Abstract
Trehalose plays an important role in stress tolerance in plants. Trehalose-producing, transgenic rice (Oryza sativa) plants were generated by the introduction of a gene encoding a bifunctional fusion (TPSP) of the trehalose-6-phosphate (T-6-P) synthase (TPS) and T-6-P phosphatase (TPP) of Escherichia coli, under the control of the maize (Zea mays) ubiquitin promoter (Ubi1). The high catalytic efficiency (Seo et al., 2000) of the fusion enzyme and the single-gene engineering strategy make this an attractive candidate for high-level production of trehalose; it has the added advantage of reducing the accumulation of potentially deleterious T-6-P. The trehalose levels in leaf and seed extracts from Ubi1::TPSP plants were increased up to 1.076 mg g fresh weight−1. This level was 200-fold higher than that of transgenic tobacco (Nicotiana tabacum) plants transformed independently with either TPS or TPP expression cassettes. The carbohydrate profiles were significantly altered in the seeds, but not in the leaves, of Ubi1::TPSP plants. It has been reported that transgenic plants with E. coli TPS and/or TPP were severely stunted and root morphology was altered. Interestingly, our Ubi1::TPSP plants showed no growth inhibition or visible phenotypic alterations despite the high-level production of trehalose. Moreover, trehalose accumulation in Ubi1::TPSP plants resulted in increased tolerance to drought, salt, and cold, as shown by chlorophyll fluorescence and growth inhibition analyses. Thus, our results suggest that trehalose acts as a global protectant against abiotic stress, and that rice is more tolerant to trehalose synthesis than dicots.
Trehalose (α-d-glucopyranosyl-[1,1]-α-d-glucopyranose) is a nonreducing diglucoside that is found in various organisms, including bacteria, algae, fungi, yeast (Saccharomyces cerevisiae), insects, and some plants (Elbein, 1974). Trehalose serves not only as a carbohydrate reserve, but also as a protective agent against a variety of physical and chemical stresses in various organisms (van Laere, 1989; Wiemken, 1990; Eleutherio et al., 1993; Strøm and Kassen, 1993). Trehalose is known to have high water retention activity, which maintains the fluidity of membranes under dry conditions (Leslie et al., 1995). Thus, this sugar allows desert plants to tolerate naturally occurring stresses during cycles of dehydration and rehydration (Drennan et al., 1993; Müller et al., 1995).
A role for trehalose in stress tolerance has been demonstrated for cryptobiotic plant species, such as the desiccation-tolerant Selaginella lepidophylla. In this case, trehalose accumulation represented 12% of the plant dry weight during dehydration, which probably protected the proteins and membrane structures. Upon rehydration, S. lepidophylla regained complete viability and the trehalose levels declined (Goddijn and van Dun, 1999). Plants accumulate a number of osmoprotective agents, such as Pro, in response to NaCl stress. During osmotic stress in rice (Oryza sativa), trehalose or similar carbohydrates appear to be more important than Pro. It has been shown that treatment of rice with exogenous trehalose caused a decrease in NaCl accumulation and growth inhibition (Garcia et al., 1997).
Transgenic plants that expressed the trehalose-6-phosphate (T-6-P) synthase (TPS) and/or T-6-P phosphatase (TPP) genes from microorganisms, not only exhibited increased drought tolerance, but also showed strong developmental alterations (Holmström et al., 1996; Goddijn et al., 1997; Romero et al., 1997; Pilon-Smits et al., 1998). These pleiotropic phenotypes were present even in the absence of trehalose accumulations (Müller et al., 1999). All of the transgenic plants reported to date have been dicot plants, which generally produce very low levels of trehalose (Holmström et al., 1996; Goddijn et al., 1997; Romero et al., 1997; Pilon-Smits et al., 1998). Interestingly, rice appears to be more tolerant to trehalose than dicot plants because exogenous application of trehalose produced no growth inhibition or visible changes in the appearance of rice plants, whereas Pro inhibited growth by approximately 15% (Garcia et al., 1997). To develop stress-tolerant transgenic plants through elevated production of trehalose, we transformed rice plants with a gene that encodes a bifunctional fusion enzyme (TPSP) of TPS and TPP from Escherichia coli (Seo et al., 2000). The catalytic efficiency of TPSP was 3.5- to 4.0-fold higher than that of a mixture of the individual enzymes, which demonstrates the kinetic advantage of the fusion enzyme (Seo et al., 2000). The resultant transgenic plants produced trehalose levels that were up to 0.1% of the fresh weight, and the plants showed no visible growth inhibition. The production of trehalose in these plants resulted in increased tolerance to drought, salt, and cold stresses.
RESULTS
Transformation of Rice with the Recombinant Fusion Gene TPSP
Overexpression of a heterologous TPS gene from E. coli or yeast in dicot plants results in significant morphological growth defects and altered metabolism (Goddijn et al., 1997; Romero et al., 1997). The yeast T-6-P inhibits hexokinase in vitro (Blazquez et al., 1993), thereby partly regulating Glc influx into glycolysis (Thevelein and Hohmann, 1995). These observations have led us to speculate that T-6-P might cause phenotypic alterations in transgenic plants. To produce high levels of trehalose while maintaining relatively low levels of T-6-P in plants, we transformed rice with a gene that encodes a bifunctional fusion (TPSP) of the TPS and TPP of E. coli (Fig. 1A). The Kcat value, the turnover number, of TPSP for UDP-Glc and Glc-6-phosphate was similar to that of TPS plus TPP. However, the catalytic efficiency of TPSP was 3.5- to 4.0-fold higher than that of a equimolar mixture of the individual enzymes, which demonstrates the kinetic advantage of the fusion (Seo et al., 2000). The high catalytic efficiency that resulted from simultaneous catalysis of two-step synthesis by a single enzyme probably reduces the accumulation of potentially deleterious T-6-P.
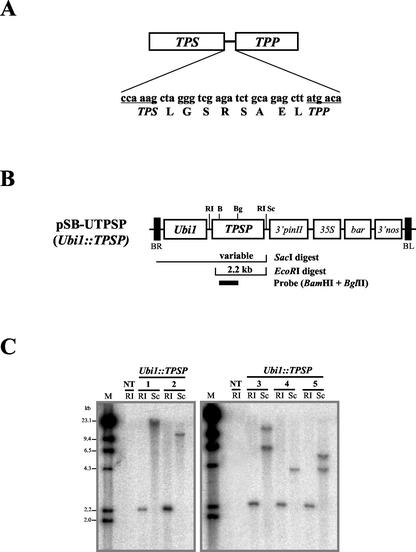
Figure 1.
The bifunctional TPSP fusion, a transformation vector, and genomic Southern-blot hybridization of transgenic rice plants. A, The predicted amino acid sequence of the fusion boundary of TPSP is shown. The TPSP construct was made by in-frame fusion of the E. coli otsA and otsB genes, which encode TPS and TPP, respectively. B, pSB-UTPSP (Ubi1::TPSP) consists of the maize (Zea mays) ubiquitin promoter (Ubi1) linked to the TPSP coding region, the 3′ region of the potato proteinase inhibitor II gene (3′pinII), and a gene expression cassette that contains the 35S promoter, the bar-coding region, and the 3′ region of the nopaline synthase gene (nos). The restriction enzymes, the expected fragment sizes, and the hybridization probe (probe) used for genomic DNA-blot analyses are shown below the map. C, Genomic Southern-blot analysis of Ubi1::TPSP transgenic rice plants. Genomic DNAs from the leaves of five Ubi1::TPSP plant lines and from untransformed control plants (NT) were digested with EcoRI (RI) or SacI (Sc), fractionated on an agarose gel, blotted onto a nylon membrane, and hybridized with the probe for TPSP coding region (described in B).
The components of the plasmid used for rice transformation are shown in Figure 1B. The maize ubiquitin promoter was linked to the recombinant fusion gene TPSP (Seo et al., 2000), which was constructed by connecting the TPS and TPP genes from E. coli after the stop codon of the TPS gene had been removed by PCR. The chimeric Ubi1::TPSP gene was then ligated to the expression cassette that carried the coding region of the phosphinothricin acetyl transferase gene (bar) under the control of the 35S promoter, thereby generating the plasmid pSB-UTPSP. Fourteen independent transgenic lines were obtained by the Agrobacterium tumefaciens-mediated method, and grown to maturity in the greenhouse. Phosphinothricin acetyl transferase can detoxify phosphinothricin-based herbicides (Duan et al., 1996). All of the transformants were herbicide resistant, as tested by painting leaves with the commercial herbicide Basta (Jang et al., 1999). Of the 14 plants, 11 were fertile, and their T1 and T2 seeds were collected. The copy numbers and integration events relating to the transgene were determined by genomic Southern blots. The 11 lines contained one to three copies of the transgene. Five homozygous T2 lines containing one or two copies of TPSP were chosen for further analysis (Fig. 1C).
Analysis of Transgenic Rice Plants
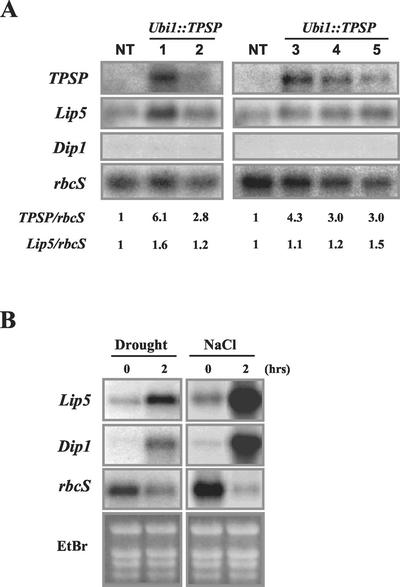
To investigate TPSP expression levels, RNA-blot hybridization was carried out using total RNA samples from leaf tissues. As shown in Figure 2A, the probe (Fig. 1B) detected a single mRNA band of approximately 2.4 kb in the five transgenic lines tested. Transcript levels of TPSP varied within a range of 2-fold among the lines, as judged by values of TPSP to rbcS ratio. To examine whether the TPSP expression could activate other stress-inducible genes in the Ubi1::TPSP plants, we analyzed transcript levels of some candidate genes including salT (Claes et al., 1990), Lip19 and Lip5 (Aguan et al., 1991), and the Arabidopsis cor47 homolog Dip1 (GenBank accession no. AU095986). Lip5 and Dip1 were the ones that were largely induced upon exposure of untransformed rice to drought and salt stresses for 2 h, as depicted in Figure 2B. Therefore, RNAs from transgenic and non-transgenic plants were hybridized with Lip5 and Dip1 probes. In the case of Ubi1::TPSP-1 and -5 plants under normal growth conditions, transcript levels of Lip5, but not those of Dip1, were increased by 1.5- and 1.6-fold, respectively, as compared with non-transgenic controls (Fig. 2A). Thus, the stress-inducible genes are partly induced by trehalose synthesis, but not as much as by stress treatments in rice.
Figure 2.
Transcript levels of TPSP and stress-inducible rice genes in the leaves of Ubi1::TPSP and untransformed plants. A, Northern-blot analysis was performed using total RNA from young leaves of five Ubi1::TPSP plant lines (shown in Fig. 1C) and from untransformed control plants (NT). The blots were hybridized with probes for TPSP (as described in Fig. 1B), Lip5 (Aguan et al., 1991), and Dip1 (GenBank accession no. AU095986). Equal loading of total RNA samples was verified by reprobing the membrane with the rice rbcS gene for Rubisco (Kyozuka et al., 1993). Transcript levels of TPSP and Lip5 in the Ubi1::TPSP lines were calculated using those of corresponding rbcS as a reference and the resultant values were then normalized to 1 for that from NT. B, Northern blots of total RNA from untransformed plants immediately before and after stress treatments. The blots were hybridized with probes for Lip5, Dip1, and rbcS. Transcript levels of rbcS were previously reported to be decreased upon exposure to drought and salt stresses (Weatherwax et al., 1996). For drought stress, 14-d-old seedlings were air dried for 2 h at 28°C; for salt stress, 14-d-old seedlings were exposed to 400 mm NaCl for 2 h at 28°C. All of the experiments were carried out under continuous 150 μmol m2 s−1 light conditions. Ethidium bromide (EtBr) staining of total RNA was used to ensure equal RNA loading.
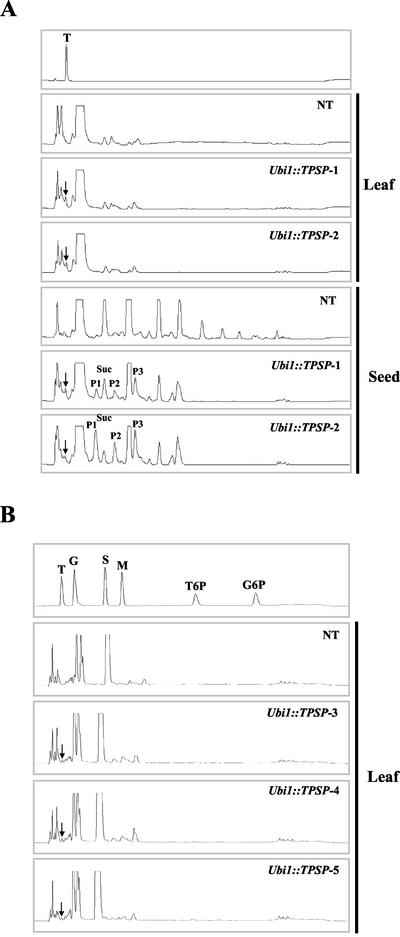
To examine the accumulation levels of trehalose and T-6-P in transgenic plants, quantitative carbohydrate analysis was carried out by high-performance ion chromatography (HPIC), as described in “Materials and Methods.” The carbohydrate profiles of Ubi1::TPSP plants were similar, but distinct from those of untransformed controls (Fig. 3). Trehalose was present in the leaf and seed extracts of transgenic plants at levels of 0.31 to 1.076 mg g fresh weight−1 depending on lines, which contrasted with the negligible levels of trehalose in untransformed control plants (Table I). The transcript levels of TPSP did not correlate with those of trehalose accumulation. For example, the Ubi1::TPSP-2 plants had lower expression of TPSP by about 2-fold than the Ubi1::TPSP-1 plants (see TPSP to rbcS ratios in Fig. 2A), but contained relatively similar levels of trehalose (Fig. 3). This is probably because transgenic plants can tolerate levels of trehalose accumulation within a limited range that allows them to grow and develop normally, thereby restricting the trehalose levels of the higher expressor. As summarized in Table I, several previous studies showed that transgenic plants expressing TPS and/or TPP, either from E. coli or yeast, had lower levels of trehalose accumulation than Ubi1::TPSP plants. Our Ubi1::TPSP plants produced trehalose at levels that were up to 200-fold higher than those reported for transgenic tobacco plants that were transformed independently with E. coli TPS or TPP expression cassettes (Goddijn et al., 1997). As shown in Figure 3B, T-6-P was not detected in leaf tissues of both transgenic and non-transgenic rice plants. We also measured trehalase activities in young rice leaves by estimating the amounts of Glc produced by hydrolysis of trehalose and corresponding decrease in trehalose. As shown in Table I, trehalase activity of rice is lower than that of tobacco and comparable with that of potato tuber. Taken together, these results suggest that the high levels of trehalose accumulation in Ubi1::TPSP plants is because of the enzymatic activity of TPSP, rather than lower activity of trehalase.
Figure 3.
HPIC analysis of trehalose accumulation in Ubi1::TPSP plants. A, The chromatograms show carbohydrate profiles from a standard containing 1 μg of trehalose (T), leaf and seed extracts that were prepared from untransformed controls (NT), and two transgenic lines (Ubi1::TPSP-1 and -2). B, Carbohydrate profiles from a standard containing 1 μg each of trehalose (T), Glc (G), Suc (S), maltose (M), T-6-P, and Glc-6-phosphate (G-6-P), leaf extracts that were prepared from untransformed controls (NT), and three transgenic lines (Ubi1::TPSP-3, -4, and -5).
Table I.
Trehalose contents and trehalase activities in monocot and dicot transgenic plants
| Transgenic Plants | Transgene | Trehalose Production | Trehalase Activitya | References |
|---|---|---|---|---|
| mg g fresh wt−1 | μmol h mg protein−1 | |||
| Rice | E. coli otsA/otsB fusion | 0.31–1.076 | 0.174 ± 0.05 | This study |
| Tobacco (Nicotiana tabacum) | Yeast TPS1 | 0.16–0.64 (0.8–3.2)b | NTc | Holmström et al. (1996) |
| Tobacco | Yeast TPS1 | 0.17 | NT | Romero et al. (1997) |
| Tobacco | E. coli otsA and otsB | 0.038–0.04 (0.19–0.20)b | NT | Pilon-Smits et al. (1998) |
| Tobacco | E. coli otsA and otsB | 0.005–0.07 | 0.442–0.679 | Goddijn et al. (1997) |
| Tobacco | E. coli otsA | 0.02–0.11 | 0.442–0.679 | Goddijn et al. (1997) |
| Potato (Solanum tuberosum) | E. coli otsA and otsB | 0.003–0.02 | 0.048 ± 0.006 (0.125 ± 0.003)d | Goddijn et al. (1997) |
Leaf tissues were ground in liquid nitrogen and extracted at 100°C with water. After the extract was clarified, trehalose was quantitated by HPIC with a Carbo-Pak PA1 column using the DX500 HPIC system (Dionex 500). Trehalase activity was measured by estimating both the glucose produced by hydrolysis of trehalose and trehalose reduced.
Tested in leaf tissues.
Nos. in parentheses refer to milligram per gram dry wt, as described in the literature.
NT, Not tested.
Tested in tuber.
Effect of Trehalose Production on the Carbohydrate Content and Growth Phenotype of Transgenic Plants
Exogenous application of trehalose to Arabidopsis strongly reduced root elongation with a concomitant increase in starch accumulation in shoots, but the soluble sugar content remained unchanged. These results suggest that trehalose interferes with carbon allocation to the sink tissues by inducing starch synthesis in the source tissues (Wingler et al., 2000). To examine the effect of trehalose production on carbohydrate content in transgenic rice, extracts from leaves and seeds of the Ubi1::TPSP plants were analyzed. Quantitative carbohydrate analysis by HPIC showed no significant changes in the carbohydrate content of the leaves, whereas several of the carbohydrate peaks were changed in the seeds. In particular, the Suc and multiglucoside concentrations in the seeds were significantly reduced. Three additional peaks (P1, P2, and P3) appeared in the HPIC profiles of the transgenic seeds (Fig. 3A).
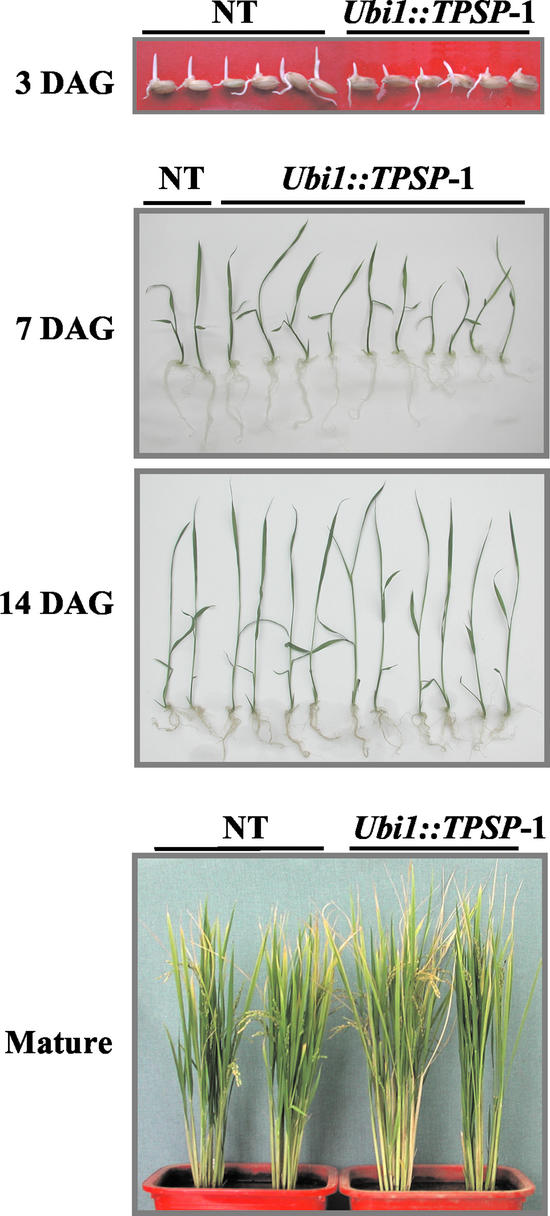
In previous reports, constitutive expression of TPS and/or TPP from either E. coli or yeast in tobacco or potato plants resulted in undesirable pleiotropic effects, including stunted growth and altered root systems under normal growth conditions (Holmström et al., 1996; Goddijn et al., 1997; Romero et al., 1997; Pilon-Smits et al., 1998). These pleiotropic growth phenotypes were present even in the absence of bulk accumulations of trehalose (Müller et al., 1999). Although the Ubi1::TPSP plants produced trehalose levels that were up to 0.1% of the plant fresh weight, they showed neither growth inhibition nor visible changes in appearance. As depicted in Figure 4, the Ubi1::TPSP plants showed normal vegetative phenotype and fertility as compared with untransformed control plants. A slight delay in germination of Ubi1::TPSP seeds was observed at 3 d after the start of germination, but the growth rates converged at later stages without notable difference in shoot and root growth. We also made 35S::TPSP potato plants that hardly grew with altered phenotypes and died prematurely (data not shown). These results lead us to speculate that the overproduction of trehalose is not as toxic for rice as it is for dicot plants.
Figure 4.
Growth phenotypes of T2 plants of Ubi1::TPSP-1 and untransformed control plants (NT), 3 d after germination (3 DAG), 7 d after germination (7 DAG), 14 d after germination (14 DAG), and in mature plants setting seeds (mature).
Stress Tolerance Is Significantly Improved in the Transgenic Plants
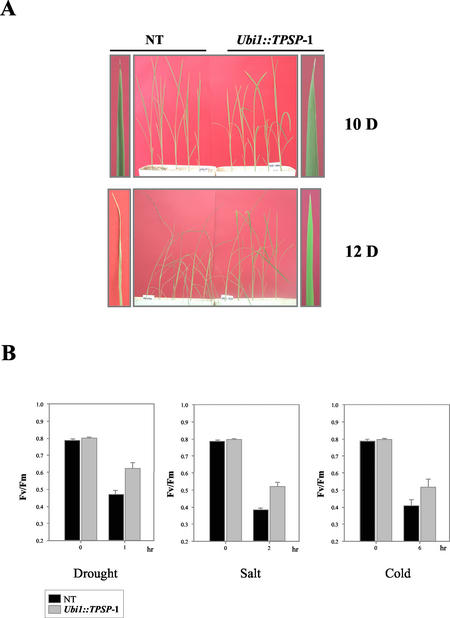
In nature, trehalose serves as a protectant against a variety of stresses in different organisms (Eleutherio et al., 1993; Strøm and Kassen, 1993; Garcia et al., 1997). To investigate whether the accumulation of trehalose in Ubi1::TPSP plants was correlated with increased stress tolerance, 6-d-old T2 seedlings were grown in a greenhouse and watering was stopped for up to 12 d. After 12 d without watering, differences in drought tolerance were evident between the untransformed control and Ubi1::TPSP plants (Fig. 5A). After prolonged exposure to drought stress, the Ubi1::TPSP plants survived and displayed vigorous root and shoot growth; over the same treatment period, the untransformed plants were nearly dead because of severe damage of leaves and concomitant loss of chlorophyll. The increased tolerance of the Ubi1::TPSP plants was confirmed by measuring changes in chlorophyll fluorescence. Most of the chlorophyll fluorescence in leaves arises from chlorophyll and is associated with the PSII. The ratio of Fv to Fm was used to estimate the quantum yield of PSII (Strasser and Butler, 1977). Environmental stresses that damage the efficiency of PSII result in decreases in the Fv/Fm ratio (Artus et al., 1996). To examine stress tolerance using the Fv/Fm ratio, 14-d-old seedlings were exposed to various stresses under continuous 150 μmol m2 s−1 light (see “Materials and Methods”). A decrease in the Fv/Fm ratio was observed after the plants were subjected to dehydration, salt, or low-temperature stresses. As shown in Figure 5B, the Fv/Fm ratios were 15% to 19% higher in Ubi1::TPSP plants than in the untransformed control plants.
Figure 5.
Stress tolerance of T2 plants of Ubi1::TPSP-1 and untransformed control plants (NT). A, Six-day-old seedlings were grown in the greenhouse for 10 d (10 D) and 12 d (12 D) after watering stopped. Photos of the upper leaves of corresponding plants are shown at either side of the figures. B, For drought stress, 14-d-old seedlings were air dried for 1 h at 28°C; for salt stress, 14-d-old seedlings were exposed to 150 mm NaCl for 2 h at 28°C; and for cold stress, 14-d-old seedlings were exposed to 4°C for 6 h. All of the experiments were carried out under continuous 150 μmol m2 s−1 light conditions. Chlorophyll fluorescence (variable fluorescence [Fv] and maximal fluorescence [Fm]) was measured using a pulse modulation fluorometer. Six seedlings were measured and averaged for each treatment protocol.
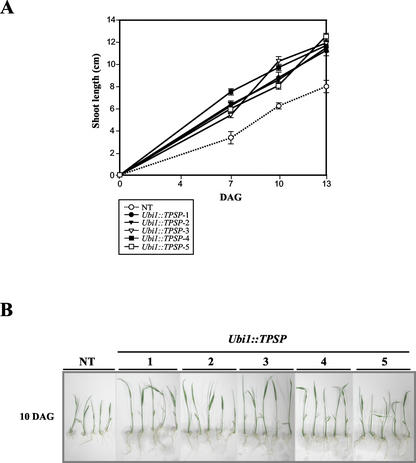
To investigate the increased tolerance of Ubi1::TPSP plants against salinity, we measured the growth during germination of five homozygous T2 seedlings in hydroponic solutions that contained 100 mm NaCl. In the absence of NaCl, Ubi1::TPSP seedlings grew similarly to non-transgenic seedlings during 13 d after germination, as shown in Figure 4. In the presence of NaCl, in contrast, both shoot and seminal root growth of the Ubi1::TPSP seedlings was much faster than that which occurred in those of the non-transgenic seedlings (Fig. 6, A and B).
Figure 6.
Salt tolerance of non-transgenic and Ubi1::TPSP seedlings grown in the presence of 100 mm NaCl. Ten T2 seeds from each of the five (1–5) Ubi1::TPSP lines and the non-transgenic (NT) plants were germinated and grown in hydroponic solutions that contained 100 mm NaCl under continuous 150 μmol m2 s−1 light conditions. A, The shoot length was scored at various intervals. Each data point represents the mean ± se of triplicate experiments (n = 10). B, Representative seedlings at 10 d after germination are shown.
Thus, the constitutive expression of TPSP in transgenic plants leads to increased levels of trehalose accumulation, which correlated with enhanced tolerance against drought, salinity, and low temperature, suggesting that trehalose acts as a global protectant against abiotic stress in rice.
DISCUSSION
Trehalose is a nonreducing disaccharide that functions as a stress protection metabolite and carbohydrate reserve in many organisms (van Laere, 1989; Wiemken, 1990; Eleutherio et al., 1993; Strøm and Kassen, 1993; Goddijn and van Dun, 1999). To generate stress-tolerant transgenic rice plants, we transformed rice with a gene encoding the bifunctional enzyme TPSP, which was derived from an in-frame fusion of TPS and TPP from E. coli. The high catalytic efficiency of the fusion enzyme (Seo et al., 2000) and the single-gene engineering strategy made this an attractive candidate for the high-level production of trehalose combined with reduced accumulations of potentially deleterious T-6-P. This is probably because physical proximity of two enzymes increases the reaction rate by facilitating transfer of the reaction intermediate T-6-P when they are present in a complex. The resultant transgenic plants (Ubi1::TPSP) produced trehalose levels that accounted for up to 0.1% of the plant fresh weight, which was 200-fold higher than the levels in transgenic tobacco plants that were cotransformed with E. coli TPS and TPP on independent expression cassettes (Goddijn et al., 1997). The fact that trehalase activity of rice is comparable with that of potato tubers (Table I) led us to conclude that the high levels of trehalose accumulation in Ubi1::TPSP plants is because of the enzymatic activity of TPSP, rather than the lower activity of trehalase. This is because trehalose was not detected at all in transgenic potato tubers with E. coli otsA and otsB even though they contained a similar level of trehalase activity to that of rice (Goddijn et al., 1997). This becomes clearer if the trehalase activity in rice was underestimated because of the usage of unpurified, non-desalted extracts in our assay conditions. Interestingly, our Ubi1::TPSP plants showed no growth inhibition or visible phenotypic alterations despite the high-level production of trehalose, in contrast with the results obtained for transgenic dicots, such as potato and tobacco (Goddijn et al., 1997; Romero et al., 1997).
Trehalose may also function as a regulator of plant metabolism and development (Goddijn and Smeekens, 1998; Vogel et al., 1998; Goddijn et al., 1999). For example, the growth of Arabidopsis seedlings on trehalose-containing medium led to the inhibition of root elongation and an accumulation of starch in the shoots (Wingler et al., 2000). An Arabidopsis mutant that was disrupted in the gene encoding TPS showed an embryo-lethal phenotype (Eastmond et al., 2002). These results are seemingly consistent with observations that overexpression of a heterologous TPS and/or TPP gene in dicot plants results in severely stunted growth (Goddijn et al., 1997; Romero et al., 1997). To date, studies of this type have been conducted in dicotyledonous plants, such as Arabidopsis, tobacco, and potato. Very little is known about the physiological roles of trehalose metabolism in monocots. Garcia et al. (1997) treated rice plants with exogenous trehalose or Pro, and found that, unlike the situation in dicots, trehalose produced no growth inhibition or visible changes in plant appearance, but instead reduced the inhibitory effects of NaCl. In contrast, Pro inhibited growth by approximately 15%. These observations led us to speculate that trehalose synthesis might not function in monocot plants as it does in dicot plants. It seems likely that monocots are more tolerant to the biosynthesis of trehalose than dicots because our Ubi1::TPSP plants produced trehalose at relatively high levels without any phenotypic alterations. This is further evidenced by our 35S::TPSP potato plants that were severely stunted and died prematurely.
In yeast, T-6-P affects glycolysis and sugar signaling through its interaction with hexokinase, which is a putative sensor (Thevelein and Hohmann, 1995; Paul et al., 2001). Although it remains to be determined whether T-6-P in plants interacts with hexokinase as it does in yeast, T-6-P appears to be important in sugar signaling in plants (Paul et al., 2001). Our bifunctional enzyme TPSP was designed in such a way that it not only gave high catalytic efficiency (Seo et al., 2000) for trehalose production, but it also restricted T-6-P accumulation to minimum levels. In our Ubi1::TPSP plants, T-6-P was present at levels below detection. Although this might be because our assay method (about 1-ng sensitivity) for T-6-P was not sensitive enough, we believe that the fusion gene assisted the transgenic rice plants in achieving normal growth.
Exogenous application of 25 mm trehalose to Arabidopsis induced strong accumulations of starch in the shoots, whereas the Glc and Fru levels were not affected and the Suc content was reduced. Thus, trehalose appears to affect starch biosynthesis by inducing directly the components of the starch biosynthetic pathway (Wingler et al., 2000). Inhibition of trehalase in vivo by validamycin A led to the accumulation of trehalose and to strong reductions in the Suc and starch contents of the flowers, leaves, and stems. Thus, Arabidopsis trehalose and trehalase may play significant roles in regulating carbohydrate allocation in plants (Müller et al., 2001). We performed carbohydrate profile analysis to examine the effect of trehalose accumulation on carbohydrate allocation in the Ubi1::TPSP plants. This method enabled us to detect significant changes in the soluble carbohydrate content of the seeds, but not of the leaves. In the transgenic seeds, the concentrations of Suc and multiple-glucoside carbohydrates were reduced, whereas three new carbohydrate peaks (P1, P2, and P3 in Fig. 3) were detected. Although the constituents of the three peaks remain to be determined, our data suggest that production of trehalose does not affect carbohydrate allocation in leaf tissues. This could be one reason why our transgenic rice plants grew normally in the presence of accumulated trehalose. One possible explanation for the difference in carbohydrate contents between seeds and leaves could be that trehalose inhibits carbon allocation to the sink tissues by increasing starch synthesis in the source tissues, as observed in trehalose-treated Arabidopsis seedlings (Wingler et al., 2000). It is also possible that trehalose affects activities of enzymes involved in starch biosynthesis in a sink-specific manner, thereby altering the pool sizes of soluble carbohydrates in rice seeds. Trehalose has been shown to interfere with carbohydrate-mediated gene regulation in soybean (Glycine max; Müller et al., 1998), barley (Hordeum vulgare; Wagner et al., 1986), and Arabidopsis (Wingler et al., 2000).
Trehalose has been found to be more effective than other sugars in increasing lipid bilayer fluidity (Crowe et al., 1984a, 1984b) and in preserving enzyme stability during drying (Colaco et al., 1992). In rice, trehalose promotes resistance to salt stress (Garcia et al., 1997). Under conditions of dehydration, and salt or cold stress, the Fv/Fm ratios of our Ubi1::TPSP plants were 15% to 19% higher than those of control plants (Fig. 5B), which indicates that the transgenic plants are performing efficient photosynthesis under the adverse conditions. Consistent with our observations is that transgenic rice plants overexpressing OsCDPK7, a gene for a protein kinase, showed 10% higher levels of Fv/Fm ratio for up to a 24-h period of cold treatment, yet the extent of tolerance to the stress was significant (Saijo et al., 2000). Moreover, under drought- and salt-stressed conditions, growth of Ubi1::TPSP seedlings was much faster than that of the non-transgenic seedlings (Figs. 5 and 6). Transcript levels for Lip5 in Ubi1::TPSP plants were slightly elevated under normal growth conditions, whereas they were greatly induced upon exposure to drought and salt stresses (Fig. 2), suggesting that the enhanced stress tolerance was not mainly because of the induction of stress-inducible genes. Taken together, these results demonstrated that trehalose functions as a global protectant against abiotic stress in rice.
MATERIALS AND METHODS
Plant Materials
Transgenic and non-transgenic rice (Oryza sativa) plants were grown in a greenhouse or in one-half-strength Murashige and Skoog solid medium. Embryogenic callus formation was initiated from mature rice cv Nakdong embryos and maintained on solid Murashige and Skoog medium (pH 5.8) that contained 1% (w/v) agarose, 30 g L−1 Suc, and 2.5 mg L−1 2,4-dichlorophenoxyacetic acid.
Vector Construction and Transformation of Rice
The recombinant fusion of the Escherichia coli genes for TPS and TPP (Seo et al., 2000) was introduced into rice plants. The pSB-UTPSP (Ubi::TPSP) plasmid consisted of the maize (Zea mays) ubiquitin promoter linked to the TPSP coding region, and the 3′ region of the potato (Solanum tuberosum) proteinase inhibitor II gene (pinII), as well as a gene expression cassette that comprised the 35S promoter, the bar-coding region, and the 3′-region of the nopaline synthase gene (nos). The plasmids were introduced into Agrobacterium tumefaciens LBA4404 by triparental mating, as previously described (Jang et al., 1999). For A. tumefaciens-mediated transformation, about 200 mature seeds of rice cv Nakdong were dehusked and sterilized with 70% (w/v) ethanol for 1 min with gentle shaking. The ethanol was discarded and the seeds were sterilized further with 100 mL of 20% (w/v) commercial bleach for 1 h with gentle shaking. The sterilized seeds were rinsed several times with sterile water. Callus induction, cocultivation with A. tumefaciens, and the selection of transformed calli were carried out as previously described (Jang et al., 1999).
Carbohydrate Analysis
The samples were ground in liquid nitrogen and extracted for 10 min at 100°C with 10 mL g fresh weight−1 water. The extract was centrifuged, and the supernatant filtered through a 0.45-μm filter unit. Quantitative carbohydrate analysis was carried out by HPIC with a Carbo-Pak PA1 column (4 × 250 nm) using the DX500 HPIC system (Dionex 500, Dionex, Sunnyvale, CA). Carbohydrate was eluted in a continuous sodium acetate gradient of 0 to 250 mm in a 150 mm NaOH solution over 30 min, and monitored with an ED40 electrochemical detector (Dionex DC Amperometry). Commercially available trehalose, Glc, Suc, maltose, T-6-P, and Glc-6-phosphate (Sigma, St. Louis) were used as the standard.
Trehalase Assay
Crude enzyme extracts were obtained by grinding frozen plant material in extraction buffer containing 50 mm Tris-HCl (pH 7.5), 250 mm Suc, 1 mm EDTA (pH 8.0), and 10 mm phenylmethylsulfonyl fluoride. The suspension was incubated for at least 2 h at 0°C and centrifuged (5,000 rpm for 5 min). The supernatant was used for the enzyme activity assays. Trehalase activity was measured by estimating both the Glc produced by hydrolysis of trehalose and trehalose reduced using HPIC with a Carbo-Pak PA1 column (4 × 250 nm) using the DX500 HPIC system (Dionex 500). The reaction mixture containing 30 mm trehalose (Sigma) was incubated at 37°C for 1, 2, and 3 h and stopped by boiling for 2 min. Soluble protein was determined with the Bradford method (Bradford, 1976). Trehalose activity represents the mean of triplicate experiments.
Chlorophyll Fluorescence under Conditions of Drought, and Salt or Cold Stress
Rice seeds were sterilized with 70% (w/v) ethanol for 1 min with gentle shaking. The ethanol was discarded and the seeds were sterilized further with 100 mL of 20% (w/v) commercial bleach for 1 h with gentle shaking. The sterilized seeds were rinsed several times with sterile water and germinated on soil in a growth chamber (16-h-light/8-h-dark cycles at 28°C). For the cold stress treatment, 14-d-old seedlings were exposed to 4°C for 6 h under continuous 150 μmol m2 s−1 light. For the salt stress treatment, 14-d-old seedlings were grown in a nutrient solution, 0.1% (v/v) Hyponex (Hyponex, Busan, Korea), for 2 d and then transferred to fresh nutrient solution containing 9% (w/v) NaCl for 2 h at 28°C under continuous 150 μmol m2 s−1 light. For the dehydration stress treatment, whole plants were air dried for 1 h at 28°C under continuous 150 μmol m2 s−1 light. The chlorophyll fluorescence levels of the untransformed control and of transgenic plants were measured using a pulse modulation fluorometer. The plants were kept in the dark for 2 h before fluorescence measurements and then subjected to a 1-h light period. Subsequently, the leaves were dark adapted for 10 min. At the beginning of each measurement, a small measuring light beam was turned on, and the minimal fluorescence level (Fo) was measured. Fm was then measured by applying a saturation light pulse. Fv/Fm represented the activity of PSII, and was used to assess functional damage to the plants (Artus et al., 1996).
ACKNOWLEDGMENT
The authors thank Dr. Takuji Sasaki (National Institute of Agrobiological Resources, Ibaraki, Japan) for providing the EST clones of Lip5 and Dip1.
Footnotes
This work was supported by the Ministry of Science and Technology through the Crop Functional Genomics Center (grants to J.-K.K. and S.I.S.), by the Korea Science and Engineering Foundation through the Plant Metabolism Research Center at Kyung-Hee University (grant to J.-K.K.), and by the Ministry of Education's Brain Korea 21 Project (fellowships to I.-C. J., S.-J.O., J.-S.S., and S.I.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.007237.
LITERATURE CITED
- Aguan K, Sugawara K, Suzuki N, Kusano T. Isolation of genes for low-temperature-induced proteins in rice by a simple subtractive method. Plant Cell Physiol. 1991;32:1285–1289. [Google Scholar]
- Artus NN, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proc Natl Acad Sci USA. 1996;93:13404–13409. doi: 10.1073/pnas.93.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez MA, Lagunas R, Gancedo C, Gancedo JM. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Claes B, Dekeyser R, Villarroel R, Van den Bulcke M, Bauw G, Van Montagu M, Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990;2:19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaco C, Sen S, Thangavelu M, Pinder S, Roser B. Extraordinary stability of enzymes dried in trehalose: simplified molecular biology. Bio/Technology. 1992;10:1007–1011. doi: 10.1038/nbt0992-1007. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Chapman D. Preservation of membranes in anhydrobiotic organism: the role of trehalose. Science. 1984a;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Whittam MA, Chapman D, Crowe LM. Interactions of phospholipid monolayers with carbohydrates. Biochim Biophys Acta. 1984b;769:151–159. doi: 10.1016/0005-2736(84)90018-x. [DOI] [PubMed] [Google Scholar]
- Drennan PM, Smith MT, Goldsworthy D, van Staden J. The occurrence of trehalose in the leaves of the desiccation-tolerant angiosperm Myrothamnus flabellifolius. J Plant Physiol. 1993;142:493–496. [Google Scholar]
- Duan X, Li X, Xue Q, Abo-El-Saad M, Xu D, Wu R. Transgenic rice plants harboring an introduced potato proteinase inhibitor II gene are insect resistant. Nat Biotechnol. 1996;14:494–498. doi: 10.1038/nbt0496-494. [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JDG, Smeekens SC, Graham IA. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002;29:225–235. doi: 10.1046/j.1365-313x.2002.01220.x. [DOI] [PubMed] [Google Scholar]
- Elbein A. The metabolism of α,α-trehalose. Adv Carbohydr Chem Biochem. 1974;30:227–256. doi: 10.1016/s0065-2318(08)60266-8. [DOI] [PubMed] [Google Scholar]
- Eleutherio ECA, Araujo PS, Panek AD. Protective role of trehalose during heat stress in Saccharomyces cerevisiae. Cryobiology. 1993;30:591–596. doi: 10.1006/cryo.1993.1061. [DOI] [PubMed] [Google Scholar]
- Garcia AB, Engler JdeA, Iyer S, Gerats T, Van Montague M, Caplan AB. Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol. 1997;155:159–169. doi: 10.1104/pp.115.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddijn OJM, van Dun K. Trehalose metabolism in plants. Trends Plant Sci. 1999;4:315–319. doi: 10.1016/s1360-1385(99)01446-6. [DOI] [PubMed] [Google Scholar]
- Holmström K-O, Mäntylä E, Welin B, Mandal A, Palva ET, Tunnela OE, Londesborough J. Drought tolerance in tobacco. Nature. 1996;379:683–684. [Google Scholar]
- Goddijn O, Smeekens S. Sensing trehalose biosynthesis in plants. Plant J. 1998;14:143–146. doi: 10.1046/j.1365-313x.1998.00140.x. [DOI] [PubMed] [Google Scholar]
- Goddijn OJM, Verwoerd TC, Voogd E, Krutwagen RWHH, de Graaf PTHM, Poels J, van Dun K, Ponstein AS, Damm B, Pen J. Inhibition of trehalase activity enhances trehalose accumulation in transgenic plants. Plant Physiol. 1997;113:181–190. doi: 10.1104/pp.113.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I-C, Nahm BH, Kim J-K. Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed. 1999;5:453–461. [Google Scholar]
- Kyozuka J, McElroy D, Hayakawa T, Xie Y, Wu R, Shimamoto K. Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion gene in transgenic rice. Plant Physiol. 1993;102:991–1000. doi: 10.1104/pp.102.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Aeschbacher RA, Wingler A, Boller T, Wiemken A. Trehalose and trehalase in Arabidopsis. Plant Physiol. 2001;125:1086–1093. doi: 10.1104/pp.125.2.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Boller T, Wiemken A. Trehalose and trehalase in plants: recent developments. Plant Sci. 1995;112:1–9. [Google Scholar]
- Müller J, Boller T, Wiemken A. Trehalose affects sucrose synthase and invertase activities in soybean (Glycine max L. Merr.) roots. J Plant Physiol. 1998;153:255–257. [Google Scholar]
- Müller J, Wiemken A, Aeschbacher R. Trehalose metabolism in sugar sensing and plant development. Plant Sci. 1999;147:37–47. [Google Scholar]
- Paul M, Pellny T, Goddijn O. Enhancing photosynthesis with sugar signals. Trends Plant Sci. 2001;6:197–200. doi: 10.1016/s1360-1385(01)01920-3. [DOI] [PubMed] [Google Scholar]
- Pilon-Smits EAH, Terry N, Sears T, Kim H, Zayed A, Hwang S, van Dun K, Voogd E, Verwoerd TC, Krutwagen RW et al. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J Plant Physiol. 1998;152:525–532. [Google Scholar]
- Romero C, Bellës JM, Vayá JL, Serrano R, Culiáñez-Maciá FA. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta. 1997;201:293–297. doi: 10.1007/s004250050069. [DOI] [PubMed] [Google Scholar]
- Saijo Y, Hata S, Kyozuka J, Shimamoto K, Izui K. Over-expression of a single Ca2+-dependent protein kinase confers both cold and salt/drought tolerance on rice plants. Plant J. 2000;23:319–327. doi: 10.1046/j.1365-313x.2000.00787.x. [DOI] [PubMed] [Google Scholar]
- Seo HS, Koo YJ, Lim JY, Song JT, Kim CH, Kim J-K, Lee JS, Choi YD. Characterization of a bifunctional fusion enzyme between trehalose 6-phosphate synthase and trehalose 6-phosphate phosphatase of Escherichia coli. Appl Environ Microbiol. 2000;66:2484–2490. doi: 10.1128/aem.66.6.2484-2490.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser RJ, Butler WL. Fluorescence emission spectra of photosystem I, photosystem II and the light-harvesting chlorophyll a/b complex of higher plants. Biochim Biophys Acta. 1977;17:307–313. doi: 10.1016/0005-2728(77)90129-3. [DOI] [PubMed] [Google Scholar]
- Strøm AR, Kassen I. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol Microbiol. 1993;8:205–210. doi: 10.1111/j.1365-2958.1993.tb01564.x. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- Vogel G, Aeschbacher RA, Müller J, Boller T, Wiemken A. Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J. 1998;13:673–683. doi: 10.1046/j.1365-313x.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wiemken A, Matile P. Regulation of fructan metabolism in leaves of barley (Hordeum vulgare L. cv Gerbel) Plant Physiol. 1986;81:444–447. doi: 10.1104/pp.81.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherwax SC, Ong MS, Degenhardt J, Bray EA, Tobin EM. The interaction of light and abscisic acid in the regulation of plant gene expression. Plant Physiol. 1996;111:363–370. doi: 10.1104/pp.111.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. J Gen Microbiol. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- Wingler A, Fritzius T, Wiemken A, Boller T, Aeschbacher RA. Trehalose induced the ADP-glucose pyrophosphorylase gene, ApL3, and starch synthesis in Arabidopsis. Plant Physiol. 2000;124:105–114. doi: 10.1104/pp.124.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]