Abstract
The sugars in the endosperm of a developing seed have many potential roles, including the supply of carbon to the developing embryo and controlling gene expression in it. Our understanding of their metabolism is, however, fragmentary and is confined to a very few species (especially Vicia spp.). To develop a quantitative understanding of the regulation of sugars in seeds of oilseed rape (Brassica napus), we measured relevant enzyme activities, the sizes of the pools of sugars in the liquid endosperm, and the flux of sugars from the endosperm into the embryo. The concentrations of hexose sugars in the liquid endosperm decreased, and sucrose (Suc) increased through development. The overall osmotic potential also fell. The timing of the changes was not precise enough to determine whether they signaled the onset of rapid accumulation of storage products. Changes in endosperm invertase activity were complex and quantitatively do not explain the changes in sugars. The embryo can metabolize hexose sugars in addition to Suc, and possibly at higher rates. Therefore, in addition to invertase, the growing embryo itself has a potential to influence the balance of sugars in the endosperm. The activity of Suc synthase in the embryo was greater than that of invertase during development. This observation and a higher activity of fructokinase than glucokinase in the embryo are both consistent with the embryo using Suc as a carbon source.
In this paper, we use measurements of enzymes, sugars, and fluxes to examine the factors responsible for the regulation of concentrations of sugars in the liquid endosperm of developing seeds of oilseed rape (canola [Brassica napus]). These concentrations are important for numerous reasons. In addition to having osmotic consequences for the seed, they may be necessary for maintaining the flow of carbohydrate and its unloading into the developing seed (Patrick and Offler, 1995). The balance of concentrations of sugars has also been implicated in control of gene expression during development.
The interaction of metabolism and seed development is probably understood better in legumes and especially Vicia spp. than in any other dicotyledonous seed (for review, see Wobus and Weber, 1999). In Vicia spp. seeds, before storage product deposition in the developing embryo, the endosperm contains largely hexose sugar. The hexose sugars are produced from Suc by an invertase bound to the cell walls of parenchymatous cells facing into the liquid part of the endosperm. The high levels of hexoses maintain cell division and expansion in the embryo (Weber et al., 1996a, 1997b; Borisjuk et al., 1998). When the embryo expands to the point where it begins to touch these cells, they die (presumably by apoptosis), and the invertase activity disappears. As a consequence, the levels of hexoses fall and Suc becomes the main endosperm sugar. This induces a change of gene expression in favor of storage product accumulation. A change from predominantly hexose to Suc content in the endosperm during development has also been reported for pea (Pisum sativum; Borisjuk et al., 2002).
The work on legumes that has identified a correlation between the changes in sugars and the disappearance of the invertase in the cellular endosperm has focused attention on the role of this endosperm invertase in controlling the development and final size of the seed (Weber et al., 1996a). How widely the determination of embryo development by sugars, as described for Vicia spp., applies to other species is unknown. In Arabidopsis, genetic evidence indicates that the endosperm plays a role in determining seed size (Scott et al., 1998), although whether sugar metabolism in the endosperm is linked to this is not known. Baud et al. (2002) have measured the Suc and hexose content of whole Arabidopsis seeds during development, but it is not possible to determine from this how the sugar composition of the endosperm changes. Therefore in species other than legumes, the underlying mechanism of the effect of the endosperm on seed development is not yet known.
Although invertase is an important enzyme in the link between metabolism, the endosperm, and control of gene expression in Vicia spp. (and probably Pisum spp.), it is not the only factor, and its role is unclear. Attempts in Vicia narbonensis to manipulate sugars by ectopic expression of an extracellular invertase in the cotyledons (presumably the site of sugar sensing in the embryo) have been difficult to interpret because of pleiotropic effects (Neubohn et al., 2000). This is almost to be expected because the sugars have multiple roles, as signals, as carbon sources, and as an osmoticum. Furthermore, other enzymes and processes have also been implicated (sugar transporters [Weber et al., 1997a]; Suc synthase and Suc phosphate synthase [Weber et al., 1996b]; and interaction with nitrogen metabolism [Weber et al., 1998]). In addition, it is unlikely that either the Vicia spp. sugar model or any other model depending only on the endosperm will provide a full explanation of seed size. Alonso-Blanco et al. (1999) found that both maternal and non-maternal factors control seed size in Arabidopsis, maternal factors affecting cell number, non-maternal affecting cell size. They also conclude that cell size is influenced continuously by genotype throughout development and is not determined by some event at one, early stage.
Sugar metabolism in seeds has an obvious agricultural context because of the importance of seed size, but it also has a developmental context. It is a potential example of a self-governing, stable cycle of control and regulation. The sugars control expression of genes encoding enzymes whose activities are responsible for the regulation of the concentrations of sugars. Metabolic regulation and control of gene expression thus work together to determine the development of the seed. We chose to investigate this in seeds of oilseed rape, an important crop species with close genetic synteny to Arabidopsis that has seeds large enough to dissect by eye. In this species, we can also build on the work of King et al. (1997), who have thoroughly investigated the interactions of carbohydrate metabolism in the silique with those in the whole seed. In contrast to their approach, we have concentrated on the internal metabolism of the seed, and therefore dissected it to separate the liquid endosperm from the seed coat and embryo. We have then studied metabolism in the individual tissues as a detailed function of development.
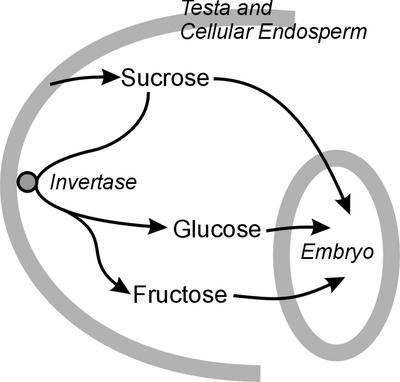
Our approach had two stages. First, we measured the sugars present in the liquid endosperm of oilseed rape seeds to study whether this species behaves similarly to Arabidopsis and Vicia spp. and to develop an accurate time course for changes in levels of sugars. Then, to explain the accurate time course, we examined the possibilities for production and consumption of each sugar. The concentration of a sugar clearly depends on its production and its use (Fig. 1). Levels of hexose sugars depend not on invertase alone but on a balance of synthesis by invertase and consumption by the tissues of the seed. Therefore, we measured invertase and the uptake of sugars by the embryo. To examine how the change in demand for sugars varies through development, we measured the major sugar-metabolizing enzymes through development. In this way, we attempted to look at as many of the processes in Figure 1 as possible.
Figure 1.
Schematic view of sugar movements within a developing seed of oilseed rape.
RESULTS AND DISCUSSION
The Endosperm Undergoes a Shift from Hexoses to Suc during Development
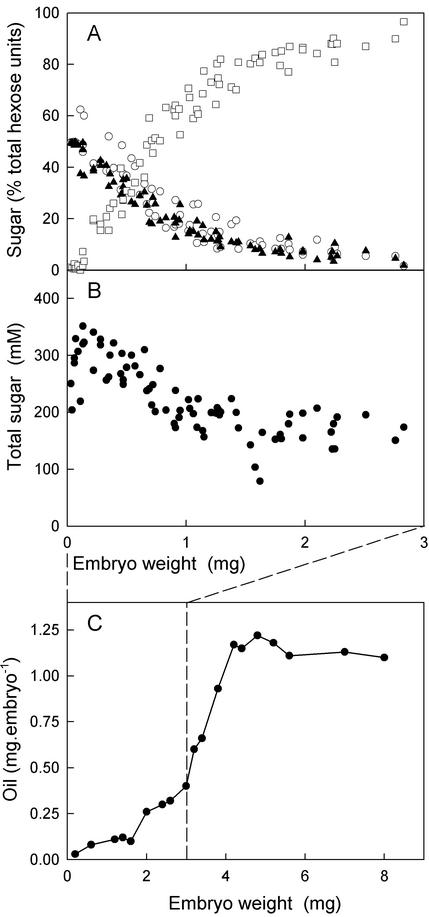
To enlarge on the understanding gained from Vicia spp. and the growing genetic evidence in Arabidopsis, we measured the sugar composition of the endosperm of oilseed rape seeds during their development. Oilseed rape seeds can easily be dissected into an embryo and an external part consisting of the testa and cellular endosperm, which we refer to here as “seed coat.” Between these is the uncellularized, liquid endosperm, which has a maximum volume of 2 μL (data not shown). This shrinks as the embryo grows and has largely disappeared by the time embryos have attained a fresh weight of 3 mg. At the beginning of embryo development, in the seed the liquid endosperm contains a large proportion of Glc and Fru and very little Suc (Fig. 2A). As the embryo develops, the hexose content of the endosperm declines while that of Suc increases, consistent with the Vicia spp. model (Wobus and Weber, 1999). This change is gradual and is confined to the endosperm. The sugar content of the embryo ranges between 0.58 and 0.88 μmol sugar embryo−1, with Suc composing more than 97% throughout development. Oil accumulation in the embryo (replotted from Eastmond and Raws-thorne [2000]; see also Murphy and Cummins, 1989; Kang et al., 1994; da Silva et al., 1997) was slower up to a fresh weight of 1.5 mg embryo−1 and then accelerated on an embryo basis reaching a maximum content of 1.25 mg lipid embryo−1 (Fig. 2C). Our measurements of the sugar composition of the endosperm stop at an embryo weight of 3 mg, because at this stage, the embryo had nearly filled the testa, and it was difficult to extract any liquid endosperm. The change in sugars in the endosperm is nearly complete before oil synthesis in the embryo accelerates (around 1.5 mg embryo weight). This suggests that some other factor triggered the onset of rapid synthesis of oil, although we cannot be certain because (a) it is possible that some relatively high threshold of Suc (or low threshold of hexose) was the trigger, and (b) we do not know how long the embryo takes to react to the trigger.
Figure 2.
Sugars in the liquid endosperm of developing seeds of oilseed rape. The weight of the embryo is used as a developmental scale (x axis), but the data in A and B relate only to the endosperm. A, Suc (□), Glc (○), and Fru (▴) are plotted as percentages of the total hexose moieties found in all three. B, The summed concentration of Suc, Glc, and Fru. Note that in A, which is intended to display the balance of forms carbohydrate, Suc counts as two hexose moieties. In B, which is concerned with the osmotic potential of endosperm solutes, Suc is counted as a single molecule. In both panels, each point represents a single silique. We harvested a range of siliques on 4 different d, and we have pooled the four data sets in this figure. C, The oil content of developing embryos, taken from Eastmond and Rawsthorne (2000). Each data point represents a value obtained from a batch of five embryos. Very similar developmental profiles have been measured previously within our laboratory (Murphy and Cummins, 1989; Kang et al., 1994; da Silva et al., 1997). Note the relative timings of the onset of rapid oil synthesis and the change in sugar content (A), taking into account the x axis scaling.
Figure 2A shows the sugars as their hexose equivalents (1 Suc = 2 hexose units), plotted as a percentage of the total hexose units present. This is more accurate than our estimate of the actual concentration of sugar (Fig. 2B) because it does not depend on accurate pipetting of a small volume of liquid under conditions where evaporation is likely. Nevertheless Figure 2B shows that the change toward Suc is accompanied by a decrease in the total concentration of sugar during development. The total amount of carbohydrate measured in hexose units per volume of liquid endosperm remains little changed, but a greater proportion of it exists as Suc. This is consistent with invertase playing a role in maintaining a high osmotic pressure inside the testa at the time when the testa is expanding.
Changes in Acid Invertase Only Partially Explain Changes in Sugar Concentrations
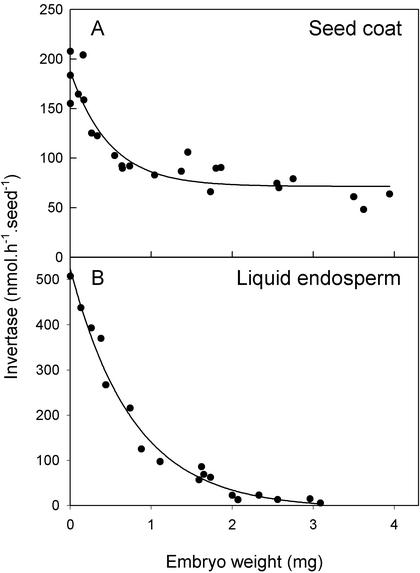
The activities of acid invertases found in the liquid endosperm and associated with the seed coat decline throughout development (Fig. 3). There was more invertase in the liquid endosperm than on the seed coat, and the invertase of the liquid endosperm fell more completely than that of the seed coat. It is therefore possible that changes in the liquid endosperm activity may be responsible for the high concentrations of hexose sugars in the endosperm. The cell wall-bound acid invertase of the seed coat, however, has historically been held responsible for the hexoses (Weber et al., 1996a). To deduce which invertase is responsible for the hexoses, we need to answer three questions. First, are the invertases sufficiently active? Both have activities that are large relative to estimated in vivo fluxes. An oilseed rape embryo can synthesize lipids at up to 78 nmol acetate embryo−1 h−1, which is equivalent to 39 nmol hexose embryo−1 h−1 assuming acetate is produced through glycolysis. This is less than the minimum activity of acid invertase found in the seed coat and much less than the activity of either invertase earlier in development. The second question is whether the invertases are in the appropriate tissues. The endosperm invertase and sugars were extracted together in a single sample, but because this sample contained some cellular material (light microscopy, results not shown), we cannot be certain that the invertase had free access to the sugars. Fractionation of the liquid endosperm to examine this material is not meaningful, because in early stages it is likely to contain genuine but fragile partially developed cellular endosperm, which would easily rupture on extraction. In contrast, the mechanical effort necessary to extract endosperm from older seeds is likely to damage adjacent tissues and to produce artifactual cell debris. The last question is whether the comparatively small change in acid invertase could explain the large changes in hexoses. We provide two explanations of how it could. First, the concentration of Suc in steady-state conditions does not vary linearly with the Vmax of the invertase consuming it. If we assume invertase has saturation kinetics, we can estimate its behavior using the Michaelis-Menten equation, which can be rearranged as follows:
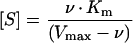 |
Under steady-state conditions, v, the rate of degradation of Suc, is equal to the rate of supply of Suc by the testa minus direct uptake of Suc by the embryo (see Fig. 1). Therefore the concentration of Suc is low and fairly constant so long as the Vmax of invertase is much higher than the supply of Suc. As we consider smaller values of Vmax, only slightly more than the rate of supply, the concentration of Suc must increase dramatically to maintain a steady state. Thus a small decrease in activity of invertase at some point in development can cause a larger increase in Suc, depending on how the activity compares with the current balance of fluxes of Suc between the mother plant and the embryo. At steady state, the flux from the mother plant is ultimately equal to the total carbohydrate demand of the embryo, and this is therefore a relevant factor in dictating the changes of sugars (see below).
Figure 3.
Acid invertase in the seed coat (A; cellular endosperm and testa) and the liquid endosperm (B) of oilseed rape seeds. Invertase was measured at pH 4.5 as moles of Suc consumed. Each point represents a single silique. Invertase was measured in different siliques on 3 different d, but the trends were similar in all three experiments. Therefore, this figure shows the combined data of all experiments. Data for liquid endosperm were initially measured per microliter of endosperm and were then converted to a seed basis using a separate curve of endosperm volume versus embryo weight. Embryo weight is used as a developmental scale. The lines have no significance except to emphasize the trend in the data and are best fit three-parameter exponential decay curves.
The second explanation is that the near-complete change in sugars may result from a near-complete loss of one (important) isoform of invertase, whereas other (irrelevant) isoforms contribute a background level of invertase to our measurements throughout development (Fig. 3). It is quite conceivable that the invertase of the liquid endosperm is actually the same as the decreasing component of the invertases of the seed coat and that both are a single isoform loosely associated with the seed coat and also present “eluted” from the seed coat into the adjacent endosperm.
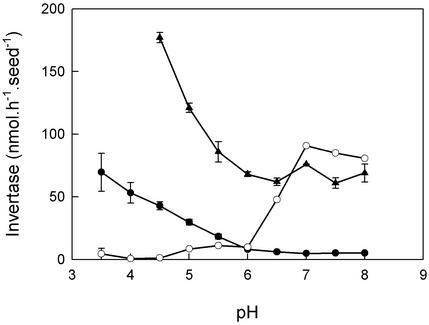
This focuses attention on the isoforms of invertase, of which there are many, in many locations (Sturm, 1999). We made no attempt to separate the isoforms, but because different isoforms have different pH maxima, we can draw some conclusions from our measurements at different pH values. We found a decrease in activity of invertase in the seed coat at all pH values (Fig. 4). The alkaline invertases actually undergo a greater proportional decrease than the acid invertases. Further, the liquid endosperm after dissection appears to have a pH greater than 6 (measured by indicator paper; results not shown). The bulk of the Suc is in this compartment, not in any hypothetical acidic subcompartment. Therefore it is possible that alkaline invertases are more relevant than the acid invertases in vivo, although we cannot be certain without knowledge of the ultrastructure of the seed and the pH of its compartments before we damaged it by dissection.
Figure 4.
The pH dependence of invertases in developing seeds of oilseed rape. Invertases were extracted from young (▴) and old (●) seed coats and from embryos (○). Young seed coats came from seeds whose embryos weighed less than 0.5 mg. Old seed coats were from seeds with embryos weighing approximately 3 mg. Note that young seed coats have a higher activity of invertase than old ones, regardless of pH. Also note that neither the (acid) invertase of the old seed coat, nor the (alkaline) invertase of the embryo has an activity at pH 6, yet young seed coats do have an isoform of invertase active at this neutral pH. Data are plotted as mean ± se of three measurements from a single experiment.
Figure 4 also shows the pH curve of a typical alkaline invertase, that of the embryo. Note that it has no activity at pH 6.0, nor does the acid invertase remaining in the seed coat at the end of development. Therefore the invertase active at pH 6.0 in young seed coats is another, neutral invertase that also declines during development and that may contribute to the changes in concentration of sugars.
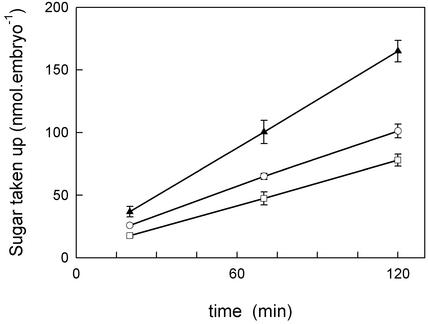
Embryos Take up Hexose Sugar Faster than Suc
The concentrations of sugars in the liquid endosperm result from a balance of synthesis and degradation (above and the introduction). It is often assumed that Suc is the major source of carbon for the embryo (e.g. King et al., 1997). It is clear, however, that hexoses must also be metabolized by some tissue in the seed. Otherwise, in the presence of invertase, they would accumulate indefinitely, and at the disappearance of invertase, they would remain constant. To determine the extent to which the balance of sugars in the liquid endosperm may be influenced by the metabolism of the embryo itself we incubated “older” embryos (approximately 2–3 mg fresh weight) in a medium designed to simulate the endosperm of a younger seed (about 0.5 mg fresh weight). The rate of uptake of sugars from the incubation medium was approximately linear for at least 2 h (Fig. 5). This indicates that the embryos were neither dying nor running out of substrate, and therefore, the rates are a true measure of the affinity of the embryo for each sugar. Suc was taken up by the embryos, but more slowly than the hexose sugars. Fru was used most rapidly (89 ± 7 nmol h−1 embryo−1, mean ± se of three separate experiments, each experiment using three separate siliques). In the same experiments, the rate of uptake of Glc was 67% ± 4% and that of Suc 51% ± 2% of the rate from Fru.
Figure 5.
The rate of uptake of Fru (▴), Glc (○), and Suc (□) by young (approximately 0.5 mg fresh weight) embryos of oilseed rape. These symbols are the same as used in Figure 2A. Note that uptake is approximately linear for 2 h. This figure shows a single experiment in which each point is the mean ± se of three measurements, each made on a single silique. Corresponding data in the text are means of these data and two other, similar experiments, not shown here.
Embryos of greater than 1 mg fresh weight probably use more Suc in vivo than our experiment would suggest, because their natural environment contains only approximately 20 mm of each hexose sugar and much more Suc (Fig. 2A). Oil synthesis from Suc does not become half-saturated until between 10 and 20 mm (Hill and Rawsthorne, 2000), and from hexoses, even higher concentrations are required. It is likely that uptake of sugars over periods of several hours will exhibit similar saturation kinetics. The saturation kinetics of oil synthesis were measured using single sugars in the absence of competition by other sugars. If competition takes place, hexose uptake may be even slower. Nevertheless, our primary conclusion is that developing embryos can take up hexose sugars rapidly, and therefore their growth is likely to lead to an increasing demand for hexose, which may also contribute to the disappearance of hexose sugars from the endosperm.
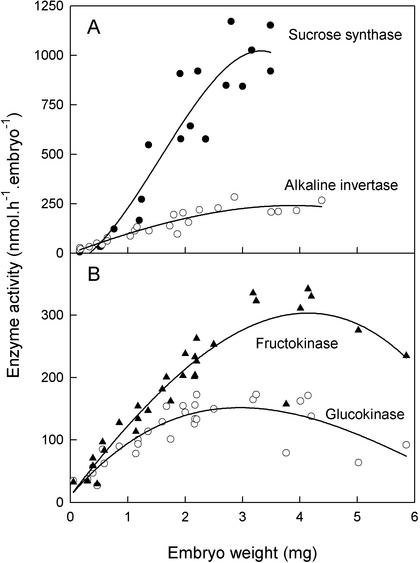
The Capacity of the Embryo to Metabolize Sugars Increases through Development; Fru Is Favored, Possibly Because Fructokinase Is More Active Than Glucokinase
The activities of the major sugar metabolizing enzymes of the embryo were measured as an indirect measure of embryo capacity to metabolize the sugars. Embryonic alkaline invertase and Suc synthase rose throughout development, Suc synthase being about four times as active as invertase (Fig. 6A). The activity of invertase exceeds that necessary to supply carbon for synthesis of oil (see above; 39 nmol h−1 embryo−1) by a factor of four. Therefore, both enzymes are in a position to catalyze the flux of carbohydrate to metabolism in vivo. The activities of hexokinases also rose through development (Fig. 6B), fructokinase being about twice as active as glucokinase. We assume these activities are attributable to different enzymes because they compete only slightly (the hexokinase activity in the presence of both hexoses is approximately 90% of the sum of the activities with each sugar alone; data not shown). The hexokinases have a dual role in metabolizing hexose taken up from the endosperm and hexoses released from Suc by Suc synthase and invertase. Invertase produces both Glc and Fru, whereas Suc synthase produces only Fru. The excess of fructokinase over glucokinase would enable metabolism of the Fru produced by Suc synthase. Consistent with our observations, the activities of Suc synthase and fructokinase increase during the stolon to tuber transition in potato (Appeldorn et al., 2002). The presence of glucokinase in the oilseed rape embryo does however suggest that in vivo it metabolizes some Glc directly or some Suc via invertase. The seed coat by contrast contains six times as much fructokinase as glucokinase (data not shown) suggesting that this tissue makes much less use of the invertase route of Suc metabolism. The activities of these enzymes alone do not tell us the full story of the capacity of the embryo to take up and metabolize each sugar, because we lack information about the actual sugar transporters that are expressed in the oilseed rape embryo. Nevertheless, they illustrate an increasing demand for all three sugars.
Figure 6.
Sugar-metabolizing enzymes in developing embryos of oilseed rape. A, Suc synthase (●) and alkaline invertase (○); B, fructokinase (▴) and glucokinase (○). Each point represents a single silique, and data are combined from three separate experiments. The lines merely indicate a trend and are best fit cubic curves.
Given a complete and accurate description of all the processes in our system, we should be able to recreate in silico the patterns of sugar content in the endosperm that we have measured in vivo. We therefore constructed a simple arithmetic model of an oilseed rape seed, with two aims. First, to use any discrepancies between the model and actual measurements to identify failures in our understanding that we might otherwise ignore. Second, we wanted to investigate whether the increasing demand of the embryo for sugars was as influential as the decrease in activity of endosperm invertase in causing the decrease in hexose sugars in the endosperm.
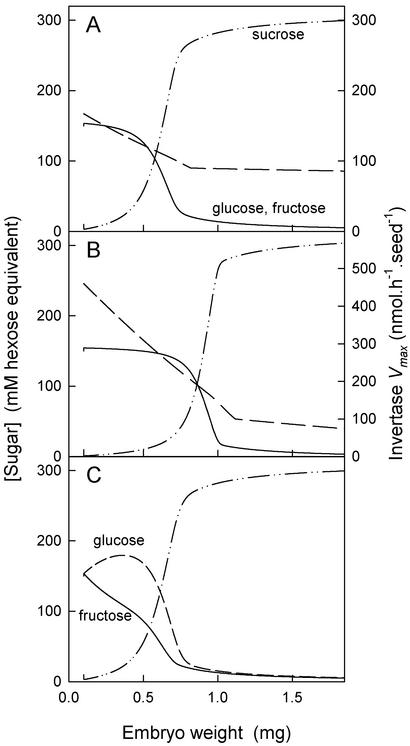
The Levels of Hexose Sugars in the Endosperm Suggest That Figure 1 Does Not Fully Describe the Metabolism of Suc in the Endosperm
Figure 7A shows the output of a model based on the scheme in Figure 1 using data presented above. The developmental model begins with an embryo of 0.1 mg in an appropriate, predominantly hexose endosperm of 2 μL volume. Invertase operates with Michaelis-Menten kinetics, Vmax progressing as shown in Figure 7A, similarly to the real rates of the seed-coat bound acid invertase (Fig. 3A). Sugar uptake by the embryo is assumed to be via processes obeying Michaelis-Menten kinetics with Km values of 20 mm (not unrealistic if uptake is similar to oil synthesis; Hill and Rawsthorne, 2000). Uptake is proportional to embryo weight, with Vmax values at 1 mg of 40 nmol h−1 embryo−1 for Suc and 100 nmol h−1 embryo−1 for the hexoses. Hexose units taken up by the embryo are replaced by the seed coat in the form of Suc, and the embryo is assumed to convert into biomass 30% of the weight of sugar it takes up.
Figure 7.
An arithmetic model of the system described in Figure 1, using values similar to those measured in vivo where possible. A, The concentrations of Suc (double-chain line) and the hexoses (superimposed, solid line) that would be expected assuming the Vmax of invertase follows that of the seed-coat acid invertase (dashed line). Note the delay before the concentrations of Glc and Fru fall and Suc increases. B, As in A, but assuming the Vmax of invertase follows that of the acid invertase of the liquid endosperm. For the critical, early period of development, this invertase has a higher activity. Note that higher activities of invertase lead to a more pronounced plateau before the change in sugar concentrations. C, As in A, but with the Vmax values of uptake of hexose adjusted, Fru 5% upward, Glc 5% downward. Note that this very modest change has large consequences for the concentrations of Glc (dashed line) and Fru (solid line), which no longer superimpose. Full details of the method of calculation are found in “Materials and Methods.”
The model describes a decline in hexose sugars in the endosperm during development. This decline occurs after an initial lag phase, which is not seen in oilseed rape in vivo (Fig. 2A). The exact timing of the decline varies with the Vmax of invertase, but it always occurs after a delay unless the Vmax of invertase is very small. The overall picture is similar if a constant Vmax (of 100 nmol h−1 embryo−1) is used throughout. It is little changed if we vary Vmax to reflect the activity of acid invertase in the liquid endosperm itself (Figs. 3B and 7B). This raises questions about the role of invertase in vivo. The sugar changes could potentially result from the increased rate of carbohydrate use by the embryo, and the model would give a better fit to actual measurements if a lot of the invertase activity was not accessible to the bulk sugars in the endosperm in vivo. The overall shape of the curve does not depend greatly on the Km of the invertase (which we did not measure) or the efficiency with which the plant converts sugar to biomass (which greatly affects the x axis, but not the shape). This suggests that the interpretation we draw from the model depends on the parts of the model about which we are confident and not on the parts we have been obliged to estimate.
In Figure 7A, we used equal rates of uptake of Glc and Fru, but in our measurements we showed that Fru is favored (Fig. 5). Figure 7B shows the same model as Figure 7A but with the Vmax value for uptake of Fru increased by 5% and that of Glc decreased by 5%. As expected, this leads to a divergence between concentrations of Glc and Fru, but note that even these very modest changes in rates of uptake lead to a huge divergence. This raises a second question about the role of invertase and its products in vivo. Glc and Fru are present in the liquid endosperm in exactly equal concentrations throughout development; out of seven independent experiments, only once did we see any significant difference (Fig. 2A shows the four largest sets of data, including the aberrant one, clearly visible as a row of higher Glc points; Hill and Rawsthorne [2000] show another set). These equal concentrations suggest that the hexoses resulted from hydrolysis of Suc catalyzed by invertase. However, if the embryo did take up Fru faster than Glc, the concentrations could not remain the same. Therefore either the embryo does not take up Fru faster or the seed coat makes up the difference by supplying Fru (perhaps generated by Suc synthase). An alternative explanation is that the pool of hexoses is created from Suc by invertase but is not in direct contact with the embryo.
CONCLUSIONS
We conclude that the role of sugars in the developing seed will only be understood when we can attach our understanding of the biochemistry and enzymology of the process to a good understanding of the ultrastructure of the seed. The relative contribution that an enzyme such as invertase might play in determining the sugar content of the environment in which the embryo develops requires detailed knowledge of the isoforms expressed in the seed and where and when during development this occurs. Furthermore, the different tissues of the seed have very different compositions and change their relative sizes through development, factors which must be considered in the interpretation of measurements of whole seeds (Baud et al., 2002). It will therefore also be important to understand the extent to which each isoform of invertase is accessible to the sugars that we are able to measure in these different tissues.
MATERIALS AND METHODS
Oilseed rape (Brassica napus L. cv Topas) was grown in greenhouses at nominal temperatures of 18°C (day) and 12°C (night) using supplementary lighting during the natural photoperiod between October and March.
Measurement of Endosperm Sugars
Developing seeds were taken from a plant and punctured with a clean needle, and drips of liquid endosperm collected. Endosperm was pooled from several seeds in the same silique, rejecting the end seeds and any others that looked unusual. Within 2 min, between 1 and 5 μL of endosperm was added to 500 μL of 0.16 g mL−1 trichloroacetic acid and 5 mm EGTA. Precipitated protein was removed by centrifugation (5 min, 11,000g) after incubation for 3 h at 0°C. Supernatant (400 μL) was washed four times with 1.2 mL of water-saturated ether to remove excess trichloroacetic acid and then neutralized with 1 m HEPES-NaOH, pH 8.2. Sugars were assayed spectrophotometrically (Stitt et al., 1989) in a mixture containing 50 mm HEPES-KOH (pH 7.0), 5 mm MgCl2, 0.8 mm NAD, 1.4 mm ATP, 0.7 unit of Glc-6-phosphate dehydrogenase (from Leuconostoc mesenteroides), 1 unit of hexokinase, 1 unit of phosphoglucoseisomerase, and approximately 30 units of invertase (all from yeast).
Measurement of Invertase
Siliques were harvested on ice and extracted within 2 h. From each silique, we took five to 10 seeds (rejecting end and unusual seeds) and dissected them into seed coat and embryo. Both parts were washed for a few seconds in water and then ground with 200 μL of buffer A (20 mm HEPES-KOH [pH 7.0], 10 mm KCl, 2 mm MgCl2, 1 mm EDTA, 5 mm dithiothreitol, and 10 g L−1 bovine serum albumin) in a glass homogenizer with 3 to 5 mg of polyvinylpolypyrrolidone. The extract was washed from the homogenizer with a further 300 μL of buffer and was used without centrifugation or desalting to avoid loss of invertase bound to solid material.
Alkaline invertase was assayed by the addition of 40 μL of extract to 40 μL of assay buffer (50 mm HEPES-NaOH [pH 7.5]) and 20 μL of 0.5 m Suc. The reaction was stopped by boiling for 5 min, and invertase activity was calculated from the appearance of hexose sugar (assayed as for endosperm sugars), after subtracting the hexose found in a zero-time incubation that had been boiled at once. Acid invertase was assayed in a similar mixture, but the assay buffer was 250 mm sodium acetate (pH 4.5). The reaction was stopped by the addition of 100 μL of 50 g L−1 ZnSO4 and left on ice for 5 min before boiling for 5 min more. The zinc was then precipitated by addition of 400 μL of 100 mm K2CO3, and hexoses released from Suc were assayed in the supernatant after centrifugation (2 min, 11,000g). The pH dependence of invertase was assayed as for alkaline invertase except that the assay buffer was a constant ionic strength buffer (Ellis and Morrison, 1982) consisting of 50 mm acetic acid, 50 mm MES, and 100 mm triethanolamine made to pH with KOH or HCl. To measure the invertase in the liquid endosperm, samples (3 μL) of endosperm were collected as for measurement of sugars and added to 100 μL of buffer A. Because the high concentrations of hexose sugars in young endosperm render invertase assays unreliable, the extract was dialyzed against buffer A without dithiothreitol or bovine serum albumin overnight at 4°C. Invertases were then measured as above, yielding results in nanomoles per hour per microliter of endosperm. We also weighed a range of developing seeds before and after dissection, subtracting to calculate the weight (and hence approximate volume) of the liquid endosperm. These values were used to convert our measurements of invertase into nanomoles per hour per seed.
Measurement of Hexokinases
Extracts were prepared as for invertase. Hexokinase was measured by continuous spectrophotometric assay following the change of A340 in a mixture containing 40 mm glycyl-Gly-KOH (pH 8.2), 4 mm MgCl2, 0.8 mm NAD, 1.2 mm ATP, 1 unit of Glc-6-phosphate dehydrogenase (from Leuconostoc mesenteroides), and 3 units of phosphoglucoseisomerase (from yeast). The reaction was started by the addition of 1.2 mm Glc, Fru, or both, which is enough to give a near-maximal rate. The assays were only partially optimized for pH. Brassica spp. hexokinases were found to prefer very alkaline conditions. The activity of fructokinase roughly doubled between pH 6 and 8 (data not shown), then remaining constant to pH 9.5. That of glucokinase increased with pH and was still increasing at pH 9.5. Our measurements of glucokinase are therefore not maximal rates, rather rates that we feel are realistic in vivo where such alkaline conditions are unlikely.
Measurement of Suc Synthase
Extracts were prepared as for invertase and desalted using NAP5 columns (Amersham Biosciences AB, Uppsala) according to the manufacturers' instructions. Suc synthase was assayed in the synthetic direction basically according to Salerno et al. (1979) in reaction mixtures (55 μL) containing 82 mm glycyl-Gly-KOH (pH 8.2), 9 mm Fru, and 9 mm UDP-[U-14C]Glc (1 GBq mol−1). The reaction was stopped by boiling for 4 min. After the addition of 100 μL of water and centrifugation (2 min, 11,000g), 140 μL of supernatant was loaded on a column consisting of 0.7 mL of slurry of Dowex 1×8–200 (Cl− form) in a 1,000-μL pipette tip, the end of which was blocked by a glass bead. Neutrals were eluted from the column in 2.4 mL of water, and [14C]Suc produced by Suc synthase was assayed by liquid scintillation counting with 3 mL of Optiphase Hisafe III (Fisher Chemicals, Loughborough, UK).
Uptake of Sugars by Embryos
Embryos were incubated in a medium suitable for the in vitro culture of oilseed rape embryos, based on Nitsch and Nitsch medium as modified by Lichter (1981). The medium, adjusted to pH 6.0 with KOH, contained: 800 mg L−1 Gln, 500 mg L−1 Ca(NO3)2·4H2O, 125 mg L−1 KH2PO4, 125 mg L−1 KNO3, 125 mg L−1 MgSO4, 100 mg L−1 Ser, 100 mg L−1 inositol, 40 mg L−1 EDTA (ferric monosodium salt), 30 mg L−1 reduced glutathione, 25 mg L−1 MnSO4·H2O, 10 mg L−1 H3BO3, 10 mg L−1 ZnSO4·7H2O, 5 mg L−1 nicotinic acid, 2 mg L−1 Gly, 500 μg L−1 folic acid, 500 μg L−1 pyridoxine-HCl, 500 μg L−1 thiamine-HCl, 250 μg L−1 Na2MoO4, 50 μg L−1 biotin, 25 μg L−1 CoCl2·6H2O, and 25 μg L−1 CuSO4·5H2O. Ten embryos from the same silique were washed for a few seconds in three changes of medium and then incubated in 60 μL of medium in a 5-mL scintillation vial cut to a length of about 1 cm. This is sufficient to keep the embryos thoroughly in contact with medium while ensuring free access to air. The incubations also contained 100 mm Glc, 100 mm Fru, and 40 mm Suc, with one of the three sugars 14C-labeled (approximately 1 GBq mol−1). At intervals, groups of three embryos were removed, washed three times for a few seconds in water, and ground in a glass homogenizer in 100 μL of water. The material was diluted to 1-mL and 100-μL portions were assayed for 14C by liquid scintillation counting. To ensure that the geometry in the incubation changed as little as possible (because this could change availability of oxygen and therefore the rate of uptake of sugars), embryos that had been removed were replaced by glass beads of approximately the same size.
Modeling of Metabolism in a Developing Seed
Starting conditions were 0.1 mg of embryo in 2 μL of endosperm (approximately correct for this cultivar; data not shown) containing 5 mm Suc and 150 mm of each hexose (compare Fig. 2). Rates for each arrow in Figure 1 were calculated as described in “Results.” The change in each pool size was then calculated assuming the rate remained constant for 0.08 h, and the changes were added to the initial pool sizes (time step = 0.08 h). This process was repeated 3,990 times. The calculated uptake of sugar was converted to weight of hexose and multiplied by 0.3 to estimate the increase in weight of the embryo. This factor is a convenience necessary to complete the arithmetic, and although probably incorrect, it only affects the absolute scaling of the x axis. Thus our model should yield an accurate relative picture of the time course of changes of sugars, but not one that is absolutely calibrated on the time axis. Crude models of this type break down if the changes during one time step become large compared with the pool sizes, particularly if the change becomes so large that it can make a pool size become negative, obviously impossible in real life, and with dire consequences for calculations of the next rate. We took care not to use the model in such a situation.
ACKNOWLEDGMENTS
We thank Chloe Sellwood for analysis of the sugar composition of developing embryos. We thank Ian Hagon and his team for their support in plant husbandry and Céline Thomasset for technical assistance.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (Core Strategic Grant). E.R.M.-S. was partially supported by a Nuffield/John Innes Foundation Scholarship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010868.
LITERATURE CITED
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:4710–4717. doi: 10.1073/pnas.96.8.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeldorn NJK, Sergeeva L, Vreugdenhil D, van der Plas LHW, Visser R. In situ analysis of enzymes involved in sucrose to hexose-phosphate conversion during stolon-to-tuber transition of potato. Physiol Plant. 2002;115:303–310. doi: 10.1034/j.1399-3054.2002.1150218.x. [DOI] [PubMed] [Google Scholar]
- Baud S, Boutin J-P, Miquel M, Lepiniec L, Rochat C. An integrated overview of seed development in Arabidopsis thaliana ecotype WS. Plant Physiol Biochem. 2002;40:151–160. [Google Scholar]
- Borisjuk L, Walenta S, Weber H, Mueller-Klieser W, Wobus U. High-resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba in relation to mitotic activity and storage processes: glucose as a possible developmental trigger. Plant J. 1998;15:583–591. [Google Scholar]
- Borisjuk L, Wang TL, Rolletscheck H, Wobus U, Weber H. A pea seed mutant affected in the differentiation of the embryonic epidermis is impaired in embryo growth and seed maturation. Development. 2002;129:1595–1607. doi: 10.1242/dev.129.7.1595. [DOI] [PubMed] [Google Scholar]
- da Silva PMFR, Eastmond PJ, Hill LM, Smith AM, Rawsthorne S. Starch metabolism in developing embryos of oilseed rape. Planta. 1997;203:480–487. [Google Scholar]
- Eastmond PJ, Rawsthorne S. Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryos. Plant Physiol. 2000;122:767–774. doi: 10.1104/pp.122.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KJ, Morrison JF. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Hill LM, Rawsthorne S. Carbon supply for storage-product synthesis in developing seeds of oilseed rape. Biochem Soc Trans. 2000;28:667–669. [PubMed] [Google Scholar]
- Kang F, Ridout CJ, Morgan CL, Rawsthorne S. The activity of acetyl-CoA carboxylase is not correlated with the rate of lipid synthesis during development of oilseed rape (Brassica napus L.) embryos. Planta. 1994;193:320–325. [Google Scholar]
- King SP, Lunn JE, Furbank RT. Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol. 1997;114:153–160. doi: 10.1104/pp.114.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter R. Anther culture of Brassica napus in a liquid culture medium. Z Pflanzenphysiol. 1981;103:229–237. [Google Scholar]
- Murphy DJ, Cummins I. Biosynthesis of seed storage products during embryogenesis in rapeseed, Brassica napus. J Plant Physiol. 1989;135:63–69. [Google Scholar]
- Neubohn B, Gubatz S, Wobus U, Weber H. Sugar levels altered by ectopic expression of a yeast-derived invertase affect cellular differentiation of developing cotyledons of Vicia narbonensis L. Planta. 2000;211:325–334. doi: 10.1007/s004250000305. [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE. Post-sieve element transport of sucrose in developing seeds. Aust J Plant Physiol. 1995;22:681–702. [Google Scholar]
- Salerno GL, Gamundi SS, Pontis HG. A procedure for the assay of sucrose synthetase and sucrose phosphate synthetase in plant homogenates. Anal Biochem. 1979;93:196–199. [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- Stitt M, Lilley RMC, Gerhardt R, Heldt HW. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- Sturm A. Invertases, primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999;121:1–7. doi: 10.1104/pp.121.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J. 1996a;10:823–834. [Google Scholar]
- Weber H, Buchner P, Borisjuk L, Wobus U. Sucrose metabolism during cotyledon development of Vicia faba L. is controlled by the concerted action of both sucrose-phosphate synthase and sucrose synthase: expression patterns, metabolic regulation and implications for seed development. Plant J. 1996b;9:841–850. doi: 10.1046/j.1365-313x.1996.9060841.x. [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell. 1997a;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Sugar import and metabolism during seed development. Trends Plant Sci. 1997b;2:169–174. [Google Scholar]
- Weber H, Golombek S, Heim U, Borisjuk L, Panitz R, Manteuffel R, Wobus U. Integration of carbohydrate and nitrogen metabolism during legume seed development: implications for storage product synthesis. J Plant Physiol. 1998;152:641–648. [Google Scholar]
- Wobus U, Weber H. Sugars as signal molecules in plant seed development. Biol Chem. 1999;380:937–944. doi: 10.1515/BC.1999.116. [DOI] [PubMed] [Google Scholar]