Abstract
For most plants survival depends upon the capacity of root tips to sense and move towards water and other nutrients in the soil. Because land plants cannot escape environmental stress they use developmental solutions to remodel themselves in order to better adapt to the new conditions. The primary site for perception of underground signals is the root cap (RC). Plant roots have positive hydrotropic response and modify their growth direction in search of water. Using a screening system with a water potential gradient, we isolated a no hydrotropic response (nhr) semi-dominant mutant of Arabidopsis that continued to grow downwardly into the medium with the lowest water potential contrary to the positive hydrotropic and negative gravitropic response seen in wild type-roots. The lack of hydrotropic response of nhr1 roots was confirmed in a system with a gradient in air moisture. The root gravitropic response of nhr1 seedlings was significantly faster in comparison with those of wild type. The frequency of the waving pattern in nhr1 roots was increased compared to those of wild type. nhr1 seedlings had abnormal root cap morphogenesis and reduced root growth sensitivity to abscisic acid (ABA) and the polar auxin transport inhibitor N-(1-naphtyl)phtalamic acid (NPA). These results showed that hydrotropism is amenable to genetic analysis and that an ABA signaling pathway participates in sensing water potential gradients through the root cap.
Survival for most plants depends upon the capabilities of a dynamic, specialized organ at the root apex—the root cap (RC)—to sense water and nutrients and to respond by transmitting signals, which result in altered growth patterns. The root tip's response to environmental cues by directed growth plays a role in all aspects of plant development by virtue of its crucial role in establishing a stable underground architecture with access to nutrients and water (Hawes et al., 2000, 2002). The notion that plant roots penetrate the soil in search of water to sustain their growth may be one of those popular ideas that persist, simply because it is so easily thought of and seems so natural. This sort of behavior, rather than the action of gravity, was first offered as the explanation for the downward orientation of roots (suggested by Dodart around 1700; in Hart, 1990). However, with greater awareness of the roles of other directional signals such as gravity and light on the general orientation of plant organs, interest in hydrotropism has fluctuated over the years. In addition to roots, rhizoids and pollen tubes have been also reported to be positively hydrotropic (Molisch, 1883; Vöchting, 1902; in Hart, 1990). By 1872, Sachs (in Hart, 1990) demonstrated that in pea (Pisum sativum) seedlings grown at an angle in a hanging sieve basket, the emergent roots bent around and grew back toward the wet substratum, thus overcoming the force of gravity. Around that time, Sachs, Darwin, Pfeffer, and Weisner (who introduced the term hydrotropism) demonstrated that moisture affected root orientation. Darwin (1881) discovered that the primary site for perception of underground signals is the root tip. He also proposed that the tip of the radicle transmit an influence to the upper part of the root so the root can bend after sensing an environmental stimulus.
The phenomenon of root responsiveness to moisture gradients is known as hydrotropism. We still do not know how hydrotropism works and how the RC senses moisture gradients in the soil. Plant scientists have paid little attention to hydrotropism, whereas gravitropism, the ability of plant organs to use gravity as a guide for growth, has been studied extensively (Takahashi, 1997). Gravity is unique among environmental signals in that it is present continuously, it is unidirectional, and it maintains constant intensity. However, evidence shows that this intrinsic growth of roots is sensitive to the microenvironment at the root tip, and, thus, gravity is continually challenged by differences in moisture gradients, distribution of nutrients, heat, light, and oxygen, among others. Studies of hydrotropism have always been difficult to achieve because the root response to gravity strongly interacts with its positive hydrotropic response (Takahashi and Suge, 1991; Takahashi et al., 1992a, 1992b, 1996). In addition, there are difficulties implicit in controlling and maintaining a continual moisture gradient adequate for both inducing a root-positive hydrotropic response and for challenging its response to gravity. For instance, pea roots are in general hydrotropically nonsensitive but when the gravitropic response is nullified by clinorotation, they show positive hydrotropism (Takahashi et al., 1996). Therefore, the observation made by Jaffe et al. (1985) on roots of the pea mutant ageotropum, which are agravitropic, but respond to hydrotropism, is significant because it indicates that perception and response of these two tropisms are separable.
To date, various screening procedures have been designed to isolate mutants affected in stimulus response for gravity, light, obstacle touching, or combinations of these stimuli. To gain insight into the poorly understood phenomenon of hydrotropism, we have developed a screening system with a water potential gradient for the isolation of Arabidopsis mutants whose roots respond negatively to hydrotropic stimulus. In this study, we report the isolation of the nhr1 mutant and its characterization under various physiological conditions.
RESULTS AND DISCUSSION
Screening System with a Water Potential Gradient for the Isolation of No Hydrotropic Response Mutants
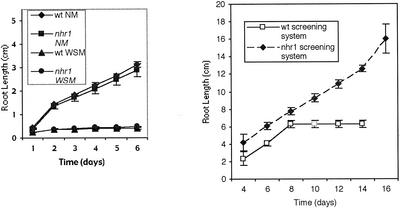
So far, hydrotropism has not been studied in Arabidopsis roots. We first developed a system with a water potential gradient for demonstrating positive hydrotropic response of Arabidopsis wild-type Columbia (Col) roots. This system comprised a square petri dish that was maintained in vertical position on one side and contained a normal nutrient medium (NM; where Arabidopsis seeds were plated) in the upper part, and in the lower part, the same NM supplemented with glycerol and alginic acid (water stress medium [WSM]; Fig. 1A). Wild-type plants grew downward and after 7 d stopped growing, or started to form a curvature responding positively to the hydrotropic stimulus by avoiding the substrate with lower water potential in the screening system; that is, roots never reached the area containing the WSM. The frequency of wild-type Arabidopsis roots that developed a hydrotropic curvature in the screening system was 48% (n = 98).
Figure 1.
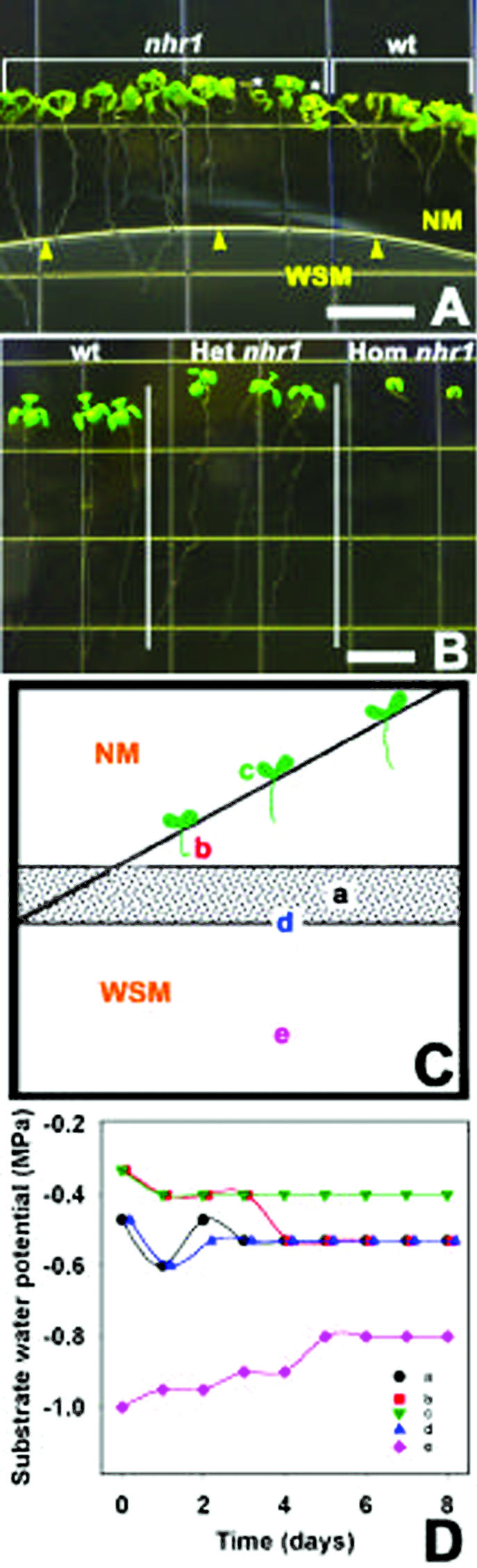
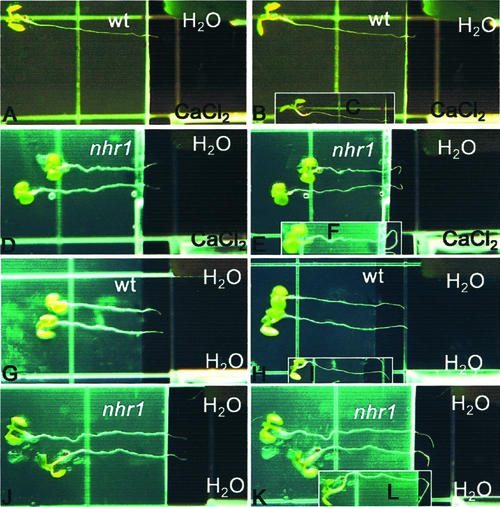
Screening system for the isolation of hydrotropic mutants, and phenotypes of wild-type and nhr1 seedlings in this system and in NM. A, Ten-day-old nhr1 and wild-type seedlings growing in the screening system. Wild-type roots arrested their growth at d 7 and developed a hydrotropic curvature. Roots of heterozygous nhr1 plants continued to grow after crossing the boundary between NM and WSM showing a lack of hydrotropic response. Homozygous nhr1 seedlings have short-root phenotype and are marked with asterisks. Arrowheads indicate the boundary between NM and WSM. Bar = 50 mm. B, Phenotype of 10-d-old, heterozygous (Het) and homozygous (Hom) nhr1 plants grown in NM. Seedlings of 7-d-old heterozygous nhr1 were selected from the screening system and then were transplanted to NM. Bar = 10 mm. C, Diagram of the screening system showing where water potential was measured. To determine the threshold level of the substrate water potential in the screening system, seeds were plated diagonally along the line drawn in the upper part of the dish and agar water potential was measured daily. Position “a” is where the two media are in contact; position “b” is where the root stopped growing or where root curvature took place in NM; position “c” is the middle position along the diagonal within NM; and position “d” is the upper limit of the WSM in the screening system. Position “e” is in the lower part of the dish containing the WSM. D, Changes in the substrate water potential over time within the screening system in the locations indicated in C. Average data of four independent experiments are given.
In the making of the screening medium for examining positive hydrotropism in wild-type Arabidopsis roots, we tested several osmolytes for the WSM such as sorbitol (5%, 8%, 10%, and 15% [v/v]), mannitol (15% and 20% [v/v]), and polyethylenglycol 8000 (10%, 15%, and 20% [v/v]) besides glycerol (data not shown). Even though both sorbitol and mannitol produced a water potential gradient in the system, they were not adequate for inducing a positive hydrotropic response in wild-type roots, particularly in long-term experiments. On the contrary, these roots continued to grow into the WSM (with either sorbitol or mannitol) for 13 to 15 d (see Fig. 1 in supplemental data available at www.plantphysiol.org). We assumed that Arabidopsis wild-type roots probably accumulated or metabolized both sorbitol and mannitol and, as a consequence, could grow in the presence of these osmolytes. Polyethylenglycol, on the other hand, did not diffuse in agar and, hence, no water potential gradient was formed in the screening system. In contrast, glycerol was capable of inducing a positive hydrotropic response in wild-type roots. However, glycerol has to be combined with alginic acid for improving medium solidification. We also tested the effect of alginic acid (0.5%, 1%, 1.5%, and 2.5% [w/v]) in the growth of wild-type roots by germinating seeds in plates with NM with different concentrations of alginic acid and by sowing seeds in a two-medium system having at the bottom of the plate NM plus alginic acid and at the top NM. Alginic acid did not influence the growth of Arabidopsis wild-type roots (data not shown).
The screening system was then used for screening a population of 26,400 ethyl methane sulfonate (EMS)-mutagenized Arabidopsis M2 seedlings. Putative mutants were selected based on their lack of hydrotropic response or their inability to sense the low water potential in the WSM, subsequently showing considerable root growth in contrast to wild-type roots (Figs. 1A and 2B).
Figure 2.
Dynamics of root growth of wild-type and heterozygous nhr1 seedlings in NM, WSM, and screening system. A, Seeds were sown in NM or WSM plates and placed vertically. B, For measuring root growth in the screening system, seedlings initially were grown for 4 d in NM and then were transferred to the screening system. Root elongation was measured on a subsequent day after germination by digital image analysis. Each point represents the mean ± sd (n = 20). Data are from three independent experiments.
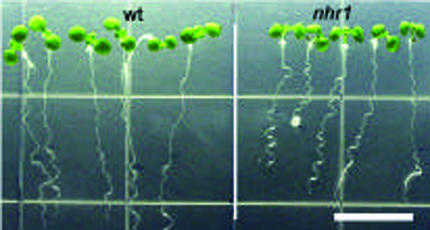
The screening resulted in the isolation of 10 putative negative hydrotropic mutants. After backcrossing three times to wild-type plants, only two lines, nhr1 and nhr2, had negative hydrotropism. nhr2 seeds germinated poorly; thus, our main attention was on the analysis of nhr1. In a backcross of nhr1 seed to wild type, 50% of the F1 seedlings grown in the screening system for 8 d showed no hydrotropic response phenotype and 50% showed positive hydrotropic response. The segregation ratio of F1 seedlings (44 wild type:31 no hydrotropic phenotype) indicates that the nhr1 mutation is partially dominant (χ2 = 2.26 < χ20.05(1) = 3.84). When the backcrossed F1 plants were selfed, and the no hydrotropic genotype of the corresponding F2 plants was determined by examining F3 families, the segregation ratios (homozygous wild type:heterozygous:homozygous mutant F2 seedlings) were consistent with the1:2:1 segregation ratio expected for mutations in a single locus. However, the homozygous mutant F2 seedlings revealed a different phenotype because these seedlings were dwarfs with a very short root that never reached the reproductive stage (Fig. 1B). The segregation ratio of selfed F2 heterozygous nhr1 individuals (113 wild type:286 no hydrotropic response phenotype:136 short-root phenotype F3 seedlings) was consistent with the 1:2:1 segregation ratio expected for a semidominant and single-locus mutation (χ2 = 4.5 < χ20.05(2) = 5.99). These results are consistent with no hydrotropic response phenotype being heterozygous nhr1, and the short-root phenotype being homozygous nhr1. The main difference between heterozygous and homozygous nhr1 roots was that the homozygous nhr1 plants never developed significant growth of their roots and shoots under any condition tested. For example, the root lengths of 10-d-old homozygous nhr1 seedlings grown in NM were on average 2.9 ± 0.04 mm, whereas those of heterozygous nhr1 and wild-type seedlings were 37.9 ± 1.7 mm and 36.2 ± 1.8 mm (mean ± sd, n = 10), respectively (Fig. 1B).
To characterize the water potential gradient in the screening system and plant responses in it, we measured the changes in water potential over time in different parts of the system (Fig. 1D). The water potential in the upper part of the dish gradually decreased by glycerol diffusion during 8 d and became more negative in positions closer to the WSM (Fig. 1C). Roots of wild type usually stopped growing or started to curve away from WSM where water potential was −0.53 MPa after 6 to 7 d (position “b,” Fig. 1C). Roots of heterozygous nhr1 continued to grow into the WSM along the gradient between −0.3 and −0.8 MPa. Notably, heterozygous nhr1 seedlings became chlorotic when their roots were left more than 4 d in the WSM. To determine the threshold level of the substrate water potential, we planted wild-type seeds diagonally in the upper part of the plate (Fig. 1C). Roots stopped growing or developed a curvature in response to hydrotropic stimuli 1.3 cm above the boundary between the two media where the water potential was approximately −0.5 MPa.
Mapping of the nhr1 Mutation
We determined the chromosomal position of the NHR gene by simple sequence length polymorphism (SSLP) linkage analysis (Lukowitz et al., 2000). We detected linkage of the recessive nhr1 (short-root phenotype) mutation, heterozygous nhr1 (no hydrotropic response), and wild type by bulked segregant analysis. The nhr mutation was induced in a Col background and crossed to Wassilewskija (Ws) to generate the following mapping population: F2 population derived from a Col/Ws F1 plant with the genotype nhr/NHR. The nga162 and nga172 molecular markers showed a clear bias toward the Col-specific band in the mutant pool. This indicated that the mutation maps to the upper arm of chromosome III. The recombination frequency across the nga162/nga172 interval obtained was 2.5% and 6%, respectively.
Root Growth Responses of nhr1 Seedlings
The growth of heterozygous nhr1 roots in NM and WSM was indistinguishable from the wild type (Fig. 2A). When both wild-type and heterozygous nhr1 seeds were sown in NM they showed similar growth rate (Fig. 2A). However, when both wild-type and heterozygous nhr1 seeds were sown in WSM, their root growth was seriously impaired (Fig. 2A). This demonstrates that heterozygous nhr1 roots were not resistant to the severe water deficit conditions of the WSM. Heterozygous nhr1 seedlings were distinguished from those of wild type in both NM and WSM by their wavy-like root growth (Fig. 1B). This wavy-like root growth is absent in the wild-type Col ecotype and frequently cosegregated with no hydrotropic response phenotype of heterozygous nhr1 seedlings. In the screening system, however, heterozygous nhr1 roots showed a faster growth rate in comparison with wild-type roots (Fig. 2B). Wild-type roots grew for only 4 d after transplanting to the screening system and no further growth was observed after d 8 (Fig. 2B). However, heterozygous nhr1 roots continued to grow for 12 d in the screening system (Fig. 2B). Heterozygous nhr1 had close to normal root growth in the screening system but could not grow from the onset in WSM. This most likely suggests that the heterozygous nhr1 mutation does not confer water deficit resistance, but rather provides a short-term capacity for root growth in the presence of a substrate water potential gradient. On the other hand, this mutation might impair perception of such a gradient.
Hydrotropic and Gravitropic Responses of nhr1 Roots in a System with Air Moisture Gradient
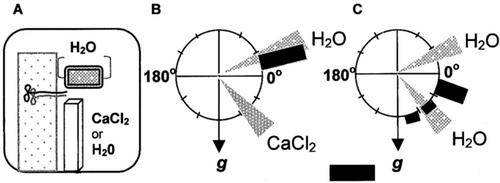
To discern whether the phenotype shown by heterozygous nhr1 roots in the screening system occurred as a consequence of an alteration in glycerol metabolism, we developed a different system for studying root hydrotropism with a gradient in air moisture (Figs. 3 and 4A). In this system, a humidity gradient was formed in a square petri dish with a saturated solution of calcium chloride. Here, heterozygous nhr1 roots responded negatively to the moisture gradient stimulus (Fig. 3, E and F), in contrast to wild-type roots that developed positive hydrotropic response toward the water source (Fig. 3, B and C). Roots of both heterozygous nhr1 and wild-type seedlings developed a curvature approximately 3 h after the exposure to a water potential gradient. The root curvature angles of all wild-type roots showed negative gravitropic values and roots grew toward the water source (Fig. 4B). On the other hand, heterozygous nhr1 roots showed either a right- or a left-handed twist in their growth direction in the same system (Fig. 3, E and F). However, without a moisture gradient, 6 (Fig. 3H) and 24 (Fig. 3I) h after the beginning of the experiments, wild-type roots responded not only to the gravity vector but also to the water source. The direction of most wild-type roots was settled in the first of the 12 30o sectors and only 16% of roots were close to the expected 90o (Fig. 4C). This indicates that the cumulative response in wild-type roots was developed to both gravitropic and hydrotropic stimuli. In contrast, the direction of approximately all heterozygous nhr1 roots was closer to 90o (Fig. 3, K and L), thus confirming their faster positive gravitropic response (Fig. 5A) in contrast with those of wild type. Besides, some heterozygous nhr1 roots showed a right- or a left-handed turn in their growth direction in the system without air moisture gradient. Hence, only wild-type roots responded positively to the water source and were capable of abrogating the effect of gravity. Both wild-type and heterozygous nhr1 roots showed no significant differences in their rate of elongation after 6 h of growth under the two conditions tested. In a system with air water potential gradient, wild-type roots grew at 59 ± 35.5 μm h−1 (n = 6), whereas heterozygous nhr1 roots grew at 70 ± 37.1 μm h−1 (n = 10). In the absence of a gradient, wild-type roots grew at 84 ± 65.2 μm h−1 (n = 11) and heterozygous nhr1 roots at 114 ± 34.7 μm h−1 (n = 8; mean ± sd). The root responses to both absence and presence of an air moisture gradient were maintained up to 48 h, indicating its physiological nature.
Figure 3.
Hydrotropic response of heterozygous nhr1 and wild-type roots in a system with a gradient in air moisture. In this system, an air moisture gradient was created around the roots between the oasis (water) and the cuvette (saturated solution of CaCal2) for testing their positive hydrotropic response (A–F). The additive effect of gravitropism and hydrotropism was tested in a control system where both the oasis and the cuvette contained water (G–L). At time 0 (A, D, G, and J), roots were placed horizontally with a distance of 2 to 3 mm from the tips growing in the air to the water source. In the system with air moisture gradient, wild-type roots were hydrotropically stimulated and, as a consequence, showed negative gravitropic response 6 and 24 h after the beginning of the experiment (B and C). Heterozygous nhr1 roots were not hydrotropically stimulated 6 and 24 h after the beginning of the experiment (E and F). These roots showed either a right or a left-handed twist in their growth direction. Root growth direction of heterozygous nhr1 ended behind the microscope slide in a loop-like structure 24 h after the beginning of the experiment (F). This root growth behavior hampered the measurements of root curvature angles. In the moisture air system, 6 (H) and 24 (I) h after the beginning of the experiment, root growth of wild-type seedlings was primarily directed toward the water source instead to the gravity vector. After 6 (K) and 24 (L) h, roots of heterozygous nhr1 seedlings grew mostly in the direction of gravity. The figures are representative of five independent experiments (n = 24).
Figure 4.
Diagram of the experimental system developed for the induction of positive root hydrotropism in Arabidopsis (A). Hydrotropic and gravitropic responses of wild-type roots grown in a system with an air moisture gradient generated with a saturated CaCl2 solution (B) or water (control; C). The frequency of root growth directions was analyzed by measuring curvature 6 h after the beginning of gravistimulation at 0o (0o angle corresponds to horizontal position of the root tip). Each hydrostimulated and/or gravistimulated root was assigned to one of 12 30o sectors. The length of each bar represents the percentage of seedlings showing direction of root growth within that sector. Bar = 100%. g, Gravity vector; H2O, water source; CaCl2, reduced air moisture source. The gray sectors are indicators of where the CaCl2 and water were placed in the plate. Data are from five independent experiments (n = 24).
Figure 5.
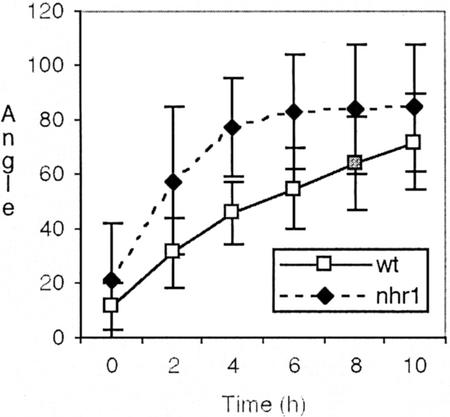
Kinetics of root gravitropic bending in wild-type and heterozygous nhr1 seedlings. Seedlings on vertical plates were turned 90o to a horizontal position. Heterozygous nhr1 seedlings were selected from the screening system and then transferred to NM plates for the gravitropism analysis. Each point represents the mean ± sd (n = 15). Student's t test demonstrates statistically significant differences between nhr1 and wild-type seedlings at 2 (P = 0.01), 4 (P = 0.00015), 6 (P = 0.0014), and 8 (P = 0.023) h after gravistimulation.
These analyses of growth and tropic responses in roots grown freely in air represent the first achieved in Arabidopsis, to our knowledge. This system has the advantage of allowing the study of directional root growth in three dimensions because previous studies have been made on flat two-dimensional agar surface systems. Furthermore, in this system basic root movement, which is regulated by a combination of right-handed and left-handed circumnutations, gravitropism, hydrotropism, obstacle avoidance, etc. (Darwin, 1881; Simmons et al., 1995 Migliaccio and Piconese, 2001) can be uncoupled from other responses to agar surface-derived environmental stimuli. In fact, when the heterozygous nhr1 roots grew freely in the moisture air gradient their directional growth pattern was unusual (Fig. 3, E and F). Heterozygous nhr1 roots moved in various planes and formed a loop or a hairpin structure at the tip, in contrast to wild-type roots. Roots (like all plant organs) grow by making oscillatory movements around the growth vector rather than by growing linearly (Sachs, 1872; Migliaccio and Piconese, 2001). However, the unusual directional growth pattern of heterozygous nhr1 roots in free air may indicate that forces other than positive gravitropism, lack of hydrotropism, circumnutation, and thigmotropism could be involved in this process. For instance, the biomechanical properties of the root might also influence the directional root growth behavior of nhr1 seedlings.
In summary, heterozygous nhr1 roots did not show positive hydrotropism in response to water potential gradients created either with glycerol or with a saturated solution of calcium chloride, thus confirming their lack of hydrotropic phenotype.
Root Gravitropism and Waving Pattern Is Affected in the nhr1 Mutant
Heterozygous nhr1 roots developed significantly faster gravitropic responses when grown on the surface of an agar medium (Fig. 5), and when embedded within the agar medium (not shown), indicating that heterozygous nhr1 roots possess positive gravitropic but no hydrotropic competence (Figs. 1A, 3, E and F, and 5). This implies that in heterozygous nhr1 roots, gravity can activate a complex signal transduction pathway that results in the production of a gravitropic root curvature at the elongation zone. Hence, in heterozygous nhr1 roots, perception of the hydrotropic stimulus is impaired but not the development of a tropic response. We also investigated the wavy growth pattern of heterozygous nhr1 roots (Okada and Shimura, 1990). These roots showed increased wavy growth, with each curve encompassing a larger angle than curves in wild-type roots, similar to wav2-1 and shy2-22 (Okada and Shimura, 1990; Tian and Reed, 1999; Fig. 6). Because this waving phenomenon is likely governed by circumnutation or spiralization patterns (Darwin, 1881; Simmons et al., 1995; Rutherford and Masson, 1996; Migliaccio and Piconese, 2001), the waviness of nhr1 roots may be because of higher right-handed helical oscillations in the root growth direction. These oscillations could be amplified by tactile stimulation, gravity, or some other characteristics of the agar surface (i.e. nutrients or surface tension; Okada and Shimura, 1990; Rutherford and Masson, 1996; Mullen et al., 1998). However, the enhanced gravitropic response and wavy growth pattern of heterozygous nhr1 in agar (Figs. 5 and 6) and the unexpected growth of elongating heterozygous nhr1 roots in air (Fig. 3, E and F) may indicate that NHR1 is also involved in the control of directional root growth.
Figure 6.
Wavy root growth pattern of wild-type and heterozygous nhr1 10-d-old seedlings. Wild-type (grown in NM) and heterozygous nhr1 (selected from the screening system) 7-d-old seedlings were transferred to 1.5% (w/v) agar plates and these were tilted to 45o for 3 d. Bar = 10 mm.
Root Tip Morphology of nhr1 Mutants
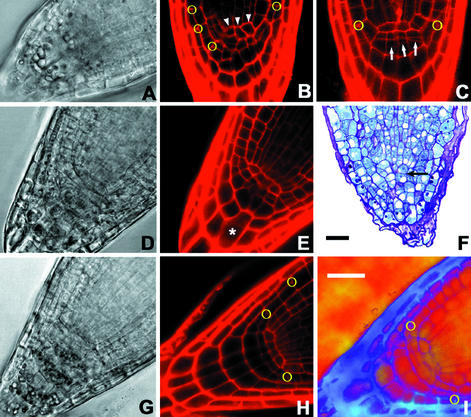
Because moisture gradients are sensed in the RC (Jaffe et al., 1985), we analyzed the root tip morphology of nhr1 mutants. By comparing root tips between wild-type and homozygous nhr1 mutants, we observed several distinct aberrations in cell patterns of 5-d-old seedlings roots (Fig. 7, compare B with H). Abnormal root apical meristems (RAMs) and RCs were observed in 99% of the seedling progeny of homozygous nhr1 mutants. In seedlings grown in either NM or screening system, the quiescent center (QC) cells were expanded (Fig. 7, B and C) and the RC was highly disorganized (Fig. 6B) compared with wild-type roots (Fig. 7, H and I). In the RC, cell divisions within the columella-initial cell layer were displaced to the first tier of the columella, where typically no periclinal divisions occur (Fig. 7C, arrows), and, as a consequence, lateral RC morphogenesis in the mutant was anomalous. The formative periclinal T divisions (giving rise to epidermal and lateral RC cells) in the RAM (Baum and Rost, 1996) were not affected. However, anticlinal divisions, both in lateral-RC (proliferating divisions) and in cells giving rise to new RC/protoderm initials (formative divisions) were not observed (Fig. 7, B and C). The inhibited root growth probably resulted from the decreased activity of QC cells because they were unusually elongated and disorganized (Fig. 7, B and C). However, the processes of cell differentiation in the RC appeared to be normal because some amyloplasts present in heterozygous nhr1 mutants were larger than in wild-type plants (Fig. 7, A and G). We assume that the inhibition of root growth in homozygous nhr1 is related to general inhibition of cell proliferation. Determinate root growth, small root size, and abnormally larger cells within the RAM indicate such a possibility.
Figure 7.
Root tip morphology in homozygous nhr1, heterozygous nhr1 and wild-type seedlings. Homozygous nhr1 seedlings grown on NM (A and B) or on screening system for 5 d (C). Homozygous nhr1 roots show abnormal lateral RC formation because after formative T divisions (marked by yellow circles), derivative cells in the epidermis and lateral RC are not formed. Successive T divisions are close one to another (B; n = 13). In homozygous nhr1, cells at the position of the wild-type quiescent center (QC) are disorganized (arrowheads on B; n = 12). Furthermore, two tiers of columella initials are found instead of a single columella tier in wild type (arrows; C; n = 6), and cell differentiation in the RC appears to be unaffected because amyloplasts were present (A). Heterozygous nhr1 8-d-old seedlings grown on NM (D and E) or on screening system (F). Images D and E were taken from the same root and the same focal plane. In heterozygous nhr1 roots, irregular anatomy of columella initials and large columella cells (asterisk) were present (n = 7; E). Amyloplasts were observed in columella cells of these roots as in wild type (G) but were larger and located at the bottom of the cell. Heterozygous nhr1 roots grown in the screening system lack a typical QC and RC (n = 16; F). Arrow indicates apparent position of a QC cell and the absence of the closed type of the Arabidopsis root apical meristem (RAM; F). Wild-type plants grown for 7 d on NM (G, H) or on screening system (I) showed normal RAM and RC structure. When grown on screening system, columella and QC cells were usually larger in size (I) compared with those roots grown in NM (G and H; n = 5). A, D, and G, Bright-field images of the confocal sections (B, E, and H). I, Image contrast was enhanced by using the “render different clouds” filter of Adobe Photoshop Elements 5.5 (Adobe Systems, Mountain View, CA). Longitudinal thin section of a heterozygous nhr1 root stained with Periodic Acid Schiff and Toludine blue of roots (F). Bar in I is the same for all images except F. The figures are representative of three independent experiments (n = 60). Bars = 25 μm.
The described root phenotype of homozygous nhr1 is unique and different from any described Arabidopsis mutants. Contrary to shr and scr, nhr1 roots contain all cell layers (Scheres et al., 1995). Contrary to hbt (Willemsen et al., 1998), nhr1 has all initial cells and a normal lateral RC is formed during embryogenesis. In contrast to rml mutants (Vernoux et al., 2000), postembryonic cell division in the RAM took place, though it was inhibited in homozygous nhr1 plants. Continuous RC ablation in transgenic DT-Atsm plants (carrying the diphtheria toxin A chain under the control of the RC-specific RCP1 promoter) produced highly abnormal short roots with reduced mitotic activity in the root tip similar to that observed in homozygous nhr1 (Tsugeki and Federoff, 1999). However, these transgenic plants displayed no evidence of a gravitropic response in contrast to homozygous nhr1 roots (data not shown). These experiments indicate that the RC contributes to the regulation of overall root growth (Tsugeki and Federoff, 1999). Physiological and genetic data suggest the involvement of auxin in pattern formation in plants. Essential components of the auxin redistribution system reside in the RC. The use of the DR5:GUS auxin response reporter construct have shown that there is a maximum auxin response in the columella initial cells (Sabatini et al., 1999). This asymmetric distribution is essential for distal patterning in the Arabidopsis RAM (Sabatini et al., 1999). PIN4, a putative auxin efflux carrier, is presumably involved in the establishment and maintenance of endogenous auxin gradients in the Arabidopsis root tip (Friml et al., 2002a). pin4 mutants displayed various patterning defects in seedling roots (Friml et al., 2002a). Interestingly, root growth in heterozygous nhr1 plants was less inhibited than in wild-type seedlings when the polar auxin transport inhibitor N-[1-naphthyl]phtalamic acid (NPA; Fig. 8A) was added exogenously. This could reflect perturbation in auxin distribution and, in turn, might explain the abnormal patterning seen in the root tip of these mutants (Fig. 7, D–F).
Figure 8.
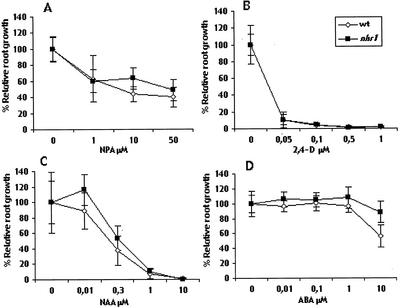
Physiological analysis of heterozygous nhr1 mutant seedlings. Effects of an inhibitor of polar auxin transport and hormones on wild-type and heterozygous nhr1 seedlings root growth. Seedlings grew on NM for 4 d and were transferred to media containing various concentrations of NPA (A), 2,4-dichlorophenoxyacetic acid (2,4-D; B), 1-naphtalene acetic acid (NAA; C), or ABA (D). Four days later, root growth was measured and plotted as a percentage of root growth on NM. A, Roots of heterozygous nhr1 seedlings were resistant to the growth-inhibiting properties of NPA. The difference was significant at 10 and 50 μm (Student's t test, P = 0.01, P = 0.0055). B, The auxin 2,4-D equally inhibited root growth in both heterozygous nhr1 and wild-type seedlings. C, The growth of heterozygous nhr1 roots showed decreased sensitivity to NAA. The difference was significant at 0.01 and 0.3 μm NAA (Student's t test, P = 0.001, P = 0.02). D, Roots of heterozygous nhr1 seedlings were resistant to the growth-inhibiting properties of ABA. The difference was significant at 1 and 10 μm ABA (Student's t test, P = 0.09, P = 4 × 10−5). Error bars represent the mean ± sd (n = 15). Absence of bar indicates that sd was less than the thickness of the symbol. The graphs are representative of three independent experiments.
The root phenotype of heterozygous nhr1 seedlings was significantly impaired in comparison with wild type when grown in NM (Fig. 7, D and E). In these roots, amyloplasts were larger than in those of the wild type (Fig. 7A) and some columella cells were unusually large (Fig. 7E, asterisk). However, when these mutants were grown in the screening system, their RC morphology was even more affected than in nhr1 homozygous roots (Fig. 7, A–C). Atypical roots were observed in 99% of the seedling progeny of heterozygous nhr 1 mutants. Thin sections were made for heterozygous nhr1 roots grown in the screening system because the confocal images of these roots were of very low resolution. Heterozygous nhr1 roots lacked a typical QC and RC because the closed type of the Arabidopsis RAM was not present (Fig. 7F). These RCs also showed abnormal columella and lateral RC cells and in both cells few amyloplasts were present. The root of this mutant was approximately 65% thicker than in those of wild type. This was because of greater cell thickness, and not because of an increase in the number of cell layers. Interestingly, these roots were capable of growth (Fig. 2B) and were not agravitropic (Fig. 1A). Because RC of heterozygous nhr1 roots had severe anomalies, we suggest that the activity of NHR1 may control the pattern formation in the RC in addition to is role in the perception of water potential gradients as seen in Figures 1A and 3, D through E. Heterozygous nhr1 roots grown in NM showed an enhanced gravity response (Fig. 5A). Amyloplasts constitute the susceptors for gravity perception (Boonsirichai et al., 2002) and because heterozygous nhr1 roots grown in NM contained unusually large amyloplasts in the RC columella (Fig. 7D), their perception and probably their response could be accelerated.
nhr1 Mutation Alters Root Growth Sensitivity to Abscisic Acid (ABA) and NPA
Many agravitropic mutants exhibit altered sensitivities to exogenously applied auxins, which are involved in the cellular growth processes accompanying organ bending and root growth (Boonsirichai et al., 2002). Furthermore, polar auxin transport plays an important role in the control of root gravitropism because mutations in genes directly implicated in this transport also affect root gravitropism (Chen et al., 1998; Lusching et al., 1998; Müller et al., 1998; Marchant et al., 1999; Friml et al., 2002b). These mutations also increase root growth insensitivity to polar auxin transport inhibitors (Chen et al., 1998; Lusching et al., 1998; Müller et al., 1998; Marchant et al., 1999; Friml et al., 2002b). The growth of the heterozygous nhr1 roots was significantly less sensitive to 10 and 50 μm NPA than those of wild type (Fig. 8A). However, this was not correlated with an absence of gravitropic response as in eir1/agr1/wav6/pin2 mutants (Chen et al., 1998; Lusching et al., 1998; Müller et al., 1998; Fig. 5) but with a lack of response to hydrostimulation (Figs. 1A and 3, E and F). Heterozygous nhr1 and wild-type seedlings were then grown on media containing various concentrations of the auxins 2,4-D (Fig. 8B), indole acetic acid (IAA; data not shown), and NAA (Fig. 8C). Heterozygous nhr1 roots responded to 2,4-D (Fig. 8B) and IAA similar to the wild type, indicating that heterozygous nhr1 roots are not altered in response to these auxins. However, heterozygous nhr 1 mutant roots grew significantly faster than those of wild type in the presence of 0.01 and 0.3 μm NAA (Fig. 8C). The membrane-permeable NAA can evade the auxin influx carrier and enter directly to the cell in contrast to both IAA and 2,4-D (Katekar and Geisler, 1977). This lower sensitivity to NAA probably suggests that the nhr1 mutation somehow affects certain aspect of auxin efflux regulation. Moreover, dark-grown nhr1 seedlings failed to maintain an apical hook, as do the auxin-response mutants axr1, tir1, bdl, and hbt (Blilou et al., 2002).
Because interactions between ABA and drought are well documented in Arabidopsis (Zhu et al., 1997), and heterozygous nhr1 roots can grow under water deficit conditions (Fig. 2B), we also examined the effect of exogenous ABA on the growth of heterozygous nhr1 roots. In wild-type plants, application of low concentrations of ABA accelerates root growth, whereas higher concentrations inhibit root development (Abeles et al., 1992). The growth of heterozygous nhr1 roots was less inhibited by increasing concentrations of ABA than those of wild type (Fig. 8D); however, seeds of this mutant were not insensitive to ABA on germination as the ABA-insensitive mutants abi1, abi2, and abi3 (Koornneef et al., 1984; data not shown). We also examined the hydrotropic response of the abi1, abi2, abi3, and aba1 (Koornneef et al., 1982) mutants in our screening system. These mutant roots showed positive hydrotropic response as those of wild type (Fig. 9 in supplemental data available at www.plantphysiol.org). Endogenous ABA concentrations can rise and fall dramatically in response to environmental cues such as water deficit (Bonetta and McCourt, 1998). Thus, if heterozygous nhr1 roots keep on growing under water deficit conditions, they might be impaired in some physiological pathway where ABA acts as a relay between the environment and the RC. Studies on how ABA is distributed, compartmentalized, and metabolized in the root tip upon gravistimulation are scarce. Moreover, some ABA mutants of Arabidopsis are agravitropic (E. Nambara, personal communication) but have not been characterized thoroughly. Our observations suggest that an ABA signaling pathway participates in the positive hydrotropic response of roots. We hypothesized that NHR1 is the first element to interact with ABA sensitiveness, which in turn control and modify the regulation of auxin efflux carriers in response to environmental parameters. This interaction might allow the RC to sense water potential gradients and to cross talk with factors implicated in the polarity of auxin transport, which ultimately determine directional root growth.
To assess whether nhr1 affects NPA, NAA, and ABA responses specifically, we tested root growth responses to other hormones. Heterozygous nhr1 mutant seedlings showed wild-type responses to the cytokinin kinetin and benzyl adenine, to the ethylene precursor 1-aminocyclopropane-1-carboxylic acid, and the GA GA3 (data not shown). These results indicated that apparently ABA responses and auxin efflux carrier regulation are predominantly altered by the nhr1 mutation.
We also tested whether the short roots of homozygous nhr1 mutants might arise from altered hormone responses by growing them in the presence of various exogenous hormones (data nor shown). However, the exogenous addition of different hormones could not stimulate growth of homozygous nhr1 roots or shoots. The only effect seen was the induction of organogenesis in the shoot after the addition of 0.1, 0.44, and 1 μm benzyl amino purine because several new leaves appeared and formed a miniature rosette. Further, both the hypocotyl and root of dark-grown homozygous nhr1 seedlings elongated considerable more than seedlings grown in the light but this growth was also limited (data not shown). It remains to be determined how the shoot apical meristem is affected in the homozygous nhr1 seedlings. The complete postembryonic growth arrest seen in homozygous nhr1 seedlings suggests that the NHR1 gene plays a role in cell proliferation. We are in the process of positional cloning the NHR1 locus. The identification of this gene and its characterization will allow us to understand its role in cell proliferation. Interestingly, the nhr1 mutation in the homozygous condition results in a lethal phenotype (complete postembryonic growth arrest) but with one functional gene copy (semidominant heterozygous condition), seedlings can grow and set seeds. Furthermore, the phenotype of heterozygous nhr1 seedlings suggests that the nhr1 mutation play a role in controlling differential root growth. Root motility is generated through the action of two discrete regions just above and separate from the RC, the RAM, and a region just above the meristem called the region of elongation (Baluska et al., 1996). When plants are supplied with basic needs, new cells are produced within the RAM more or less continuously, and these cells are the cells, which give rise to root growth. New cells derived from the RAM proceed through a transition phase before entering in to a period of rapid elongation. The elongation zone is a small region of the root tip where cell expansion occurs. Cells in the elongation zone are the first ones involved in root responses to several environmental stimuli and in the differential growth that accompanies a gravitropic curvature (Boonsirichai et al., 2002). These two linked but independent activities—cell division and cell elongation—enable root movement. However, the primary site of signal perception leading to directional movement is the RC, not the two tissues (RAM and region of elongation) that actually generate growth (Darwin, 1880; Baluska et al., 1996). Therefore, in the heterozygous nhr1 seedlings one wild-type NHR1 gene copy enables cell proliferation but one nhr1 gene copy resulted in impaired differential root growth (lack of hydrotropism and enhanced gravitropism) and abnormal patterning in the RC. This might suggest that cell proliferation and cell elongation leading to directional movement in the root are coordinately regulated with perception in the RC.
Our study demonstrates that a complex phenomenon such as hydrotropism can be genetically dissected. The isolated heterozygous nhr1 mutants are unique in their defective response to positive hydrotropism. RC morphogenesis in these mutants was disorganized, implying that NHR1 is required for plant roots to sense water and to control pattern formation in the RC. It remains to be shown if the lack of organization of the RC is the cause of the failure in the hydrotropic response or vice versa. Cloning of NHR1 loci and the isolation of new negative hydrotropic mutants will unravel the molecular nature of the mutation and clarify the mechanisms involved in root-positive hydrotropism.
MATERIALS AND METHODS
Plant Growth and Manipulation
Wild-type Arabidopsis seeds of the ecotypes Col and Ws were provided by the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Seeds were surfaced sterilized and kept at 4°C for 4 d. The screening system was prepared by pouring NM (1× Murashige and Skoog salts supplemented with 0.5% [w/v] Suc) in one-half (upper part) of a square plate; an agarose-coated polyester gelBond film (FMC BioProducts, Rockland, ME) was introduced into the dish to separate it in two equal parts. After solidification of the NM, the same medium supplemented with 2.5% (v/v) glycerol and 0.5% (w/v) alginic acid (WSM) was poured into the other one-half (lower part) of the dish. Alginic acid was added to improve medium solidification. Seeds were placed in the upper one-half of the plate, 1.3 cm above the division between both media, and subsequently the piece of gelBond film was removed. Square plates were wrapped in Parafilm, placed in vertical position at 23°C, and subjected to 16-h-d/8-h-night cycles in a growth chamber for 8 to 10 d.
Mutagenesis
Arabidopsis Col ecotype seeds (10,000) were allowed to imbibe overnight in water at room temperature, and then were soaked in 0.3% (v/v) EMS solution for 16 h. Seeds were washed extensively with water and planted in eight independent populations in flats at 1,250 seeds flat−1. M2 seeds were harvested in bulk.
Screening for Arabidopsis Mutants Affected in Positive Hydrotropic Response
Approximately 3,300 Arabidopsis seeds from each of the independent EMS-mutagenized eight populations (a total of 26,400 seeds) were sterilized and plated in the screening medium described above for 8 to 10 d. Mutants without tip curvature up to the 10th d that continued to grow toward the WSM were selected. Seedlings that showed no hydrotropic response were transferred to soil and allowed to self-fertilize for four generations. The nhr1 mutant was characterized genetically and physiologically after backcrossing to wild type at least three times to remove unlinked mutations.
Water Potential Analysis of the Screening System
The water potential of the screening system was measured at time zero and every 24 h for 8 d in different sections of the plate by the dew point psychometric combined method. The HR-337 Dew Point Microvoltimeter (Wescor Inc., Logan, UT) was used.
Assay for Hydrotropism in Arabidopsis Roots with an Air Moisture Gradient
For the hydrotropic assay, 7-d-old wild-type and heterozygous nhr1 seedlings grown in NM were transferred to a microscope slide containing a 2-mm layer of NM. The slide was placed vertically in a square petri dish next to the water source (a piece of 2.5- × 1.4-cm oasis [Hummert International, St. Louis] saturated with 3 mL of water) and a saturated solution of calcium chloride (3 mL) in a plastic cuvette placed in the bottom of the dish. We used a saturated solution of calcium chloride for creating a gradient in air moisture as reported by Takahashi and Scott (1991). The seedling was placed horizontally onto the microscope slide leaving 2 mm of the root tip in the air. The distance between the water source and the root tip was approximately 2.5 to 3.2 mm. The dish was sealed with Parafilm and maintained in a vertical position for 48 h. An air moisture gradient thus was created between the oasis and the cuvette. All manipulations were done in the presence of a room humidifier. Root growth rate was measured with the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Photographs were taken at the indicated times.
Analysis of Root Growth in the Presence or Absence of Hormones and Auxin Polar Transport Inhibitors
Wild-type and heterozygous nhr1 mutant seedlings were grown on vertically oriented NM plates for 4 d in a growth chamber. After 4 d of growth, seedlings were carefully transferred (to avoid damage of the roots) onto vertically oriented NM plates supplemented with 2,4-D, NAA, ABA, and NPA cited in the figure at the indicated concentrations. Heterozygous nhr1 seedlings were selected from those of wild type because of their wavy-like root growth in NM. Auxins (2,4-D, IAA, and NAA) and an auxin polar transport inhibitor (NPA) were purchased from Sigma (St. Louis) and Chem Service (West Chester, PA), prepared as 10 mm stock solutions in diluted NaOH/ethanol, and added to the medium at the concentrations defined in the text. Plates were incubated in the growth chamber for 5 d, with pictures taken every 12 h. Root length data were standardized against lengths on unsupplemented medium. Root lengths were measured using digital image analysis and statistically analyzed. We used a Morpho Expert image analyzer (Explora Nova, La Rochelle, France), with a monochrome CCD camera (COHU 4815, Jessop Canon UK Limited, Neasden, London) and a macrolens (FD 50 mm, 1:3.5, Canon, Tochigi, Japan) attached to a video wide-angle 0.5× high-resolution lens (Jessop, London). The plates were trans-illuminated in a macrostand (AMS, London).
Root Gravitropism and Wavy Growth Pattern Examination in the nhr1 Mutant
For the gravitropic response experiments, plates were also kept in a vertical position. Wild-type seedlings were grown in NM at 90o either on the surface of 0.9% (w/v) agar NM or submerged within the medium for 5 d, and then manually rotated in a clockwise direction so that the roots were parallel to the surface of the Earth. Heterozygous nhr1 seedlings were selected from the screening system after 5 d, then transferred to a NM plate and manually rotated as described above. The direction of new root growth was analyzed using digital image analysis every 2 h. Then, plates were reoriented to the vertical (initial) position and the recovery of root growth directions was recorded after 24 h. Root elongation was assayed at 4 d after germination. For the waving pattern, wild-type seedlings were grown on plates containing 0.5× Murashige and Skoog medium, plus 1% (w/v) Suc and 1.5% (w/v) agar, and placed in a vertical position for 7 d in a growth chamber (23°C with 16 h of light). Heterozygous nhr1 seedlings were selected from the screening system after 7 d and then transferred to plates containing the medium described above. Then, the plates were tilted to 45o and the seedlings were grown for 3 d and were photographed as described by Okada and Shimura (1990).
Confocal Imaging of nhr1 Mutant Root Tips
For microscopic examination, 5- to 8-d-old Arabidopsis seedlings were stained with propidium iodide (PI; 10 μg mL−1) for 10 min and then were observed with an MRC-600 laser scanning confocal microscope (Bio-Rad Laboratories, Hercules, CA) or bright-field microscopy using a 63× C-Apochromat W Korr (1.2 numerical aperture and 0.25 water difraction) water immersion objective (Zeiss, Jena, Germany). Seedlings grown in screening system when stained with PI had very low apoplast penetration and low contrast images were obtained. To improve image contrast, we used PI supplemented with 1% to 2% (w/v) glycerol. This staining still gave less contrasted images than seedlings grown in NM. Selection of heterozygous nhr1 seedlings grown in NM was based upon their wavy-like root growth phenotype, and those grown in the screening system because of their lack of hydrotropic response.
Determination of Genetic Map Position of the nhr1 Mutation
Genetic mapping was done according to Lukowitz et al. (2000). Mapping populations were generated by manually crossing heterozygous nhr1 mutants (selected from the screening system) to the Ws ecotype. F1 plants were allowed to self-pollinate and set seed. Tissue from homozygous F2 plants was collected for DNA isolation. DNA pools were bulked and used to assign a rough position on the genetic map by identifying linked genetic markers (a set of SSLP markers spaced at regular intervals throughout the genome). For more fine-scale mapping, DNA was prepared from individual F2 plants (62 plants, 124 chromosomes) and recombination frequencies were obtained with flanking SSLP markers from the region.
Supplementary Material
ACKNOWLEDGMENTS
We warmly thank Lewis Feldman, Patrick Masson, Jorge Nieto-Sotelo, and two anonymous reviewers for critically reviewing the manuscript; Stewart Guillmor for valuable suggestions on the mapping of nhr1; Juan Manuel Hurtado for helpful computer support; Xóchitl Alvarado for marvelous help with the confocal microscope; and Natasha Doktor, Yoloxóchitl Sánchez, and Manuel Saucedo for wonderful help with figures.
Footnotes
This research was supported by the Mexican Council for Science and Technology (grant nos. CONACYT 25186N and 36071N), by the Universidad Nacional Autónoma de México (Dirección General de Asuntos del Personal Académico grant no. IN208999), by University of California (Mexus), and by DeGAPA IN204496 (scholarships to M.L.B. and D.E.).
The online version of this article contains Web-only data. The supplemental material is available at www.plantphysiol.org.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011841.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME., Jr . Ethylene in Plant Biology. San Diego: Academic Press; 1992. [Google Scholar]
- Baluska F, Volkmann D, Barlow PW. Specialized zones of development in roots. View from the cellular level. Plant Physiol. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SF, Rost TL. Root apical organization in Arabidopsis thaliana: I. Root cap and protoderm. Protoplasma. 1996;192:178–188. [Google Scholar]
- Blilou I, Frugier F, Folmer S, Serralbo O, Willemsen V, Wolkenfelt H, Eloy NB, Ferreira PCG, Weisbeek P, Scheres B. The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev. 2002;16:2566–2575. doi: 10.1101/gad.237302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetta D, McCourt P. Genetic analysis of ABA signal transduction pathways. Trends Plant Sci. 1998;3:231–235. [Google Scholar]
- Boonsirichai K, Guan C, Chen R, Masson PH. Root gravitropism: an experimental tool to investigate basic cellular and molecular process underlying mechanosensing and signal transmission in plants. Annu Rev Plant Biol. 2002;53:421–447. doi: 10.1146/annurev.arplant.53.100301.135158. [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis AGRAVITROPIC 1 gene encodes a component of the polar-auxin transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The Power of Movement in Plants. New York: Da Capo Press; 1881. [Google Scholar]
- Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002a;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002b;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Hart JW. Plant Tropisms and Growth Movements. London: Unwin Hyman; 1990. pp. 176–202. [Google Scholar]
- Hawes MC, Bengough G, Cassab GI (2002) Root caps and rhizosphere. J Plant Growth Regul (in press)
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–132. doi: 10.1016/s1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- Jaffe MJ, Takahashi H, Biro RL. A pea mutant for the study of hydrotropism in roots. Science. 1985;230:445–447. doi: 10.1126/science.230.4724.445. [DOI] [PubMed] [Google Scholar]
- Katekar GF, Geisler AE. Auxin transport inhibitors. Plant Physiol. 1977;60:826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karseed CM. The isolation of abscisic acid (ABA)-deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible W-R. Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol. 2000;123:795–805. doi: 10.1104/pp.123.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusching C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Müller P, Delbarré A, Perrot-Rechenmann C, Bennett MJ. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio F, Piconese S. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6:561–565. doi: 10.1016/s1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- Mullen JL, Turk E, Johnson K, Wolverton C, Ishikawa H, Simmons C, Söll D, Evans ML. Root growth behavior of the Arabidopsis mutant rgr1. Plant Physiol. 1998;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 2. 1990;50:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- Rutherford R, Masson PH. Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiol. 1996;111:987–998. doi: 10.1104/pp.111.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamay J, Benfey P, Leyser O, Bechtold N, Weisbeek P et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Scheres B, Di Laurenzio L, Willemsen V, Hauser M-T, Janmaat K, Weisbeek P, Benfey PN. Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121:53–62. [Google Scholar]
- Simmons C, Migliaccio F, Masson PH, Caspar T, Söll D. Circumnutation and gravitropism cause root waving in Arabidopsis thaliana. J Exp Bot. 1995;46:143–150. [Google Scholar]
- Takahashi H, Brown CS, Dreschel TW, Scott TK. Hydrotropism in pea roots in a porous-tube water delivery system. HortScience. 1992a;27:430–432. [PubMed] [Google Scholar]
- Takahashi H, Scott TK, Suge H. Stimulation of root elongation and curvature by calcium. Plant Physiol. 1992b;98:246–252. doi: 10.1104/pp.98.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Scott TK. Hydrotropism and its interaction with gravitropism in maize roots. Plant Physiol. 1991;96:558–564. doi: 10.1104/pp.96.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Suge H. Root hydrotropism of an agravitropic pea mutant, ageotropum. Physiol Plant. 1991;82:24–31. doi: 10.1093/oxfordjournals.pcp.a029015. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Takano M, Fuji N, Yamashita M, Suge H. Induction of hydrotropism in clino-rotated seedling roots of Alaska pea, Pisum sativum L. J Plant Res. 1996;109:335–337. doi: 10.1007/BF02344481. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Hydrotropism: the current state of our knowledge. J Plant Res. 1997;110:163–169. doi: 10.1007/BF02509304. [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Tsugeki R, Federoff NV. Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:12941–12946. doi: 10.1073/pnas.96.22.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld J-P, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE 2 gene defines a glutathione-dependent pathway involve in initiation and maintenance of cell division during post-embryonic root development. Plant Cell. 2000;12:97–109. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen V, Wolkenfelt H, Vrieze, G de, Weisbeek P, Scheres B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 1998;125:521–531. doi: 10.1242/dev.125.3.521. [DOI] [PubMed] [Google Scholar]
- Zhu JK, Hasegawa PM, Bressan RA. Molecular aspects of osmotic stress in plants. Crit Rev Plant Sci. 1997;16:253–277. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.