Abstract
Persimmon (Diospyros kaki Thunb.) fruit are usually classified as climacteric fruit; however, unlike typical climacteric fruits, persimmon fruit exhibit a unique characteristic in that the younger the stage of fruit detached, the greater the level of ethylene produced. To investigate ethylene induction mechanisms in detached young persimmon fruit, we cloned three cDNAs encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (DK-ACS1, 2, and -3) and two encoding ACC oxidase (DK-ACO1 and -2) genes involved in ethylene biosynthesis, and we analyzed their expression in various fruit tissues. Ethylene production was induced within a few days of detachment in all fruit tissues tested, accompanied by temporally and spatially coordinated expression of all the DK-ACS and DK-ACO genes. In all tissues except the calyx, treatment with 1-methylcyclopropene, an inhibitor of ethylene action, suppressed ethylene production and ethylene biosynthesis-related gene expression. In the calyx, one ACC synthase gene (DK-ACS2) exhibited increased mRNA accumulation accompanied by a large quantity of ethylene production, and treatment of the fruit with 1-methylcyclopropene did not prevent either the accumulation of DK-ACS2 transcripts or ethylene induction. Furthermore, the alleviation of water loss from the fruit significantly delayed the onset of ethylene production and the expression of DK-ACS2 in the calyx. These results indicate that ethylene biosynthesis in detached young persimmon fruit is initially induced in calyx and is modulated by water loss through transcriptional activation of DK-ACS2. The ethylene produced in the calyx subsequently diffuses to other fruit tissues and acts as a secondary signal that stimulates autocatalytic ethylene biosynthesis in these tissues, leading to a burst of ethylene production.
The gaseous plant hormone ethylene plays an important role in the regulation of fruit ripening and senescence (Lelièvre et al., 1997a; Jiang and Fu, 2000). Fruits have been classified as climacteric and non-climacteric based on their patterns of respiration and ethylene production during maturation and ripening (Biale and Young, 1981). Persimmon (Diospyros kaki Thunb.) fruit are classified as climacteric because they produce a small but significant amount of ethylene during ripening and are induced to ripen with autocatalytic ethylene production by exogenously applied ethylene (Abeles et al., 1992; Wills et al., 1998; Kubo et al., 2003). However, unlike other climacteric fruit species, ethylene production in persimmon is substantially greater in fruit harvested at younger stages (Takata, 1983) and is induced only when fruit are detached from the parent tree (Nakano, 2002). For example in persimmon cv Hiratanenashi, detached young fruit produce more than 10 nL g−1 h−1 of ethylene within a few days after detachment accompanied with rapid softening and calyx abscission. Whereas fruit harvested at the mature stage do not always produce ethylene soon after harvest. They produce as little as 0.5 nL g−1 h−1 of ethylene when they are held in ambient condition for more than 25 d. Similar ethylene production in detached young fruit has been observed in citrus, typical non-climacteric fruit (Aharoni, 1968; Eaks, 1970) and considered to be related to fruit abscission (Hyodo and Murata, 1972). However, mechanisms or factors that induce ethylene production in detached young fruit have not been determined.
Apart from internal factors that regulate ethylene biosynthesis developmentally in plant tissues such as ripening fruit, senescing flower, and germinating seed, a variety of biotic and abiotic external factors induce ethylene production (Yang and Hoffman, 1984; Abeles et al., 1992; Morgan and Drew, 1997). These external factors include mechanical wounding, plant hormones, heavy metals, water logging, water deficit, chilling, hypoxia, and elevated carbon dioxide levels. Attached fruit maintain vascular continuity with the parent tree, from which they receive water; however, once detached, the fruit have no renewable source of water to compensate for that lost through transpiration. Detached fruit therefore experience water stress, which might be involved in ethylene induction. Induction of ethylene production by water stress was reported in detached leaves of wheat (Triticum aestivum; Apelbaum and Yang, 1981).
In recent years, genes encoding two key enzymes in ethylene biosynthesis, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase, have been cloned and characterized from a number of species (Zarembinski and Theologis, 1994). These studies have revealed that ethylene biosynthesis is typically regulated at the transcriptional level of both ACC synthase and ACC oxidase genes (Kende, 1993), although there is some evidence for posttranscriptional regulation (Spanu et al., 1994; Vogel et al., 1998; Woeste et al., 1999). It has also been demonstrated that both ACC synthase and ACC oxidase are encoded by multigene families, members of which are differentially expressed during specific developmental stages and in response to various environmental stresses known to induce ethylene (Kende, 1993; Zarembinski and Theologis, 1994; Fluhr and Mattoo, 1996). Some of the ACC synthase (Lincoln et al., 1993; Peck and Kende, 1998; Mathooko et al., 2001) and ACC oxidase (Barry et al., 1996; Bouquin et al., 1997) genes can be induced by more than one stimuli. Moreover, it is well known that expression of these genes is often subjected to either positive or negative regulation (Kende, 1993). For example, the expression of a subset of ACC synthase and ACC oxidase genes has been shown to increase with the onset of ripening in climacteric fruit (Lelièvre et al., 1997a; Nakatsuka et al., 1998; Liu et al., 1999; Barry et al., 2000; Jiang and Fu, 2000) and in wounded or auxin-treated zucchini (Cucurbita pepo; Huang et al., 1991) and winter squash (Cucurbita maxima) fruits (Nakajima et al., 1990; Kato et al., 2000), wounded or touched tomato (Lycopersicon esculentum) fruit (Yip et al., 1992; Lincoln et al., 1993; Tatsuki and Mori, 1999), chilling-treated citrus (Wong et al., 1999) and pear (Pyrus communis) fruit (Lelièvre et al., 1997b) and cucumber (Cucumis sativus) fruit exposed to high concentrations of carbon dioxide (Mathooko et al., 1999). Despite these many reports dealing with expression of ACC synthase and ACC oxidase genes in fruit, little information is available concerning differential expression of these genes in individual fruit tissues and/or inter-tissue regulation of ethylene biosynthesis within fruit.
In this present study, we describe the isolation, expression, and regulation of cDNAs encoding three ACC synthases and two ACC oxidases from young persimmon fruit and that the initiation and propagation of ethylene biosynthesis in detached young persimmon fruit are regulated by the temporally and spatially coordinated expression of these genes. Moreover, we reveal that initial ethylene induction occurred in the calyx of persimmon fruit in correlation to water loss from the fruit and that this ethylene in turn acts as a secondary signal to stimulate autocatalytic ethylene in the other tissues of fruit, leading to burst of ethylene production.
RESULTS
Isolation and Identification of cDNAs Encoding ACC Synthase and ACC Oxidase
Three cDNA fragments encoding ACC synthase (DK-ACS1, DK-ACS2, and DK-ACS3) and two encoding ACC oxidase (DK-ACO1 and DK-ACO2) were isolated from pulp and calyx tissues of young persimmon fruit harvested at 62 d after full bloom (AFB) and held for 4 d at 20°C. These cDNA fragments were used to screen a persimmon pulp cDNA library, and the full-length cDNAs for DK-ACS1 (accession no. AB073005), DK-ACS2 (accession no. AB073006), DK-ACO1 (accession no. AB073008), and DK-ACO2 (accession no. AB073009) were cloned. The DK-ACS3 fragments identified no positive clone from the library screen, and the full-length cDNA sequence for DK-ACS3 (accession no. AB073007) was therefore obtained by RACE-PCR.
DK-ACS1 (1,660 bp), DK-ACS2 (1,839 bp) and DK-ACS3 (1,829 bp) encode predicted open reading frames of 471, 488, and 486 amino acids, respectively, and alignment of the three deduced amino acid sequences, together with that of a tomato ACC synthase gene Le-ACS2 (Rottmann et al., 1991), showed a high degree of sequence identity over seven previously described conserved regions (Kende, 1993), including the dodecapeptide reported to be a constituent of the active site (Yip et al., 1992). Eleven invariant amino acid residues that are conserved in the subgroup I aminotransferases and ACC synthases (Tarun and Theologis, 1998) are also well conserved and located at corresponding positions in all three persimmon ACC synthases, including the five amino acid residues verified to be enzymatically important by site-directed mutagenesis (White et al., 1994; Tarun and Theologis, 1998; Zhou et al., 1999). This indicates that the cloned cDNAs represent genes encoding active ACC synthase isozymes.
Alignment of the open reading frames of the tomato ACC oxidase gene LE-ACO1 (Barry et al., 1996) with the two persimmon ACC oxidases, DK-ACO1 (1,239 bp) and DK-ACO2 (1,316 bp), which encode predicted open reading frames of 319 and 321 amino acids, respectively, revealed that the DK-ACO1 and DK-ACO2 polypeptides share a high degree of sequence homology with LE-ACO1 (83% and 78%, respectively). Moreover, both DK-ACO1 and DK-ACO2 polypeptides contain conserved amino acids that are shared with all members of the Fe(II) ascorbate family of dioxygenases (Lasserre et al., 1996), including three amino acid residues reported to form a Fe(II) binding site (Shaw et al., 1996).
Genomic DNA gel-blot hybridization analysis with the probes containing the 3′-untranslated region of the cDNAs showed that each probe hybridized to distinct and unique restriction fragments, indicating that each probe hybridized to unique sequences under the hybridization conditions used in this study (data not shown).
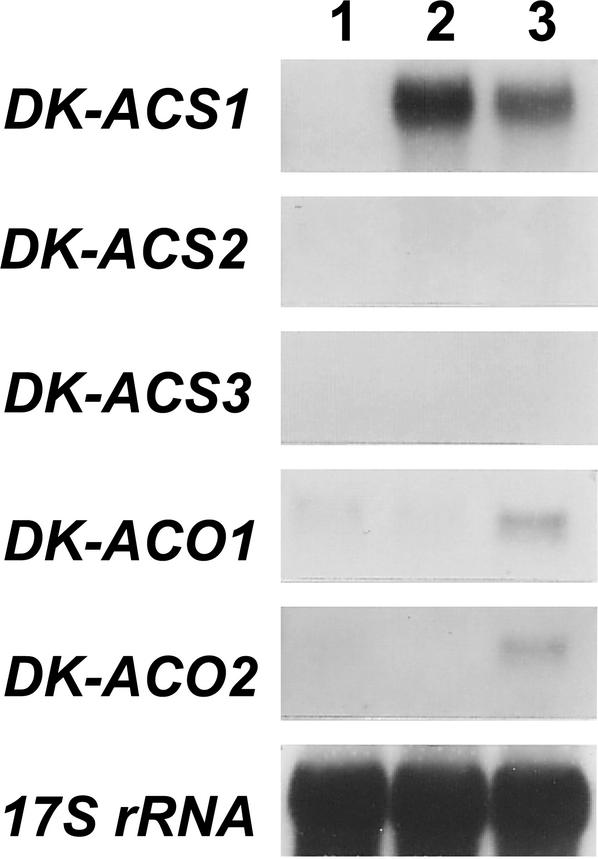
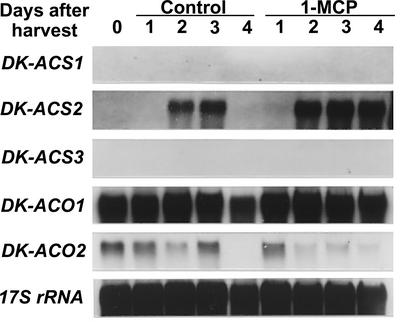
RNA gel-blot analysis with the same specific probes showed that, in fruit harvested at mature stage and held in ambient condition for 30 d until ripening-associated ethylene was produced, accumulation of DK-ACS1 mRNA but not DK-ACS2 and DK-ACS3 mRNA was detected with faint constitutive expression of DK-ACO1 (Fig. 1). In addition, when harvested mature fruit were treated with exogenous ethylene, the expression of DK-ACS1 was induced with the slight increase in the level of the abundance of the two ACC oxidase mRNAs. These results suggest that, within the three ACC synthase genes tested, DK-ACS1 was predominantly expressed in ripening fruit and was thus responsible for the ethylene production associated with fruit ripening.
Figure 1.
Expression of DK-ACS and DK-ACO gene families during ripening in persimmon fruit. Fruit harvested at mature stage (156 d AFB) were held at 20°C in ambient laboratory humidity for 30 d or treated with 50 μL L−1 of exogenous ethylene for 2 d. Lane 1, Fruit at harvest; lane 2, fruit held for 30 d; lane 3, ethylene-treated fruit. Each lane contained 5 μg of total RNA, and the transcript levels of 17S rRNA are shown as an internal loading control.
Effect of the Ethylene Action Inhibitor 1-Methylcyclopropene (1-MCP) on Ethylene Production in Different Tissues of Detached Young Persimmon Fruit
To characterize ethylene biosynthesis induced in detached young persimmon, fruit detached at 65 d AFB were treated with or without 1-MCP, an effective inhibitor of ethylene action, and then ethylene production and related gene expression were determined in the individual fruit tissues shown in Figure 2.
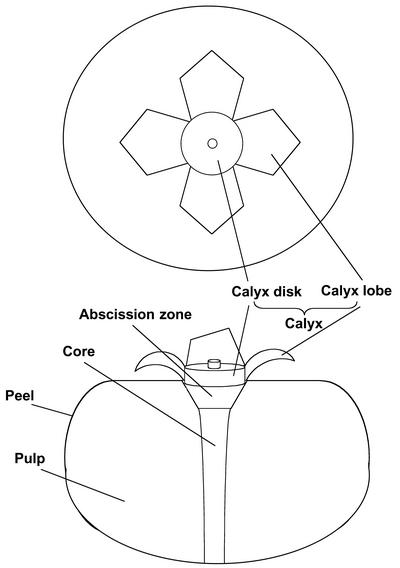
Figure 2.
Schematic diagrams showing a perspective view and a longitudinal section of persimmon fruit.
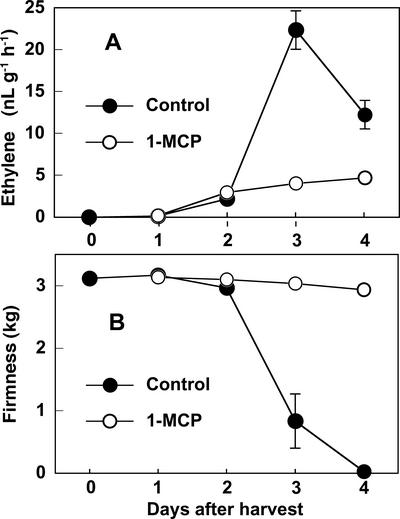
Ethylene production from whole control fruit was detectable at 1 d after detachment and increased to a peak at 3 d (Fig. 3A), followed by rapid fruit softening at 3 d (Fig. 3B) and calyx abscission at 4 d. The application of 1-MCP substantially suppressed ethylene production and virtually eliminated fruit softening and calyx abscission, indicating the involvement of ethylene action in these characteristic aspects of fruit senescence.
Figure 3.
Effects of 1-MCP treatment on the rate of whole-fruit ethylene production (A) and flesh firmness (B) in detached young persimmon fruit during storage at 20°C. Fruit were detached at young stage (65 d AFB) and held at 20°C in ambient laboratory humidity (40%–60% RH). Immediately after detachment, fruit were treated with 200 nL L−1 1-MCP for 3 h. Each point represents the mean value for three fruits and vertical bars represent ±se.
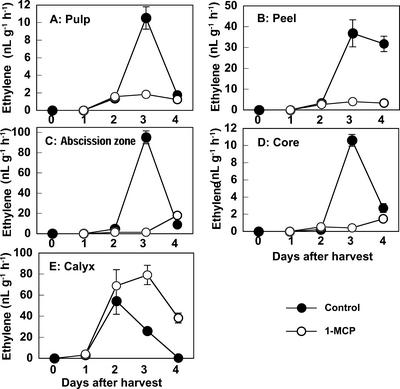
Figure 4 shows the rate of ethylene production from different fruit tissues. In control fruit, ethylene production by the pulp was detectable at 2 d after detachment and peaked at 3 d (Fig. 4A) and was markedly suppressed by 1-MCP. Ethylene production in abscission zones and core and peel tissues showed similar patterns (Fig. 4, B–D, respectively). In contrast, in the calyx of control fruit, ethylene production started to increase and peaked 1 d earlier than in the other tissues, and 1-MCP did not suppress ethylene production, but rather prolonged elevated ethylene levels (Fig. 4E). The calyx tissue was further divided into two parts, calyx lobes and calyx discs, and ethylene production by each was measured (data not shown). Both parts of the calyx showed a similar pattern of ethylene production with regard to timing at the peak and response to 1-MCP; however, the calyx discs produced a much larger amount of ethylene than the calyx lobes.
Figure 4.
Effect of 1-MCP treatment on the rate of ethylene production in various tissues of young persimmon fruit during storage at 20°C. Each point represents the mean of three replications and vertical bars represent ±se.
Expression of the ACC Synthase and ACC Oxidase Genes in Individual Tissue of Fruit and the Effect of 1-MCP Treatment
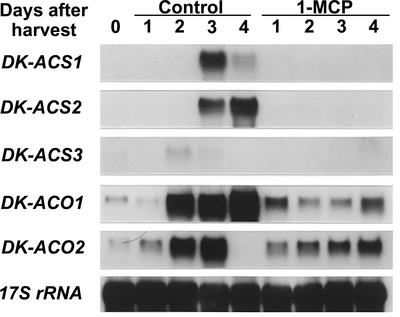
RNA gel-blot analysis with the specific probes containing the 3′-untranslated region of the cDNAs showed that in the pulp of control fruit, low levels of DK-ACS3 mRNA accumulated transiently at 2 d after detachment, whereas DK-ACS1 and DK-ACS2 mRNA levels were more abundant and peaked at 3 and 4 d after detachment, respectively (Fig. 5). Accumulation of DK-ACO1 and DK-ACO2 mRNAs was detectable at harvest, and their levels increased dramatically at 2 d after detachment. 1-MCP suppressed the mRNA levels of all three ACC synthase genes to undetectable levels and the ACC oxidase genes to lower levels. These observations indicate that the induction of ACC synthase genes and the elevated levels of ACC oxidase mRNA in the pulp are mediated by the action of ethylene, although accumulation of DK-ACO2 mRNA increased slightly even in the 1-MCP-treated pulp.
Figure 5.
Northern-blot analysis of the expression of DK-ACS and DK-ACO genes in the pulp of young persimmon fruit treated with or without 1-MCP during storage at 20°C. Each lane contained 5 μg of total RNA, and the transcript levels of 17S rRNA are shown as an internal loading control.
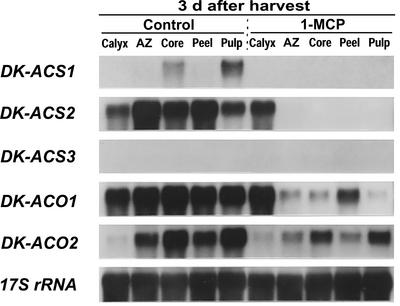
Figure 6 shows expression of ACC synthase and ACC oxidase genes in various fruit tissues at 3 d after detachment. Accumulation of DK-ACS2, DK-ACO1, and DK-ACO2 mRNAs was detected in each control fruit tissue, whereas accumulation of DK-ACS1 mRNA was detected only in the core and pulp. Other than in calyx, the accumulation of these mRNAs was substantially suppressed by 1-MCP.
Figure 6.
Expression of DK-ACS and DK-ACO gene families in various tissues of young persimmon fruit at 3 d after detachment with or without 1-MCP treatment. Each lane contained 5 μg of total RNA, and the transcript levels of 17S rRNA are shown as an internal loading control. AZ, Abscission zone.
In the total calyx of control fruit, elevated levels of DK-ACS2, but not DK-ACS1 and DK-ACS3, were detected at 2 and 3 d, and in the calyx of 1-MCP-treated fruit, higher levels of DK-ACS2 mRNA were maintained until 4 d after detachment (Fig. 7). Abundant levels of DK-ACO1 mRNA and low levels of DK-ACO2 mRNA were somewhat constitutive irrespective of 1-MCP treatment. Thus, expression patterns of the genes related to ethylene biosynthesis in the calyx were completely different from those in the other fruit tissues. RNA gel-blot analysis with subdivided parts of calyx showed that the accumulation levels of DK-ACS2 mRNA were much higher in calyx discs than calyx lobes (data not shown), correlating to the rates of ethylene production. On the other hand, the levels of DK-ACO1 and DK-ACO2 mRNAs were almost equal between the two parts.
Figure 7.
Changes in the accumulation of mRNAs corresponding to DK-ACS and DK-ACO genes in the calyx of young persimmon fruit treated with or without 1-MCP during storage at 20°C. Each lane contained 5 μg of total, and the transcript levels of 17S rRNA are shown as an internal loading control.
Effect on Ethylene Production of Alleviation of Water Loss by Packaging in a Perforated Polyethylene Bag
To investigate the potential involvement of water loss from fruit in ethylene induction in detached young persimmon fruit, we investigated the response of fruit to various rates of water loss by packaging the fruit detached at a young stage (70 d AFB) in polyethylene bags with different numbers of holes, representing from 0.03% to 0.3% of the total film surface area (TFSA).
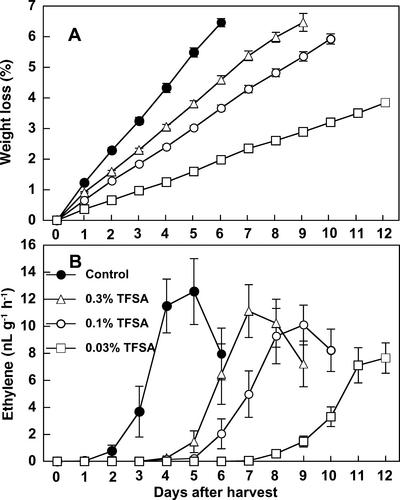
Packaging the fruit effectively alleviated the weight loss, and average daily weight loss values of 0.76%, 0.61%, and 0.33% were observed in the fruit packaged in 0.3%, 0.1%, and 0.03% TFSA polyethylene bag, respectively, compared with 1.1% in non-packaged control fruit (Fig. 8A). Ethylene production in control fruit began at 2 or 3 d after detachment when the weight loss reached 2.5% to 3.0%, whereas packaging significantly delayed the onset of ethylene production (Fig. 8B). This delay was correlated with the reduced rate of weight loss, and irrespective of hole number, most fruit started to produce ethylene when the weight loss reached 2.5% to 3.0%. Thus, the packaging retarded ethylene induction in the fruit. Because the gas compositions inside the bags were virtually identical to ambient atmosphere (data not shown), this was apparently independent of the so-called controlled atmosphere effect.
Figure 8.
Effects of packaging fruit in perforated polyethylene bags with different numbers of holes on weight loss (A) and the rate of ethylene production (B) in persimmon fruit detached at a young stage (70 d AFB). Ten fruit per treatment were packaged individually in perforated polyethylene bags (150 × 130 mm) with two, eight, or 18 holes (4-mm diameter) equivalent to 0.03%, 0.15%, or 0.3% of the TFSA, respectively. Non-packaged fruit were used as control. Each column represents the mean of 10 fruits and vertical bars represent ±se.
Expression of the ACC Synthase and ACC Oxidase Genes in Fruit Kept in Low- or High- Humidity Conditions
To determine whether enhancement of ethylene induction by water loss is regulated at transcriptional level of DK-ACS2 in the calyx, young fruit (65 d AFB) were detached and held in high- or low-humidity conditions, and the expression of the ACC synthase and the ACC oxidase genes in the pulp and calyx were compared.
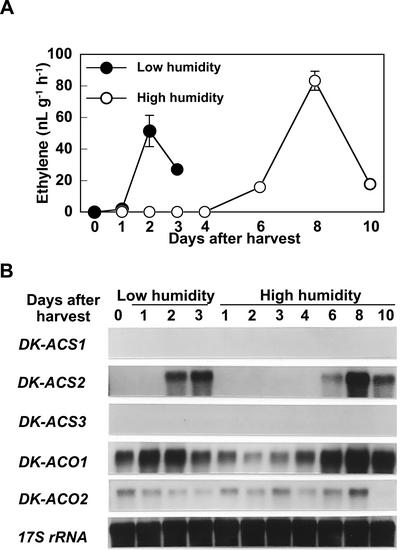
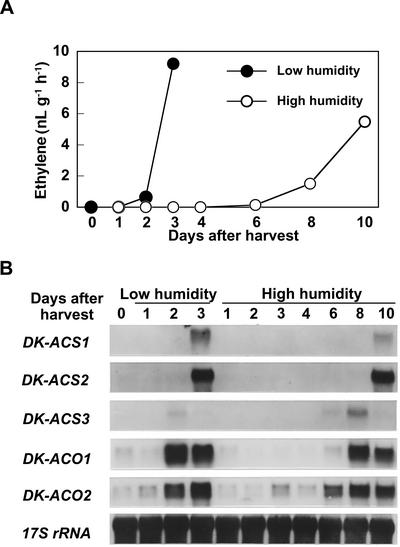
By keeping the fruit in high-humidity conditions, weight loss from the fruit was substantially reduced, and initiation of ethylene production was delayed by approximately 5 d (data not shown). Fruit held in low or high humidity initiated ethylene production at 1 or 6 d after detachment, respectively.
The induction of ethylene production and accumulation of DK-ACS2 mRNA in the calyx of fruit held in high humidity were also delayed for more than 4 d compared with those in low humidity (Fig. 9). DK-ACS2 expression in calyx was detected at 2 or 6 d in low- or high-humidity conditions, respectively. In parallel with the delay in ethylene biosynthesis and ethylene-related gene expression in the calyx, the induction of ethylene production and the accumulation of three DK-ACS and two DK-ACO mRNAs in the pulp were delayed in fruit held in high humidity (Fig. 10). In the pulp of fruit held in low humidity, substantial levels of DK-ACS1 and DK-ACS2 mRNAs accumulated at 3 d after detachment and transient accumulation of DK-ACS3 mRNA and elevated levels of DK-ACO1 and DK-ACO2 mRNAs were detected from 2 d onward, whereas in the pulp under high humidity, similar changes in the accumulation of these mRNAs were not detected until 6 d after detachment.
Figure 9.
Changes in the rate of ethylene production (A) and the accumulation of mRNAs corresponding to members of the DK-ACS and DK-ACO gene families (B) in the calyx of young persimmon fruit held in low- or high-humidity conditions. Low humidity was equivalent to ambient laboratory conditions (40%–60% RH), and high-humidity conditions (95% RH) were maintained in a container through which humidified air was passed at 250 mL min−1. Each point in A represents the mean of three replications and vertical bars represent ±se. Each lane in B contained 5 μg of total RNA, and the transcript levels of 17S rRNA are shown as an internal loading control.
Figure 10.
Changes in the rate of ethylene production (A) and the accumulation of mRNAs corresponding to DK-ACS and DK-ACO genes (B) in the pulp of young persimmon fruit held in low- (40%–60% RH) or high- ( 95% RH) humidity conditions. Each point in A represents the mean of three replications and vertical bars represent ±se. Each lane in B contained 5 μg of total RNA, and the transcript levels of 17S rRNA are shown as an internal loading control.
DISCUSSION
Tissue-Specific Regulation of Ethylene Biosynthesis in Detached Young Persimmon Fruit and the Effect of the Ethylene Action Inhibitor 1-MCP
It has been reported that ethylene production in persimmon fruit is greater in fruit detached at younger stages (Takata, 1983). In this study, we confirmed that young persimmon fruit generate large quantities of ethylene immediately after detachment, accompanied with rapid fruit softening and calyx abscission (Fig. 3). This ethylene was produced from a variety of fruit tissues (Fig. 4); however, ethylene production in the calyx initiated and reached a peak 1-d earlier than in other fruit tissues (Fig. 4). RNA gel-blot analysis of three ACC synthase gene (DK-ACS1, DK-ACS2, and DK-ACS3) and two ACC oxidase gene (DK-ACO1 and DK-ACO2) revealed that different members of these gene families exhibit distinct spatial patterns of expression. For example, in pulp, marked accumulation of three ACC synthase and two ACC oxidase mRNAs was detected with increase in ethylene production (Fig. 5), while in the calyx, only DK-ACS2 mRNA accumulated with ethylene production but two ACC oxidase genes were expressed constitutively (Fig. 7).
Because 1-MCP effectively blocks ethylene receptors and represses ethylene-mediated effects in plant tissues (Sisler and Serek, 1997), it provides a useful tool to study ethylene-regulated processes, such as feedback mechanisms that control ethylene biosynthesis (Bouquin et al., 1997; Nakatsuka et al., 1998). In pulp, abscission zones, core, and peel, 1-MCP treatment markedly inhibited ethylene production and the expression of all ACC synthase gene tested (Figs. 4–6), suggesting that ethylene biosynthesis in these tissues is modulated by ethylene action and that the expression of these genes is regulated by a positive feedback system. In contrast, ethylene production in the calyx was not inhibited but rather was maintained at elevated levels by the 1-MCP treatment (Fig. 4), and expression of DK-ACS2 in the calyx of 1-MCP-treated fruit was enhanced compared with control fruit (Fig. 7). These results indicate that induction of ethylene biosynthesis in the calyx is regulated in an ethylene-independent manner.
Interestingly, the expression of DK-ACS2 was regulated both in an ethylene-dependent and independent manner, as observed in the pulp and calyx, respectively (Figs. 5–7), suggesting that both regulatory systems coordinately regulate this single ethylene biosynthesis-related gene in persimmon fruit. A similar observation has been reported for LE-ACS2, a tomato ACC synthase gene, which is inducible by both ripening and wound stress (Rottmann et al., 1991). The ripening-related expression of this gene is suppressed by an inhibitor of ethylene action, suggesting positive feedback regulation (Nakatsuka et al., 1998), whereas the wound-induced expression is ethylene independent (Tatsuki and Mori, 1999). In detached young persimmon fruit, however, the regulatory system is much more complicated because both ethylene-dependent and -independent regulations operate at the same time in different tissues of the same fruit.
In general, ripening-related ethylene biosynthesis in climacteric fruit is considered to be under positive feedback regulation, and the suppression of ethylene production by 1-MCP has been reported in various ripening fruit, including apple (Malus domestica; Fan et al., 1999), pear (Lelièvre et al., 1997b), tomato (Nakatsuka et al., 1998), and avocado (Persea americana; Feng et al., 2000). Ethylene biosynthesis and the expression of ACC synthase genes, which is stimulated by external factors such as wounding in fruit (Nakajima et al., 1990; Mullins et al., 1999; Tatsuki and Mori, 1999), application of exogenous auxin to vegetative tissues (Yoon et al., 1997; Peck and Kende, 1998) and pollination of orchid flowers (Bui and O'Neill, 1998), is conversely under negative feedback regulation or independent of ethylene action.
Therefore, the factor that stimulates DK-ACS2 expression in the calyx might not be an internal ripening-related factor but be an external stress-related factor. In the harvested mature fruit, only the expression of DK-ACS1 was detected during fruit ripening or in response to exogenous ethylene treatment, suggesting that DK-ACS1 but not DK-ACS2 is a ripening-regulated gene (Fig. 1). Because ethylene induction in the calyx preceded that in other fruit tissues (Fig. 4), ethylene biosynthesis in detached young persimmon is suggested to be initiated by a stimulus that induces DK-ACS2 expression in the calyx in an ethylene-independent manner.
Involvement of Water Loss from Fruit in Ethylene Induction in Detached Young Persimmon Fruit
After detachment from the parent tree, harvested fruit experience water loss (Wills et al., 1998), and we accordingly hypothesized that water stress may act as an external stress-related factor that modulates ethylene biosynthesis in persimmon calyx tissue. The alleviation of water loss from the fruit by packaging in perforated polyethylene bags or exposure to humidified airflow markedly delayed the initiation of ethylene production and expression of DK-ACS2 in the calyx (Figs. 8 and 9). Interestingly, the initiation of ethylene production was delayed, together with a reduced rate of water loss, and irrespective of the reduced rate, the fruit started to produce ethylene when a specific amount of water was lost (Fig. 8). These results demonstrate the involvement of water loss in the initiation of ethylene biosynthesis in detached young persimmon.
On the basis of the results presented in this study, a model can be proposed in which initiation and propagation of ethylene biosynthesis in detached young persimmon fruit is regulated by temporally and spatially coordinated expression of the ACC synthase and ACC oxidase genes. As the harvested fruit lose water through transpiration, water stress occurs in the fruit when water loss reaches a critical point. The water stress signal stimulates the expression of DK-ACS2 in the calyx, particularly in the calyx disc, and the newly formed ACC is oxidized to ethylene by basal levels of constitutive pre-existing ACC oxidase, resulting in initial ethylene induction in the calyx. This ethylene diffuses into the other fruit tissues and acts as a secondary signal to stimulate the expression of the three ACC synthase and the two ACC oxidase genes, leading to autocatalytic ethylene production within these tissues.
Similar models of sequential expression of ACC synthase and ACC oxidase genes and inter-tissue regulation of ethylene biosynthesis have been reported in auxin-treated etiolated pea (Pisum sativum) seedlings (Peck and Kende, 1995) and pollinated orchid flowers (O'Neill et al., 1993; Bui and O'Neill, 1998). In orchid flowers, it has been demonstrated that a primary pollination signal induces the expression of two ACC synthase genes in the stigma and ovary, respectively, and the resultant ACC and ethylene are translocated to perianth and labelum. In the perianth, translocated ACC is oxidized to ethylene by ACC oxidase, whereas in the labelum, translocated ethylene stimulates the expression of an ACC synthase and an ACC oxidase gene, leading to further ethylene biosynthesis.
Persimmon possesses a relatively large calyx compared with other fruits. The calyx contains chlorophyll and shows high photosynthetic ability equivalent to leaves (Nakano et al., 1997). Moreover, unlike the fruit skin, the calyx has many stomata and is considered to be the “gas exchange organ” of the persimmon (Kitagawa and Glucina, 1984). Removal of calyx lobes and sealing the scar with Vaseline actually reduced the fruit carbon dioxide exchange rate markedly, which resulted in a remarkable inhibition of fruit development (Yonemori et al., 1996; Nakano et al., 1998). In addition to these functions, the calyx may have a role as stress sensor for the fruit as shown in this study. Because weight loss was observed in every tissue of the fruit (data not shown), it seems that calyx has a higher sensitivity to the water stress than the other parts of persimmon fruit and thus acts as a water stress sensor for the fruit. In the case of exposure of fruit to elevated levels of carbon dioxide, initial ethylene production is also detected in the calyx (R. Nakano, P. Kubo, and A. Inaba, unpublished data), suggesting that the calyx is responsible for sensing not only water stress, but also for other environmental stresses. However, further experiments will be required to investigate whether the enhanced expression of DK-ACS2 by water loss is a unique phenomenon in the calyx or a general phenomenon shown in other tissues such as leaf and seedling.
In conclusion, we propose a model in which ethylene production by detached young persimmon fruit is triggered by water loss through the induction of DK-ACS2 expression in the calyx. This ethylene diffuses into other parts of the fruit where it induces autocatalytic ethylene biosynthesis, resulting in a burst of ethylene production. In some cultivars of Japanese persimmon such as cvs Tonewase and Saijo, even the fruit harvested at optimal maturity retain the characteristics of young fruit and thus produce ethylene in correlation with water loss, which in turn causes the rapid fruit softening, a major problem in marketing of these cultivars in Japan (Nakano et al., 2001, 2002). We are now establishing the method to prolong the post-harvest life of these persimmon cultivars by using perforated polyethylene film or a corrugated cardboard container coated with the water-impervious material.
MATERIALS AND METHODS
Plant Material and Treatments
Persimmon (Diospyros kaki Thunb. cv Hiratanenashi) fruit were harvested at young stage (65 d AFB) and held at 20°C in ambient laboratory humidity (40%–60% relative humidity [RH]). Immediately after harvest, some fruit were treated with 200 nL L−1 of 1-MCP for 3 h according to the method described by Nakatsuka et al. (1997). The rates of ethylene production and flesh firmness in whole fruit were measured daily. Fruit were then cut and divided into pulp, peel, abscission zone, core, and calyx tissues (Fig. 2), and in another experiment, the calyx was subdivided into lobe and disc tissues (Fig. 2). Individual tissues were measured for ethylene production and subsequently frozen in liquid nitrogen and stored at −80°C before RNA extraction.
To investigate the involvement of water loss in ethylene production by young persimmon, fruit harvested at a young stage (70 d AFB) were packaged individually in perforated polyethylene bags (150 × 130 mm) with two, eight, or 18 holes (4 mm in diameter), representing 0.03%, 0.15%, or 0.3% of the TFSA, respectively. The packaged fruit and non-packaged control fruit were held at 20°C in ambient laboratory humidity (40%–60% RH), and weight loss and rate of ethylene production were monitored daily. In another experiment, fruit harvested at a young stage (65 d AFB) were held at 20°C in low humidity, which was equivalent to ambient laboratory conditions (40%–60% RH), or high humidity (greater than 95% RH), achieved by placing the fruit in a container through which humidified air was passed at the rate of 250 mL min−1. The rates of ethylene production and flesh firmness values by whole fruit were measured at one or 2-d intervals and then ethylene production by individual fruit tissues was determined before freezing and storing the tissue as described above.
To analyze expression of the ACC synthase and ACC oxidase genes during fruit ripening, fruit harvested at mature stage (156 d AFB) were held at 20°C in ambient laboratory humidity for 30 d until ripening-associated ethylene was produced. Furthermore, after harvest, some fruit were treated with 50 μL L−1 of exogenous ethylene for 2 d to induce autocatalytic ethylene production. Pulp tissues were frozen in liquid nitrogen and stored at −80°C before RNA extraction.
Determination of Ethylene Production, Flesh Firmness, and Weight Loss
The rate of ethylene production by whole fruit was measured by enclosing samples in 1.5-L airtight containers for 1 h at 20°C, withdrawing 1 mL of the headspace gas, and injecting it into a gas chromatograph (model GC-4CMPF, Shimadzu, Kyoto) fitted with a flame ionization detector and an activated alumina column. To determine ethylene production in individual fruit tissues, immediately after dividing the fruit into individual tissue, each tissue was enclosed in a 30- or 150-mL airtight chamber and incubated for 10 min at 20°C to avoid contamination with wound-induced ethylene. Flesh firmness was measured using a penetrometer (model SMT-T-50, Toyo Baldwin, Tokyo) fitted with a cylindrical plunger (8 mm in diameter) and corresponded to the force required to puncture the peeled flesh at equatorial regions of the fruit on two opposite sides. Weight loss was expressed as a percentage of initial fruit weight.
RNA Extraction and Reverse Transcriptase (RT)-PCR
RNA was extracted by the hot borate method (Wan and Wilkins, 1994), and poly(A+) RNA was isolated using Oligotex-dT30 (Takara, Kyoto) according to the manufacturer's protocol. The first-strand cDNAs, synthesized by RT from 2 μg of the poly(A+) RNA isolated from pulp or calyx tissues of ethylene-producing young persimmon fruits, were used as templates for the RT-PCR using degenerate oligonucleotide primers for ACC synthase and ACC oxidase. These primers were designed based on conserved amino acid sequences of ACC synthases (MGF/LAENQ and WFRVT/CFA) and ACC oxidases (Nakatsuka et al., 1998). Reactions for the RT-PCR were subjected to 30 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 3 min. The amplified cDNAs were cloned into the pGEM-T Easy vector (Promega, Madison, WI) and sequenced using a model DSQ-1000 DNA sequencer (Shimadzu) with either the −21M13 or M13 sequencing primers, according to the manufacturer's protocol (Amersham Biosciences UK, Ltd., Little Chalfont, UK).
cDNA Library Construction and Screening
A persimmon fruit cDNA library was constructed by using 5 μg of poly(A+) RNA from pulp tissue of detached young fruit and a ZAP-cDNA synthesis kit (Stratagene, La Jolla, CA). cDNAs were cloned into a Uni-ZAP XR vector (Stratagene) and packaged in Gigapack III gold packaging extract (Stratagene), and the unamplified library was used directly for screening. For the library screening of each cDNA, 1.5 × 104 plaques were plated, and the corresponding filters were hybridized overnight at 68°C in standard buffer with DIG-labeled probes of the cDNA fragments obtained from the RT-PCR described below (Roche Diagnostics, Mannheim, Germany). After low-stringency washes (twice at 37°C in 5× SSC and 0.1% [w/v] SDS for 15 min and twice at 68°C in 2× SSC and 0.1% [w/v] SDS for 30 min), the membranes were subjected to immunological assay according to manufacturer's instructions (Roche Diagnostics). Positive plaques were carried through a second screening and then in vivo-excised and sequenced as described above. Both strands of the clones of interest were sequenced after subcloning.
Amplification of Full-Length cDNA by RACE-PCR
To determine the full-length nucleotide sequences for DK-ACS3, RACE-PCR was performed using a cDNA amplification kit (Marathon, CLONTECH, Palo Alto, CA) according to the manufacturer's protocol. To amplify 5′-end and 3′-end fragments, specific primers were designed based on the nucleotide sequences of the cDNA fragments for DK-ACS3.
Probe Preparation
DIG-labeled probes were synthesized using a PCR DIG probe synthesis Kit (Roche Diagnostics). For the screening of the cDNA library, DIG-labeled probes were amplified using pGEM-T Easy plasmids containing the cDNA fragments obtained from the RT-PCR as templates and T7 and SP6 primers corresponding to vector sequences adjoining the multiple cloning site. For northern and Southern analysis, gene-specific probes containing the 3′-untranslated region of the cDNAs were amplified using plasmids obtained from the cDNA library screening and the RACE-PCR as templates together with the gene-specific primers. These primers were designed to amplify the regions corresponding to bp 1,174 to 1,612 of DK-ACS1, bp 1,365 to 1,821 of DK-ACS2, bp 1,367 to 1,802 of DK-ACS3, bp 819 to 1,154 of DK-ACO1, and bp 800 to 1,298 of DK-ACO2.
DNA Gel-Blot Hybridization
Genomic DNA was isolated from immature persimmon leaves according to Kanzaki et al. (2001) and a 5-μg sample of DNA digested with the restriction enzymes NcoI, EcoRI and HindIII was separated on 0.8% (w/v) agarose gels, and then blotted onto nylon membranes (Hybond N+, Amersham Biosciences UK). The filters were hybridized with the DIG-labeled gene-specific probes containing the 3′-untranslated region of the cDNAs described above in high SDS buffer (7% [w/v] SDS, 5× SSC, 50 mm sodium-phosphate, pH 7.0, 2% [w/v] blocking reagent, and 0.1% [w/v] N-lauroylsarcosine) containing 50% (v/v) formamide (Roche Diagnostics) overnight at 42°C. After hybridization, filters were washed twice at 37°C in 2× SSC and 0.1% (w/v) SDS for 15 min and twice at 55°C in 0.1× SSC and 0.1% (w/v) SDS for 30 min. The membranes were then subjected to immunological detection according to the manufacturer's instructions using CDP-star as a chemiluminescent substrate for alkaline phosphatase (Roche Diagnostics).
RNA Gel-Blot Hybridization
Aliquots of total RNA (5 μg) were separated by electrophoresis on 1% (w/v) agarose gels containing 2.2 m formaldehyde and blotted onto nylon membranes (Hybond N+, Amersham Biosciences UK). The filters were then hybridized, washed, and subjected to immunological detection as described above. The hybridization and final washes were at 42°C and 58°C, respectively.
ACKNOWLEDGMENTS
We would like to thank Dr. Jocelyn Rose (Cornell University, Ithaca, NY) for his helpful discussion and Mr. Willis Owino (Okayama University, Okayama, Japan) for his careful reading of the manuscript.
Footnotes
This work was supported in part by the Ministry of Education, Science, Sports and Culture of Japan (Grant-in-Aid for Young Scientists no. 13760023 to R.N. and Grant-in-Aid for Scientific Research no. 14560023 to Y.K.) and by the Ministry of Agriculture, Forestry and Fisheries of Japan (research project for utilizing advanced technologies in agriculture, forestry and fisheries no. 1421 to R.N.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010462.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. San Diego: Academic Press; 1992. [Google Scholar]
- Aharoni Y. Respiration of oranges and grapefruits harvested at different stages of development. Plant Physiol. 1968;43:99–102. doi: 10.1104/pp.43.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A, Yang SF. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981;68:594–596. doi: 10.1104/pp.68.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biale JB, Young RE. Respiration and ripening in fruits: retrospect and prospect. In: Friend J, Rhodes MJC, editors. Recent Advances in the Biochemistry of Fruits and Vegetables. London: Academic Press; 1981. pp. 1–39. [Google Scholar]
- Bouquin T, Lasserre E, Pradier J, Pech J-C, Balague C. Wound and ethylene induction of the ACC oxidase melon gene CM-ACO1 occurs via two direct and independent transduction pathways. Plant Mol Biol. 1997;35:1029–1035. doi: 10.1023/a:1005902226054. [DOI] [PubMed] [Google Scholar]
- Bui AQ, O'Neill SD. Three 1-aminocyclopropane-1-carboxylate synthase genes regulated by primary and secondary pollination signals in orchid flowers. Plant Physiol. 1998;116:419–428. doi: 10.1104/pp.116.1.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaks IL. Respiratory response, ethylene production, and response to ethylene of citrus fruit during ontogeny. Plant Physiol. 1970;45:334–338. doi: 10.1104/pp.45.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Blankenship SM, Mattheis JP. 1-Methylecyclopropene inhibits apple ripening. J Am Soc Hortic Sci. 1999;124:690–695. [Google Scholar]
- Feng X, Apelbaum A, Sisler EC, Goren R. Control of ethylene responses in avocado fruit with 1-methylcyclopropene. Postharvest Biol Technol. 2000;20:143–150. [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene: biosynthesis and perception. CRC Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- Huang PL, Parks JE, Rottmann PW, Theologis A. Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated. Proc Natl Acad Sci USA. 1991;88:7021–7025. doi: 10.1073/pnas.88.16.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H, Murata T. Ethylene production by Satsuma mandarin fruit harvested at the various stages of development. J Jpn Soc Hortic Sci. 1972;41:405–410. [Google Scholar]
- Jiang Y, Fu J. Ethylene regulation of fruit ripening: molecular aspects. Plant Growth Regul. 2000;30:193–200. [Google Scholar]
- Kanzaki S, Yonemori K, Sugiura A, Sato A, Yamada M. Identification of molecular markers linked to the trait of natural astringency loss of Japanese persimmon. J Am Soc Hortic Sci. 2001;126:51–55. [Google Scholar]
- Kato M, Hayakawa Y, Hyodo H, Ikoma Y, Yano M. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurubita maxima. Plant Cell Physiol. 2000;41:440–447. doi: 10.1093/pcp/41.4.440. [DOI] [PubMed] [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Kitagawa H, Glucina PG. Persimmon Culture in New Zealand. Wellington, New Zealand: Science Information Publishing Center; 1984. [Google Scholar]
- Kubo Y, Nakano R, Yamamoto M, Inaba A (2003) Cloning of genes encoding cell wall modifying enzymes and their expression in persimmon fruit. Acta Hortic (in press)
- Lasserre E, Bouquin T, Hernandez JA, Bull J, Pech J-C, Balague C. Structure and expression of three genes encoding ACC oxidase homologs from melon (Cucumis melo L.) Mol Gen Genet. 1996;251:81–90. doi: 10.1007/BF02174348. [DOI] [PubMed] [Google Scholar]
- Lelièvre J-M, Latchè A, Jones B, Bouzayen M, Pech J-C. Ethylene and fruit ripening. Physiol Plant. 1997a;100:727–739. [Google Scholar]
- Lelièvre J-M, Tichit L, Dao P, Fillion L, Nam Y-W, Pech J-C, Latchè A. Effects of chilling on the expression of ethylene biosynthetic genes in Pass-Crassane pear (Pyrus communis L.) fruits. Plant Mol Biol. 1997b;33:847–855. doi: 10.1023/a:1005750324531. [DOI] [PubMed] [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Liu X, Shiomi S, Nakatsuka A, Kubo Y, Nakamura R, Inaba A. Characterization of ethylene biosynthesis associated with ripening in banana fruit. Plant Physiol. 1999;121:1257–1265. doi: 10.1104/pp.121.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathooko FM, Mwaniki MW, Nakatsuka A, Shiomi S, Kubo Y, Inaba A, Nakamura R. Expression characteristics of CS-ACS1, CS-ACS2 and CS-ACS3, three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in cucumber (Cucumis sativus L.) fruit under carbon dioxide stress. Plant Cell Physiol. 1999;40:164–172. doi: 10.1093/oxfordjournals.pcp.a029524. [DOI] [PubMed] [Google Scholar]
- Mathooko FM, Tsunashima Y, Owino WZO, Kubo Y, Inaba A. Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunus persica Basch.) fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biol Technol. 2001;21:265–281. [Google Scholar]
- Morgan PW, Drew CM. Ethylene and plant responses to stress. Physiol Plant. 1997;100:620–630. [Google Scholar]
- Mullins ED, McCollum TG, McDonald RE. Ethylene: a regulator of stress-induced ACC synthase activity in nonclimacteric fruit. Physiol Plant. 1999;107:1–7. [Google Scholar]
- Nakajima N, Mori H, Yamazaki K, Imaseki H. Molecular cloning and sequence of a complementary DNA encoding 1-aminocyclopropane-1-carboxylate synthase induced by tissue wounding. Plant Cell Physiol. 1990;31:1021–1029. [Google Scholar]
- Nakano R. Involvement of water stress-induced ethylene in postharvest softening in Japanese persimmon (Diospyros kaki Thunb.) fruit. PhD thesis. Kyoto: Kyoto University; 2002. [Google Scholar]
- Nakano R, Harima S, Ogura E, Inoue S, Kubo Y, Inaba A. Involvement of stress-induced ethylene biosynthesis in fruit softening of ‘Saijo’ persimmon. J Jpn Soc Hortic Sci. 2001;70:581–585. [Google Scholar]
- Nakano R, Inoue S, Kubo Y, Inaba A. Water stress-induced ethylene in the calyx triggers autocatalytic ethylene production and fruit softening in ‘Tonewase’ persimmon grown in a heated plastic-house. Postharvest Biol Technol. 2002;25:293–300. [Google Scholar]
- Nakano R, Yonemori K, Sugiura A. Fruit respiration for maintaining sink strength during final swell at growth stage III of persimmon fruit. J Hortic Sci Biotechnol. 1998;73:341–346. [Google Scholar]
- Nakano R, Yonemori K, Sugiura A. Photosynthesis by calyx lobes has no contribution to early fruit development in persimmon. Acta Hortic. 1997;436:345–354. [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Shiomi S, Kubo Y, Inaba A. Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol. 1997;38:1103–1110. doi: 10.1093/oxfordjournals.pcp.a029094. [DOI] [PubMed] [Google Scholar]
- O'Neill SD, Nadeau JA, Zhang XS, Bui AQ, Halevy AH. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell. 1993;5:419–432. doi: 10.1105/tpc.5.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC, Kende H. Sequential induction of the ethylene biosynthetic enzymes by indole-3-acetic acid in etiolated peas. Plant Mol Biol. 1995;28:293–301. doi: 10.1007/BF00020248. [DOI] [PubMed] [Google Scholar]
- Peck SC, Kende H. Differential regulation of genes encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase in etiolated pea seedlings: effects of indole-3-acetic acid, wounding, and ethylene. Plant Mol Biol. 1998;38:977–982. doi: 10.1023/a:1006033030081. [DOI] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Shaw J-F, Chou Y-S, Chang R-C, Yang SF. Characterization of the ferrous ion binding sites of apple 1-aminocyclopropane-1-carboxylate oxidase by site-directed mutagenesis. Biochem Biophys Res Commun. 1996;225:697–700. doi: 10.1006/bbrc.1996.1237. [DOI] [PubMed] [Google Scholar]
- Sisler EC, Serek M. Inhibitors of ethylene responses in plants at the receptor level: recent developments. Physiol Plant. 1997;100:577–582. [Google Scholar]
- Spanu P, Grosskopf DG, Felix G, Boller T. The apparent turnover of 1-aminocyclopropane-1-carboxylate synthase in tomato cells is regulated by protein phosphorylation and dephosphorylation. Plant Physiol. 1994;106:529–535. doi: 10.1104/pp.106.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M. Respiration, ethylene production and ripening of Japanese persimmon fruit harvested at various stages of development. J Jpn Soc Hortic Sci. 1983;52:78–84. [Google Scholar]
- Tarun AS, Theologis A. Complementation analysis of mutants of 1-aminocyclopropane-1-carboxylate synthase reveals the enzyme is a dimer with shared active sites. J Biol Chem. 1998;273:12509–12514. doi: 10.1074/jbc.273.20.12509. [DOI] [PubMed] [Google Scholar]
- Tatsuki M, Mori H. Rapid and transient expression of 1-aminocyclopropane-1-carboxylate synthase isogenes by touch and wound stimuli in tomato. Plant Cell Physiol. 1999;40:709–715. doi: 10.1093/oxfordjournals.pcp.a029597. [DOI] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA. 1998;95:4766–4771. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C-Y, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Anal Biochem. 1994;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- White MF, Vasquez J, Yang SF, Kirsch JF. Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization of wild-type and active-site mutant forms. Proc Natl Acad Sci USA. 1994;91:12489–12432. doi: 10.1073/pnas.91.26.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills R, McGlasson B, Graham D, Joyce D. Postharvest: An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals. New York: CAB International; 1998. [Google Scholar]
- Woeste KE, Ye C, Kieber JJ. Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol. 1999;119:521–529. doi: 10.1104/pp.119.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WS, Ning W, Xu PL, Kung SD, Yang SF, Li N. Identification of two chilling-regulated 1-aminocyclopropane-1-carboxylate synthase genes from citrus (Citrus sinensis Osbeck) fruit. Plant Mol Biol. 1999;41:587–600. doi: 10.1023/a:1006369016480. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yip WK, Moore T, Yang SF. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci USA. 1992;89:2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemori K, Itai A, Nakano R, Sugiura A. Role of calyx lobes in gas exchange and development of persimmon fruit. J Am Soc Hortic Sci. 1996;121:676–679. [Google Scholar]
- Yoon IS, Mori H, Kim JH, Kang BG, Imaseki H. VR-ACS6 is an auxin-inducible 1-aminocyclopropane-1-carboxylate synthase gene in mungbean (Vigna radiata) Plant Cell Physiol. 1997;38:217–224. doi: 10.1093/oxfordjournals.pcp.a029156. [DOI] [PubMed] [Google Scholar]
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- Zhou H, Wang HW, Zhu K, Sui SF, Xu P, Yang SF, Li N. The multiple roles of conserved arginine 286 of 1-aminocyclopropane-1-carboxylate synthase: coenzyme binding, substrate binding, and beyond. Plant Physiol. 1999;121:913–919. doi: 10.1104/pp.121.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]