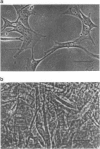
Abstract
1. We have studied the action of different transmitters on the transmembrane 42K or 86Rb efflux from tissue cultures of the BC3H1 muscle cell line. 2. The effect of catecholamines and carbachol (CCh) on the isotope efflux rate was measured by addition of the drugs at different times during the washout. 3. Noradrenaline (NA), phenylephrine (Phe), isoprenaline (Iso) and CCh increased 42K and 86Rb efflux rate in a dose-dependent manner. 4. The action of NA seems to be due exclusively to the stimulation of alpha-receptors, since its effect was blocked by phentolamine but not by propranolol. The effects of Iso on the 86Rb efflux were inhibited by propranolol. The beta-receptors in the BC3H1 cells seem to be the beta2-type since they are stimulated by Iso and insensitive to NA. 5. The effect of CCh was blocked (+)-tubocurarine but not by atropine. This result confirms the presence of nicotinic receptors in BC3H1 cells.
Full text
PDF
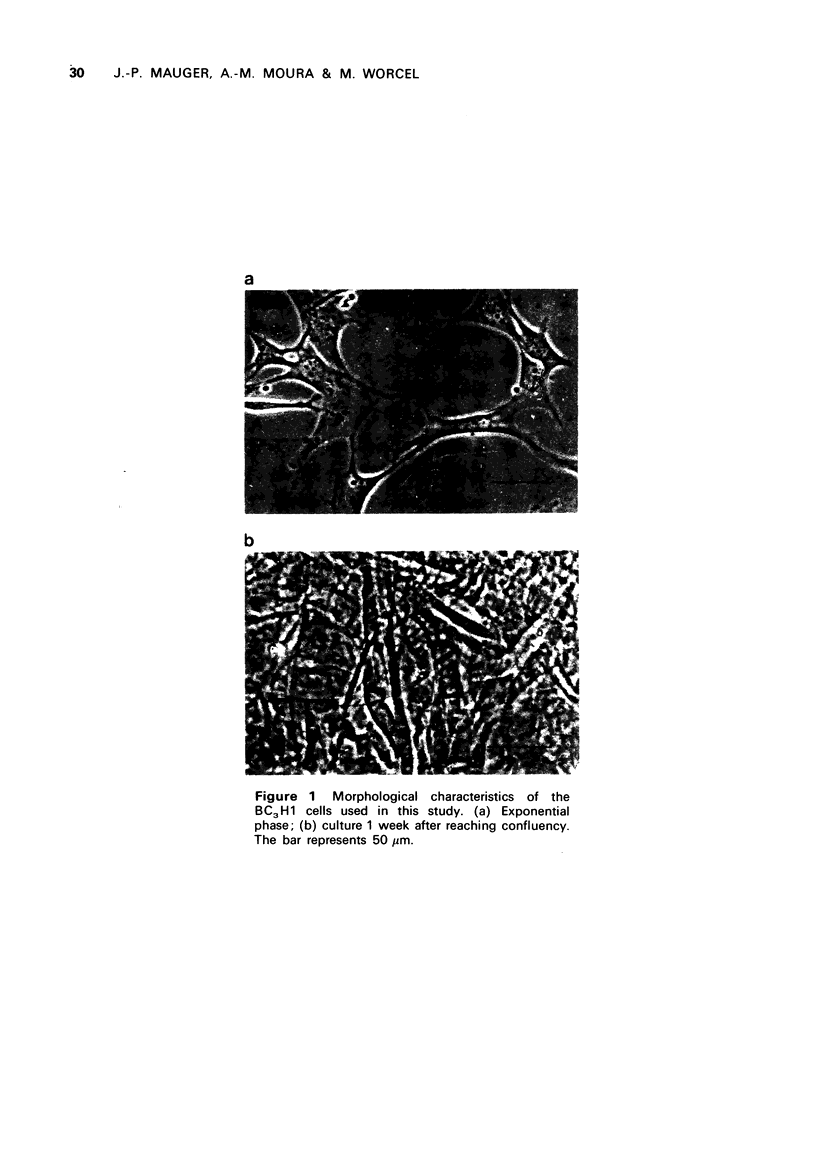
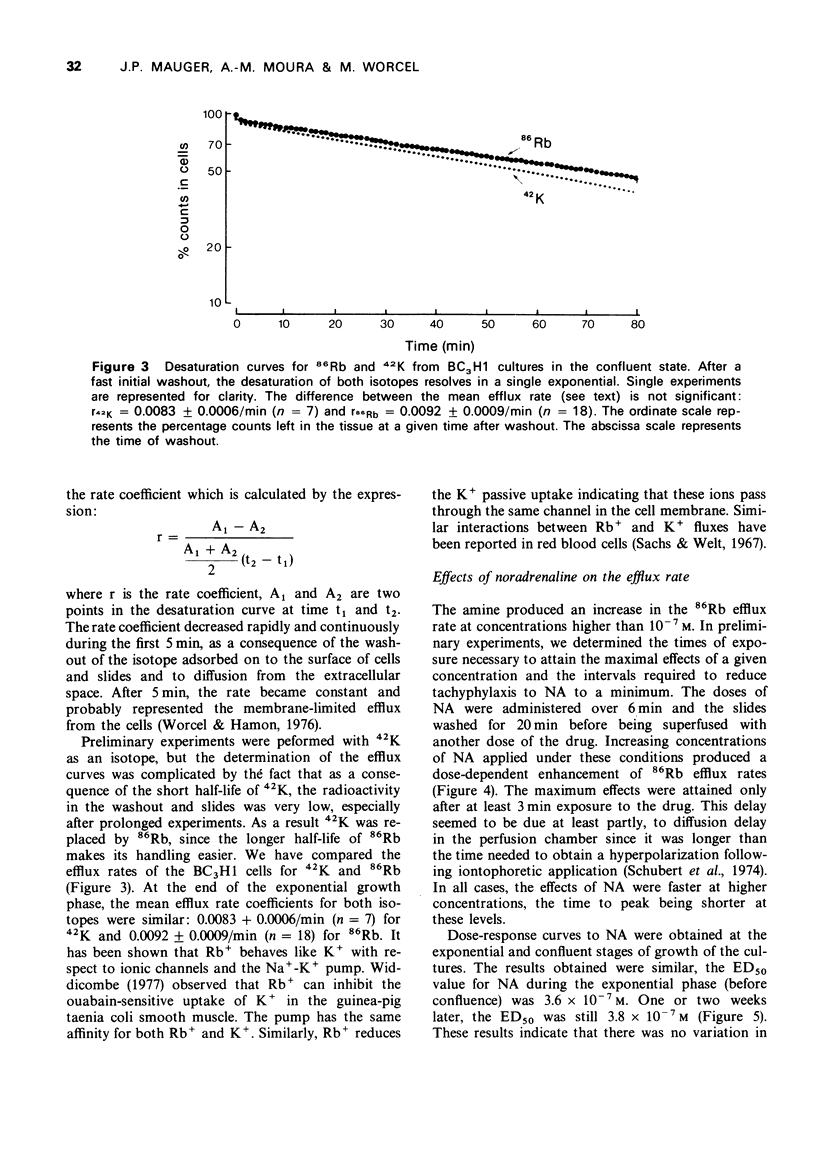
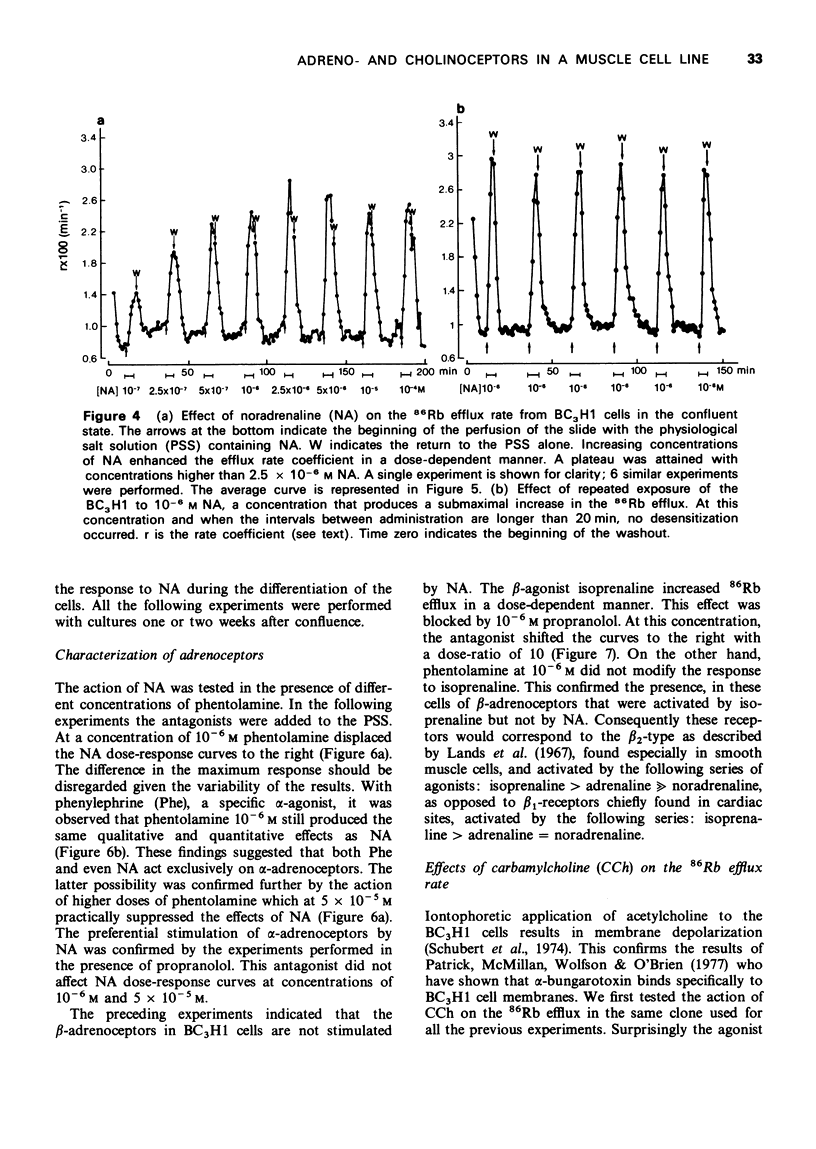
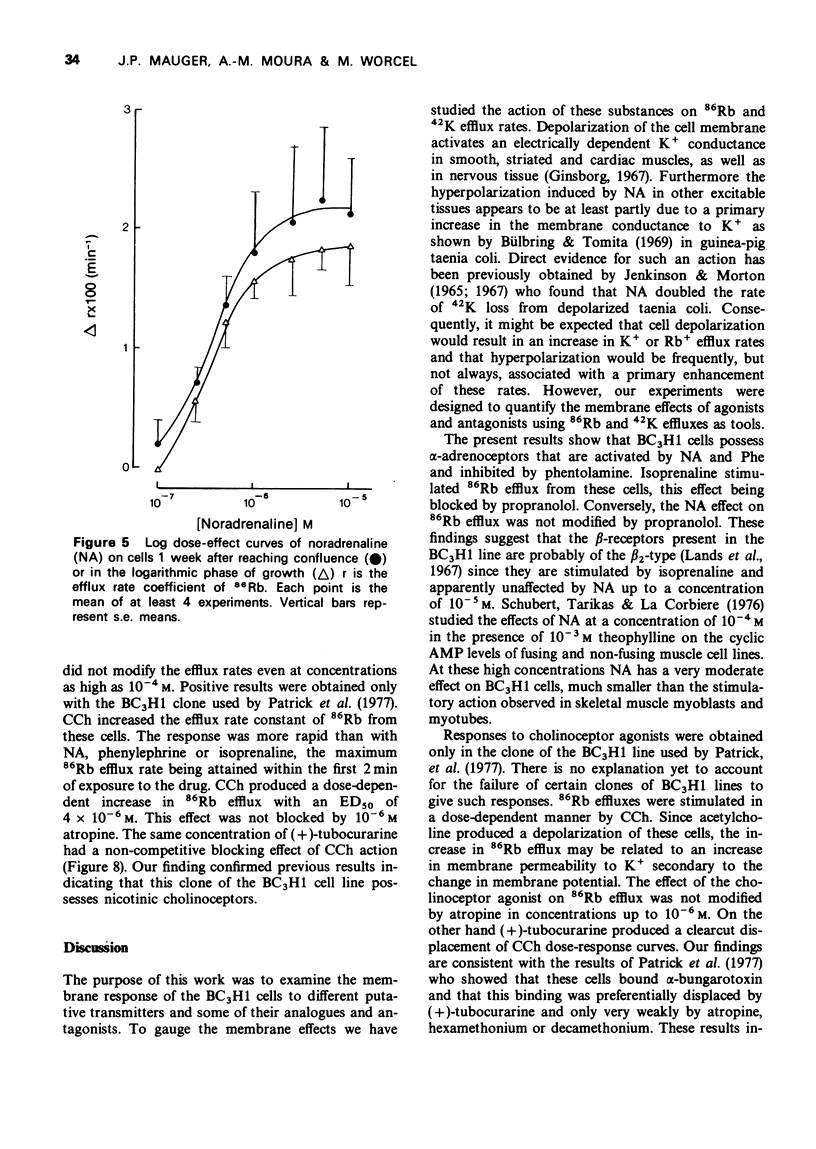
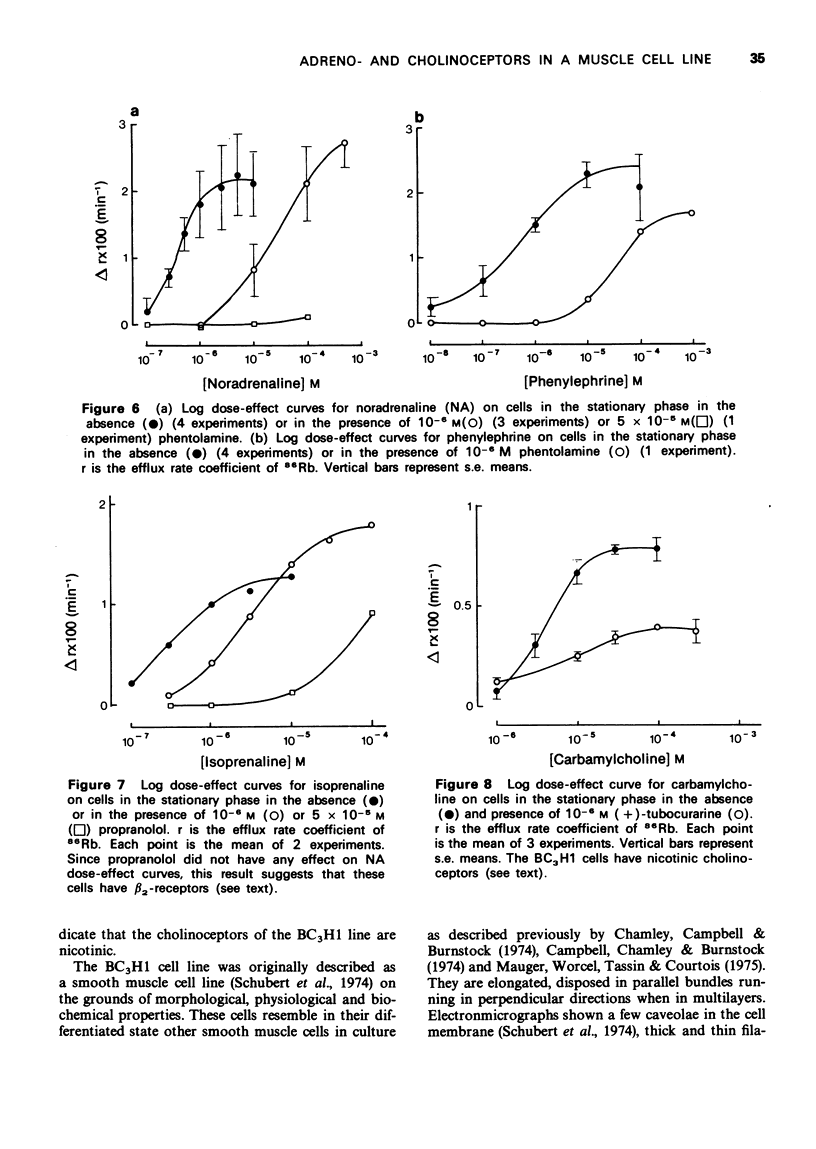

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Chamley J. H., Burnstock G. Development of smooth muscle cells in tissue culture. J Anat. 1974 Apr;117(Pt 2):295–312. [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., Burnstock G. Dedifferentiation, redifferentiation and bundle formation of smooth muscle cells in tissue culture: the influence of cell number and nerve fibres. J Embryol Exp Morphol. 1974 Oct;32(2):297–323. [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Cellular structures and electrophysiological behaviour. Fine structure of smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):7–16. doi: 10.1098/rstb.1973.0004. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Hamon G., Papadimitriou A., Worcel M. Ionic fluxes in rat uterine smooth muscle. J Physiol. 1976 Jan;254(2):229–243. doi: 10.1113/jphysiol.1976.sp011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic S., Druckrey H. Transplacentare Erzeugung maligner Tumoren des Nervensystems. I. Athyl-nitroso-harnstoff (ANH) an BD IX-Ratten. Z Krebsforsch. 1968;71(4):320–360. [PubMed] [Google Scholar]
- JENKINSON D. H., MORTON I. K. EFFECTS OF NORADRENALINE AND ISOPRENALINE ON THE PERMEABILITY OF DEPOLARIZED INTESTINAL SMOOTH MUSCLE TO INORGANIC IONS. Nature. 1965 Jan 30;205:505–506. doi: 10.1038/205505a0. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Morton I. K. The effect of noradrenaline on the permeability of depolarized intestinal smooth muscle to inorganic ions. J Physiol. 1967 Feb;188(3):373–386. doi: 10.1113/jphysiol.1967.sp008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Worcel M., Tassin J., Courtois Y. Contractility of smooth muscle cells of rabbit aorta in tissue culture. Nature. 1975 May 22;255(5506):337–338. doi: 10.1038/255337a0. [DOI] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Tarikas H., LaCorbiere M. Neurotransmitter regulation of adenosine 3',5'-monophosphate in clonal nerve, glia, and muscle cell lines. Science. 1976 Apr 30;192(4238):471–473. doi: 10.1126/science.176728. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Steps in the neoplastic transformation of hamster embryo cells by polyoma virus. Proc Natl Acad Sci U S A. 1963 Feb 15;49:171–179. doi: 10.1073/pnas.49.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. H. Ouabain-sensitive ion fluxes in the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1977 Apr;266(2):235–254. doi: 10.1113/jphysiol.1977.sp011766. [DOI] [PMC free article] [PubMed] [Google Scholar]