Abstract
Kinins are important mediators in cardiovascular homeostasis, inflammation, and nociception. Two kinin receptors have been described, B1 and B2. The B2 receptor is constitutively expressed, and its targeted disruption leads to salt-sensitive hypertension and altered nociception. The B1 receptor is a heptahelical receptor distinct from the B2 receptor in that it is highly inducible by inflammatory mediators such as bacterial lipopolysaccharide and interleukins. To clarify its physiological function, we have generated mice with a targeted deletion of the gene for the B1 receptor. B1 receptor-deficient animals are healthy, fertile, and normotensive. In these mice, bacterial lipopolysaccharide-induced hypotension is blunted, and there is a reduced accumulation of polymorphonuclear leukocytes in inflamed tissue. Moreover, under normal noninflamed conditions, they are analgesic in behavioral tests of chemical and thermal nociception. Using whole-cell patch-clamp recordings, we show that the B1 receptor was not necessary for regulating the noxious heat sensitivity of isolated nociceptors. However, by using an in vitro preparation, we could show that functional B1 receptors are present in the spinal cord, and their activation can facilitate a nociceptive reflex. Furthermore, in B1 receptor-deficient mice, we observed a reduction in the activity-dependent facilitation (wind-up) of a nociceptive spinal reflex. Thus, the kinin B1 receptor plays an essential physiological role in the initiation of inflammatory responses and the modulation of spinal cord plasticity that underlies the central component of pain. The B1 receptor therefore represents a useful pharmacological target especially for the treatment of inflammatory disorders and pain.
Diseases of the cardiovascular system as well as inflammatory diseases that often lead to pain are of increasing importance for health care in aging populations. Kinins have been known for some time to be important mediators of cardiovascular homeostasis, inflammation, and nociception (1). They are probably the first mediators released in injured tissue from kininogens either by plasma kallikrein, which is activated early in the coagulation cascade, or tissue kallikrein, which is activated by proteases released at injured sites. Surprisingly, the therapeutic value of intervention in the kallikrein–kinin system has not been fully explored. The two known receptors for kinins, B1 and B2, might be suitable pharmacological targets to treat chronic inflammatory and cardiovascular diseases. The B2 receptor binds the major effector peptide of the kallikrein–kinin system, which is bradykinin (BK) in rodents and kallidin in humans. Deletion of the B2 receptor in mice leads to salt-sensitive hypertension and altered nociception (2–4). Like the B2 receptor, the B1 receptor is a heptahelical receptor, but unlike B2 receptors, it is not widely expressed in normal tissue but is highly inducible by inflammatory mediators like bacterial lipopolysaccharide (LPS) and cytokines and does not desensitize after agonist binding (5–9). Agonists for the B1 receptor are derived from BK and kallidin by carboxypeptidase action, generating des-Arg9-BK and des-Arg10-kallidin, respectively. Using specific antagonists, the B1 receptor has been implicated in nociception and the accumulation of leukocytes in inflamed tissue (3, 5, 10). However, despite the detection of mRNA for the B1 receptor in dorsal root ganglia, its role in pain transmission has remained elusive (11). To clarify the functions of the B1 receptor, we have cloned the murine B1-receptor gene (12) and generated mice lacking this protein by using gene targeting technology. In B1-deficient mice, the hypotensive response to LPS injection, a model for sepsis, was significantly blunted. Furthermore, in inflammatory disease models, invasion of polymorphonuclear leukocytes (PMN) into inflamed tissue was markedly reduced. B1-deficient mice showed hypoalgesia in two different chemical nociception models, and the activity-dependent facilitation of a spinal nociceptive reflex evoked by electrical stimulation of afferent nociceptive fibers was reduced.
Materials and Methods
Disruption of the Kinin B1-Receptor Gene.
The B1-receptor gene was cloned from a genomic library of 129/SvJ mice in λFIXII (12). The targeting vector was generated by flanking the neomycin-resistance gene with a 1.0-kb genomic fragment 5′ of the B1-coding region and a 7.0-kb fragment 3′ of the B1-coding region. For negative selection, the HSV-tk gene was inserted into the 5′polylinker. The construct was linearized with NotI and transfected into E14–1 embryonic stem cells by electroporation as described (13). Gancyclovir- and G418-resistant clones were selected and identified by PCR. Two positive clones were microinjected into C57BL/6 blastocysts, which gave rise to two germ-line chimeras with offspring heterozygous for the targeted mutation. Transmission of the mutant allele was determined by Southern blot analysis of tail DNA. B1-deficient mice were established by mating the heterozygotes.
Receptor Expression and Function.
B1-receptor, IL-1β, and β-actin expression was analyzed with the RNase protection assay by using the RPAII kit (Ambion, Austin, TX) with total RNA (50 μg) extracted from animals after treatment for 5 h with 10 μg/kg LPS injected intravenously under anesthesia (ketamine 50 μg/g and inactin 100 μg/g i.p.) and control animals receiving saline. The three antisense probes used were: (i) a 387-nt RNA transcribed from a vector containing a PCR fragment of the B1-receptor gene and complementary to 258 nucleotides of the native B1-receptor mRNA; (ii) a 497-nt IL-1β probe complementary to 368 nucleotides of the IL-1β mRNA; and (iii) a 304-nt RNA complementary to 250 nucleotides of the β-actin mRNA (Ambion). Functionality of B1 receptors was determined by testing the contractile response of smooth muscle strips (stomach, ileum, and uterus) to B1 agonists. Briefly, strips were extracted from wild-type and knockout mice and placed in oxygenated (95% O2/5% CO2) Krebs solution (37°C). The strips were stretched with a resting tension of 0.5 g. Experiments were initiated after an equilibration period of 90–120 min by applying submaximal concentrations of kinin agonists. Changes in tension were measured with isometric transducers and displayed on a polygraph.
Blood Pressure Measurements.
For blood pressure measurements, the animals were initially anesthetized with thiobutabarbital sodium salt (100 mg/kg) and placed on a heated table to maintain body temperature. Cannulas (PE10) were inserted into the carotid artery and exteriorized at the neck of the animal. After the animals were allowed to recover, blood pressure and heart rate were recorded on a computer system (Technical & Scientific Equipment, Bad Homburg, Germany). For blood pressure evaluation after LPS shock, the animals were maintained under anesthesia while LPS (250 μg/kg) was being administered intravenously via a femoral vein and blood pressure was measured in the contralateral femoral artery.
Acute Inflammatory Responses.
Pleurisy was produced by intrapleural injection of 100 μl of carrageenan (1%) or des-Arg9-BK (30 nmol per site) in the right pleural space. Control animals received a similar injection of sterile saline. Animals received Evans blue (25 mg/kg, 0.2 ml, i.v.) 1 h before the experiments. One hour after injection, total fluid exudation was measured, and 3 h later the infiltrating leukocytes were differentiated and counted by means of an optical microscope as described (14, 15).
Responses to Noxious Stimuli.
Nonfasted female mice (16 weeks of age), housed at 22 ± 2°C were used. In the formalin test, the animals received an intraplantar injection of 20 μl of 2.5% formalin whereas the contralateral paw received the same volume of 0.9% NaCl solution. The amount of time spent licking the injected paw, between 0 to 5 min (early phase) and 15 to 30 min (late phase), was determined with a chronometer as described (16). Another group of animals received an intraplantar injection of 20 μl of capsaicin solution (1.6 μg per paw) or the same volume of saline in the contralateral paw. The amount of time spent licking the injected paw was recorded with a chronometer for 5 min (17). In the hot plate test (Ugo Basile, Varese, Italy; DS-37), animals were placed in a 24-cm diameter cylinder with the heated surface adjusted to either 52.5, 55.5, or 58.5°C. A latency period of 30 s before the first signs of discomfort was defined as complete analgesia and the cutoff time used was 30 s. In the tail-flick test, a radiant heat tail-flick analgesiometer was used, and the animal responded to a focused intensity heat stimulus (150 W) by flicking or removing their inflected tail, thereby exposing a photocell in the apparatus immediately below the tail.
Whole-Cell Recordings in Isolated Nociceptors.
Dorsal root ganglia were removed from adult B1-deficient mice (n = 7) and littermate controls (n = 8), isolated and cultured as described in the absence of added growth factors (18). Recordings were made within 24 h of isolation. Whole-cell recordings were made from the soma of isolated small-diameter neurons (<26-μm diameter) by using standard procedures and solutions (18). Neurons were treated with isolectin B4 (IB4) coupled directly to fluorescein for 10 min either directly before or after recordings were made. IB4 staining was visualized with standard FITC filters. Heat-ramp stimuli (24–49°C in 10–15 s) were applied to the cell soma by heating the extracellular solution immediately before it entered the bath. The criterion for a response was ≥100 pA inward current evoked by heat.
In Vitro Spinal Cord Experiments.
Spinal cords, caudal to midthoracic level, were removed from neonatal (P5-P9) B1-deficient mice (n = 18) and wild-type controls (n = 15) under urethane anesthesia. Cords were hemisected down the midline, placed in a Perspex recording chamber, and superfused at 5 ml/min at room temperature with oxygenated modified Krebs solution (138 mM NaCl/1.35 mM KCl/21 mM NaHCO3/0.58 mM NaH2PO4/1.16 mM MgCl2/1.26 mM CaCl2/10 mM glucose). Recordings (dc) were made after a 2-h recovery period with a close-fitting glass suction electrode attached to the L5 ventral root. The L5 dorsal root was stimulated via a glass suction electrode at constant current sufficient to activate C-fibers (500 μA, 500 μs), and wind-up was evoked by repetitive stimulation at 1 Hz for 20 s (see ref. 19). The A-fiber-mediated component of the ventral root potential (VRP) was measured as the maximal response to dorsal root stimulation, and C-fiber responses were measured as the integrated area of the VRP. Wind-up was determined by normalizing the increase in size of the VRP to the initial magnitude and expressing it as a percentage increase. Des-Arg9-BK was dissolved in Krebs solution (200 nM) and recirculated for 20 min. Recordings were made before agonist application, immediately after application and at 20-min intervals during an additional hour of washout with Krebs solution.
Results
Generation of Kinin B1-Receptor Knockout Mice.
The mouse kinin B1-receptor gene was cloned (12), and B1-deficient mice were generated by gene-targeting technology (Fig. 1 A and B). Absence of the receptor was shown at the mRNA level in several tissues that normally express the B1 receptor. Even after induction with LPS, which markedly increased B1 mRNA levels in normal mice, there was no evidence for B1-receptor expression (Fig. 1C). In contrast, induction of IL-1β mRNA after LPS was normal in mice of both genotypes. Furthermore, the specific B1 agonist des-Arg9-BK did not elicit contractile responses in smooth muscle tissues, such as ileum (Fig. 1D), stomach, and uterus (data not shown) or potentiate the synaptically evoked VRP in isolated spinal cords from B1-deficient mice (see below).
Figure 1.
Targeting of the kinin B1-receptor gene. (A) Schematic representation of the targeting strategy with the wild-type B1 locus, the recombination vector, and the predicted structure of the targeted gene. The probe used for Southern blot and the length of the EcoRI restriction fragments are indicated. (B) Southern blot analysis of tail DNA from offspring of a cross between two heterozygous B1-deficient mice digested with EcoRI and hybridized with the aforementioned probe. (C) RNase protection assay detecting B1, IL-1β, and β-actin mRNA in untreated animals or 5 h after injection of 10 μg/kg LPS. (D) Isometric responses of isolated ileum from wild-type (WT) and B1-deficient mice (KO) to BK and des-Arg9-BK (1 μM each). Only the B2 receptor-dependent contractile response to BK is preserved in B1-deficient mice.
Blood Pressure.
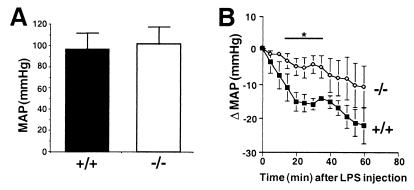
B1-deficient animals are grossly normal, fertile, and normotensive (Fig. 2A). Nevertheless, the hypotensive response to LPS injection is markedly reduced in these mice during the first 35 min (Fig. 2B). In the later phase of hypotension (>40 min), there was no longer a significant difference between wild-type and B1-deficient mice (Fig. 2B).
Figure 2.
Blood pressure. (A) Mean arterial pressure (MAP) measured by carotid artery cannulation in conscious animals is not different between wild-type (+/+, n = 8) and B1-deficient mice (−/−, n = 8). (B) Blood pressure decline after i.p. injection of 250 μg/kg LPS in wild-type (+/+, n = 11) and B1-deficient mice (−/−, n = 9) is significantly different until 35 min after LPS administration but not thereafter. Data are means ± SEM and were analyzed by analysis of variance followed by Dunnetts' test. *, P < 0.05 compared with +/+ animals.
Inflammation.
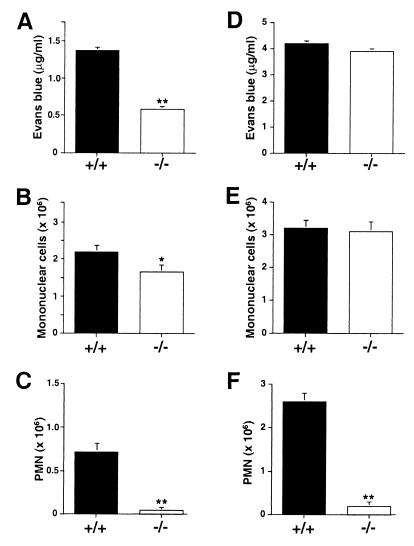
Intrathoracic injection of carrageenan or the specific B1 agonist, des-Arg9-BK, produces pleurisy with plasma extravasation and leukocyte infiltration in normal rodents (14, 15, 20). As expected, the response to des-Arg9-BK is abolished in mice that lack the B1 receptor (Fig. 3 A–C). However, in the carrageenan-induced pleurisy model, the invasion of PMN is also virtually absent in B1-deficient mice (Fig. 3F), whereas endothelial permeabilization (Fig. 3D) and mononuclear cell infiltration (Fig. 3E) are preserved.
Figure 3.
Acute inflammatory response induced by intrapleural injection of des-Arg9-BK (A-–C) or carrageenan (D–F) obtained in wild-type (+/+) or B1-deficient mice (−/−). Plasma extravasation (A and D) and the invasion of mononuclear (B and E) and PMN (C and F) leukocytes was determined. Data are means ± SEM and were analyzed by analysis of variance followed by Dunnetts' test (n = 5). **, P < 0.01; *, P < 0.05 compared with +/+ animals.
Nociception.
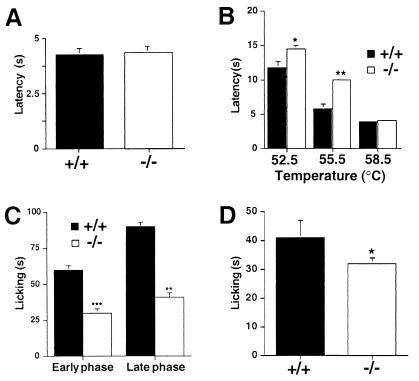
Kinins exert a critical role in acute pain by acting directly on Aδ- and C-fiber sensory neurons or indirectly by releasing inflammatory mediators that sensitize or activate these nociceptors (21). Here we tested whether mice lacking B1 receptors exhibited hypoalgesia in acute behavioral assays. Acute nociceptive responses initiated with a noxious heat stimulus in the tail-flick assay were not different between mutant and wild-type mice (Fig. 4A). However, by using the hot plate assay, which is a behavior strongly modulated at spinal and supraspinal levels, the B1 receptor-deficient mice showed significant hypoalgesia when mildly painful heat stimuli were used (52.5 and 55.5°C) but with more painful stimuli (58.5°C), no difference was observed between the genotypes (Fig. 4B). In addition, capsaicin, an algogen that powerfully activates polymodal nociceptors (nociceptors responding to heat), evoked reduced nocifensive behaviors in B1-deficient compared with wild-type mice (Fig. 4D). Finally, in a model of acute inflammatory pain, the formalin test, B1-deficient mice showed significantly less nocifensive behaviors in the early and late phase of this model (Fig. 4C).
Figure 4.
Responses to thermal (A and B) and chemical (C and D) noxious stimuli. Thermal nociception was tested in the tail-flick (A) and the hot-plate test at three different temperatures (B) and chemical nociception by intradermal injection of formalin (2.5%) (C) and capsaicin (1.6 mg) (D) in wild-type (+/+) and B1-deficient mice (−/−). Data are means ± SEM and were analyzed by analysis of variance followed by Dunnetts' test (n = 4–6). ***, P < 0.001; **, P < 0.01; *, P < 0.05 compared with +/+ animals.
The noxious stimulus for most of these tests was heat, which is a natural stimulus for nociceptors in vivo. Noxious heat has been shown to directly induce inward currents in isolated nociceptors that can be potentiated by kinins (22, 23). To test whether the B1 receptor directly modulates this acute heat-induced current, we used whole-cell patch clamp techniques to analyze small-diameter dorsal root ganglion sensory neurons, which are presumably nociceptors, in B1 receptor-deficient mice. Recordings were made from a total of 97 neurons from at least 7 mice of each genotype. Both the proportions of small-diameter sensory neurons exhibiting a heat-activated current and the magnitude of these currents were normal in B1 receptor-deficient mice (Fig. 5 A–D). The heat responses of nociceptors that bind the lectin IB4 and those that do not bind IB4 have been shown to differ so that IB4-negative nociceptors exhibit larger heat-activated inward currents (18). These differences were still present in mice lacking the B1 receptor (Fig. 5D). The normal responses of nociceptors to heat in the absence of the B1 receptor are consistent with the lack of change in the tail-flick latency.
Figure 5.
Sensory neuron and spinal cord electrophysiology. Examples are shown of inward currents to a heat ramp (top bar from 24 to 49°C) in an isolated nociceptor from a wild-type (A) and B1 receptor-deficient neuron (B). The proportion of both IB4-positive and -negative nociceptors responding to the heat stimulus was unchanged in the mutants (C) and the mean magnitude of the inward current was also not altered (D). Application of des-Arg9-BK to isolated neonatal spinal cord from wild-type mice increased the C-fiber-evoked component of the VRP (F). The magnitude of the VRP returned to control values 20 min after washout of des-Arg9-BK. In B1-deficient mice, wind-up was significantly reduced compared with control (G). Examples of VRP wind-up from wild-type (+/+) and B1 −/− mice are shown in E. Data are means ± SEM and were analyzed by using the t test. **, P < 0.005; *, P < 0.05 compared with control.
We wished to test the hypothesis that a change in the spinal processing of nociceptive input from the periphery might underlie the hypoalgesia seen in the hot plate, capsaicin, and formalin tests. To do this, we used an in vitro spinal cord preparation where the strength and excitability of a spinal reflex can be assayed without complicating systemic factors. Electrical stimulation (500 μA, 500 μs) of the L5 dorsal root (sensory afferent fibers) evokes a prolonged reflex response that is recorded from the corresponding ventral root. This ventral root potential consists of an early peak evoked by rapidly conducting A-β afferents and a late component activated by unmyelinated nociceptive afferent fibers (C-fibers). Application of the specific B1-agonist des-Arg9-BK selectively increased the prolonged C-fiber component of the VRP (P < 0.05 paired t test) (Fig. 5F) without altering the initial A-fiber response (data not shown) (n = 5), suggesting that B1 receptors are present in mouse spinal cord and function specifically in nociceptive synaptic pathways. Application of des-Arg9-BK to B1 receptor-deficient spinal cords (n = 7) never produced an increase in the C-fiber-evoked potential at the same time point (mean value 100.1%) or at any other time point. Recordings were also made from B1-deficient mice and the A-fiber fast component was not different in the spinal cords of wild-type and B1-deficient mice (peak response was 0.68 ± 0.09 mV and 0.54 ± 0.05 mV in wild type and B1 −/−, respectively, P > 0.2). However, the VRP to C-fiber stimulation appeared reduced, but this was not significantly different (integrated response was 4.24 ± 0.73 mVS and 2.82 ± 0.35 mVS in wild type and B1 −/−, respectively, P = 0.1). The B1 receptor might be important for the acute (seconds) activity-dependent facilitation of the VRP that probably underlies some forms of central sensitization (24). Repetitive electrical stimulation (500 μA, 500 μs) of the L5 dorsal root at 1 Hz for 20 s induces an increase in the size of the VRP. This type of plasticity has been termed wind-up. In B1-deficient mice, wind-up was significantly reduced by ≈50% compared with wild-type animals (Fig. 5 E and G), confirming that this receptor modifies nociceptor-induced plasticity of synaptic transmission at the spinal level. Thus, the B1 receptor functions as a critical mediator of the central sensitization in the spinal cord that follows sustained activation of nociceptors.
Discussion
Through the generation and physiological characterization of B1-receptor knockout mice, we have shown that the B1 receptor is critically required for a number of important physiological functions in vivo. These include blood pressure homeostasis, inflammation, and nociception.
In a model of sepsis (LPS injection), the initial hypotensive response was severely curtailed in mice lacking the B1 receptor. Kinins have been suggested to be mediators of the vasodilatation observed in septic shock (25–27), and our results indicate that the B1 receptor is involved in the pathophysiological mechanism. The profound vasodilatation seen after LPS may be elicited by nitric oxide (NO), due to B1 receptors in the vasculature releasing NO via calcium-mediated activation of the endothelial NO synthase (27–29). Because it has been shown that endothelial cells release NO immediately after administration of LPS (30), endothelial NO synthase activated by kinins via the B1 receptor may cause the early hypotensive response to LPS in intact animals. In support of this notion, we found significantly reduced levels of endothelial NO synthase activity in B1-deficient mice (data not shown). In the later phase of septic shock, inducible NO synthase is expressed in the vessel wall and becomes essential for the vascular endotoxin effects because animal models lacking this enzyme are protected from the vasodilatory and lethal effects of LPS (31, 32). For this later phase of hypotension, our results suggest a limited importance of B1 receptors, but the high variability in the blood pressure measurements 40 min after LPS administration precludes a definitive answer.
There is considerable evidence from pharmacological studies that kinins are involved in the recruitment of leukocytes to inflamed tissue (1). B2 as well as B1 receptors (5, 10) have been implicated in this process, but the mechanisms involved are still unclear. In B1 receptor-deficient mice, a drastic reduction in the accumulation of PMN at sites of inflammation was observed in models of pleurisy (Fig. 3) and peritonitis (data not shown). Thus, it appears that the recruitment of PMN to inflamed tissue is mediated by kinins mostly via B1 receptors. In contrast, plasma extravasation and mononuclear cell infiltration are not affected by the absence of B1 receptors in the pleurisy model, despite the fact that these parameters are stimulated by B1-agonist injection into the pleural cavity of normal mice (ref. 14; this study). However, we know from earlier studies (15) that vascular permeability changes and mononuclear cell infiltration occur mainly in the late phase of carrageenan-induced pleurisy (after 48 h) that was not analyzed in B1-deficient mice. Further studies will be necessary to elucidate whether the B1 receptor is involved in this late phase of carrageenan action. It is conceivable that some of the effects of B1 receptors in PMN recruitment might require the release of neurokinins, calcitonin gene-related peptide, or NO, because inhibitors of these inflammatory mediators can block the effects of kinins in several model systems (5, 14).
In this study, we have shown that the B1 receptor is required for normal pain behavior in models of both thermal and chemical nociception. Furthermore, by using two electrophysiological preparations, we show that the action of B1 receptors in the spinal cord underlies, at least in part, the function of this receptor in nociception. In resting nociceptive sensory neurons, it has been shown that the B1 receptor is expressed but does not apparently mediate the depolarizing action of kinins (11). Kinins have long been described as powerful regulators of the noxious heat sensitivity of nociceptors (33). Because the noxious heat sensitivity of two functional classes of isolated nociceptors (differentiated by their IB4 binding) (18) was normal, we can conclude that the B1 receptor is not required for the development or maintenance of the noxious heat sensitivity. Consistent with these results, acute thermal nociception in B1 receptor-deficient mice was unaltered. However, in the hot-plate test, B1-receptor knockout animals showed significant analgesia compared with wild-type controls. For this reason, we asked whether the synaptic connectivity between nociceptive sensory neurons and spinal reflex circuits was altered in B1 receptor-deficient mice. We used an isolated hemisected spinal cord preparation from neonatal mice to record ventral root potentials in response to electrical stimulation of primary afferents. The behavior of spinal circuits in this neonatal preparation has been found to accurately reflect that found in more mature animals (34). The VRP has two major components, a short A-fiber-mediated monosynaptic component and a much longer lasting polysynaptic component that can be blocked in part by N-methyl-d-aspartate- and neurokinin-receptor antagonists (34, 35). The long-lasting VRP is evoked only after stimulation of nonmyelinated (C-fibers) presumably nociceptive sensory afferent fibers. In the present study, we have shown that the size of this component is selectively facilitated in the presence of des-Arg9-BK, a specific agonist of the B1 receptor. Unlike neurokinin-receptor activation (36), des-Arg9-BK did not directly depolarize the ventral root when applied in vitro (37). The facilitation, which recovered within 20 min after washout of des-Arg9-BK, was not observed in isolated spinal cords obtained from B1-receptor knockout mice. Thus, activation of B1 receptors in spinal cord neurons can produce an increase in spinal cord reflex excitability that would directly underlie a proalgesic role of this receptor in vivo. This potentiation of reflex excitability is similar to, but shorter lasting than, that seen with brain-derived neurotrophic factor, which is thought to be released by nociceptive C-fibers after injury or inflammation (35). Repetitive stimulation of C-fiber inputs (>1 Hz) can produce an activity-dependent increase in reflex excitability that has characteristics in common with a phenomenon first described in the spinal cord dorsal horn called wind-up (38, 39). We directly measured this synaptic plasticity in the spinal cord from B1 receptor-deficient mice and demonstrate that the magnitude of C-fiber-evoked reflex facilitation is dramatically reduced in the absence of the B1 receptor. These data indicate that endogenous B1 receptors are essential for the expression of normal central sensitization after activation of C-fibers. This is direct evidence for a role for central kinin receptors in nociception and suggests that the hypoalgesia seen in B1-receptor knockout mice is largely caused by a reduced central sensitization in the spinal cord.
In further behavioral tests, such as capsaicin-induced paw licking, nocifensive behavior was also significantly attenuated in B1-receptor knockout mice. In the formalin test, the mice exhibited analgesia in both the early (up to 5 min) and late phase (15–30 min). Both these algogens powerfully activate nociceptors (certainly at frequencies greater than 1 Hz), and it is likely that the attenuated behavioral responses are caused by a reduced central sensitization in the spinal cord in the absence of the B1 receptor. The functional status of B1 receptors expressed by resting sensory neurons is at present unclear while functional B2 receptors are expressed (11). However, in one of the behavioral tests used here, the formalin test, in which the B1 receptor is required, mice lacking the B2 receptor do not display hypoalgesia (3, 16). It is possible that B1 receptors located on the nociceptor terminals in the spinal cord or on spinal cord interneurons could mediate the central effects of the receptor. More studies on the B1-receptor expression will be needed to clarify this issue.
B1 receptors are markedly up-regulated during inflammation by IL-1β and NFκB-dependent mechanisms (5–9, 40). This study shows that B1 receptors are of great importance as mediators of leukocyte accumulation at sites of tissue injury. Furthermore, we demonstrate here that it is probably B1 receptors, expressed in the spinal cord, that are required for normal acute nociception. Therefore, the phenotype of the B1-receptor knockout mice described here suggests that orally active B1 antagonists may have a very favorable therapeutic spectrum exhibiting both analgesic and antiinflammatory actions.
Acknowledgments
We thank R. Förster and O. Lockley for critical reading of the manuscript and A. Böttger for excellent technical assistance. This work was supported by grants of the Volkswagenstiftung and the Deutsche Akademische Austauschdienst to M.B., J.B.P., and J.L.P. Additional support was obtained from a Deutsche Forschungsgemeinschaft grant (SPP 1026) to G.R.L., a grant of the Fundação de Amparo à Pesquisa do Estado de São Paulo (Proc. 99/10659-2) to J.B.P. and a Marie Curie fellowship to P.A.H.
Abbreviations
- BK
bradykinin
- IB4
isolectin B4
- LPS
bacterial lipopolysaccharide
- NO
nitric oxide
- PMN
polymorphonuclear leukocytes
- VRP
ventral root potential
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120035997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120035997
References
- 1.Bhoola K D, Figueroa C D, Worthy K. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 2.Borkowski J A, Ransom R W, Seabrook G R, Trumbauer M, Chen H, Hill R G, Strader C D, Hess J F. J Biol Chem. 1995;270:13706–13710. doi: 10.1074/jbc.270.23.13706. [DOI] [PubMed] [Google Scholar]
- 3.Rupniak N M, Boyce S, Webb J K, Williams A R, Carlson E J, Hill R G, Borkowski J A, Hess J F. Pain. 1997;71:89–97. doi: 10.1016/s0304-3959(97)03343-5. [DOI] [PubMed] [Google Scholar]
- 4.Madeddu P, Varoni M V, Palomba D, Emanueli C, Demontis M P, Glorioso N, Dessi Fulgheri P, Sarzani R, Anania V. Circulation. 1997;96:3570–3578. doi: 10.1161/01.cir.96.10.3570. [DOI] [PubMed] [Google Scholar]
- 5.Ahluwalia A, Perretti M. J Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- 6.Schanstra J P, Bataille E, Marin Castano M E, Barascud Y, Hirtz C, Pesquero J B, Pecher C, Gauthier F, Girolami J P, Bascands J L. J Clin Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ni A, Chao L, Chao J. J Biol Chem. 1998;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Polgar P, Taylor L. Biochem J. 1998;330:361–366. doi: 10.1042/bj3300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marceau F, Hess J F, Bachvarov D R. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- 10.Perron M-S, Gobeil F J, Pelletier S, Regoli D, Sirois P. Eur J Pharmacol. 1999;376:83–89. doi: 10.1016/s0014-2999(99)00348-9. [DOI] [PubMed] [Google Scholar]
- 11.Seabrook G R, Bowery B J, Heavens R, Brown N, Ford H, Sirinathsinghi D J S, Borkowski J A, Hess J F, Strader C D, Hill R G. Neuropharmacology. 1997;36:1009–1017. doi: 10.1016/s0028-3908(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 12.Pesquero J B, Pesquero J L, Oliveira S M, Roscher A A, Metzger R, Ganten D, Bader M. Biochem Biophys Res Commun. 1996;220:219–225. doi: 10.1006/bbrc.1996.0384. [DOI] [PubMed] [Google Scholar]
- 13.Walther T, Balschun D, Voigt J-P, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M. J Biol Chem. 1998;273:11867–11873. doi: 10.1074/jbc.273.19.11867. [DOI] [PubMed] [Google Scholar]
- 14.Vianna R M, Calixto J B. Br J Pharmacol. 1998;123:281–291. doi: 10.1038/sj.bjp.0701590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleh T S, Calixto J B, Medeiros Y S. Br J Pharmacol. 1996;118:811–819. doi: 10.1111/j.1476-5381.1996.tb15472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrêa C R, Calixto J B. Br J Pharmacol. 1993;110:193–198. doi: 10.1111/j.1476-5381.1993.tb13791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beirith A, Santos A R, Rodrigues A L, Creczynski Pasa T B, Calixto J B. Eur J Pharmacol. 1998;345:233–245. doi: 10.1016/s0014-2999(98)00026-0. [DOI] [PubMed] [Google Scholar]
- 18.Stucky C L, Lewin G R. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson S W, Gerber G, Sivilotti L G, Woolf C J. Brain Res. 1992;595:87–97. doi: 10.1016/0006-8993(92)91456-o. [DOI] [PubMed] [Google Scholar]
- 20.Di Rosa M. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 21.Dray A. Can J Physiol Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- 22.Cesare P, McNaughton P. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesare P, Moriondo A, Vellani V, McNaughton P A. Proc Natl Acad Sci USA. 1999;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon S B, Lewin G R, Wall P D. Curr Opin Neurobiol. 1993;3:602–610. doi: 10.1016/0959-4388(93)90062-4. [DOI] [PubMed] [Google Scholar]
- 25.Carretero O A, Nasjletti A, Fasciolo J C. Experientia. 1970;26:63–65. doi: 10.1007/BF01900394. [DOI] [PubMed] [Google Scholar]
- 26.Katori M, Majima M, Odoi Adome R, Sunahara N, Uchida Y. Br J Pharmacol. 1989;98:1383–1391. doi: 10.1111/j.1476-5381.1989.tb12688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mombouli J V, Vanhoutte P M. Annu Rev Pharmacol Toxicol. 1995;35:679–705. doi: 10.1146/annurev.pa.35.040195.003335. [DOI] [PubMed] [Google Scholar]
- 28.Drummond G R, Cocks T M. Br J Pharmacol. 1995;116:2473–2481. doi: 10.1111/j.1476-5381.1995.tb15098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pruneau D, Luccarini J M, Defrene E, Paquet J L, Belichard P. Eur J Pharmacol. 1996;297:53–60. doi: 10.1016/0014-2999(95)00720-2. [DOI] [PubMed] [Google Scholar]
- 30.Salvemini D, Korbut R, Anggard E, Vane J. Proc Natl Acad Sci USA. 1990;87:2593–2597. doi: 10.1073/pnas.87.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchinson N, et al. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 32.Wei X Q, Charles I G, Smith A, Ure J, Feng G J, Huang F P, Xu D, Muller W, Moncada S, Liew F Y. Nature (London) 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 33.Beck P W, Handwerker H O. Pflügers Arch Eur J Physiol. 1974;347:209–222. doi: 10.1007/BF00592598. [DOI] [PubMed] [Google Scholar]
- 34.Thompson S W, Dray A, Urban L. J Neurosci. 1994;14:3672–3687. doi: 10.1523/JNEUROSCI.14-06-03672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerr B J, Bradbury E J, Bennett D L H, Trivedi P M, Dassan P, French J, Shelton D B, McMahon S B, Thompson S W N. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox A J, Naeem S, Patel I A, Walpole C, Urban L. Acta Biol Hung. 1996;47:129–144. [PubMed] [Google Scholar]
- 37.Davis C L, Naeem S, Phagoo S B, Campbell E A, Urban L, Burgess G M. Br J Pharmacol. 1996;118:1469–1476. doi: 10.1111/j.1476-5381.1996.tb15562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendell L M. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 39.Woolf C J, Thompson S W. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 40.Campos M M, Souza G E P, Calixto J B. Br J Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]