aux/IAA family members to form homo- and heterodimers, as well as heterodimers with DNA-binding auxin response factors, supports their role as regulators of auxin responses (for review, see Reed, 2001). In Arabidopsis, 29 Aux/IAA genes have been identified (Reed, 2001), some of which show differences in gene expression kinetics, tissue specificity, and responsiveness to auxin induction (Abel et al., 1995; Abel and Theologis, 1996; Kim et al., 1997). Characterization of mutant phenotypes for nine of the Arabidopsis Aux/IAA genes has provided functional evidence for the importance of Aux/IAA genes as regulators of various auxin responses (Timpte et al., 1992; Kim et al., 1996; Leyser et al., 1996; Reed et al., 1998; Rouse et al., 1998; Hamann et al., 1999; Tian and Reed, 1999; Nagpal et al., 2000; Reed, 2001; Rogg et al., 2001).
Several Aux/IAA Arabidopsis mutants also exhibit reproductive alterations as part of their phenotypes. The axr2-1 mutant has short inflorescences because of reduced cell length and cell number (Timpte et al., 1992). In contrast, the single unbranched inflorescence of axr3 plants is shorter than wild type because of reduced internode number (Leyser et al., 1996). The axr3 mutant also exhibits reduced seed set compared with wild-type plants (Leyser et al., 1996). Similarly, the iaa28-1 mutant has a lower seed yield, smaller siliques, and shorter inflorescence internodes (Rogg et al., 2001), whereas shy2-2 mutants flower early (Reed et al., 1998). Eleven members of the Aux/IAA gene family are expressed in tomato vegetative tissues (Nebenführ et al., 2000), but whether any of these genes influence tomato fruit development is unknown.
The involvement of ethylene in the ripening stage of tomato fruit is well documented (Olson et al., 1991; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993). However, the importance of ethylene in regulating early stages of tomato fruit growth has only recently been examined (Nakatsuka et al., 1998; Barry et al., 2000). The enzyme 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) catalyzes the first regulatory step in the ethylene biosynthesis pathway, conversion of S-adenosyl-l-Met into ACC, whereas ACC oxidase (ACO) catalyzes the final step, conversion of ACC into ethylene (Yang and Hoffman, 1984; Kende, 1993). Both ACS and ACO are encoded by multigene families (Fluhr and Mattoo, 1996). The eight tomato ACS (LeACS) genes characterized so far have differences in their tissue-specific expression patterns, developmental control, and kinetics of ethylene induction (Van der Straeten et al., 1990; Olson et al., 1991, 1995; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993; Spanu et al., 1993; Terai, 1993; Oetiker at al., 1997; Nakatsuka et al., 1998; Shiu et al., 1998) but it is not yet known how or if they interact with auxin to regulate fruit development.
The auxin-resistant dgt (diageotropica) mutant of tomato provides a tool to further investigate the interactions between auxin and ethylene in regulating several aspects of plant development. Plants that are homozygous for any of three independent alleles of dgt result in the same pleiotropic phenotype, which includes: reduced apical dominance and gravitropic response, hyponastic leaves, retarded vascular development, high levels of anthocyanin and chlorophyll, and lack of lateral roots (Zobel, 1973, 1974). Although endogenous levels of IAA are the same in both dgt and wild-type shoot apices (Fujino et al., 1988b), dgt hypocotyl segments do not elongate or produce ethylene in response to exogenously applied auxin (Kelly and Bradford, 1986). Roots of the dgt mutant are more resistant to growth inhibition by exogenously applied IAA, auxin transport inhibitors, and ethylene than wild-type roots (Muday et al., 1995). Very low ethylene concentrations can restore the reduced gravitropic response of dgt to wild-type levels but not with wild-type kinetics (Madlung et al., 1999).
In hypocotyls, the dgt mutation reduces auxin-induced expression of a subset of auxin-regulated genes such as LeSAUR and the LeIAA5, 8, 10, and 11 members of the tomato Aux/IAA gene family. However, the dgt mutation has no effect on the expression of other auxin-inducible genes such as Lepar and several other members of the LeIAA gene family (Mito and Bennett, 1995; Nebenführ et al., 2000). The expression of two auxin-regulated ACS (LeACS) gene family members is also reduced in response to applied auxin in dgt, but not wild-type seedlings, whereas that of another auxin-regulated LeACS gene is not (Coenen and Lomax, 2003). The means by which subsets of auxin- and ethylene-regulated genes are affected by the dgt mutation has not yet been determined.
Although the pleiotropic effects of the dgt mutation on a variety of auxin responses during vegetative development are well studied, the only published reports of dgt reproductive development state briefly that it is normal (Fujino et al., 1988a; Ludford, 1995). Here, we document profound differences in fruit development in dgt versus wild-type plants. The expression of a subset of LeACS and LeIAA gene family members is also altered in dgt fruits. The observed changes are specific to early fruit development and different from those observed in vegetative tissues, indicating developmental specificity in the regulation of members of these auxin- and ethylene-responsive gene families by the DGT gene product.
RESULTS
The dgt Mutation Affects Fruit Size and Internal Anatomy of Tomato Fruit
We investigated the effects of the dgt mutation on fruit development using three different dgt alleles (dgt 1-1, dgt 1-2, and dgt dp) produced by three different mechanisms (spontaneous, ethyl methanesulfonate, and x-ray induced, respectively). The presence of the three different dgt alleles in four different isogenic and near-isogenic tomato varieties allowed us to evaluate possible allele- and background-specific effects. The dgt mutation affects the size, weight, and internal anatomy of tomato fruit. Fruit size is clearly reduced in dgt plants irrespective of genetic background (Fig. 1). Fruit weight, number of locules, and number of seeds per fruit varies for each genetic background, but with few exceptions, these characteristics are significantly reduced by the dgt mutation in each genetic background in both greenhouse and growth chamber experiments (Table I). The largest difference between dgt and wild-type fruits is in number of seeds per fruit, followed by fruit weight, and then by number of locules per fruit. The dgt mutation has less of an effect on all of these characteristics when plants are grown in growth chambers. The largest differences between greenhouse and growth chamber results are observed for the number of seeds per fruit, whereas the smallest differences are in fruit weight (Table I).
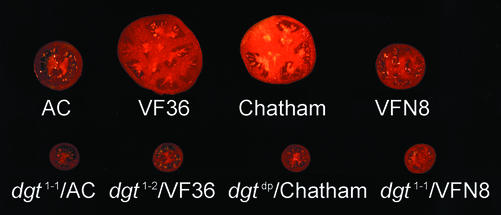
Figure 1.
The dgt mutation affects fruits characteristics irrespective of genetic background. Top, Ripe wild-type tomato fruits (Alisa Craig [AC]). Bottom, Ripe dgt tomato fruits in corresponding genetic backgrounds. Fruits were obtained from the greenhouse experiment.
Table I.
The dgt mutation affects fruit size and internal anatomy of tomato fruit
| Genotype | Fruit Wt
|
No. of Locules per Fruit
|
No. Seeds per Fruit
|
|||
|---|---|---|---|---|---|---|
| Greenhouse | Growth chamber | Greenhouse | Growth chamber | Greenhouse | Growth chamber | |
| g | ||||||
| AC | 32.6 bc | 47.5 c | 2.1 cd | 2.1 d | 82.5 a | 128 a |
| dgt1-1/AC | 6.2 d | 23.0 de | 1.1 e | 2.0 d | 13.2 cd | 61.9 df |
| VFN8 | 38.2 b | 69.8 b | 4.9 b | 5.6 b | 70.5 ba | 96.6 bc |
| dgt1-1/VFN8 | 9.8 d | 23.3 de | 1.3 ed | 3.5 c | 14.6 cd | 62.5 ef |
| VF36 | 64.9 a | 99.3 a | 7.3 a | 7.1 a | 48.6 bc | 101.9 b |
| dgt1-2/VF36 | 22.2 c | 44.4 cd | 3.1 cd | 5.1 b | 27.4 cd | 82.4 bf |
| Chatham | 28.8 c | 29.3 ce | 4.5 b | 5.0 b | 72.3 ba | 93.1 bde |
| dgtdp/Chatham | 8.4 d | 20.7 e | 2.7 cd | 3.7 c | 23.0 cd | 75.2 cef |
Effect of the dgt mutation on fruit weight and internal anatomy of tomato fruits. Mean fruit weight, locules, and seed nos. were measured at ripening in both greenhouse and growth chamber experiments from at least 30 fruits. Values within a column followed by the same letter are not significantly different at the P = 0.05 level by Tukey's Studentized Range (HSD, honest significant difference) test.
Relative growth rate is also significantly lower in dgt compared with wild-type fruits. For example, in a typical experiment comparing VFN8 and dgt 1-1/VFN8 fruits, the wild-type relative growth rate was 0.10 cm d−1, whereas the dgt relative fruit growth rate was 0.04 cm d−1 (P = 0.05; data not shown). Fruit set is also dramatically decreased by the dgt mutation, with 70% to 93% reduction under greenhouse conditions and 11% to 64% reduction in plants grown in growth chambers (data not shown).
Mutation of the Dgt Gene Delays the Onset of Fruit Development
In addition to affecting fruit characteristics, the dgt lesion delays the onset of reproductive development, measured as the number of days from planting to anthesis. Depending on the allele and parent line tested, the dgt lesion delays first anthesis from 35 to 70 d in the greenhouse and from 23 to 49 d in growth chambers. Figure 2 shows a representative developmental time course for dgt 1-1/VFN8 and the wild-type isogenic parent line, VFN8. The number of internodes produced before flowering also increases in the dgt mutant. For example, dgt 1-1/VFN8 produced eight more internodes before flowering than did wild-type VFN8 plants in a typical greenhouse experiment (data not shown). Similar results were seen with the other dgt alleles and genetic backgrounds.
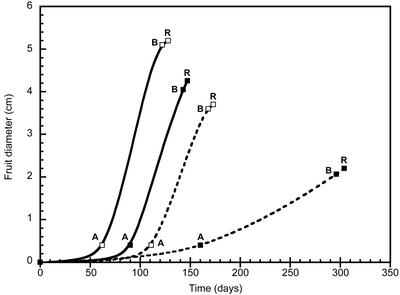
Figure 2.
The dgt mutation delays the onset of reproductive development and reduces fruit size. Fruit diameters were measured three times per week from the time of fruit set until ripeness and increases were plotted against time from planting to ripeness. Values represent means of at least six fruits. Diameters at anthesis (A), breaker (B), and red ripe (R) are shown. —▪—, VFN8, greenhouse; —□—, VFN8, growth chamber; --▪--, dgt 1-1/VFN8, greenhouse; --□--, dgt 1-1/VFN8, growth chamber.
The time necessary for fruits to progress from anthesis (A) to breaker (B) stage, the first appearance of orange color at the blossom end of fruit, is also dramatically increased in all dgt mutant alleles under greenhouse conditions. For example, dgt 1-1/VFN8 requires 83 more d to develop from anthesis to B than wild-type VFN8 (Fig. 2). When grown in growth chambers, the time from anthesis (A) to breaker (B) is similar for wild-type and corresponding dgt fruits. The time from B to red ripe (R) is not changed significantly by the dgt lesion for any mutant allele background comparison in either condition tested (Fig. 2; data not shown).
Ethylene Evolution during dgt and Wild-Type Fruit Development
To test whether the dgt mutation affects ethylene production, the rate of ethylene evolution was measured in mutant and wild-type fruits at several stages of development. In all cases, ethylene production is low in preclimateric fruit and increases at the onset of ripening. A peak in ethylene production occurs at the orange (O) stage and declines slightly later. Although minor differences are observed at certain stages, no clear pattern of differential ethylene production between mutant and wild-type fruits is found in the four genetic backgrounds at any stage of fruit growth or ripening (Table II).
Table II.
Ethylene evolution during dgt and wild-type fruit development
| Genotype | Fruit Stages
|
|||||
|---|---|---|---|---|---|---|
| IG | MG | B | O | R | FR | |
| nL g h−1 | ||||||
| AC | 0.11 ± 0.26 | 2.99 ± 1.78 | 3.44 ± 0.83 | 5.12 ± 1.70 | 4.63 ± 2.99 | 0.89 ± 0.53 |
| dgt1-1/AC | 0 | 2.74 ± 0.42 | 7.56 ± 2.90 | 9.38 ± 4.27 | 2.03 ± 0.82 | 0.97 ± 0.29 |
| VFN8 | 0 | 2.25 ± 0.62 | 5.21 ± 2.62 | 2.96 ± 1.75 | 4.09 ± 2.94 | 3.71 ± 2.46 |
| dgt1-1/VFN8 | 0.18 ± 0.21 | 1.32 ± 0.91 | 5.74 ± 2.49 | 11.06 ± 2.61 | 2.71 ± 1.47 | 5.90 ± 2.90 |
| VF36 | 0 | 0.46 ± 0.09 | 3.68 ± 0.32 | 6.79 ± 2.40 | 6.11 ± 1.39 | 2.50 ± 1.70 |
| dgt1-2/VF36 | 0.04 ± 0.09 | 4.30 ± 2.10 | 4.24 ± 1.13 | 8.77 ± 2.58 | 5.90 ± 1.70 | 1.17 ± 0.11 |
| Chatham | 0 | 0.37 ± 0.17 | 9.64 ± 3.43 | 11.96 ± 0.55 | 3.24 ± 0.99 | 1.29 ± 0.31 |
| dgtdp/Chatham | 0.07 ± 0.18 | 1.60 ± 0.6 | 12.1 ± 3.6 | 12.22 ± 2.9 | 12.29 ± 4.76 | 6.00 ± 1.70 |
Ethylene production from dgt and wild-type tomato fruits during development. Fruits were harvested at the following stages: immature green (IG), mature green (MG), breaker (B), orange (O), red ripe (R), and full ripe (FR). Values are expressed in nanoliters per gram per hour (±se of the mean, n = 4–12 fruits). Fruits were obtained from the growth chamber experiment.
Differential Expression of ACS Genes during Fruit Development in dgt and Wild-Type Tomato Plants
To investigate whether the dgt mutation affects the expression of ethylene-responsive and nonresponsive genes from a single gene family during fruit development, relative RNA expression patterns were determined using real-time reverse transcriptase (RT)-PCR for each LeACS gene family member in dgt and wild-type tomato fruits. Transcript levels of LeACS genes were normalized to transcript levels of RPL2 (ribosomal protein large subunit 2; Fleming et al., 1993) to allow quantification of gene expression relative to an endogenous control.
Among the eight members of the LeACS gene family, transcripts from LeACS 2, 4, and 6 are detected in both wild-type and dgt fruits (Fig. 3, A–C). LeACS7 mRNA is detected in wild-type fruits at 15 DPA and the immature green (IG) stage, but not in dgt fruits at any stage (Fig. 3D). Similarly, LeACS7 transcripts are also present in wild-type hypocotyls but not detected in dgt hypocotyls (data not shown). LeACS1B, 3, and 5 transcripts were detected at the IG stage in both wild-type and dgt fruits only when higher concentrations of template were used (0.25 versus 0.025 μg μL−1; data not shown), indicating that transcripts from these genes occur at lower abundance than LeACS2, 4, and 6 transcripts in tomato fruits. The LeACS1A gene was expressed in wild-type fruits at low relative levels in all developmental stages evaluated. In dgt fruits, transcripts of the LeACS1A gene were detected only at the mature green (MG) and O stages, where their relative levels were comparable with those found in the wild-type fruits (data not shown).
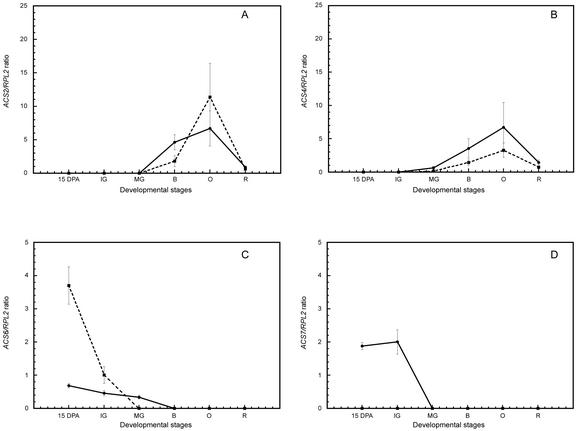
Figure 3.
Differential expression of LeACS genes during fruit development in wild-type and dgt plants. Fruits of both dgt (dotted lines) and wild-type (solid lines) were harvested at the following stages: 15 DPA, IG, MG, B, O, and R. Expression levels of LeACS transcripts relative to RPL2 were measured via real-time RT-PCR as described in “Materials and Methods.” A, LeACS2. B, LeACS4. C, LeACS6. D, LeACS7. Note different scales.
Expression of LeACS2 and 4 is similar in dgt and wild-type fruits (Fig. 3, A and B) and follows the well-documented ripening-related patterns of expression (Olson et al., 1991; Rottmann et al., 1991; Yip et al., 1992; Lincoln et al., 1993). Transcripts of these genes are not detected in preclimateric stages of fruit development (15 DPA, IG, and MG), increase from the B to the O stage, and decline thereafter (Fig. 3, A and B). In contrast, the LeACS6 gene is expressed at 15 DPA and the IG and MG stages in wild-type fruits, but is not detected during ripening (Fig. 3C). In dgt fruits, LeACS6 is expressed at 15 DPA and IG stage, and the expression level at 15 DPA is 5-fold higher than in wild-type fruits (Fig. 3C). The LeACS6 gene is expressed at lower relative levels than LeACS2 and 4 in wild-type fruits (Fig. 3, A–C), whereas in dgt, the relative expression levels of LeACS6 at 15 DPA are comparable with those of LeACS4 in the O stage (Fig. 3, B and C).
Differential Expression of Members of the LeIAA Gene Family during Fruit Development in Wild-Type and dgt Tomato Plants
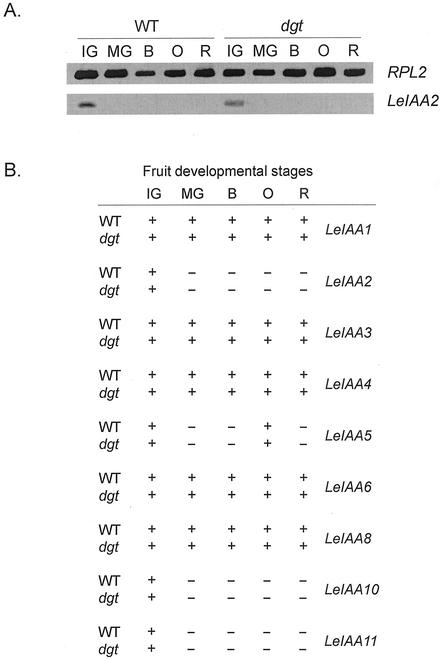
To determine how the expression of the LeIAA genes in fruits compares with patterns previously reported in seedlings (Nebenführ et al., 2000), we analyzed endogenous expression of LeIAA genes in wild-type and dgt fruits using RT-PCR. Although LeIAA1, 3, 4, 6, and 8 are constitutively expressed in all five fruit developmental stages, LeIAA2, 10, and 11 transcripts are only present at the IG stage of tomato development. LeIAA5 is expressed only at the IG and O developmental stages (Fig. 4). No differences between dgt and wild-type expression were observed for any of the genes using this technique.
Figure 4.
Differential expression of members of the LeIAA gene family during fruit development in wild-type and dgt plants. Fruits were harvested at the following stages: IG, MG, B, O, and R. A, Gene expression was analyzed by RT-PCR. Total RNA (2.5 μg) was used in the RT reaction in a final volume of 20 μL. The cDNAs generated were subsequently used in a 25-μL PCR reaction in the presence of specific primers for each LeIAA gene as well as the RPL2 control. The RT-PCR products were separated on a 1.5% (w/v) agarose gel stained with ethidium bromide. A representative experiment is shown. B, Presence (+) or absence (−) of LeIAA transcripts at different stages of fruit development in wild-type and dgt tomatoes. Data was obtained as described for A. No cDNA clone has been isolated for LeIAA7. LeIAA9 exhibited very low and erratic expression levels, precluding accurate analysis.
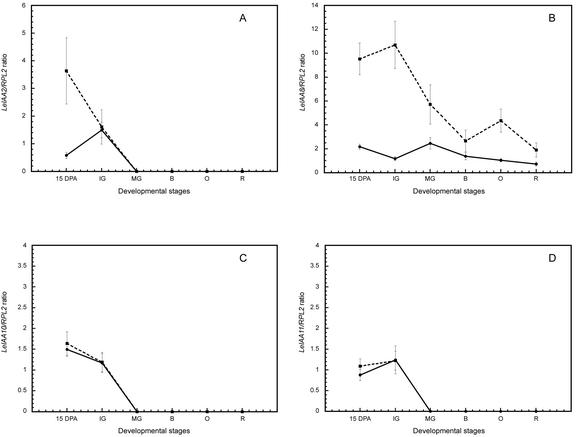
Further investigation of LeIAA2, 8, 10, and 11 using real-time quantitative RT-PCR revealed significant differences in the relative expression levels of LeIAA2 and 8. Transcripts from LeIAA2, 10, and 11 are detected in both wild-type and dgt fruits only at the 15-DPA and IG stages (Fig. 5, A, C, and D). In contrast, LeIAA8 is constitutively expressed in both wild-type and dgt fruits throughout all developmental stages evaluated (Fig. 5B). Although there are no significant differences in the relative expression levels of LeIAA10 and 11 between dgt and wild-type fruits, dgt fruits contain considerably higher levels of LeIAA2 transcript at the 15-DPA stage compared with wild-type fruits (Fig. 5, A, C, and D). Relative transcript levels of LeIAA8 are higher in dgt than in wild-type fruits during several stages of fruit development, most notably at the early stages (Fig. 5B).
Figure 5.
Differential expression of four members of the LeIAA gene family during fruit development in wild-type and dgt plants. Fruits of both dgt (dotted lines) and wild type (solid lines) were harvested at the same stages as those in Figure 3. Expression levels of LeIAA transcripts relative to RPL2 were measured via real-time RT-PCR as described in “Materials and Methods.” A, LeIAA2. B, LeIAA8. C, LeIAA10. D, LeIAA11. Note different scales.
DISCUSSION
Although the auxin-resistant dgt mutant has been reported to exhibit normal reproductive behavior (Fujino et al., 1988a; Ludford, 1995), we found that the dgt lesion dramatically reduces fruit size (Fig. 1), fruit weight, number of locules, number of seeds (Table I), and fruit set (data not shown), irrespective of genetic background or mutant allele.
Final fruit size results from the number of cells within the ovary before fertilization, the number of seeds, the number of cell divisions that occur in the developing fruit after fertilization, and the extent of cell expansion (Gillaspy et al., 1993). Locule number is also positively correlated with final fruit weight in tomato (Houghtaling, 1935; Yeager, 1937; MacArthur and Butler, 1938; Lippman and Tanksley, 2001). The involvement of all these factors in determining final fruit size clearly indicates the complexity of this phenomenon. The reduction in fruit weight in dgt—between 29% and 81% depending on the allele, parent line, and growth conditions (Table I)—may be explained in part by the reduced number of seeds. Seed number is proposed to enhance fruit growth by controlling cell division in the surrounding tissue (Varga and Bruinsma, 1986).
Relative fruit growth rate, measured from the time of fruit set (roughly defined as the point at which the ovary diameter triples from that at anthesis) until the B stage, is significantly lower in dgt fruits. Because cell division in tomato ovaries reportedly occurs for only 7 to 10 d after fertilization (Mapelli et al., 1978; Varga and Bruinsma, 1986) and most cell expansion stops at the B stage, the measured period roughly corresponds to the cell expansion phase of fruit development. The lower relative fruit growth rate found in dgt fruits suggests that reduced cell expansion also plays a role in the smaller final size of dgt fruits. When the growth of wild-type and dgt developing fruits was followed by measuring the increase in ovary diameter at 5-d intervals from pre-anthesis to 20 DPA, the larger size of wild-type ovaries was evident by 5 DPA (data not shown). Taken together, our results suggest that both cell division and cell expansion are involved in the generation of the smaller fruit size in dgt.
Fruit set is also greatly reduced by the dgt mutation. Auxins are implicated as part of the signal transduction pathway that controls fruit set in tomato (Gillaspy et al., 1993). We did not perform comparative studies of the auxin responsiveness of dgt fruits; therefore, dgt effects on fruit set cannot be directly tied to auxin. Because flowers were not manually pollinated, the lower fruit set may also be a result of an effect of the dgt mutation on pollen release rather than on fruit set directly.
Time to flowering, measured by the number of internodes produced before the reproductive switch and by the number of days from planting to anthesis, was significantly longer in dgt than in wild-type plants (Fig. 2). Of the several Arabidopsis Aux/IAA mutants exhibiting reproductive phenotypes as part of their related but distinct pleiotropic phenotypes, only the shy2-2 mutant, which exhibits early flowering, is reported to affect developmental time (Tian and Reed, 1999).
The dgt lesion also affects the developmental timing of the early stages of fruit growth. Although the time required for fruits to progress from anthesis to B is dramatically increased by the dgt mutation under greenhouse conditions, it is comparable with wild-type fruits under more controlled growth chamber conditions (Fig. 2). Taken together with the effects of growth conditions on fruit weight, number of locules, and seeds (Table I), these results indicate that reproductive development is more environmentally plastic in dgt plants. The average temperature varied between the greenhouse (28.2°C ± 3°C, days; and 15°C ± 2.3°C, nights; with a diurnal temperature range over the growing season between 23.3°C and 37.7°C) and the growth chambers (25°C ± 1.5°C, days; and 15°C ± 1.5°C, nights). However, differences in humidity, light levels, photoperiod, and/or CO2 levels may also influence the increased environmental plasticity of reproductive development in dgt. Detailed measurements of water potential, photosynthesis, and leaf area were outside the scope of this study, but will be needed to more exactly identify the environmental conditions that influence the plasticity of the dgt reproductive phenotype.
It is possible that some effects of the dgt mutation on fruit set, seed number, fruit size, and developmental time are indirect results of the reduced leaf area, and root and vascular systems typical of dgt plants. When reciprocal graftings between wild-type and dgt plants were performed, however, the mutant phenotype was maintained even in the presence of wild-type root stock (data not shown), indicating that the root biomass or structure is not responsible for the fruit differences. Photosynthetic rates are similar between dgt and wild-type plants (Lomax et al., 1993), but total leaf biomass may influence the final fruit characteristics.
Induction of ethylene synthesis via auxin has been reported (Yang and Hoffman, 1984; Yip et al., 1992). No clear pattern of differential behavior in total ethylene evolution between dgt and wild-type fruits was found at any stage of fruit development (Table II), which is consistent with the lack of significant change in developmental time from breaker to ripening in mutant versus wild-type fruits (Fig. 2). However, it remained possible that more subtle differences in isoform-specific ethylene evolution are involved in altered early fruit development in the dgt mutant.
Two systems have been proposed to explain the regulation of ethylene during plant development (for review, see Lelievre et al., 1997). System 1 provides for the basal level of ethylene present in vegetative tissues and preclimateric and non-climateric fruits, whereas system 2 is responsible for the high levels of ethylene production associated with ripening of climateric fruits and flower senescence (Oetiker and Yang, 1995). Specific members of the LeACS and LeACO gene families are proposed to regulate the transition from system 1 to 2 ethylene production (Nakatsuka et al., 1998; Barry et al., 2000). Based on their gene expression patterns, as well as their regulation by ethylene, LeACS2 and 4 are proposed to mediate system 2 ethylene production, whereas LeACS1A and 6 function in system 1 in green fruit and vegetative tissue (Nakatsuka et al., 1998; Barry et al., 2000).
To investigate whether either system 1 or 2 is altered by the dgt mutation, we measured the expression levels of all known LeACS gene family members relative to RPL2 from six developmental stages in both dgt and wild-type fruits using real-time RT-PCR (Fig. 3). The high sensitivity and specificity of real-time RT-PCR is important when analyzing the often low expression levels associated with the expression pattern of individual members of large gene families (Freeman et al., 1999). Our results generally agree with previous studies with respect to transcript occurrence; however, more subtle variations were observed via this more sensitive assay. Transcripts of LeACS1B, 3, and 5 were only detected when higher concentrations of template were used, indicating that the abundance of these transcripts is low (data not shown). Previous studies that did not detect expression of LeACS1B, LeACS5 (Nakatsuka et al., 1998; Barry et al., 2000), and LeACS7 (Barry et al., 2000) in fruits used ribonuclease protection assays and northern-blot analysis, which are less sensitive and specific than real-time RT-PCR.
Expression of LeACS2 and 4 was not detected in either wild-type or dgt fruits at the preclimateric stages, but increased equally in both genotypes with the onset of ripening (Fig. 3, A and B), indicating that system 2 is intact in mutant fruits and agreeing with our observations that ripening is unaffected by the dgt lesion. LeACS6 is only expressed early in the development in both dgt and wild-type fruits, a pattern that has been linked to the regulation of system 1 ethylene synthesis in tomato fruit (Nakatsuka et al., 1998; Barry et al., 2000). Transcript levels of LeACS6 were 4- to 5-fold higher in dgt versus wild-type fruits at 15 DPA (Fig. 3C). Expression of the LeACS6 gene is subject to negative feedback regulation by ethylene (Nakatsuka et al., 1998; Barry et al., 2000) and it will be interesting to determine whether the intact DGT gene product plays a role in its regulation by ethylene or represses LeACS6 expression via an alternate pathway. The greatly increased relative expression level of LeACS6 in dgt at 15 DPA also suggests that alterations in early fruit development result, at least in part, from changes in system 1 ethylene production. The LeACS1A gene, which also participates in system 1 ethylene production, is expressed in wild-type fruits at all developmental stages evaluated. Very low relative levels of LeACS1A transcript found in dgt fruits precluded definitive conclusions regarding the effect of dgt on LeACS1A expression (data not shown).
The LeACS7 gene, which has not previously been associated with system 1, is also exclusively expressed early in fruit development (15 DPA and IG). However, LeACS7 transcripts were not detected in dgt fruits at any stage (Fig. 3D). LeACS7 expression was also not detected in dgt hypocotyls but was present in wild-type hypocotyls (data not shown). LeACS7 has been reported to play an early and transient role during flooding and wounding responses (Shiu et al., 1998); however, the significance of LeACS7 in fruit development is not known. The accumulation of mRNAs from the auxin-inducible LeACS3 and 5 genes requires a wild-type Dgt gene in hypocotyls (Coenen and Lomax, 2003). It may be that intact Dgt expression is also necessary for the expression of LeACS7 and that the LeACS7 gene product is also involved in system 1 regulation of early fruit development.
The significant changes in LeACS transcript accumulation in dgt fruit take place when ethylene production is not reliably detected. There could potentially be posttranscriptional regulation such that activity of the ACS protein is not directly related to the steady-state levels of ACS mRNA. Evidence does exist for posttranslational regulation of ACS (Woeste et al., 1999; Vogel et al., 1998). Alternatively, the activity of ACO may be insufficient to allow significant ethylene production at that point in development. However, the correlation with the altered dgt fruit phenotype makes it most likely that the normal activity of LeACS1A, 6 and 7 during early fruit ontology is sufficient to modulate normal development, but too subtle to produce significant ethylene to be measured via gas chromatography (note the difference in scale between Fig. 3, C and D, and A and B).
Because dgt mutants are not affected in overall auxin metabolism or transport and auxin responsiveness is not completely abolished (Muday et al., 1995; Rice and Lomax, 2000), it has been proposed that the dgt lesion disrupts a specific step during early auxin signal transduction (Nebenführ et al., 2000). In hypocotyls, the dgt lesion specifically disrupts expression of a subset of Aux/IAA gene family members (LeIAA5, 8, 10, and 11) while not affecting others (e.g. LeIAA1–3). The developmental specificity of LeIAA gene expression in fruits differs from that previously found in seedlings (Figs. 4–6). For example, although LeIAA2 and LeIAA10 are constitutively expressed in seedlings (Nebenführ et al., 2000), both genes are only expressed at the IG stage in fruits (Fig. 4). This finding suggests that specific LeIAA family members play different functional roles during fruit development versus seedling growth. Interestingly, all LeIAA genes measured are expressed at the IG stage of fruit development (Fig. 4). This result may indicate that participation of all LeIAA gene members is required during the IG stage of tomato fruit development when cell expansion is the primary process driving fruit growth (Gillaspy et al., 1993).
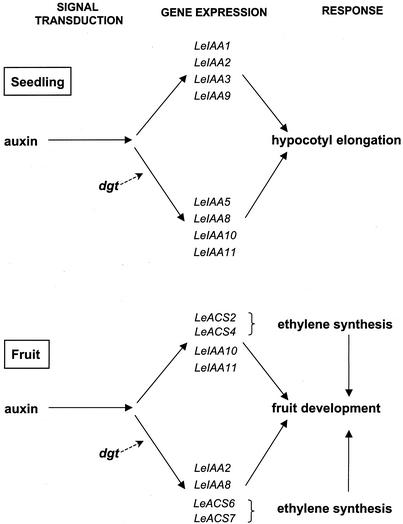
Figure 6.
Diagram of gene expression of members of the LeIAA and LeACS gene families in tomato seedlings and fruits. Note the differential developmental specificity in the expression of members of the LeIAA gene family during hypocotyl elongation and fruit development in wild-type and dgt plants. The broken arrows labeled dgt indicate that the expression of the genes in that pathway is either enhanced or lowered by the dgt lesion.
Real-time RT-PCR was used to more precisely measure relative expression levels of a subset of LeIAA genes that differ both in their endogenous expression patterns during fruit development (Fig. 4) and in the effects of the DGT gene product on their auxin regulation in seedlings (Nebenführ et al., 2000). Both LeIAA2 and LeIAA8 are expressed at higher levels in dgt at early stages of fruit development compared with wild-type fruits (Fig. 5, A and B). In contrast, expression of LeIAA10 and 11 is similar in wild-type and dgt fruits (Fig. 5, C and D). This is the opposite result from that found in hypocotyls, where the dgt mutation reduces transcript levels of LeIAA8, 10, and 11 but has no effect on LeIAA2 (Nebenführ et al., 2000). Thus, in different tissues and/or during distinct developmental processes, the DGT gene product regulates different members of the LeIAA gene family. The differences in expression between fruits (this study) and seedlings (Nebenführ et al., 2000) indicate either different functional roles for the Aux/IAA genes in regulating fruit and seedling development or different roles of DGT in these processes (Fig. 6). The dgt mutation does not seem to affect developmental specificity of LeIAA genes in terms of transcript occurrence but rather in terms of relative levels of expression.
Aux/IAA-mediated negative feedback has been proposed to allow tight regulation between auxin abundance and target gene expression in different cells (Reed, 2001), thus mediating tissue-specific responses to auxin during plant development. The dgt lesion may disrupt the function of Aux/IAA genes early in signal transduction and alter their role as tissue-specific mediators during the regulation of various developmental processes. This hypothesis could explain the highly pleiotropic phenotype of the dgt mutant. In this context, the up-regulation of LeIAA2 and 8 in dgt fruits indicates that the intact Dgt-gene product functions as a negative regulator in wild-type fruit tissues (Fig. 5, A and B). Although expression of LeIAA10 and 11 requires a functional DGT protein in hypocotyls, they appear to be regulated by a DGT-independent pathway during fruit development (Fig. 5, C and D).
To our knowledge, this study provides the first analysis of Aux/IAA gene expression in fruits, as well as the first comparison of gene expression patterns between the LeIAA and LeACS gene families during fruit development. The altered expression of specific members of the LeIAA and LeACS gene families in the dgt mutant suggests a role for those genes not only in generating the dgt reproductive phenotype, possibly as downstream targets of DGT, but also in the early development of wild-type fruits.
The correlation between altered expression of specific LeIAA and LeACS gene family members and differences in dgt and wild-type reproductive development indicates that auxin responsiveness and ethylene biosynthesis play significant roles in early fruit development and demonstrates the importance of the early stages of ovary/fruit development as determinants of mature fruit characteristics in tomato. Further studies should elucidate the complex mechanisms that regulate final fruit size and morphology in tomato. It will be especially interesting to determine whether dgt reproductive characteristics are determined pre- or postanthesis, as well as the relative importance of cell number and size in determining final fruit size.
MATERIALS AND METHODS
Plant Material
Four varieties of wild-type tomato (Lycopersicon esculentum Mill.)—Alisa Craig (AC), Chatham, VFN8, and VF36—as well as three alleles of the dgt mutation were tested under greenhouse and growth chamber conditions in facilities at Oregon State University. The mutant alleles used in this study were dgt 1-1, a spontaneous mutation in VFN8 (the isogenic parent) that was also extensively backcrossed into AC (a near-isogenic parent); dgt 1-2, an ethyl methanesulfonate-induced mutation in VF36 (the isogenic parent); and dgt dp (formerly called droopy; Jones and Jones, 1996), an x-ray-induced mutation in Chatham (the isogenic parent).
Growth Conditions and Phenotypic Measurements
For the greenhouse experiment, 10 plants of each mutant and corresponding parent were transplanted 2 weeks after germination and grown under greenhouse conditions with supplemental lighting (14 h of light and 10 h of dark). Greenhouse air temperature was set at 25°C during the day and 16°C at night. For the growth chamber experiment, four plants of each line were transferred from the greenhouse to growth chambers 4 weeks after germination. One plant of each mutant and its corresponding parent were placed in each of four identical growth chambers. An additional mutant/parent pair was assigned to each chamber to test for chamber-variety interactions. Light was supplied by a 1,000-W metal halide lamp in each chamber; photon flux densities averaged 400 μmol m−2 s−1. Plants in the growth chambers were subjected to the same day length and fertilizing conditions as used in the greenhouse experiment. Air temperatures were set at 25°C during the day and 15°C at night. Plants were watered twice daily by drip irrigation.
Individual flowers were tagged on the day of anthesis (flower opening) and dates from anthesis to B and ripening were recorded for each tagged flower. In addition, the dates for anthesis of the first flower on each plant, B, and ripening were recorded for at least five fruits from each plant for all varieties. Ripe fruits were individually analyzed with respect to fruit weight, number of locules, and number of seeds. The total number of flowers and fruits per plant were recorded at biweekly intervals to identify peak flowering times and to calculate percent fruit set (no. of fruits/no. of flowers). In the greenhouse experiment, fruit diameters were measured three times per week (about six fruits per plant) from the time of fruit set (roughly defined as the point at which the ovary triples in diameter compared with the diameter at anthesis) until the B stage to allow calculation of relative growth rate (diameter at B − diameter at fruit set/no. of days from fruit set to B). Finally, the number of internodes to first flower was recorded for each plant in both experiments.
Ethylene Evolution Measurements
Ethylene evolution in dgt and wild-type fruits was measured using a gas chromatograph (model GC-14A, Shimadzu, Kyoto) equipped with a flame ionization detector and a Poropak Q column (Waters, Milford, MA). Measurements were taken from growth chamber-grown fruits harvested at the following stages: IG (about 2–3 weeks after flowering), MG (pale-green color in fruit surface), B, O, R (red color and firm texture), and FR (red color and soft texture). At least four fruits from each variety and developmental stage were used. Fruits were harvested and maintained in open 135-mL containers for 1 h to reduce the effect of wound ethylene production caused by harvesting. The containers were then sealed with airtight serum stoppers (Fisher Scientific, Pittsburgh) and allowed to equilibrate for 1 h. A 1-mL headspace sample was withdrawn from the airtight container using a 1-mL gas-tight syringe (Hamilton Co., Reno, NV) and injected into the gas chromatograph.
RNA Extraction, Reverse Transcription, and RT-PCR
Total RNA was extracted from the pericarp of fruits at six developmental stages–15 DPA, IG, MG, B, O, and R—using a hot phenol method (Verwoerd et al., 1989). After extraction, RNA samples were treated with DNaseI (RQ1, Promega, Madison, WI). Complementary DNA was synthesized from 2.5 μg of total RNA using oligo(dT) primers and modified Moloney murine leukemia virus RT (SuperScript II; Life Technologies/Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Expression of the LeIAA gene family was analyzed by RT-PCR in the presence of specific primers for each LeIAA gene, as well as the RPL2 control essentially as described by Nebenführ and Lomax (1998). Expression analysis of LeIAA gene family members was performed in VFN8 and dgt 1-1/VFN8 and repeated in Chatham and dgt dp with similar results.
Analysis of Gene Expression by Real-Time RT-PCR
Real-time RT-PCR was performed with an ABI Prism 7700 sequence detection system (Central Services Laboratory, Oregon State University) using the SYBR Green PCR master mix kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. All real-time RT-PCR reactions were performed in Chatham and dgt dp fruits. Transcript levels of LeACS and LeIAA genes in the RNA samples were normalized with transcript levels of RPL2 to allow quantification of gene expression relative to an endogenous control. Primers for specific amplification of each cDNA were designed using the Primer Express software (Applied Biosystems), taking into account criteria such as product length, optimal PCR annealing temperature, and likelihood of primer self-annealing (Table III).
Table III.
Sets of real-time RT-PCR primers used to amplify gene-specific regions
| Gene | Primer Sequence (5′ → 3′) | Gene | Primer Sequence (5′ → 3′) |
|---|---|---|---|
| LeACS1A | LeACS7 | ||
| Sense | AGTATGCGATGAAATCTATGCTGCTA | Sense | TCTGGCACTGTTTTTAACTCACCTAA |
| Antisense | TCTGAATCCTGGAAATCCCAAG | Antisense | GGCACCAACTCGAAATCCTG |
| LeACS1B | LeIAA2 | ||
| Sense | TTCTTGACAAGGACACGCTACG | Sense | AAGCGAGCTATGTTAAAGTGAGCA |
| Antisense | ATTCAATCATCTCCTCAACCATTTC | Antisense | CCGTTGTATCCATCTGTTTCTGAA |
| LeACS2 | LeIAA8 | ||
| Sense | CTACGCAGCCACTGTCTTTGAC | Sense | CAAATACGTGAAGGTAGCAGTTGAC |
| Antisense | TGATTCCGACTCTAAATCCTGGTAA | Antisense | ACACCATTTGTAAGGTCCATAAGCT |
| LeACS3 | LeIAA10 | ||
| Sense | CCAGGCCTCGTTAGTGTCATG | Sense | GACTTCTCAAAAGCTTGATCGAGAG |
| Antisense | ATCTCATCGTTGGAATAGATTGCA | Antisense | TGAAATCTTTCATTCCTTGGACAA |
| LeACS4 | LeIAA11 | ||
| Sense | TTGCGACGAAATATATGCTGCT | Sense | AAAGAACAGTTTTAACGGACGTGAA |
| Antisense | CACTCGAAATCCTGGAAAACCT | Antisense | GACTTATCTGCATCCTCCAATGCT |
| LeACS5 | RPL2 | ||
| Sense | CACAGTATTCGATTGGCCAAAAT | Sense | CAGCGGATGTCGTGCTATGAT |
| Antisense | AAATCATGCCAACTCTGAAACCTG | Antisense | GGGATGCTCCACTGGATTCA |
| LeACS6 | |||
| Sense | TATGCAGCAACCGCGTTTAGT | ||
| Antisense | TGTACGAGTAAATAATCCCAACCCTAA |
PCR reactions were performed in triplicate in a 25-μL volume using 500 nm each forward and reverse primers, 12.5 μL of SYBR green master mix, 5 μL of a 1:10 (v/v) dilution of cDNA: 25 μL water. Reactions were performed in MicroAmp 96-well plates (Applied Biosystems) covered with optical adhesive covers (Applied Biosystems). Samples were subjected to a two-temperature thermal cycling consisting of denaturation at 95°C for 15 s, followed by anneal extension at 60°C for 1 min. To distinguish specific product from nonspecific products and primer dimers, a melting curve was obtained immediately after amplification by using the ABI PRISM Dissociation Analysis software (Applied Biosystems). The melting curve results were verified by subjecting PCR products to agarose gel electrophoresis and identifying the bands by DNA sequence analysis (Central Services Laboratory, Oregon State University).
Statistical Analysis
All statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Data were subjected to one-way ANOVA for comparison of means. The statistical significance of differences between means was determined using Tukey's Studentized Range (HSD, honest significant difference) test.
ACKNOWLEDGMENTS
The analysis of fruit development in dgt was initiated by Andreas Nebenführ. We are grateful to John Fowler, Patrick Breen, and Andreas Madlung for their insightful reviews of this manuscript. The excellent technical support of Kevin Smith, London Losey, and Kristi Barckley is gratefully acknowledged. Thanks are extended to TJ White for editing this manuscript.
Footnotes
This work was supported by the National Science Foundation Integrative Plant Biology Program. V.B. was supported by a Fulbright fellowship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010132.
LITERATURE CITED
- Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indole acetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. J Biol Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- Abel S, Oeller P, Theologis A. Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA. 1994;91:326–330. doi: 10.1073/pnas.91.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous I, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EM, Quebedeaux B. Parthenocarpy in cucumber: mechanism of action of auxin transport inhibitors. J Am Soc Hortic Sci. 1974;99:385–390. [Google Scholar]
- Coenen C, Lomax TL (2003) Cytokinin inhibits a subset of DGT-dependent primary auxin responses in tomato. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Fleming AJ, Mandel T, Roth I, Kuhlemeier C. The patterns of gene expression in the tomato shoot apical meristem. Plant Cell. 1993;5:297–309. doi: 10.1105/tpc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene: biosynthesis and perception. Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- Freeman WM, Walker SJ, Vrana KE. Quantitative RT-PCR: pitfalls and potential. Biotechniques. 1999;26:112–125. doi: 10.2144/99261rv01. [DOI] [PubMed] [Google Scholar]
- Fujino DW, Burger DW, Yang SF, Bradford KJ. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. cultivar VFN8) Plant Physiol. 1988a;88:774–779. doi: 10.1104/pp.88.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino DW, Nissen SJ, Jones AD, Burger DW, Bradford KJ. Quantification of indole-3-acetic acid in dark-grown seedlings of the diageotropica and epinastic mutants of tomato (Lycopersicon esculentum Mill.) Plant Physiol. 1988b;88:780–784. doi: 10.1104/pp.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ. Aux/IAA proteins and auxin signal transduction. Trends Plant Sci. 1998;3:205–207. [Google Scholar]
- Gustafson FG. Parthenocarpy induced by pollen extracts. Am J Bot. 1937;24:102–107. [Google Scholar]
- Hamann T, Mayer U, Jurgens G. The auxin-insensitive bodenlos mutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development. 1999;126:1387–1395. doi: 10.1242/dev.126.7.1387. [DOI] [PubMed] [Google Scholar]
- Hocher V, Sotta B, Maldiney R, Bonnet M, Miginiac E. Changes in indole-3-acetic acid levels during tomato (Lycopersicon esculentum Mill.) seed development. Plant Cell Rep. 1992;11:253–256. doi: 10.1007/BF00235076. [DOI] [PubMed] [Google Scholar]
- Houghtaling HB. A developmental analysis of size and shape in tomato fruits. Bull Torrey Bot Club. 1935;62:243–252. [Google Scholar]
- Jones DA, Jones JDG. is allelic to com, dp is allelic to dgt and pu-2 is allelic to al. Tomato Genet Coop Rep 46: 15. 1996. Allelism tests: [Google Scholar]
- Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiol. 1986;82:713–717. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Kim BC, Soh MS, Kang BJ, Furuya M, Nam HG. Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J. 1996;9:441–456. doi: 10.1046/j.1365-313x.1996.09040441.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre J-M, Latche A, Jones B, Bouzayen M, Pech J-C. Ethylene and fruit ripening. Physiol Plant. 1997;101:727–739. [Google Scholar]
- Leyser HM, Pickett FB, Dharmasiri S, Estelle M. Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J. 1996;10:403–413. doi: 10.1046/j.1365-313x.1996.10030403.x. [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottman WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Lippman Z, Tanksley SD. Dissecting the genetic pathway to extreme fruit size in tomato using a cross between the small-fruited species Lycopersicon pimpinellifolium and L. esculentum var. Giant Heirloom Genetics. 2001;158:413–422. doi: 10.1093/genetics/158.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Coenen C, Gaiser JC, Hopkins R, Rayle DL, Rice MS. Auxin perception and the regulation of tomato growth and development. In: Yoder JI, editor. Molecular Biology of Tomato: Fundamental Advances and Crop Improvement. Technomic Publishing Co., Inc., PA: Lancaster; 1993. pp. 129–138. [Google Scholar]
- Ludford P. Post-harvest hormone changes in vegetables and fruits. In: Davies PJ, editor. Plant Hormones, Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 725–750. [Google Scholar]
- MacArthur JW, Butler L. Size inheritance and geometric growth processes in the tomato fruit. Genetics. 1938;23:253–268. doi: 10.1093/genetics/23.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Behringer FJ, Lomax TL. Ethylene plays multiple non-primary roles in modulating the gravitropic response in tomato. Plant Physiol. 1999;120:897–906. doi: 10.1104/pp.120.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli SC, Frova G, Torti G, Soressi G. Relationship between set, development and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978;19:1281–1288. [Google Scholar]
- Mapelli SC, Lombardi L. A comparative auxin and cytokinin study in normal and to-2 mutant tomato plants. Plant Cell Physiol. 1982;23:751–757. [Google Scholar]
- Mito N, Bennett BA. The diageotropica mutation and synthetic auxins differentially affect the expression of auxin-regulated genes in tomato. Plant Physiol. 1995;116:271–278. doi: 10.1104/pp.109.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday GK, Lomax TL, Rayle DL. Characterization of the growth and auxin physiology of roots of the tomato mutant diageotropica. Planta. 1995;195:548–553. doi: 10.1007/BF00195714. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123:563–573. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, of 1-aminocyclopropane-1-carboxylase oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Lomax TL. Multiplex titration RT-PCR: rapid determination of gene expression patterns for a large number of genes. Plant Mol Biol Rep. 1998;16:323–339. doi: 10.1023/a:1007523305132. [DOI] [PubMed] [Google Scholar]
- Nebenführ A, White TJ, Lomax TL. The diageotropica mutation alters auxin induction of a subset of the Aux/IAA gene family in tomato. Plant Mol Biol. 2000;44:73–84. doi: 10.1023/a:1006437205596. [DOI] [PubMed] [Google Scholar]
- Oeller PW, Theologis A. Induction kinetics of the nuclear proteins encoded by the early indoleacetic acid-inducible genes, PS-IAA4/5 and PS-IAA6, in pea (Pisum L.) Plant J. 1995;7:37–48. doi: 10.1046/j.1365-313x.1995.07010037.x. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Olson DC, Shiu OY, Yang SF. Differential induction of seven 1-aminocyclopropane-1-carboxylate synthase genes by elicitor in suspension cultures of tomato (Lycopersicon esculentum) Plant Mol Biol. 1997;34:275–286. doi: 10.1023/a:1005800511372. [DOI] [PubMed] [Google Scholar]
- Oetiker JH, Yang SF. The role of ethylene in fruit ripening. Acta Hortic. 1995;398:167–178. [Google Scholar]
- Olson DC, Oetiker JH, Yang SF. Analysis of LE-ACS3, a 1-aminocyclopropane-1-carboxylic acid synthase gene expressed during flooding in the roots of tomato plants. J Biol Chem. 1995;270:14056–14061. doi: 10.1074/jbc.270.23.14056. [DOI] [PubMed] [Google Scholar]
- Olson DC, White JA, Edelman L, Harkins RN, Kende H. Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci USA. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001;6:420–425. doi: 10.1016/s1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- Reed JW, Elumalai RP, Chory J. Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics. 1998;148:1295–1310. doi: 10.1093/genetics/148.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MS, Lomax TL. The auxin-resistant diageotropica mutant of tomato responds to gravity via an auxin-mediated pathway. Planta. 2000;210:906–913. doi: 10.1007/s004250050696. [DOI] [PubMed] [Google Scholar]
- Rogg LE, Lasswell J, Bartel B. A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell. 2001;13:465–480. doi: 10.1105/tpc.13.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. Changes in auxin response from mutations in an AUX/IAA gene. Science. 1998;279:1371–1373. doi: 10.1126/science.279.5355.1371. [DOI] [PubMed] [Google Scholar]
- Shiu OY, Oetiker JH, Yip WK, Yang SF. The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc Natl Acad Sci USA. 1998;95:10334–10339. doi: 10.1073/pnas.95.17.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanu P, Boller T, Kende H. Differential accumulation of transcripts of 1-aminocyclopropane-1-carboxylate synthase genes in tomato plants infected with Phytophthora infestans and in elicitor-treated tomato cell suspensions. J Plant Physiol. 1993;141:557–562. [Google Scholar]
- Terai H. Behaviors of 1-aminocyclopropane-1-carboxylic acid (ACC) and ACC synthase responsible for ethylene production in normal and mutant (nor and rin) tomato fruits at various ripening stages. J Jpn Soc Hortic Sci. 1993;61:805–812. [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Timpte CS, Wilson AK, Estelle M. Effects of the axr2 mutation of Arabidopsis on cell shape in hypocotyl and inflorescence. Planta. 1992;188:271–278. doi: 10.1007/BF00216824. [DOI] [PubMed] [Google Scholar]
- Van der Straeten D, Van Wiemeersch L, Goodman HM, Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci USA. 1990;87:4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, Bruinsma J. Tomato. In: Monselise SP, editor. CRC Handbook of Fruit Set and Development. Boca Raton, FL: CRC Press; 1986. pp. 461–480. [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989;17:2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JE, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA. 1998;95:4766–4771. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Chen Y, Kieber JJ. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–530. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yeager AF. Studies on the inheritance and development of fruit size and shape in tomato. J Agric Res. 1937;55:141–152. [Google Scholar]
- Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiol. 1973;52:385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Control of morphogenesis in the ethylene-requiring tomato mutant, diageotropica. Can J Bot. 1974;52:735–741. [Google Scholar]