Abstract
The carbon isotope composition (δ13C) of CO2 produced in darkness by intact French bean (Phaseolus vulgaris) leaves was investigated for different leaf temperatures and during dark periods of increasing length. The δ13C of CO2 linearly decreased when temperature increased, from −19‰ at 10°C to −24‰ at 35°C. It also progressively decreased from −21‰ to −30‰ when leaves were maintained in continuous darkness for several days. Under normal conditions (temperature not exceeding 30°C and normal dark period), the evolved CO2 was enriched in 13C compared with carbohydrates, the most 13C-enriched metabolites. However, at the end of a long dark period (carbohydrate starvation), CO2 was depleted in 13C even when compared with the composition of total organic matter. In the two types of experiment, the variations of δ13C were linearly related to those of the respiratory quotient. This strongly suggests that the variation of δ13C is the direct consequence of a substrate switch that may occur to feed respiration; carbohydrate oxidation producing 13C-enriched CO2 and β-oxidation of fatty acids producing 13C-depleted CO2 when compared with total organic matter (−27.5‰). These results are consistent with the assumption that the δ13C of dark respired CO2 is determined by the relative contributions of the two major decarboxylation processes that occur in darkness: pyruvate dehydrogenase activity and the Krebs cycle.
Photosynthetic CO2 assimilation of C3 plants discriminates against 13CO2 so that organic matter is, on average, 20‰ depleted in 13C compared with atmospheric carbon dioxide (for recent review, see Brugnoli and Farquhar, 2000). Respiratory carbon fluxes in light (i.e. photorespiration and “day” respiration) are often assumed to be negligible or weakly fractionating processes. However, the carbon isotope signature of organic matter may be modified by nighttime respiration depending on the δ13C of the evolved CO2 because respiratory carbon lost by many plants has been shown to be within 30% to 60% of the carbon fixed through photosynthesis (Evans, 1993; Amthor, 2000).
In vitro studies using protoplasts have shown that respired CO2 isotope composition is identical to that of the Suc supplied to the culture medium, indicating that no fractionation occurs during respiration in the dark (Lin and Ehleringer, 1997). A similar result was also obtained in long-term experiments with animals, where the isotope composition of CO2 expired by mice (Mus musculus) reflected that of the diet (Perkins and Speakman, 2001). In contrast, it has been shown previously that CO2 produced by respiration in the dark is 6‰ 13C enriched when compared with Suc in intact French bean (Phaseolus vulgaris) leaves (Duranceau et al., 1999). Similar results were also obtained in Nicotiana sylvestris and sunflower (Helianthus annuus), although CO2 was less 13C enriched with δ13C values of 4‰ and 3‰, respectively (Ghashghaie et al., 2001). Moreover, it has been demonstrated that the δ13C value of CO2 evolved in the dark decreased in sunflower leaves subjected to drought (Ghashghaie et al., 2001). Assuming carbohydrates as the main respiratory substrate, Ghashghaie and coworkers suggested that discrimination occurred during dark respiration in C3 plants, but it varied among species and with drought conditions. When compared with total organic matter, respired CO2 was found to be enriched in 13C in wheat (Triticum aestivum; Troughton et al., 1974), tomato (Lycopersicon esculentum; Park and Epstein, 1961), and Cucurbita moschata (Smith, 1971), whereas it was depleted in 13C in Pinus radiata and maize (Zea mays; Smith, 1971). Moreover, in sliced potato (Solanum tuberosum) tubers, it varied with time, presumably reflecting some metabolic shifts (Jacobson et al., 1970). Interestingly, there seems to be a linear relationship between the carbon isotope composition of non-lipid plant material and the fraction of lipids in plants or algae (Park and Epstein, 1961), suggesting that the isotopic composition of carbohydrates subsequently oxidized into CO2 is a function of general carbon metabolism. Carbon isotope composition of the CO2 evolved in the dark may reflect both the discrimination of the carbon by cell metabolism in the dark, which at least occurs during the pyruvate dehydrogenase (PDH) reaction (O'Leary, 1976; Melzer and Schmidt, 1987) and the isotope composition of the carbon source feeding the Krebs cycle.
In this work, we address the question of the metabolic origin of the carbon isotope signature of CO2 evolved by French bean leaves in the dark. Respiratory activity was modified by changing both leaf temperature and by increasing dark period length from 1 h to several days. The carbon isotope composition of the respired CO2 was related to the respiratory quotient (RQ) and the carbon isotope composition of carbohydrates, heat-precipitated proteins, and fatty acids.
RESULTS
Effect of Temperature on RQ and δ13C of CO2 Evolved in Darkness
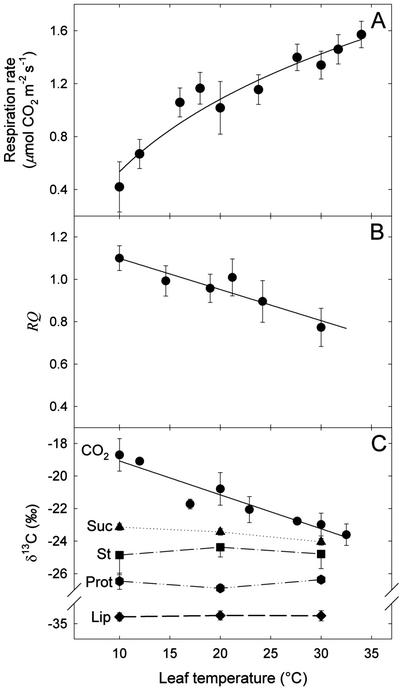
Leaf respiration increased with temperature with Q10 values of 2 (when leaf temperature shifted from 10°C to 20°C) and 1.5 (from 20°C to 30°C; Fig. 1A). RQ decreased from about 1.1 at 10°C to 0.8 at 30°C (Fig. 1B). The δ13C value of the dark-respired CO2 decreased linearly as temperature increased. The slope of the regression line was approximately 0.2‰ °C−1 (Fig. 1C). The δ13C values of Suc (−23.5‰), starch (−24.7‰), heat-precipitated proteins (−27.2 ‰), and lipids (−33.5‰) are also given (Fig. 1C). For leaf temperatures ranging from 10°C to 30°C, the respired CO2 was always 13C enriched compared with the analyzed metabolites.
Figure 1.
Effect of temperature on leaf respiration in French bean. Respiration rate (A), RQ (B), and δ13C of CO2 (circles), Suc (triangles), starch (St, squares), heat-precipitated proteins (Prot, hexagons), and lipids (Lip, diamonds; C) of intact leaves are plotted as a function of temperature. CO2 was collected in a closed system for respiration measurements and isotope analyses. Data are means of three independent replicates ± ses. Linear regressions for the RQ and δ13C value of respired CO2 give y = −1.47 10−2 x + 1.24 (r2 = 0.87) and y = −0.21x −16.98 (r2 = 0.91), respectively. Regressions are significant for both RQ and δ13C (F = 26.30, P < 0.0068, and F = 61.16, P < 0.0002, respectively).
Effect of a Prolonged Period of Darkness
Variation of Some Leaf Metabolites in Continuous Darkness
As expected, a prolonged period of darkness caused a general depletion in leaf metabolites. The amounts (relative to the internal standard) of the major metabolites extracted and detected by gas chromatography-mass spectrometry (GC-MS) are shown in Table I for each temperature condition after 1 h, 6 h, and several days in darkness (5 d at 20°C and 30°C and 14 d at 10°C). It should be noted that the Glc chromatographic signal integrates free Glc, but also Glc molecules from methanolysis of starch and Suc obtained during the extraction procedures. Fru was not detected because of its degradation during methanolysis. Despite the observed variability, the amount of the major carbon metabolites, malate, and total Glc decreased dramatically in darkness. In contrast, myo-inositol content increased slightly after 1 h of darkness. Citrate, Gal, and the fatty acids palmitate and linolenate were variable during the dark treatment. In addition, gluconate, an intermediary product of the pentose-phosphate cycle, which was not present in the control plants, was detected at the end of the dark treatment for each temperature investigated.
Table I.
Metabolite amounts of French bean leaves maintained in darkness at three temperatures
| Metabolite | Initial Composition | 1 h in Dark
|
6 h in Dark
|
End of Dark Period
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10°C | 20°C | 30°C | 10°C | 20°C | 30°C | 10°C | 20°C | 30°C | ||
| Citrate | 50 ± 7 | 54 ± 1 | 50 ± 5 | 40 ± 2 | 13 ± 3 | 100 ± 9 | 61 ± 14 | 64 ± 41 | 52 ± 19 | 41 ± 16 |
| Malate | 310 ± 112 | 230 ± 11 | 270 ± 43 | 157 ± 6 | 83 ± 22 | 256 ± 10 | 73 ± 5 | 10 ± 7 | 140 ± 39 | 13 ± 1 |
| Gluconate | 0 | 0 | 3 ± 1 | 0 | 2 ± 1 | 0 | 2 ± 1 | 9 ± 1 | 4 ± 1 | 4 ± 1 |
| Glc | 1,467 ± 565 | 1,198 ± 18 | 1,418 ± 140 | 1,436 ± 22 | 686 ± 234 | 1432 ± 26 | 455 ± 118 | 84 ± 18 | 273 ± 85 | 51 ± 5 |
| Gal | 120 ± 49 | 243 ± 4 | 354 ± 29 | 286 ± 1 | 137 ± 44 | 338 ± 31 | 187 ± 52 | 100 ± 29 | 179 ± 62 | 143 ± 20 |
| Myoinositol | 7 ± 2 | 16 ± 1 | 18 ± 1 | 18 ± 1 | 8 ± 3 | 17 ± 1 | 61 ± 9 | 7 ± 2 | 51 ± 11 | 0 |
| Glycerol | 82 ± 10 | 114 ± 5 | 181 ± 32 | 100 ± 8 | 44 ± 7 | 143 ± 4 | 87 ± 18 | 46 ± 1 | 69 ± 17 | 51 ± 5 |
| Linolenate | 17 ± 3 | 30 ± 1 | 31 ± 1 | 29 ± 1 | 10 ± 5 | 32 ± 1 | 22 ± 2 | 10 ± 1 | 20 ± 2 | 9 ± 1 |
| Palmitate | 5 ± 1 | 10 ± 1 | 11 ± 1 | 10 ± 1 | 4 ± 1 | 11 ± 1 | 10 ± 2 | 5 ± 1 | 8 ± 2 | 6 ± 1 |
Metabolites were simultaneously identified and quantified with the GC-MS procedure. Their amounts are given relative to an internal standard added in each sample. Glc signal integrates free Glc and also Glc from starch and Suc. Data are means of three independent measurements ± se.
Variation of Carbohydrate Pool in Continuous Darkness
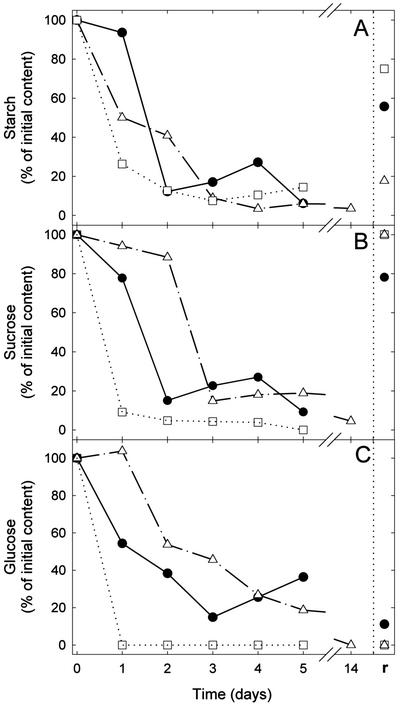
The time course of starch, Suc, and Glc content as a percentage of the initial values are shown in Figure 2, A through C, respectively. Their initial contents in a control leaf were about 5, 20, and 150 μg mg−1 of dry matter respectively. Starch content decreased similarly under the three temperature conditions and was about 10% of its initial value after 3 d (Fig. 2A). On the other hand, the decline in the amounts of Suc and Glc were the fastest at 30°C and the slowest at 10°C (Fig. 2, B and C), presumably indicating the regulation of carbohydrate consumption by temperature. It should also be noted that after 1 d darkness at 10°C, Glc slightly increased (Fig. 2C), whereas starch strongly decreased (Fig. 2A). Similarly, it has been observed that Glc-6-phosphate increased in cambial cells of Acer pseudoplatanus subjected to low temperature (R. Bligny, personal communication).
Figure 2.
Variation in metabolite contents in intact French bean leaves maintained in darkness for several days at different temperatures (circles, 20°C; triangles, 10°C; and squares, 30°C) and then subjected to 6 h of light at a photosynthetic photon flux density (PPFD) of about 500 μmol m−2 s−1 (r). Starch (A), Suc (B), and Glc (C) amounts are expressed as percentage of initial content and are plotted as a function of time.
Variation of Respiration Rate, RQ, and δ13C-Respired CO2 in Continuous Darkness
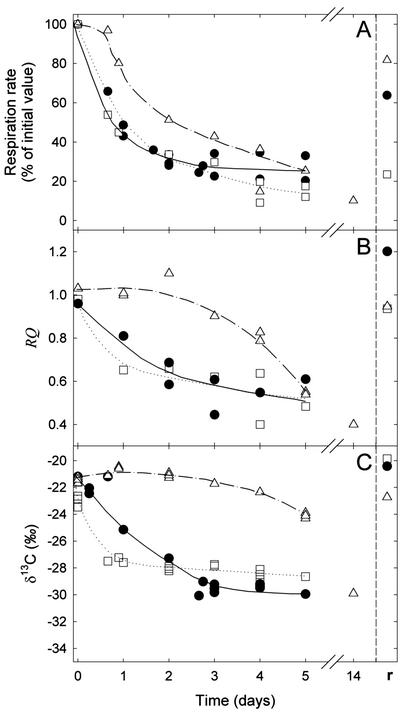
The time course of respiration (as percentage of the initial value), RQ, and carbon isotope composition of CO2 respired by intact leaves kept in darkness for some days at 10°C, 20°C, or 30°C are shown in Figure 3. Initial respiration rates were around 1.2, 0.8, and 0.6 μmol m−2 s−1 at 30°C, 20°C, and 10°C, respectively. Leaf respiration progressively decreased, reaching around 20% of its initial value at 30°C and around 30% at 10°C and 20°C after 5 d of darkness (Fig. 3A). RQ was about 1 at the beginning of the dark period and then decreased, reaching 0.6 after 2 d at 20°C and 30°C. The decrease in RQ was the slowest at 10°C, remaining more or less constant during the first 3 d in the dark and reaching 0.4 after 14 d (Fig. 3B). The δ13C of respired CO2 also decreased from −21‰ to about −28‰ after 1 and 2 d at 30°C and 20°C, respectively, and eventually reached values between −28‰ and −30‰ after 5 d (Fig. 3C). At 10°C, such low values were observed after 14 d in the dark. It is notable that after 5 d at 10°C, the δ13C was around −24‰ when the RQ was only 0.6. Obviously, the metabolism in the dark was altered at low temperature. The carbon isotope composition of lipids and carbohydrates such as starch remained almost constant in darkness at around −33‰ and −25‰, respectively. Respired CO2 was 13C enriched compared with the analyzed leaf metabolites and when compared with total leaf organic matter at low temperature during 5 d of continuous darkness. At 20°C and 30°C, CO2 was 13C enriched compared with leaf metabolites but it became 13C depleted (except compared with lipids) with increasing dark period length.
Figure 3.
Variation of respiration rate in intact French bean leaves maintained in darkness for several days at different temperatures (circles, 20°C; triangles, 10°C; and squares, 30°C) and then subjected to 6 h of light at a PPFD of about 500 μmol m−2 s−1 (r). Leaf respiration (A), RQ (B), and δ13C of respired CO2 (C) are plotted as a function of time. δ13C of starch (−24.7‰), heat-precipitated proteins (−26.7‰), total organic matter (−27.5‰), and lipids (−33‰) are constant. CO2 was collected in a closed system for respiration measurements and isotope analyses. Data points were obtained on two individual plants.
Plants were subjected to a light period of 6 h in the greenhouse after continuous darkness. Immediately after this light treatment, the leaf respiration, RQ, and the δ13C value of dark-respired CO2 were measured at 20°C (the symbol r in Figs. 2 and 3), allowing us to check if there was any long-term effect of the temperature treatments at 10°C and 30°C on respiration. Both RQ and δ13C of CO2 almost completely recovered for each temperature treatment. It should be noted that leaf respiration after light treatment was similar for the three temperature conditions (around 0.5 μmol m−2 s−1) because the measurements after the light treatment were all carried out at 20°C, but the r values of leaf respiration on Figure 3A were different because they were expressed in percentage of initial respiration rate.
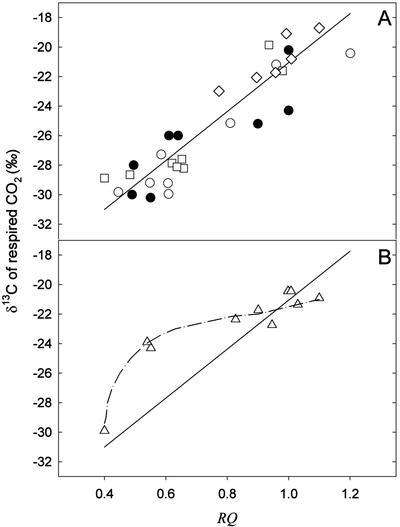
Relationship between RQ and δ13C of CO2
RQ and δ13C data from “temperature” (Fig. 1, B and C) and “continuous darkness” (Fig. 3, B and C) experiments have been replotted in Figure 4 (white symbols). The relationship between RQ and δ13C, obtained without data from the continuous darkness experiment at 10°C, is clearly linear (r2 = 0.87) and gives a slope of 16.57‰ per RQ unit (Fig. 4A). δ13C of respired CO2 (from Smith, 1971; Park and Epstein, 1961) and RQ values (from James, 1953) of sunflower, wheat, castor bean (Ricinus communis), and squash (Cucurbita pepo) seedlings and leaves of tomato and pea (Pisum sativum), grown in the greenhouse at ambient temperature, have also been plotted in Figure 4A (black symbols). Data from “continuous darkness” experiments at 10°C show a nonlinear relationship (Fig. 4B).
Figure 4.
Relationship between δ13C of dark-respired CO2 and the RQ. A, Data are taken from Figures 1 (diamonds) and 3 (white circles, 20°C; and squares, 30°C) and from the literature (James, 1953; Park and Epstein, 1961; Smith, 1971; black circles). The linear regression does not take into account data from the literature. The regression equation is: y = 16.57x − 37.62 (r2 = 0.87). The regression is significant (F = 144.21, P < 0.0001). B, Data taken from the “continuous darkness” experiment at 10°C of Figure 3 (triangles). The solid line represents the regression line obtained in A.
DISCUSSION
The carbon isotope signature of CO2 produced in darkness linearly decreased when temperature increased, from around −19‰ at 10°C to −24‰ at 35°C (Fig. 1C). It also progressively decreased, from −21‰ to −30‰, when leaves were maintained in continuous darkness for several days (Fig. 3C). Under “normal” conditions (temperature less than 30°C and a usual dark period duration), the evolved CO2 was enriched in 13C compared with carbohydrates, the most 13C-enriched metabolites. This is in agreement with previous results (Duranceau et al., 1999; Ghashghaie et al., 2001), suggesting that a fractionation occurs during dark respiration. However, at the end of a long dark period (carbohydrate starvation), respired CO2 was 13C depleted even when compared with the composition of total organic matter (Fig. 3C).
Interestingly, the temperature induced decrease in δ13C was paralleled by a decrease in RQ (Fig. 1), the ratio of CO2 production to oxygen consumption. Because RQ values close to 1 indicate highly oxygenated substrates (e.g. carbohydrates) with a relative high level of 13C and RQ values near to 0.6 indicate weakly oxygenated substrates (e.g. fatty acids) with a relative low level of 13C, it follows that the decline in δ13C is best explained by the isotope composition of the carbon of the products feeding the respiratory processes. RQ values lower than 0.6 may be observed when respiration is low and coupled to gluconeogenesis from lipids (Lundegardh, 1966). RQ values higher than 1 may be observed when metabolites such as malate or citrate are oxidized. Thus, it is possible that newly synthesized malate and citrate were oxidized together with carbohydrates in a dark period that immediately follows a period of photosynthesis. As shown in Table I, under such conditions, the amount of malate is substantial. δ13C of malate has not been measured in these experiments; however, it has been shown using tobacco (Nicotiana tabacum; Jamin et al., 1997) that the 13C to 12C ratio of malate is close to that found for carbohydrates. As expected, the decline in RQ as leaf temperature increases (Fig. 1B) is consistent with a much faster decrease in starch, Suc, and Glc contents observed with increasing temperature (Fig. 2). This is presumably because of an increase of dark respiration rate and Suc loading under these conditions. As a result, leaves maintained at an elevated temperature rapidly consume respiratory carbohydrates and use proteins and fatty acids to feed respiration, and this causes a decline in the δ13C of CO2.
Similarly, when leaves are maintained in darkness, RQ, which progressively decreases from 1 to around 0.6 after 5 d, parallels the δ13C decrease. Dark-induced sugar starvation (Fig. 2) is coupled to an increased contribution of fatty acid β-oxidation. Amino acids may also contribute to oxidative metabolism because the isotope composition of respired CO2 decreased to −30‰, which is between that of proteins (around −27‰) and lipids (around −33‰). A switch to fatty acid catabolism as a consequence of sugar starvation is well documented and has been described in tomato leaves maintained in darkness (Park and Epstein, 1961), cell suspensions of A. pseudoplatanus (Aubert et al., 1996), and maize root tips (Dieuaide-Noubhani et al., 1997).
When plotted together, the results give a linear relationship between the carbon isotope composition of CO2 and the RQ (Fig. 4A, white symbols), thus pointing out that the variability of CO2 signature mainly originates from substrate switching (r2 = 0.87). The residual variability observed in Figure 4A may come from a natural variation in isotope signature from one plant line to another. However, there is an obvious qualitative change in dark metabolism in leaves maintained under continuous darkness at 10°C because for RQ values from 1.1 to 0.6, the δ13C of CO2 remained high (Fig. 4B), with a “carbohydrate signature” despite the starch, Suc, and Glc contents being very low. This might presumably indicate the occurrence of a dark metabolism involving gluconeogenesis from 13C-enriched molecules like amino acids, e.g. Ala and Ser (Abelson and Hoering, 1961). Variability may also be partly because of the temperature dependence of isotope effects of enzymes involved in dark metabolism such as decarboxylases. In vitro, yeast (Saccharomyces cerevisiae) PDH discriminates during CO2 production with a positional isotope effect on the C-1 of pyruvate around 1.006 at 15°C and 1.008 at 35°C (DeNiro and Epstein, 1977). It can be assumed that temperature has the same effect on α-ketoglutarate decarboxylase, which could be another fractionating enzyme because it has an enzymatic mechanism that bears much similarities with that of PDH. Further experiments are needed to investigate dark metabolism at low temperature.
When the RQ is around 1, the δ13C value of respired CO2 is higher than that of carbohydrates (around −24‰ for Suc). This raises the question of the origin of the 13C-enrichment of CO2 compared with substrates oxidized through respiration. This has already been discussed in Ghashghaie et al. (2001); they emphasized that carbon atom positions C-1, C-2, C-5, and C-6 of Glc-feeding glycolysis are 13C depleted when compared with the C-3 and C-4 positions (Rossmann et al., 1991). This contributes to induce both a 13C depletion of acetyl-CoA and subsequent fatty acids, and a 13C enrichment of carbon dioxide produced by PDH. Moreover, PDH isolated in vitro discriminates against 13C during acetyl-CoA formation (DeNiro and Epstein, 1977; Melzer and Schmidt, 1987). This also contributes to 13C depletion in acetyl-CoA. Thus, there are two main origins of metabolic CO2 sources: one 13C enriched from pyruvate decarboxylation, and another 13C depleted from acetyl-CoA degradation through the Krebs cycle. The imbalance between these two sources may be responsible for the prevalence of 13C in respired CO2.
Relative carbon fluxes involved in the respiratory pathway may change depending on the cell's metabolic status. For example, acetyl-CoA (light carbons) may be used for anabolic purposes (e.g. fatty acids synthesis) when carbohydrates are degraded (RQ around 1 and 13C-enriched CO2 around −21‰). In contrast, acetyl-CoA is produced by β-oxidation of fatty acids when lipids are degraded (RQ around 0.6 and 13C-depleted CO2 around −30‰).
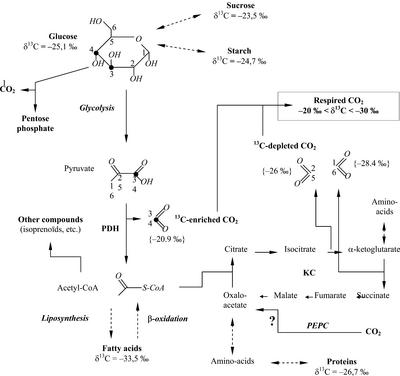
The isotope composition of CO2 produced by respiratory metabolism taking into account heterogeneous isotope distribution in Glc (Rossmann et al., 1991) is shown in Figure 5 (values in parentheses). Enzymatic isotope effects have not been used for calculations because of uncertainty in their values measured in vitro only (PDH) or because they are unknown (isocitrate dehydrogenase and α-ketoglutarate dehydrogenase). If dark carbon fixation (through phosphoenolpyruvate carboxylase, phosphoenolpyruvate carboxykinase, and carbamylphosphate synthase) and CO2 production through the pentose phosphate cycle are neglected, the isotope composition of dark respired CO2 should be close to −21‰ (mean value of C-3 and C-4 in Glc) when pyruvate dehydrogenation predominates and close to −27‰ (mean value of C-1, C-2, C-5, and C-6 in Glc) when the Krebs cycle is coupled to β-oxidation of fatty acids. This interval fits well the observed variation range of evolved CO2 δ13C values (between −20‰ and −30‰).
Figure 5.
Carbon metabolism in darkness in relation to δ13C of leaf-respired CO2. δ13C values of metabolites are those measured in French bean leaves. δ13C of CO2 in brackets are derived from positional δ13C values in natural Glc as given by Rossmann et al. (1991). PEPC, Phosphoenolpyruvate carboxylase; KC, Krebs cycle. The symbol “?” points out the uncertainty about the amplitude of the PEPC reaction in darkness.
It is concluded that the isotope signature of respired CO2 in C3 plants is not constant and is determined mainly by: (a) the carbon source used for respiration, i.e. the relative metabolic activities in the cell (glycolysis, β-oxidation, and gluconeogenesis); (b) the non-statistical carbon isotope distribution in Glc; and (c) the possible isotope effects of respiratory enzymes. Although all the fractionating (enzymatic) steps involved in respiration are not well known, the observed 13C enrichment in CO2 compared with substrate does suggest that there is a “fractionation” during dark respiration (Duranceau et al., 1999).
Moreover, our data show that the δ13C of dark-respired CO2 is modulated by environmental conditions such as temperature. Presumably, respiration during nighttime, particularly at low temperature when CO2 is strongly 13C enriched, may have a significant 13C-depleting effect on the remaining leaf organic material. Some studies are now needed on other plant organs to determine if the carbon isotope composition of dark-respired CO2 is similar at the whole-plant level.
MATERIALS AND METHODS
Plant Material
French beans (Phaseolus vulgaris L. cv Contender, Vilmorin, La Verpillière, France) were grown from seed in 1-L pots of potting mix in a greenhouse. Minimum PPFD during a 16-h photoperiod was maintained at approximately 500 μmol m−2 s−1 at leaf level by supplementary lighting from high-pressure sodium lamps. Temperature and the leaf-to-air vapor pressure deficit were maintained at approximately 25.5°C/18.5°C and 1.2/1.4 kPa day/night, respectively. Carbon isotope composition (δ13C) of CO2 in greenhouse air was −9.5‰ ± 0.3‰. All experiments were carried out on the first trifoliar mature leaf. The harvested leaves were immediately frozen in liquid nitrogen, lyophilized, and powdered.
Carbon Isotope Analysis and Gas Exchange Measurements
Dark-respired CO2 was analyzed on-line with a closed system directly coupled to an elemental analyzer NA-1500 (Carlo-Erba, Milan) through a 15-mL loop. After placing intact leaves in the respiration chamber, CO2 was removed with soda lime columns; when the CO2 level remained stable, flow from the soda lime columns was switched to the loop and carbon dioxide was accumulated. Molar fractions of respiratory CO2 were measured with an infrared gas analyzer (Finor, Maihak, Germany) placed in the closed system. When CO2 reached around 300 μL L−1, the loop was shunted and the gas inside was introduced into the elemental analyzer with helium for GC. The connection valve between the elemental analyzer and the isotope ratio mass spectrometer (VG Optima, Micromass, Villeurbanne, France) was opened when the CO2 peak emerged from the elemental analyzer. Isotope analysis of metabolites and total organic matter was conducted using the same elemental analyzer and isotope ratio mass spectrometer. Carbon isotope compositions were calculated as deviations of the carbon isotope ratio (13C to 12C, called R) from international standards (Pee Dee Belemnite) according to Farquhar et al. (1982): δ13C = 103 [(Rsample − Rstandard)/Rstandard].
Leaf temperature in the chamber was measured with a thermocouple and controlled with a water bath. The RQ was calculated from the ratio of carbon production [v(CO2)] to oxygen consumption [v(O2)]: RQ = v(CO2)/v(O2). The CO2 production in darkness was measured with the infrared gas analyzer as described above. Oxygen consumption of leaf discs from one leaflet of the same leaf was measured with an oxygen electrode (Hansatech, King's Lynn, UK).
“Temperature” experiments were started after a light period of 8 to 10 h. The δ13C value of CO2 and respiration rate were measured at 20°C before shifting leaf temperature to either 10°C or 30°C. The duration of this experiment was approximately 2 h. For each temperature, after δ13C measurement, some leaf blades were sampled for metabolite analysis. “Continuous darkness” experiments were done at different temperatures (5 d at 20°C and 30°C and 14 d at 10°C) and started after a light period of 10 to 12 h. For each temperature, δ13C of CO2 was continuously measured on the same leaf. Leaves of other plants maintained under similar conditions were sampled for metabolite analysis.
Metabolite Extraction and Quantification
The starch and Suc extraction procedures were taken from Duranceau et al. (1999). In brief, 50 mg of leaf powder was suspended with 1 mL of distilled water in an Eppendorf tube (Eppendorf Scientific, Hamburg, Germany). After centrifugation, starch was extracted from the pellet by HCl solubilization. Soluble proteins of the supernatant were heat denatured and precipitated, and soluble sugars of the protein-less extract were separated by HPLC. In most samples, Glc and Fru contents were low and only Suc was isotopically analyzed. After lyophylization, purified metabolites were suspended in distilled water and transferred to tin capsules (Courtage Analyze Service, Mont Saint-Aignan, France) for isotope analysis.
The lipid extraction procedure was carried out as in Deléens et al. (1984). Fifty milligrams of leaf powder was placed in glass tubes with 2 mL of hot ethanol (70°C) during 3 min and then cooled on ice. Two milliliters of chloroform was added and after 15 min at 0°C, 2 mL of distilled water was added and the tubes centrifuged at 2,000 rpm for 10 min at 15°C. The lower phase was collected with a Pasteur pipette and transferred to a new glass tube. Chloroform was evaporated at 60°C under a nonoxidizing atmosphere (N2). Fatty acids were methyl esterified with 2 mL of methanol-BF3; 0.5 mL of water and 3 mL of pentane were then added for chlorophyll/lipid separation. The upper phase was transferred to another glass tube and pentane was evaporated at 50°C with an N2 stream. Methyl-esters were dissolved in 1.5 mL of methanolic sodium hydroxide (0.5 mol L−1 NaOH in methanol). After 1 h at 40°C, pH was neutralized with 0.2 mL of HCl (6 mol L−1), and free fatty acids were separated with 2 mL of pentane. The resulting pentane phase was transferred to a glass tube. After pentane evaporation, fatty acids were dissolved in 70 μL of pentane and transferred to tin capsules for isotope analysis.
GC-MS
For metabolite identification and quantification, samples were prepared for GC-MS analysis, as described in Bleton et al. (1996). In brief, 2 mg of leaf powder was suspended in 0.5 mL of methanolysis reagent (methanol-acetyl chloride-HCl) at 80°C during 24 h. After centrifugation, pH was neutralized with pyridine, and methanol was evaporated using an N2 stream. Trimethylsilylation reagent (0.5 mL), composed of hexamethyldisilazane and trimethylchlorosilane (Hydrox-Sil Regis, Interchim, Montluçon, France), was then added and the glass tube maintained at 80°C for 2 h. After vacuum evaporation, samples were suspended in hexane for GC-MS analysis. GC-MS was conducted with a gas chromatograph 5890 A (Hewlett-Packard, Palo Alto, CA) coupled to a mass spectrometer Incos 50 quadrupole (Finnigan, San Jose, CA). Because of differential response coefficients of the gas chromatograph detector, signals were normalized to an internal standard molecule introduced to the samples (C19 methyl ester) allowing a relative quantification of metabolites.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Richard Bligny and Dr. Michael Hodges for critical reading of the manuscript, and Alain Tchapla and Marc Berry for access to GC-MS and for setting up the gas exhange-IRMS coupling, respectively. The technical assistance of Caroline Lelarge and Max Hill for isotope ratio MS and HPLC procedures, respectively, is acknowledged.
Footnotes
This work was supported by the European Research Training Network for Ecophysiology in Closing Terrestrial Carbon Budget (contract no. HPRN–CT–1999–00059).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013078.
LITERATURE CITED
- Abelson H, Hoering TC. Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proc Natl Acad Sci USA. 1961;47:623–632. doi: 10.1073/pnas.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor JS. The McCree-de Wet-Penning de Vries-Thornley respiration paradigms: 30 years later. Ann Bot London. 2000;86:1–20. [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrate. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleton J, Mejanelle P, Sansoulet J, Goursaud S, Tchapla A. Characterization of neutral sugars and uronic acids after methanolysis and trimethylsilylation for recognition of plant gums. J Chromatogr A. 1996;720:27–49. [Google Scholar]
- Brugnoli E, Farquhar GD. Photosynthetic fractionation of carbon isotopes. In: Leegood RC, Sharkey TD, von Caemmerer S, editors. Photosynthesis: Physiology and Metabolism. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 399–434. [Google Scholar]
- Deléens E, Schwebel-Dugué N, Trémolières A. Carbon isotope composition of lipidic classes isolated from tobacco leaves. FEBS Lett. 1984;178:55–58. [Google Scholar]
- DeNiro MJ, Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977;197:261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Canioni P, Raymond P. Sugar starvation induced changes of carbon metabolism in excised maize root tips. Plant Physiol. 1997;115:1505–1513. doi: 10.1104/pp.115.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranceau M, Ghashghaie J, Badeck F, Deleens E, Cornic G. δ13C of CO2 respired in the dark in relation to δ13C of leaf carbohydrates in Phaseolus vulgaris L. under progressive drought. Plant Cell Environ. 1999;22:515–523. [Google Scholar]
- Evans LT. Crop Evolution, Adaptation and Yield. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- Farquhar GD, O'Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol. 1982;9:121–137. [Google Scholar]
- Ghashghaie J, Duranceau M, Badeck F, Cornic G, Adeline MT, Deleens E. δ13C of CO2 respired in the dark in relation to leaf metabolites: comparisons between Nicotiana sylvestris and Helianthus annuus under drought. Plant Cell Environ. 2001;24:505–515. [Google Scholar]
- Jacobson BS, Smith BN, Epstein S, Laties GG. The prevalence of carbon 13 in respiratory carbon dioxide as an indicator of the type of endogenous substrate. J Gen Physiol. 1970;55:1–17. doi: 10.1085/jgp.55.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WO. Plant Respiration. London: Oxford University Press, Amen House; 1953. [Google Scholar]
- Jamin E, Naulet N, Martin GJ. Stable isotope analysis of components from tobacco leaves. Phytochem Anal. 1997;8:105–109. [Google Scholar]
- Lin G, Ehleringer JR. Carbon isotopic fractionation does not occur during dark respiration of C3 and C4 plants. Plant Physiol. 1997;114:191–194. doi: 10.1104/pp.114.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundegardh H. Plant Physiology. Edinburgh: Oliver and Boyd; 1966. [Google Scholar]
- Melzer E, Schmidt HL. Carbon isotope effects on the pyruvate dehydrogenase reaction and their importance for relative carbon-13 depletion in lipids. J Biol Chem. 1987;262:8159–8164. [PubMed] [Google Scholar]
- O'Leary MH. Carbon isotope effect on the enzymatic decarboxylation of pyruvic acid. Biochem Biophys Res Commun. 1976;73:614–618. doi: 10.1016/0006-291x(76)90854-8. [DOI] [PubMed] [Google Scholar]
- Park R, Epstein S. Metabolic fractionation of 13C and 12C in plants. Plant Physiol. 1961;36:133–138. doi: 10.1104/pp.36.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SE, Speakman JR. Measuring natural abundance of 13C in respired CO2 variability and implications for non-invasive dietary analysis. Funct Ecol. 2001;15:791–797. [Google Scholar]
- Rossmann A, Butzenlechner M, Schmidt HL. Evidence for a non-statistical carbon isotope distribution in natural glucose. Plant Physiol. 1991;96:609–614. doi: 10.1104/pp.96.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN. Carbon isotope ratios of respired CO2 from castor bean, peanut, pea, radish, squash, sunflower and wheat seedlings. Plant Cell Physiol. 1971;12:451–455. [Google Scholar]
- Troughton JH, Card KA, Hendy CH. Photosynthetic pathways and carbon isotope discrimination by plants. Carnegie Inst Washington Yearb. 1974;73:768–779. [Google Scholar]