Abstract
Zinc (Zn) is an essential micronutrient for plants. The ability of plants to maintain significant yields under low Zn is termed Zn efficiency (ZE) and its genetic and mechanistic basis is still not well understood. Previously, we showed that root Zn uptake did not play a role in ZE. In the current study, Zn-efficient and -inefficient wheat (Triticum aestivum) genotypes were grown for 13 d in chelate buffer nutrient solutions at low (0.1 pm), sufficient (150 pm), and high (1 μm) Zn2+ activities and analyzed for root-to-shoot translocation of Zn, subcellular leaf Zn distribution, and activity and expression of the Zn-requiring enzymes in leaves. No correlation between ZE and Zn translocation to the shoot was found. Furthermore, total and water-soluble concentrations of leaf Zn were not associated with ZE, and no differences in subcellular Zn compartmentation were found between Zn-efficient and -inefficient genotypes. However, the expression and activity of the Zn-requiring enzymes copper (Cu)/Zn superoxide dismutase (SOD) and carbonic anhydrase did correlate with differences in ZE. Northern analysis suggested that Cu/ZnSOD gene expression was up-regulated in the Zn-efficient genotype, Kirgiz, but not in inefficient BDME. Under Zn deficiency stress, the very Zn-efficient genotype Kirgiz and moderately Zn-efficient Dagdas exhibited an increased activity of Cu/ZnSOD and carbonic anhydrase when compared with Zn-inefficient BDME. These results suggest that Zn-efficient genotypes may be able to maintain the functioning of Zn-requiring enzymes under low Zn conditions; thus, biochemical Zn utilization may be an important component of ZE in wheat.
Crop yields are often limited by low soil levels of mineral micronutrients such as zinc (Zn), especially in calcareous soils of arid and semiarid regions (Graham et al., 1992; Cakmak et al., 1999). There is significant genetic variation both within and between plant species in their ability to maintain significant growth and yield under Zn deficiency conditions; this has been termed Zn efficiency (ZE; Graham and Rengel, 1993). Differences in ZE have been demonstrated particularly for cereal species in both field and greenhouse experiments (Graham et al., 1992; Kalayci et al., 1999). In recent years, research has been carried out in several different laboratories to elucidate the physiological mechanisms that confer ZE; however, these mechanisms are still poorly understood. A number of different wheat (Triticum aestivum) genotypes have been screened for their response to low Zn in Zn-deficient calcareous soils and significant differences in ZE among certain wheat genotypes have been consistently found in both field and growth chamber experiments (Cakmak et al., 1999; Kalayci et al., 1999; Hacisalihoglu et al., 2001).
We recently conducted a detailed characterization of root Zn2+ influx in wheat genotypes differing in ZE (Hacisalihoglu et al., 2001). The presence of two Zn transport systems mediating high-affinity (Km = 0.6–2 nm) and low-affinity (Km = 2–5 μm) uptake was demonstrated. However, no significant differences in root Zn2+ uptake between the efficient and inefficient bread wheat genotypes were found. These findings were similar to those previously reported by Erenoglu et al. (1999) for different bread wheat genotypes. In addition, because it often is speculated that phytosiderophores may play a role in root Zn uptake, it has been shown that root phytosiderophore release from bread wheat genotypes differing in ZE did not correlate with ZE (Cakmak et al., 1998). Finally, results from several laboratories have shown that when Zn-efficient and -inefficient wheat cultivars are grown under low Zn conditions that produce Zn deficiency symptoms only in the inefficient genotypes, no significant differences in leaf and shoot Zn concentrations are found (Rengel and Graham, 1995; Cakmak et al., 1999; Hacisalihoglu et al., 2001). All of these findings indicate that root Zn uptake is not a major determinant of ZE.
Zn is an essential mineral nutrient and a cofactor of over 300 enzymes and proteins involved in cell division, nucleic acid metabolism, and protein synthesis (Marschner, 1986). There are several well-known Zn-requiring enzymes that have been studied in plants. Copper (Cu)/Zn superoxide dismutase (SOD) plays an important role in protecting plants against oxidative damage catalyzed by reactive oxygen species (Marschner and Cakmak, 1989). Because Zn is directly involved in both gene expression and protein synthesis, Cakmak (2000) has speculated that Zn deficiency stress may inhibit the activities of a number of antioxidant enzymes, resulting in extensive oxidative damage to membrane lipids, proteins, chlorophyll, and nucleic acids. A second well-characterized Zn-requiring enzyme is carbonic anhydrase (CA); in fact, it has been suggested that CA activity could be used as an indicator for diagnosing Zn deficiency in plants (Bar-Akiva and Lavon, 1969). Zn deficiency induces a decrease in the activity of CA, especially in Zn-inefficient durum wheat genotypes (Rengel, 1995). In a study with rice (Oryza sativa) plants, Sasaki et al. (1998) found that the level of CA mRNA decreased under Zn deficiency.
In the present study, we employed several different experimental approaches with Zn-efficient and -inefficient wheat genotypes to gain insight into possible physiological mechanisms of ZE. Because it is possible that more efficient Zn transport to the shoot under low Zn conditions could be involved in ZE, this was one of the processes studied. Second, because ZE could involve altered cellular Zn compartmentation in the leaf such that Zn-efficient cultivars could maintain higher cytoplasmic Zn levels under low Zn conditions, this was also studied. Finally, the role of biochemical Zn utilization was examined by studying the expression and activity of Zn-requiring enzymes. The findings presented here indicate that the ability of Zn-efficient genotypes to maintain higher activity of Zn-requiring enzymes in the face of Zn deficiency is correlated with ZE.
RESULTS
Leaf Symptoms and Shoot Zn Concentrations
When grown under low Zn conditions (0.1 pm), wheat cv BDME exhibited stunted growth, with small leaves and considerable leaf necrosis, whereas wheat cv Dagdas and cv Kirgiz showed normal growth with no Zn deficiency symptoms on the leaves.
The chemical composition of the xylem sap was determined for Zn-efficient and -inefficient cultivars grown under the low Zn conditions that generated the differences in Zn deficiency symptoms described above to compare root-to-shoot Zn translocation between the genotypes. Xylem sap of Zn-deficient plants for all three genotypes showed similar Zn concentrations ranging from 0.71 to 0.85 μg g−1 (Table I). Furthermore, no difference between the efficient and inefficient genotypes was found with regard to Zn concentrations in leaves (Table I). In fact, the inefficient wheat cv BDME maintained somewhat higher Zn concentrations in the shoot than did the Zn-efficient genotypes.
Table I.
The Zn concentrations in 13-d-old wheat genotypes growing in a nutrient solution with 0.1 pm Zn2+ activity
| Genotype | Phenotype | Severity of Leaf Symptoms | [Zn] in Xylem | Total [Zn] in Shoots | [Zn] in Apoplast | [Zn] in Cell Walls | Soluble [Zn] in Leaves | [Zn] in Membranes |
|---|---|---|---|---|---|---|---|---|
| μg g−1 | μg g−1 fresh wt | μg g−1 apoplastic fluid | μg g−1 cell wt | μg g−1 fresh wt | μg mg−1 protein | |||
| BDME | Inefficient | Severe | 0.71 (0.19) | 1.71 (1.04) | 14.9 (3.02) | 11.7 (2.44) | 0.64 (0.05) | 32.3 (6.81) |
| Dagdas | Efficient | Slight | 0.85 (0.34) | 1.01 (0.13) | 12.5 (4.61) | 3.35 (0.16) | 0.47 (0.01) | 15.9 (0.11) |
| Kirgiz | Efficient | Absent | 0.74 (0.13) | 1.16 (0.11) | 19.01 (8.77) | 11.9 (5.89) | 0.56 (0.11) | 28.9 (2.41) |
Nos. in the parentheses represent se values. Each value is the mean of at least three replicates.
Cellular Distribution of Zn
The Zn concentrations in several different cell fractions isolated from leaves of Zn-deficient plants are summarized in Table I. As seen in Table I, there were no differences between the genotypes in apoplastic and cell wall Zn, total soluble Zn extracted from the leaves, and membrane-associated Zn that could consistently account for differences in ZE.
Leaf 65Zn2+ Compartmental Analysis
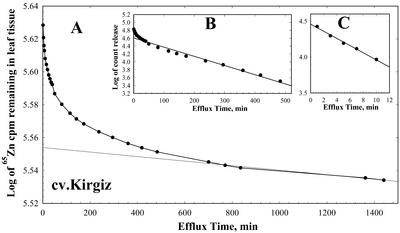
The cellular compartmentation of leaf Zn was also studied by conducting radiotracer (65Zn) efflux studies in leaves loaded with 65Zn for long periods to achieve a pseudo-steady state for 65Zn labeling of the major cellular compartments (cell wall, cytoplasm, and vacuole). Leaves of young plants in which Zn deficiency symptoms had not yet appeared were used in an attempt to minimize variation in cell size, a concern raised by Bell et al. (1994). Figure 1 illustrates a graphical representation of the data from a typical 65Zn efflux experiment for leaves of wheat cv Kirgiz. The curves were analyzed as described by Bell et al. (1994). The total efflux curve (Fig. 1) was dissected into three components, representing vacuole (slow efflux rate, Fig. 1A), cytoplasm (intermediate efflux rate, Fig. 1B), and cell wall (fast efflux rate, Fig. 1C). The slope and y axis intercepts of each line were used to calculate half-times of exchange (t1/2) and apparent Zn content (%) at the end of the loading period, respectively (Table II).
Figure 1.
A representative semilogarithmic plot of the amount of 65Zn remaining in leaf tissue versus time of efflux. The linear component in A, which represents vacuolar Zn efflux, was subtracted from the data points in A to obtain the points shown in B, which represent cytoplasmic Zn efflux. A similar procedure was used to derive the points in C, which represent cell wall Zn efflux, from the curve in B. Lines represent regression of the linear portion of each curve and were extrapolated to the y axis. Data points in A represent means ± se of four replicates.
Table II.
Zn content and t1/2 of efflux in leaves of wheat genotypes grown under low-Zn (0.1 pm) conditions
| Genotypes | Vacuole
|
Cytoplasm
|
Cell Wall
|
|||
|---|---|---|---|---|---|---|
| Zn | t1/2 | Zn | t1/2 | Zn | t1/2 | |
| % | h | % | min | % | min | |
| BDME | 83.3 (0.25) | 193 (41.6) | 10.8 (0.25) | 106 (15.9) | 6.01 (0.41) | 6.76 (2.31) |
| Dagdas | 82.3 (2.29) | 289 (44.1) | 12.3 (2.14) | 89.7 (6.03) | 5.25 (0.25) | 3.96 (0.66) |
| Kirgiz | 84.5 (0.51) | 388 (23.3) | 9.01 (0.01) | 131 (2.01) | 6.51 (0.51) | 6.01 (0.01) |
Nos. in parentheses represent se values.
Efflux curves for all three genotypes yielded similar kinetics with similar apparent Zn content for the vacuole and cytoplasm (Table II). These data suggest that there are no major differences in Zn compartmentation in leaves between efficient and inefficient genotypes. It was interesting to note that the half-time for vacuolar exchange of Zn was greater for the Zn-efficient genotypes (Kirgiz and Dagdas) compared with Zn-inefficient BDME (although the difference was statistically significant only for Kirgiz compared with BDME). It is not clear whether this could play a role in ZE because the findings suggest that the efficient genotypes would tend to retain Zn in the vacuole more effectively than in the inefficient genotype. No differences were found among half-time values for the genotypes when grown on adequate levels of Zn (data not shown).
Expression of Genes Encoding Zn-Requiring Enzymes
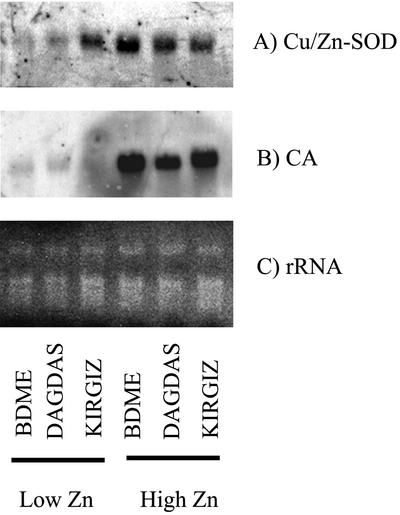
Northern-blot analysis was conducted for both SOD1.1 and CA genes with total RNA and mRNA isolated from leaf tissue for all three genotypes grown under low, sufficient, and high Zn levels. It was found that SOD1.1 and CA were expressed in shoots of all three genotypes, but not in root tissues (data not shown). Analysis of gel blots loaded with total RNA revealed no significant differences in the expression of SOD1.1 and CA among efficient and inefficient genotypes (Fig. 2). Transcripts of both genes were detected in shoots of all three genotypes grown under Zn-sufficient conditions (150 pm Zn) but not in shoots of Zn-deficient seedlings (grown on 0.1 pm Zn; Fig. 2). It was also found that when plants were grown on an excess level of Zn (1 μm Zn), expression of both genes increased over that seen in Zn-sufficient seedlings (data not shown).
Figure 2.
Expression pattern of Cu/ZNSOD [SOD1.1] (A) and CA transcripts (B). Wheat total RNA was isolated from shoots of wheat cv BDME, cv Dagdas, and cv Kirgiz grown in low-Zn (0.1 pm) or sufficient Zn (150 pm) medium. The northern blot was equally loaded with 15 μg of total RNA per lane. C, Ethidium bromide-stained rRNA is shown as a loading control. Filters were hybridized with radiolabeled ([α-32P]dCTP) SOD1.1 or CA probes overnight, washed under high-stringency conditions, and exposed to x-ray film, as described in “Materials and Methods.” Similar results were obtained in three independent experiments.
Subsequently, the RNA blots were repeated using mRNA isolated from leaves of the three genotypes to study SOD1.1 and CA gene expression patterns in more detail, specifically in Zn-deficient plants (Fig. 3). SOD1.1 was more highly expressed in Kirgiz and Dagdas than in BDME shoots in low Zn-grown plants, and SOD1.1 expression in the very Zn-efficient Kirgiz was more pronounced than in moderately Zn-efficient Dagdas (Fig. 3A). In the case of CA, it was not possible to detect differences in expression in the three genotypes grown under Zn-deficient conditions. As was the case for SOD1.1, CA expression was much greater in shoots of high Zn-grown plants (Fig. 3B).
Figure 3.
Expression analysis of SOD1.1 (A) and CA (B) transcripts. Wheat poly(A+) mRNA was directly isolated from shoots of wheat cv BDME, cv Dagdas, and cv Kirgiz grown in low Zn (0.1 pm) or high Zn (1 μm) medium. Equal amounts of mRNA (2.5 μg) were loaded per lane. C, Ethidium bromide-stained rRNA band included to show RNA loading. Filters were hybridized with radiolabeled ([α-32P]dCTP) SOD1.1 or CA probes overnight, washed under high-stringency conditions, and exposed to x-ray film, as described in “Materials and Methods.” Each experiment was repeated at least three times with similar results.
Enzyme Activities
SODs
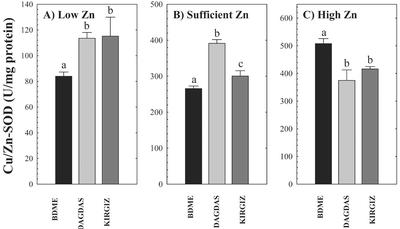
Zn-efficient and -inefficient wheat genotypes were examined for any relationship between SOD activity and ZE under low, sufficient, and high Zn supply conditions (Fig. 4). Activities of total SOD, Mn-SOD, and Cu/ZnSOD were measured in the leaves of three wheat genotypes differing in ZE. Because the Zn status of plants was increased due to growth on higher levels of Zn, the activity of total SOD (not shown) and Cu/ZnSOD activity increased in both efficient and inefficient plants. Compared with its activity in low Zn-grown plants, Cu/ZnSOD activity increased 3- to 4-fold in plants supplied with sufficient and high Zn, respectively (Fig. 4, A–C). In low Zn-grown plants, the more Zn-efficient wheat cultivars Dagdas and Kirgiz maintained about a 50% higher Cu/ZnSOD activity compared with Zn-inefficient BDME (Fig. 4A). It is interesting to note that at high Zn supply, this trend revered in that Cu/ZnSOD activity was about 25% higher in BDME compared with the efficient genotypes.
Figure 4.
Cu/ZnSOD activity in three wheat genotypes (cv BDME, cv Dagdas, and cv Kirgiz) grown under three Zn regimes: A, low Zn (0.1 pm); B, sufficient Zn (150 pm); and C, high Zn (1 μm) for 13 d. Each value represents the mean of four independent measurements. Error bars indicate se values. Means followed by different letters are significantly different at P ≤ 0.05 (Student's t test).
Activity of CA
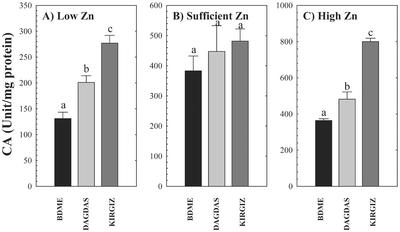
Activity of CA was also correlated with differences in ZE (Fig. 5). In low Zn-grown seedlings, CA activity was significantly lower in the Zn-inefficient genotype compared with the efficient genotypes. The activity of CA was 50% and 100% higher in moderately Zn-efficient Dagdas and very Zn-efficient Kirgiz, respectively, compared with Zn-inefficient BDME under Zn deficiency (Fig. 5). Similar to the response of SOD, exposing plants to sufficient or high Zn supply resulted in increasingly higher CA activity in the leaves of all three genotypes when compared with low Zn-grown plants (Fig. 5, B and C). In low and high Zn-grown plants, the inefficient genotype BDME had the lowest CA activity and Kirgiz exhibited the highest CA activity. Dagdas was intermediate between the other two genotypes.
Figure 5.
CA activity in three wheat genotypes (cv BDME, cv Dagdas, and cv Kirgiz) grown under three Zn regimes: A, low Zn (0.1 pm); B, sufficient Zn (150 pm); and C, high Zn (1 μm) for 13 d. Each value represents the mean of four independent measurements. Error bars indicate se values. Means followed by different letters are significantly different at P ≤ 0.05 (Student's t test).
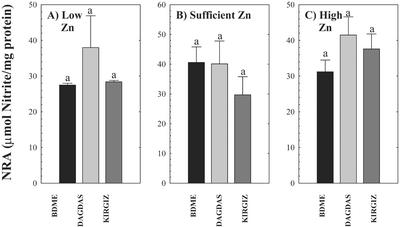
Activity of Nitrate Reductase (NR)
NR activity was determined as a representative non-Zn-requiring enzyme in the leaves of the three wheat genotypes grown under the different Zn regimes. At all three Zn levels, the wheat genotypes exhibited similar NR activities (Fig. 6). The differences in NR activities between Zn-inefficient BDME and Zn-efficient Dagdas and Kirgiz were not significantly different, suggesting that the differences in SOD and CA activity were not a general response of all enzymes (Fig. 6, A–C).
Figure 6.
NR activity in three wheat genotypes (cv BDME, cv Dagdas, and cv Kirgiz) grown under three Zn regimes: A, low Zn (0.1 pm); B, sufficient Zn (150 pm); and C, high Zn (1 μm) for 13 d. Each value represents the mean of four independent measurements. Error bars indicate se values. Means followed by different letters are significantly different at P ≤ 0.05 (Student's t test). The presence of the same letter indicates the absence of significant differences among genotypes.
DISCUSSION
In this study, we investigated physiological and biochemical mechanisms that may be related to the differential ZE expressed in the three bread wheat genotypes. Several experimental approaches were taken to elucidate ZE mechanisms. Particular attention has been paid previously to root Zn uptake and root-to-shoot translocation of Zn (Rengel and Graham, 1995; Erenoglu et al., 1999). In a recent study, we characterized uptake of Zn2+ in wheat roots. The short-term Zn2+ influx experiments that quantified unidirectional Zn2+ influx across the root cell plasma membrane revealed the presence of two separate transport systems mediating high- and low-affinity Zn influx. However, the results demonstrated that the uptake of Zn by roots was similar among the wheat genotypes differing in ZE, suggesting that Zn uptake does not confer ZE in wheat (Hacisalihoglu et al., 2001).
Alternatively, ZE mechanisms might be related to mobility and distribution of Zn within the leaf tissues. In the current study, we found that in low Zn-grown plants, Zn translocation to leaves and total leaf Zn concentrations were similar between Zn-efficient and -inefficient genotypes (Table I). This result is in good accordance with previous results showing a lack of correlation between total leaf Zn concentration and ZE (Cakmak et al., 1997). Together with these previous findings, it can be concluded that Zn translocation and accumulation in leaves are probably not involved in expression of high ZE in wheat.
Subcellular compartmentation of Zn was also examined as a candidate ZE mechanism. We tested the hypothesis that ZE is related to a decreased Zn sequestration in leaf vacuoles, providing more Zn for biochemical processes in the cytoplasm. The results presented in Table II do not, however, support this hypothesis. Zn-efficient and -inefficient wheat genotypes were not different in compartmentation of Zn between the cytoplasm and vacuole. The Zn compartmentation values calculated from the analysis of the Zn efflux experiments were in good agreement with values obtained previously by Santa Maria and Cogliatti (1988) in wheat. Those researchers showed that the proportion of Zn was 8% to 14% in the apoplasm, 8% in the cytoplasm, and 76% in the vacuole. Interestingly, the Zn-inefficient wheat cv BDME exchanged Zn from vacuoles rather rapidly, with a half-time for Zn exchange of 193 h compared with 289 and 388 h for Dagdas and Kirgiz, respectively (Table II). These results suggest that both efficient and inefficient genotypes maintain a fairly constant cytoplasmic Zn level (9%–12% of total tissue Zn). Based on these results, it can be hypothesized that genotypic differences in ZE are not associated with the differences in Zn allocation between subcellular compartments in leaf cells.
To further test for differences in subcellular compartmentation, we separated cellular components by differential centrifugation and measured the amounts of Zn associated with different subcellular compartments or components (cell wall, membrane-associated Zn, and soluble Zn [as a crude measure of symplastic Zn]). Measurements taken with low Zn-grown plants showed that Zn-efficient and -inefficient wheat genotypes displayed no consistent differences that correlated with ZE. These results agree with the Zn efflux findings (Table II) and suggest that subcellular localization of Zn is not the primary physiological mechanism that confers ZE. However, the results presented here do not rule out the possibility that subcellular compartment(s) unresolved by our methodology could affect Zn availability and functional activity of Zn-requiring enzymes.
The substantial genotypic variation in the severity of Zn deficiency symptoms and ZE coupled with the very similar rates of root Zn uptake and translocation of Zn in efficient and inefficient genotypes suggest that another process, such as biochemical utilization of Zn, may be important in conferring ZE in wheat. To test this hypothesis, we examined the expression of genes encoding the Zn-requiring enzymes Cu/ZnSOD and CA in these contrasting wheat genotypes in response to Zn deficiency. In general, the abundance of transcripts encoding both enzymes was up-regulated with elevated tissue Zn levels. This is consistent with previous findings, which reported a decrease in CA expression in Zn-deficient rice plants (Sasaki et al., 1998). In shoot tissue from plants grown under Zn-deficient conditions, we found that the expression of SOD1.1 was up-regulated in the very Zn-efficient Kirgiz compared with Zn-inefficient BDME (Fig. 3A). Although these findings are not definitive, because we did not detect higher SOD expression in the moderately Zn-efficient cv Dagdas, it is possible that regulation of expression of Zn-requiring enzymes by plant Zn status may be one component of ZE in wheat. The apparent absence of higher levels of expression of CA in the Zn-efficient cultivars suggests that the higher CA activity seen in these cultivars may involve posttranscriptional regulation of this enzyme in relation to plant Zn status.
The significantly lower activity of the Cu/ZnSOD enzyme in the Zn-inefficient genotype under Zn deficiency conditions (Fig. 4) suggests that ZE might also be related to activity of this enzyme. Previous reports also showed a positive correlation between Cu/ZnSOD activity and ZE among and within cereal species (Cakmak et al., 1997; Yu et al., 1999). A similar pattern was also found with the other Zn-containing enzyme studied, CA (Fig. 5). Previously, higher CA activity in a Zn-efficient bread wheat compared with a Zn-inefficient durum wheat has been reported (Rengel, 1995).
The differential effects of Zn deficiency on the activity of SOD and CA in wheat genotypes seem to be specific because the activity of a non-Zn-containing enzyme, NR, was not affected by Zn deficiency (Fig. 6). Irrespective of their differential ZE, all three wheat genotypes showed more or less similar activities of NR under Zn-deficient and -sufficient conditions. This result supports the hypothesis that the genotypic variation in the expression and activity of Zn-requiring enzymes is closely related to ZE.
Taken together, we have presented evidence that there is a correlation between the expression and activities of Zn-requiring enzymes and ZE in wheat. Previous work (Hacisalihoglu et al., 2001) showed that there is no correlation between ZE and root Zn uptake and there do not appear to be any correlations between ZE and Zn compartmentation or xylem translocation in wheat. It is interesting to note that although both Kirgiz and Dagdas are classified as Zn efficient, Kirgiz has been found to be more Zn efficient than Dagdas in field studies (I. Cakmak, unpublished data). This difference in ZE correlates with higher levels of SOD gene expression and higher levels of CA enzyme activity under Zn-deficient conditions in Kirgiz (Figs. 3 and 5) We propose that the greater activities of SOD and CA in Zn-efficient genotypes under Zn-deficient conditions may be representative of a more general response that allows for more efficient biochemical utilization of cytoplasmic Zn in efficient genotypes, and this may be an important contributor to the Zn-efficient phenotype in wheat.
MATERIALS AND METHODS
Plant Growth and Analysis
Three genotypes of wheat (Triticum aestivum; BDME, Dagdas, and Kirgiz) were used in the experiments. These genotypes differ in their ZE when grown in Zn-deficient calcareous soils under field conditions as BDME a Zn-inefficient cultivar; Kirgiz, a Zn-efficient cultivar; and Dagdas, a moderately Zn-efficient cultivar (Kalayci et al., 1999). Seeds were germinated and grown hydroponically under low Zn2+ (0.1 pm), sufficient Zn2+ (150 pm), and high Zn2+ (1 μM), conditions, in chelate-buffered solution culture as described elsewhere (Hacisalihoglu et al., 2001). Chemical speciation of all compounds in the nutrient solutions was calculated using GEOCHEM-PC (Parker et al., 1995). Plants were grown in a growth chamber under controlled climatic conditions with a 400 μmol m−2 s−1 photon flux density and 20°C/15°C (16/8 h) day/night temperature. Plants were harvested after growing in the different Zn treatments for 13 d. Shoots were oven dried at 65°C for 4 d, weighed, digested, and analyzed for Zn content using inductively coupled (ICP) argon-plasma emission spectrometry (ICP 61E trace analyzer, Thermo-Jarrel Ashe, Franklin, MA) as described previously (Hacisalihoglu et al., 2001).
Xylem Sap Analysis
Plants were decapitated just below the first leaf node with a razor blade. A silicon tube was inserted over the decapitated stem and sealed, and xylem sap exuded over a 24-h period was collected and analyzed via ICP.
Apoplastic Fluid Analysis
A centrifugal method for extracting apoplastic sap from leaves was used (Mimura et al., 1996). Leaves were cut, placed in a 60-mL syringe, and infiltrated with a solution containing 0.1 m sorbitol and 1 mm CaCl2 until the whole infiltrated leaves became darker in color (about 90 s). After blotting the surfaces, leaves were placed in a double-layered tube (50 mL) with all cut ends oriented toward the bottom of the tube and centrifuged for 2 min at 1,000g. The apoplastic fluid was collected at the bottom of the tube, analyzed for Zn with ICP spectrometry, and the concentrations were calculated as described by Mimura et al. (1996). Assumptions about the volume of apoplastic space were as described by Mimura et al. (1996).
Cell Fractionation
A step-wise centrifugation method was used to separate cellular components based on their density. Leaves (0.6 g) were homogenized with 1 mm MES (pH 6.0) and centrifuged for 10 min at 3,000g to yield precipitated cell walls. The supernatant was centrifuged again at 100,000g for 30 min to yield precipitated membranes and the supernatant represented the symplastic solution. The Zn concentrations of cell walls, membranes, and cell solutions were determined by ICP.
Leaf Compartmental Analysis
The protocol for efflux analysis was modified from Bell et al. (1994). Fifty leaf sections (10 mm2, each piece) of 10-d-old plants were submerged in aerated 65Zn-loading solution (2 mm MES-Tris buffer [pH 6.0], 0.5 mm CaCl2, and 10 μm 65Zn2+ [1.5 μCi]) for 24 h. Leaves of 10-d-old plants did not yet exhibit Zn deficiency symptoms. After rinsing in deionized water for 1 min, the sections were transferred to efflux solution (identical solution without 65Zn). Subsequently, at various time intervals, 1-mL aliquots of efflux solution were collected and solution exchanged with fresh efflux solution. After 24 h, leaf sections were collected and 65Zn activity in combined efflux solution samples and leaf sections was totaled. The 65Zn remaining in tissue was calculated and plotted against time on a semilogarithmic plot. The resulting linear component drawn through later time points represented first order efflux from a slowly efflux in the compartment and was extrapolated to the y axis. This line was subtracted from the original curve and resultant data were plotted against time. Similarly, subsequent linear components were extracted. Zn contents (%) were estimated as the y intercepts and t1/2 was the slope of each curve.
RNA Extraction and Northern-Blot Analysis
Leaves (0.5 g) were ground and total RNA preparation was performed by the TRIzol method (Life Technologies/Gibco-BRL, Cleveland) according to the protocol provided by the manufacturer. Poly(A+) mRNA was directly isolated from leaf tissues (0.5 g) using the Poly(A+) Pure Kit (Ambion, Austin, TX) according to the Ambion protocol. Samples were separated by 1% (w/v) agarose gel electrophoresis in glyoxal buffer and transferred to Hybond N+ nylon membrane (Amersham, Buckinghamshire, UK) and probed with radiolabeled ([α-32P]dCTP) wheat SOD1.1 or CA genes according to standard procedures. In brief, membranes were hybridized overnight at 65°C in Perfect Hyb Plus (Sigma, St. Louis) hybridization buffer and SOD1.1 (about 0.88 kb) or CA probe (about 0.49 kb), which was labeled by the random priming method following the kit manufacturer's instructions (Ambion). Hybridized membranes were washed in 10% (w/v) SDS and 20× SSC for a total of 60 min. Dried filters were exposed Biomax-MS x-ray film (Eastman-Kodak, Rochester, NY) for 5 h at −80°C. cDNA for the Cu/ZnSOD probe was kindly provided by Dr. Lawrence Gusta (University of Saskatchewan, Canada; Wu et al., 1999). A cDNA fragment for the CA gene was amplified by PCR and cloned into the pCR2.1 plasmid. DNA was isolated from the clones and sequenced. cDNA fragment showing 99% similarity with the putative CA gene from wheat (accession no. BE213573) was used as the CA probe.
Activity of SODs
Leaves were homogenized with 50 mm HEPES buffer (pH 7.6) containing 0.1 mm Na2EDTA and centrifuged at 15,000g for 15 min at 4°C. The supernatant was used for protein and SOD assays. The activity of the different SODs was assayed by the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) as described by Giannopolitis and Ries (1977) with some modifications. For the total SOD assay, a 5-mL reaction mixture contained 50 mm HEPES (pH 7.6), 0.1 mm EDTA, 50 mm Na2CO3 (pH 10.4), 13 mm Met, 75 μm NBT, 0.5 mL of enzyme extract, and 2 μm riboflavin. The reaction mixtures were illuminated for 15 min at 350 μmol m−2 s−1 light intensity. One unit of SOD activity was defined as the amount of enzyme required to cause 50% inhibition of reduction of NBT measured at 560 nm. Activities of Cu/ZnSOD were calculated by subtracting SOD activity in the presence of KCN from total SOD because KCN inhibits Cu/ZnSOD.
The analysis for protein estimation was carried out according to Bradford (1976) using bovine serum albumin as a standard.
Activity of CA
Leaf tissues (0.2 g) were ground with solution that contained 0.1 m Tris-HCl (pH 8.3), 0.01 m Na2EDTA, and 0.05 m 1,1,1,-trichloro-2,2-bis(p-chlorophenyl)ethane. The homogenate was centrifuged at 11,000g for 20 min and the supernatant was used for the determination CA activity, based on the method described by Ohki (1976) with some modifications. CA activity was assayed at 0°C to 4°C in an 8-mL reaction containing 3 mL of 0.025 m Veronal buffer (5,5-diethylbarbituric acid; pH 8.2), 1 mL of sample, and 4 mL of CO2-saturated water. The CA activity was expressed as units per milligram protein (units mg−1 protein = 10 × [T0 − Te]/Te), where T0 and Te represent the time(s) measured for the pH change (8.3–7.0) with buffer alone (T0) and with sample (Te).
Activity of NR
Leaves (0.5 g) were ground in a mortar and pestle with 1.5 mL of extraction buffer containing 0.1 m Tris-HCL (pH 8.5), 20 μm FAD, 2 μm Na2MoO4, 2 mm EDTA, 1 mm 1,1,1,-trichloro-2,2-bis(p-chlorophenyl)ethane, and 0.01 mm leupeptin. The extract was centrifuged for 15 min at 14,000g at 4°C. NR was determined as described by Mann et al. (1999) with some modifications: 0.4 mL of supernatant was added to 0.6 mL of reaction buffer (50 mm K3PO4, 20 mm KNO3, and 3 mm NADH) and incubated for 15 min at 27°C. The reaction was stopped by adding 0.5 mL of 1% (w/v) sulfanilamide and 0.5 mL of 0.02% (w/v) 2-N-(naphtyl)ethylenamine hydrochloride. After 20 min, the nitrite was measured colorimetrically at 540 nm and NR was expressed as micromoles nitrite per milligram protein.
ACKNOWLEDGMENTS
We acknowledge the Republic of Turkey and M. Kemal University (Antakya, Turkey) for supporting G.H. during his PhD. studies. We also thank Drs. Michael Grusak (U.S. Department of Agriculture-Agricultural Research Service, Houston, TX), John Cram (University of Newcastle, Newcastle upon Tyne, UK), Tetsuro Mimura (Hitotsubashi University, Tokyo), Ross Welch (Cornell University, Ithaca, NY), and Levent Ozturk (Cukurora University, Adana, Turkey) for their valuable scientific advice, and Dr. Lawrence Gusta (University of Saskatchewan, Canada) for providing the SOD1.1 gene.
Footnotes
This work was supported by The Republic of Turkey (graduate fellowship to G.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011825.
LITERATURE CITED
- Bar-Akiva A, Lavon R. Carbonic anhydrase activity as an indicator of Zn deficiency in citrus leaves. J Hortic Sci. 1969;44:359–362. [Google Scholar]
- Bell CI, Cram WJ, Clarkson DT. Compartmental analysis of 35SO42− exchange kinetics in roots and leaves of a tropical legume M. atropurpureum cv Sirato. J Exp Bot. 1994;45:879–886. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:48–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000;146:185–205. doi: 10.1046/j.1469-8137.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Erenoglu B, Gulut KY, Derici R, Romheld V. Light mediated release of phytosiderophores in wheat and barley under iron or zinc deficiency. Plant Soil. 1998;202:309–315. [Google Scholar]
- Cakmak I, Kalayci M, Ekiz H, Braun HJ, Kilinc Y, Yilmaz A. Zn deficiency as a practical problem in plant and human nutrition in Turkey: a NATO-Science for stability project. Field Crop Res. 1999;60:175–188. [Google Scholar]
- Cakmak I, Ozturk L, Eker S, Torun B, Kalfa H, Yilmaz A. Concentration of Zn and activity of Cu/Zn-SOD in leaves of rye and wheat genotypes differing in sensitivity to Zn deficiency. J Plant Physiol. 1997;151:91–95. [Google Scholar]
- Erenoglu B, Cakmak I, Romheld V, Derici R, Rengel Z. Uptake of zinc by rye, bread wheat and durum wheat cultivars differing in zinc efficiency. Plant Soil. 1999;209:245–252. [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases-occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RD, Ascher JS, Haynes SC. Selecting zinc-efficient cereal genotypes for soils of low Zn status. Plant Soil. 1992;146:241–250. [Google Scholar]
- Graham RD, Rengel Z. Genotypic variation in Zn uptake and utilization by plants. In: Robson AD, editor. Zinc in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 107–114. [Google Scholar]
- Hacisalihoglu G, Hart JJ, Kochian LV. High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol. 2001;125:456–463. doi: 10.1104/pp.125.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayci M, Torun B, Eker S, Aydin M, Ozturk L, Cakmak I. Grain yield, zinc efficiency and zinc concentration of wheat genotypes grown in a zinc-deficient calcareous soil in field and greenhouse. Field Crops Res. 1999;63:87–98. [Google Scholar]
- Mann HM, Khallaf G, Baki A, Stegman P, Weiner H, Kaiser WM. The activation state of nitrate reductase is not always correlated with total nitrate reductase activity in leaves. Planta. 1999;209:462–468. doi: 10.1007/s004250050749. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. New York: Academic Press; 1986. [Google Scholar]
- Marschner H, Cakmak I. High light intensity enhances chlorosis and necrosis in leaves of Zn, K and Mg deficient bean plants. J Plant Physiol. 1989;134:308–315. [Google Scholar]
- Mimura T, Sakano K, Shimmen T. Studies on the distribution, re-translocation and homeostasis of inorganic phosphate in barley leaves. Plant Cell Environ. 1996;19:311–320. [Google Scholar]
- Ohki K. Effect of zinc nutrition on photosynthesis and carbonic anhydrase activity in cotton. Physiol Plant. 1976;38:300–304. [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. GEOCHEM-PC: a chemical speciation program for IBM and compatible personal computers. In: Parker DR, Norvell WA, Chaney RL, editors. Chemical Equilibrium and Reaction Models (special publication No.2). Madison, WI: Soil Science Society of America; 1995. pp. 253–269. [Google Scholar]
- Rengel Z. Carbonic anhydrase activity in leaves of wheat genotypes differing in Zn efficiency. J Plant Physiol. 1995;147:251–256. [Google Scholar]
- Rengel Z, Graham RD. Wheat genotypes differ in Zn efficiency when grown in the chelate-buffered nutrient solution: I. Growth Plant Soil. 1995;176:307–316. [Google Scholar]
- Santa Maria GE, Cogliatti DH. Bidirectional Zn-fluxes and compartmentation in wheat seedling roots. J Plant Physiol. 1988;132:312–315. [Google Scholar]
- Sasaki H, Hirose T, Watanabe Y, Ohsuki R. Carbonic anhydrase activity and CO2-transfer resistance in Zn-deficient rice leaves. Plant Physiol. 1998;118:929–934. doi: 10.1104/pp.118.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GH, Wilen RW, Robertson AJ, Gusta LV. Isolation, chromosomal localization, and differential expression of mitochondrial Mn-SOD and Cu/Zn-SOD genes in wheat. Plant Physiol. 1999;120:513–520. doi: 10.1104/pp.120.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Worth C, Rengel Z. Using capillary electrophoresis to measure Cu/Zn-SOD concentration in leaves of wheat genotypes differing in tolerance to Zn deficiency. Plant Sci. 1999;143:231–239. [Google Scholar]