Abstract
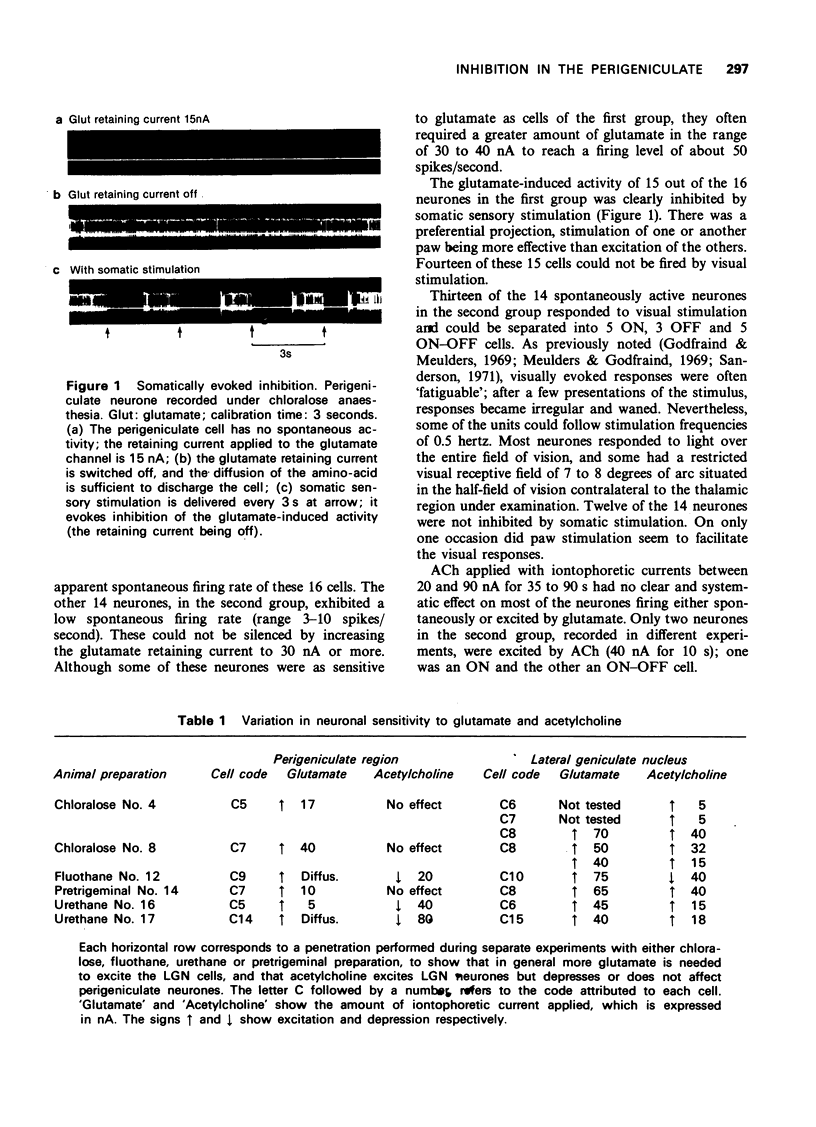
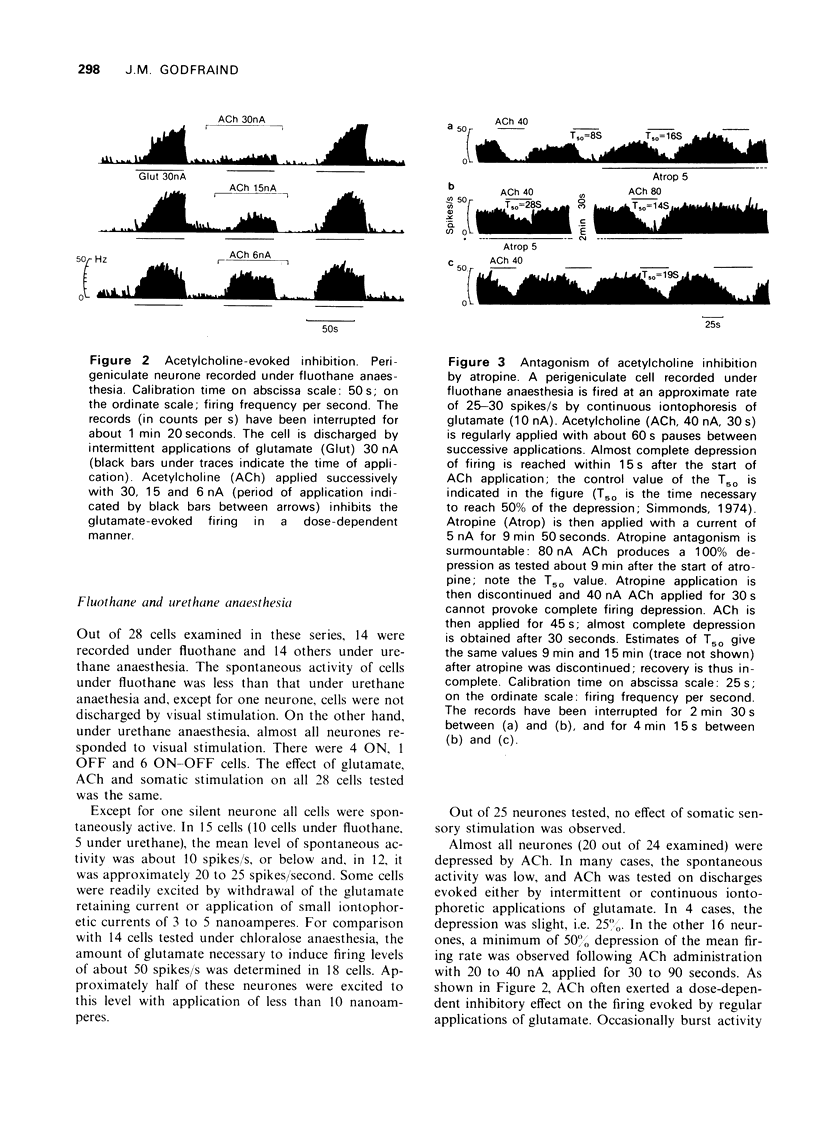
1 Perigeniculate neurones in cats were found to be inhibited by iontophoretically applied acetylcholine (ACh) and some of them by somatic sensory stimulation under certain experimental conditions. 2 Under chloralose anaesthesia, perigeniculate neurones could be divided into two groups with regard to their spontaneous activity, sensitivity to glutamate and reaction to sensory inputs. Somatic sensory stimulation clearly inhibited the glutamate discharges of those perigeniculate neurones which were characterized by a high sensitivity to glutamate and the absence of spontaneous activity. ACh had no clear inhibitory effect. 3 Under fluothane and urethane anaesthesia, no somatic sensory influence was noticed but ACh depressed almost all perigeniculate neurones. 4 In an unanaesthetized midpontine pretrigeminal preparation, the inhibitory effect of ACh was confirmed. 5 No conditions were found which the inhibitory influences of ACh and those of somatic sensory stimulation could be observed simultaneously on the same neurone. Therefore, it could not be established whether ACh mediates the somatic sensory influences on perigeniculate cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGEL A. THE EFFECT OF PERIPHERAL STIMULATION ON UNITS LOCATED IN THE THALAMIC RETICULAR NUCLEI. J Physiol. 1964 May;171:42–60. doi: 10.1113/jphysiol.1964.sp007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A., Knox G. V. The effect of anaesthesia on units in the thalamic reticular formation. J Physiol. 1970 Sep;210(2):167P–168P. [PubMed] [Google Scholar]
- Angel A., Unwin J. The effect of urethane on transmission along the dorsal column sensory pathway in the rat. J Physiol. 1970 May;208(1):32P–33P. [PubMed] [Google Scholar]
- Bagshaw E. V., Evans M. H. Measurement of current spread from microelectrodes when stimulating within the nervous system. Exp Brain Res. 1976 Jun 30;25(4):391–400. doi: 10.1007/BF00241729. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Dingledine R., Kanazawa I., Kelly J. S. Inhibitory effects of acetylcholine on neurones in the feline nucleus reticularis thalami. J Physiol. 1976 Oct;261(3):647–671. doi: 10.1113/jphysiol.1976.sp011579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher D. Reticular projections to lateral geniculate in cat. Brain Res. 1970 Oct 13;23(2):247–249. doi: 10.1016/0006-8993(70)90043-0. [DOI] [PubMed] [Google Scholar]
- DeFrance J. F., Yoshihara H., McCrea R. A., Kitai S. T. Pharmacology of the inhibiton in the lateral septal region. Exp Neurol. 1975 Sep;48(3 Pt 1):502–523. doi: 10.1016/0014-4886(75)90009-6. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Kelly J. S. Brain stem stimulation and the acetylcholine-evoked inhibition of neurones in the feline nucleus reticularis thalami. J Physiol. 1977 Sep;271(1):135–154. doi: 10.1113/jphysiol.1977.sp011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R. W., Wilson P. D., Bartlett J. R., Pecci-Saavedra J. Mesencephalic control of lateral geniculate nucleus in primates. I. Electrophysiology. Exp Brain Res. 1973 Sep 29;18(2):189–203. doi: 10.1007/BF00234723. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Iwama K. Reticular inhibition of internuncial cells in the rat lateral geniculate body. Brain Res. 1971 Dec 10;35(1):107–118. doi: 10.1016/0006-8993(71)90597-x. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M. Further studies on perigeniculate neurons: effects of glutamate, acetylcholine and somatic stimulation. Arch Int Pharmacodyn Ther. 1976 Sep;223(1):166–167. [PubMed] [Google Scholar]
- Godfraind J. M. Localisation de l'extrémité de microélectrodes de verre dans le système nerveux central par électrophorèse de pontamine. J Physiol (Paris) 1969;61 (Suppl 2):436–437. [PubMed] [Google Scholar]
- Godfraind J. M., Meulders M. Effets de la stimulation sensorielle somatique sur les champs visuels des neurones de la gégion gonouillée chez le chat anesthésié au chloralose. Exp Brain Res. 1969;9(3):183–200. doi: 10.1007/BF00234454. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M., Meulders M., Veraart C. Visual properties of neurons in pulvinar, nucleus lateralis posterior and nucleus suprageniculatus thalami in the cat. I. Qualitative investigation. Brain Res. 1972 Sep 29;44(2):503–526. doi: 10.1016/0006-8993(72)90316-2. [DOI] [PubMed] [Google Scholar]
- Godfraind J. M. Micro-electrophoretic studies in the cat pulvinar region: effect of acetylcholine. Exp Brain Res. 1975 Mar 27;22(3):243–254. doi: 10.1007/BF00234767. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Phillis J. W. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol. 1963 May;166(2):328–350. doi: 10.1113/jphysiol.1963.sp007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent J. P., Guerrero F. A., Jouvet M. Reversible suppression of the geniculate PGO waves and of the concomitant increase of excitability of the intrageniculate optic nerve terminals in cats. Brain Res. 1974 Dec 13;81(3):558–563. doi: 10.1016/0006-8993(74)90852-x. [DOI] [PubMed] [Google Scholar]
- McIlwain J. T. Nonretinal influences on the lateral geniculate nucleus. Invest Ophthalmol. 1972 May;11(5):311–322. [PubMed] [Google Scholar]
- Melzack R., Konrad K., Dubrovsky B. Prolonged changes in visual system activity produced by somatic stimulation. Exp Neurol. 1968 Mar;20(3):443–459. doi: 10.1016/0014-4886(68)90086-1. [DOI] [PubMed] [Google Scholar]
- Meulders M., Boisacq-Schepens N., Godfraind J. M., Colle J. Etude macro- et microphysiologique des projections sensorielles somatiques au niveau du corps genouillé latéral du chat anesthesié au chloralose. Arch Ital Biol. 1966 Dec;104(4):480–502. [PubMed] [Google Scholar]
- Meulders M., Godfraind J. M. Influence du réveil d'origine réticulaire sur l'étendue des champs visuels des neurones de la région genouillée chez le chat avec cerveau intact ou avec cerveau isolé. Exp Brain Res. 1969;9(3):201–220. doi: 10.1007/BF00234455. [DOI] [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G., Tradardi V. Spontaneous activity of neurones of nucleus reticularis thalami in freely moving cats. J Physiol. 1970 Oct;210(3):651–667. doi: 10.1113/jphysiol.1970.sp009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y., Domino E. F. Reticular facilitation of visually evoked responses by optic tract stimulation before and after enucleation. Exp Neurol. 1968 Dec;22(4):532–544. doi: 10.1016/0014-4886(68)90147-7. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Takaori S. Influence of norepinephrine-containing neurons derived from the locus coeruleus on lateral geniculate neuronal activities of cats. Brain Res. 1974 May 10;71(1):47–60. doi: 10.1016/0006-8993(74)90190-5. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., McMurtry J. G., Maekawa K. Synaptic events in ventrolateral thalamic neurons during suppression of recruiting responses by brain stem reticular stimulation. Brain Res. 1966 Jan;1(1):63–76. doi: 10.1016/0006-8993(66)90105-3. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Richards C. D. On the mechanism of halothane anaesthesia. J Physiol. 1973 Sep;233(2):439–456. doi: 10.1113/jphysiol.1973.sp010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. J. The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol. 1971 Sep;143(1):101–108. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Specialized organizational patterns within the nucleus reticularis thalami of the cat. Exp Neurol. 1972 Feb;34(2):316–322. doi: 10.1016/0014-4886(72)90177-x. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Characteristics of unit responses in nucleus reticularis thalami. Brain Res. 1970 Jul 14;21(2):286–288. doi: 10.1016/0006-8993(70)90371-9. [DOI] [PubMed] [Google Scholar]
- Schlag J., Waszak M. Electrophysiological properties of units of the thalamic reticular complex. Exp Neurol. 1971 Jul;32(1):79–97. doi: 10.1016/0014-4886(71)90167-1. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Quantitative evaluation of responses to microiontophoretically applied drugs. Neuropharmacology. 1974 Jun;13(6):401–406. doi: 10.1016/0028-3908(74)90127-0. [DOI] [PubMed] [Google Scholar]
- Singer W. Brain stem stimulation and the hypothesis of presynaptic inhibition in cat lateral geniculate nucleus. Brain Res. 1973 Oct 26;61:55–68. doi: 10.1016/0006-8993(73)90515-5. [DOI] [PubMed] [Google Scholar]
- Singer W. The effect of mesencephalic reticular stimulation on intracellular potentials of cat lateral geniculate neurons. Brain Res. 1973 Oct 26;61:35–54. doi: 10.1016/0006-8993(73)90514-3. [DOI] [PubMed] [Google Scholar]
- Sumitomo I., Nakamura M., Iwama K. Location and function of the so-called interneurons of rat lateral geniculate body. Exp Neurol. 1976 Apr;51(1):110–123. doi: 10.1016/0014-4886(76)90056-x. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. Lateral geniculate body structure and eye movement. Bibl Ophthalmol. 1972;82:178–188. [PubMed] [Google Scholar]
- Tatton W. G., Crapper D. R. Central tegmental alteration of cat lateral geniculate activity. Brain Res. 1972 Dec 12;47(2):371–387. doi: 10.1016/0006-8993(72)90646-4. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Suzuki D. A. Effects of frontal eye field stimulation upon activities of the lateral geniculate body of the cat. Exp Brain Res. 1976 Jun 18;25(3):291–306. doi: 10.1007/BF00234020. [DOI] [PubMed] [Google Scholar]


