Abstract
Graminan-type fructans are temporarily stored in wheat (Triticum aestivum) stems. Two phases can be distinguished: a phase of fructan biosynthesis (green stems) followed by a breakdown phase (stems turning yellow). So far, no plant fructan exohydrolase enzymes have been cloned from a monocotyledonous species. Here, we report on the cloning, purification, and characterization of two fructan 1-exohydrolase cDNAs (1-FEH w1 and w2) from winter wheat stems. Similar to dicot plant 1-FEHs, they are derived from a special group within the cell wall-type invertases characterized by their low isoelectric points. The corresponding isoenzymes were purified to electrophoretic homogeneity, and their mass spectra were determined by quadrupole-time-of-flight mass spectrometry. Characterization of the purified enzymes revealed that inulin-type fructans [β-(2,1)] are much better substrates than levan-type fructans [β-(2,6)]. Although both enzymes are highly identical (98% identity), they showed different substrate specificity toward branched wheat stem fructans. Although 1-FEH activities were found to be considerably higher during the fructan breakdown phase, it was possible to purify substantial amounts of 1-FEH w2 from young, fructan biosynthesizing wheat stems, suggesting that this isoenzyme might play a role as a β-(2,1)-trimmer throughout the period of active graminan biosynthesis. In this way, the species and developmental stage-specific complex fructan patterns found in monocots might be determined by the relative proportions and specificities of both fructan biosynthetic and breakdown enzymes.
Starch is the most prominent storage carbohydrate in plants, but about 15% of flowering plant species use fructan (a Fru polymer) as a storage compound (Hendry, 1993). Inulin-type fructan consists of linear β-(2,1)-linked fructofuranosyl units and occur mainly in dicotyledonous species. Levan consists of linear β-(2,6)-linked fructofuranosyl units, but more complex and branched fructan types (graminan, inulin neoseries, and levan neoseries) are common in monocotyledonous species (Vijn and Smeekens, 1999; Pavis et al., 2001b) mainly belonging to the Poaceae (e.g. wheat [Triticum aestivum], barley [Hordeum vulgare], oat [Avena sativa], and temperate fodder grasses) and the Liliaceae (e.g. onion, asparagus [Asparagus officinalis]).
Next to their obvious role as reserve compounds, fructan might have other functions in plants like stress protectants (drought and cold) or osmoregulators (Vergauwen et al., 2000; Hincha et al., 2002, and refs. therein). Unlike starch, fructans are water soluble and are believed to be stored in the vacuole (Wiemken et al., 1986), although the exclusive vacuolar localization has been questioned (Livingston and Henson, 1998).
Although the metabolism of inulin has become clear in dicotyledonous species and the respective biosynthetic and breakdown enzymes have been cloned (Edelman and Jefford, 1968; Van den Ende and Van Laere, 1996a; van der Meer et al., 1998; Hellwege et al., 2000; Van den Ende et al., 2000, 2001), fructan metabolism in monocots is not yet completely unraveled. So far, four different fructosyltransferases, each with their own specificity, are believed to be involved in monocot fructan biosynthesis. In addition to inulin biosynthesis by Suc:Suc 1-fructosyl transferase (1-SST) and fructan:fructan 1-fructosyl transferase (1-FFT), the 1-SST product 1-kestose is used as a substrate by the key enzyme fructan:fructan 6G-fructosyl transferase to produce neokestose, which in turn can be further elongated by the action of 1-FFT or Suc:fructan 6-fructosyl transferase (6-SFT) to produce inulin or levan neoseries, respectively (Vijn and Smeekens, 1999). Furthermore, it has become clear that 1-SST and 6-SFT are the key enzymes for graminan biosynthesis in cereals like barley and wheat (Sprenger et al., 1995; Kawakami and Yoshida, 2002). 6-SFT prefers 1-kestose as an acceptor substrate, and thus mainly produces bifurcose (1&6 kestotetraose) from Suc and 1-kestose. 1-FFT fulfills an elongation role during graminan biosynthesis (Jeong and Housley, 1992), and its cDNA has recently been cloned from wheat (Kawakami et al., 2002). For a long time, it has been a matter of debate whether the 6-kestose necessary for levan-type fructan biosynthesis in cereals originates from the direct action of a specific 6-SST (Penson and Cairns, 1994; Chatterton and Harrison, 1997) rather than by the β-(2,1)-hydrolysis of bifurcose (Bancal et al., 1992), the main product of 6-SFT (Sprenger et al., 1995). To explain the biosynthesis of the levan neoseries in ryegrass (Lolium perenne; Pavis et al., 2001a) and the authentic levan series in Poa secunda (Wei et al., 2002), these authors suggest the existence of 6-SST or 6-FT-like enzymes that might prefer fructans other than 1-kestose (e.g. neokestose or 6-kestose) as acceptor substrates. However, so far these kinds of enzymes have never been fully characterized.
In wheat stems, a typical seasonal accumulation of fructan is observed. Accumulation continues during stem growth and anthesis but fructan content in stems strongly decreases during the later stages and contributes to grain filling (Pollock and Cairns, 1991; Bancal and Triboï, 1993; Schnyder, 1996). Wheat stems accumulate low degree of polymerization (DP) levan- and graminan-type fructans that are well characterized both on C18 and anion-exchange chromatography with pulsed amperometric detection (AEC-PAD; Bancal et al., 1993). In excised and induced wheat leaves (Bancal et al., 1992) but not in field-grown wheat stems (Bancal and Triboï, 1993), a trimming of graminan-type fructans by (a) specific 1-FEH(s) was suggested. Both fructan biosynthetic and breakdown enzymes can be measured during graminan biosynthesis in wheat stems. This paper reinforces the work on fructan metabolism in wheat stems with a special attention to the putative role of 1-FEHs not only during the period of fructan breakdown but also as a putative (2,1) trimmer during the period of active fructan biosynthesis.
Plant 1-FEHs have been studied extensively in dicots like chicory (Cichorium intybus) and Jerusalem artichoke (Helianthus tuberosus; Van Laere and Van den Ende, 2002, and refs. therein). From monocots, only partially purified preparations of 1-FEH were obtained from wheat (Jeong, 1991), barley (Henson, 1989), and Lolium rigidum (Bonnett and Simpson, 1993). A fructan 6-exohydrolase (6-FEH) from ryegrass (Marx et al., 1997b) and an FEH that preferentially hydrolyzes β-(2→6) (oat; Henson and Livingston, 1996) or multiple fructofuranosidic linkages (barley; Henson and Livingston, 1998) were purified more recently.
To our knowledge, no FEH cDNA has so far been cloned from a monocot species. Three 1-FEHs have recently been cloned from chicory (Van den Ende et al., 2000, 2001). It is surprising that in severe contradiction to fructan biosynthetic enzymes that evolved from vacuolar-type invertases, dicot 1-FEHs apparently evolved from cell wall-type invertases (Van den Ende et al., 2002a).
To elucidate a putative role for 1-FEHs, not only during the period of fructan breakdown but also as a putative (2,1) trimmer during the period of active fructan biosynthesis, two isoforms of 1-FEH enzymes from wheat stems were purified and characterized. Their cDNAs were cloned and compared with other monocot glycosyl hydrolases and dicot 1-FEHs.
RESULTS
1-FEH Activities during Wheat Stem Development
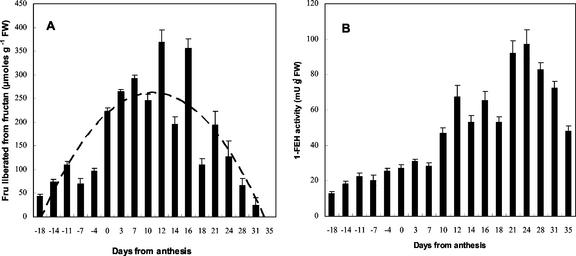
Fructans in wheat stems, as estimated by the increase of Fru after mild acid hydrolysis, accumulate to well after anthesis (green stems) but disappear during further ripening (stems turning yellow; Fig. 1A). 1-FEH activity can clearly be detected during the period of fructan biosynthesis but increases temporarily during the disappearance of fructans from the stems (Fig. 1B).
Figure 1.
A, Fru liberated by mild acid hydrolysis of total carbohydrate throughout wheat stem development. A trend line is indicated. B, 1-FEH activity throughout wheat stem development, as measured by the Fru production from 3% (w/v) commercial chicory inulin. Incubation time, 1 h. Incubation temperature, 30°C.
Enzyme Characterization
Purification of 1-FEHs
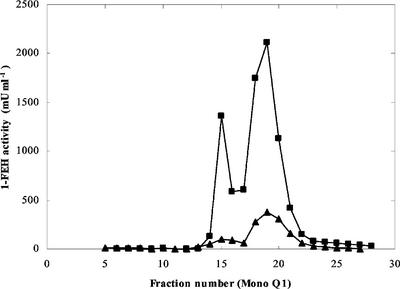
Compared with young wheat seedlings, senescing wheat stems contain much lower invertase activities, which make them interesting tissues for 1-FEH purification. Two isoforms of 1-FEH (termed 1-FEH w1 and w2) were purified from senescing wheat stems (24 d after anthesis) by a combination of Concanavalin A (Con A) affinity chromatography and AEC at different pH. 1-FEH w1 and w2 were already separated after the first Mono Q column (Fig. 2), but for removal of contaminating bands after SDS-PAGE, one or two more runs at different pH were necessary (Table I). Comparable results were obtained with younger stems (at anthesis) except that, not surprisingly, enzyme activities were much lower (Fig. 2).
Figure 2.
Separation of wheat stem 1-FEH w1 and w2 on Mono Q1 at pH 7.0. Wheat stems 24 d after anthesis (▪) and at anthesis (▴), respectively. Activity profile of the different fractions. 1-FEH activity was measured by the Fru production from 3% (w/v) commercial chicory inulin.
Table I.
A typical purification of 1-FEH w1 (left part of column) and w2 (right part of column) from 1 kg of wheat stems
| Purification Step | Protein | Total Activity | Recovery | Specific Activity | Purification | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg | units | % | units mg−1 protein | -fold | ||||||
| Crude extract | 185.6 | 30.0 | 100 | 0.16 | 1 | |||||
| 30% to 80% (w/v) (NH4)2SO4 | 43.1 | 32.2 | 107 | 0.75 | 4.6 | |||||
| Con A | 6.0 | 16.5 | 54.9 | 2.7 | 16.9 | |||||
| Mono Q (7.0) | 0.18 | 0.48 | 1.36 | 4.99 | 4.5 | 16.7 | 7.7 | 10.5 | 48.0 | 64.8 |
| Mono Q (6.0) | 0.01 | 0.10 | 0.15 | 1.10 | 0.50 | 3.67 | 14.6 | 11.0 | 90.2 | 68.1 |
| Mono Q (5.2) | 0.05 | 0.62 | 2.06 | 12.2 | 75.6 | |||||
Elution pHs on Mono Q are indicated in parentheses.
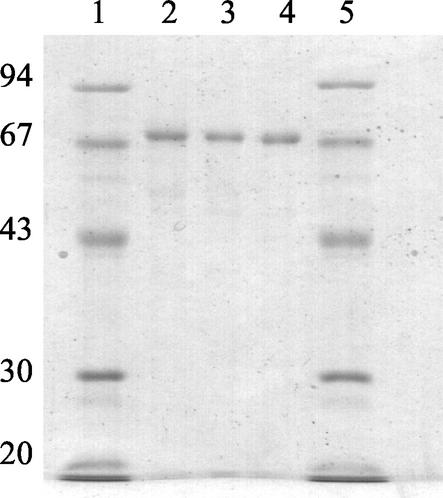
A summary of a typical purification is given in Table I. The slight increase in activity after ammonium sulfate precipitation suggests the removal of an inhibitor. Considerable activity losses occurred on Con A, and on the different Mono Q columns. A maximal purification of about 90-fold was obtained for 1-FEH w1 and about 76-fold for 1-FEH w2 yielding a specific activity of 14.6 and 12.2 units mg−1 protein, respectively. The concentration of 1-FEH w2 was apparently much higher than that of 1-FEH w1 (Fig. 2; Table I). Invertase and 6-FEH activities were also detected (not shown), but these could be separated from the two 1-FEH isoforms. The molecular mass of both purified enzymes was estimated at about 70 kD by SDS-PAGE (Fig. 3).
Figure 3.
Purification of wheat stem 1-FEH w1 and 1-FEH w2. SDS-PAGE analysis of 5 μg of the purified wheat enzymes 1-FEH w1 (lane 2, 24 d after anthesis) and 1-FEH w2 (lane 3, 24 d after anthesis; lane 4, anthesis) Lanes 1 and 5 contain molecular mass marker proteins. Numbers on the left indicate their mass in kilodaltons.
Enzymatic Properties
The pH optimum of both purified enzymes was between pH 4.5 and 5.5, and they were inactive above pH 7.5 (not shown). Both 1-FEHs had optimal activities between 30°C and 40°C, but activities remained surprisingly high at the lower temperature range (not shown). Similar properties were observed for a 1-FEH from barley (Henson, 1989) and a 6-FEH from ryegrass (Marx et al., 1997b).
When using 1-kestose as a substrate, a Km of 7 mm and a Vmax of 23.2 milliunits μg−1 protein were obtained for both 1-FEHs (Table II). The Km is much lower and the Vmax higher compared with the purified 1-FEH II from chicory (Km, 57.8 mm; Vmax, 11.1 milliunits μg−1 protein; De Roover et al., 1999). If inulin is used as a substrate, Km could not be determined accurately because no sign of saturation was observed at 10% (w/v) inulin, which is the maximum concentration that can be obtained in vitro. Both enzymes were severely inhibited by Suc with an apparent inhibition constant (Ki) of 0.90 mm at 12 mm inulin (not shown). This Suc inhibition is much stronger than observed for chicory 1-FEH II (Ki, 5.9 mm; De Roover et al., 1999).
Table II.
Substrate specificity of 1-FEH w1 and w2 from wheat
| Substrate | DP | Relative Activity
|
|
|---|---|---|---|
| w1 | w2 | ||
| % | |||
| Suc | 2 | 0.1 | 0.3 |
| 1-Kestosea | 3 | 100 | 100 |
| 6-Kestose | 3 | 0.7 | 1 |
| Neokestose | 3 | 4 | 3 |
| 1,1-Nystose | 4 | 72 | 83 |
| Inulin | >10 | 39 | 22 |
| Levan | Nd | 4 | 3 |
Results are shown as values relative to the activity with the substrate 1-kestose. Nd, Not determined.
With 1-kestose as a substrate, a Km of 7 mm and a Vmax of 23.2 milliunits μg−1 protein were obtained for both 1-FEH forms.
Substrate Specificities
Both isoforms were exohydrolases using a multichain mechanism of hydrolysis because no products other than Fru could be detected by using commercially available inulin as a substrate (not shown). Activity against Suc is minimal, indicating that these enzymes are fructan exohydrolases and not β-fructofuranosidases or invertases (Table II). From all fructans tested, the purified enzymes most efficiently hydrolyzed β-(2,1)-linkages: 1-Kestose and 1,1-nystose were the best substrates. 6-Kestose was hydrolyzed at least 100 times slower than 1-kestose (Table II). Therefore, these enzymes can be designated as 1-FEHs.
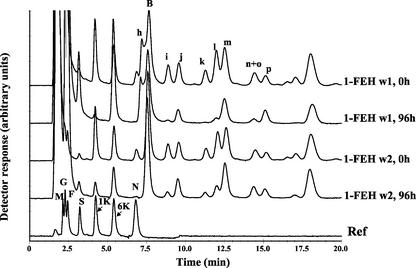
Incubation of graminans, isolated from wheat stems at anthesis, with FEH w1 and w2 revealed a different degradation profile (Fig. 4A). Fructan structures are shown in Figure 4B, available at www.plantphysiol.org. 1-FEH w1 hydrolyzed 6,1 kestotetraose (peak i), 6&1,1 kestopentaose (peak k), and 1&6,1 kestopentaose (peak l) faster than 1-FEH w2. Moreover, 6;6,1 kestopentaose and/or 6;1&6 kestopentaose (peak n+o; these compounds cannot be separated on AEC-PAD) is readily hydrolyzed by 1-FEH w1 but not by 1-FEH w2 (Fig. 4A).
Figure 4.
A, AEC-PAD chromatograms of incubation mixtures of purified 1-FEH w1 and w2 together with endogenous wheat stem fructan after 0 and 96 h of incubation, respectively.
Cloning
Reverse transcriptase (RT)-PCR was performed on total RNA derived from senescing winter wheat stems (24 d after anthesis). Primers based on conserved cell wall invertase and dicot 1-FEHs were used to obtain two partial clones with high amino acid identity to cell wall-type invertases and 1-FEHs. A mixture of the latter was subsequently used as hybridization probe to screen a winter wheat cDNA library. Full-length 1-FEH w2 and partial 1-FEH w1 were obtained. A 1-FEH w2-based primer was subsequently used to derive the missing DNA sequence part of 1-FEH w1.
The 1-FEH w1 and 1-FEH w2 cDNAs encode polypeptides of 597 and 596 amino acids, respectively. The deduced amino acid sequences for wheat 1-FEH w1 and w2 are presented in Figure 5. The cDNA-derived pIs of 1-FEH w1 and w2 are calculated at 4.79 and 4.78, respectively. These values match the chromatographic behavior of the native proteins. Furthermore, both mature proteins contain four potential glycosylation sites (N-X-S/T; Fig. 5). The cDNA-derived molecular mass of both mature enzymes (61.2 kD) is lower than the 70 kD estimated from SDS-PAGE (Fig. 3), but this discrepancy can probably be explained by the glycosylation on at least two of four potential N-glycosylation sites (see below).
Figure 5.
Alignment of the deduced amino acid sequences of wheat stem 1-FEH w1 and w2, chicory root 1-FEH I and 1-FEH IIa (all low pI), and wheat cell wall invertase (high pI). Potential glycosylation sites are italic and underlined. For 1-FEH w1 and 1-FEH w2, tryptic fragments found after Q-TOF MS/MS analysis are presented in bold. The N-terminal amino acid of the mature protein is underlined. Amino acids that are different between 1-FEH w1 and w2 are double underlined. Consensus line, Asterisks indicate identical residues; colons indicate conserved substitutions; and periods indicate semiconserved substitutions.
Quadrupole-Time-of-Flight (TOF) Mass Spectrometric (MS) Analyses
Theoretical tryptic digests on the cDNA-derived 1-FEH w1 and w2 protein sequences yielded 49 and 48 peptides, respectively. These are designated T1 to T49 from N to C terminus. Masses of tryptic peptides were determined by Q-TOF and compared (Tables III and IV) with the masses of theoretical cDNA-derived peptides (allowing for one possible missed cleavage site and with the consideration of oxidized Met). For 1-FEH w1, all except seven of the detected masses matched one of the theoretical fragments within the acceptable mass measurement error of ±0.1 D (see supplemental Table III, available at www.plantphysiol.org). For 1-FEH w2, all except two masses matched the theoretical ones (see supplemental data Table IV, available at www.plantphysiol.org). Collision-induced dissociation MS/MS analysis yielded a number of sequence tags (Mann and Wilm, 1994), which proved the identity of the tryptic peptides (Tables III and IV). Five unexplained fragments of 1-FEH w1 proved to be glycosylated peptides after fragmentation. These fragments fit perfectly with two of four potential N-glycosylation sites (Fig. 5). The other unexplained masses of 1-FEH w1 and w2 can be understood by the posttranslational cleavage of the prepeptide region from the cDNA-derived sequence. For 1-FEH w2, both peptides DPSPAVSTMYK or PSPAVSTMYK are the expected N-terminal sequences of the mature enzyme because no K or R is directly in front of this peptide region in the translated cDNA (Fig. 5). DPSPAVSTMYK is accordingly the presumptive N-terminal sequence of the 1-FEH w1 enzyme.
Comparison with Other Glycosyl Hydrolases
The cDNA-derived amino acid sequences of 1-FEH w1 and w2 are 98% identical. Similarities to chicory 1-FEH I (50% identity) and 1-FEH IIa and b (48% identity) are lower. 1-FEH w1 and w2 are more similar to cell wall invertases (46%–54% identical amino acids) than to vacuolar invertases (39%–45% identity) and fructan biosynthetic enzymes (34%–41% identity). Similarities to microbial fructan hydrolases are much lower (13%–28% identity).
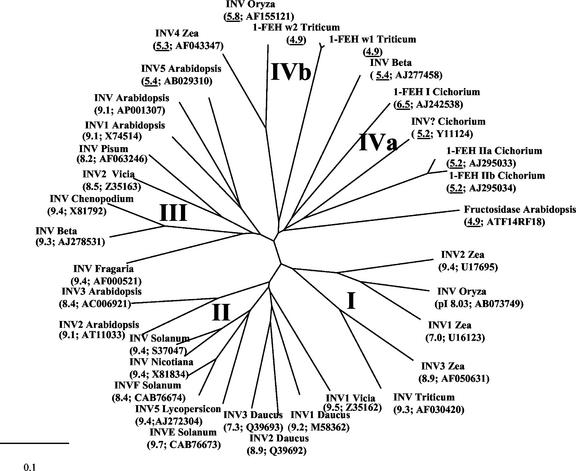
An unrooted radial tree of some members of cell wall-type glycosyl hydrolases is presented in Figure 6. Four distinct groups can be discerned: The first group (I) contains monocotyledonous and mainly basic cell wall invertases. The second group (II) contains dicotyledonous, basic cell wall-type invertases. A third group (III) also contains dicotyledonous and mainly basic cell wall-type invertases, with the exception of an acid cell wall invertase from Arabidopsis (INV5). A fourth group contains dicotyledonous (IVa) and monocotyledonous (IVb) enzymes. The IVa subgroup harbors the three chicory 1-FEH cDNAs and a fructosidase from Arabidopsis. 1-FEH w1 and w2 cluster together with cell wall invertases from rice and maize. All group IV members typically have an acidic pI, as is usually observed for vacuolar-type invertases.
Figure 6.
Unrooted phylogenetic tree containing cell wall-type invertase-like cDNA-derived amino acid sequences. Four groups can be discerned. First group (I): maize (Zea mays) INV1, 2, and 3; rice (Oryza sativa) INV; and wheat INV. Second group (II): broad bean (Vicia faba) INV1; carrot (Daucus carota) INV 1, 2, and 3; potato (Solanum tuberosum) INV, INVE, and INVF; tomato (Lycopersicon esculentum) INV5; Arabidopsis CW INV2 and 3; and tobacco (Nicotiana tabacum) INV. Third group (III): Arabidopsis INV, INV1, and 5; broad bean INV2; pea (Pisum sativum) INV; Chenopodium rubrum INV 1; sugar beet (Beta vulgaris) INV; and Fragaria × ananassa INV; Fourth group (IVb): maize INV4; rice INV; and wheat 1-FEH w1 and w2. Fourth group (IVa): Arabidopsis fructosidase; chicory 1-FEH I, IIa, and IIb and INV; and sugar beet INV. Isoelectric points and accession numbers are presented between brackets. Acid isoelectric points are underlined. The scale bar indicates a distance value of 0.1. INV, Cell wall invertase.
DISCUSSION
Properties of Plant FEHs
For the first time, to our knowledge, a completely purified monocot 1-FEH was obtained as shown by the single protein band on SDS-PAGE (Fig. 3). Purity was confirmed by Q-TOF MS analysis where all observed peaks could be explained (Tables III and IV; Fig. 5). Also, 6-FEH and invertase activities were present in crude extracts, but they eluted in different fractions. As observed for most other plant FEH enzymes (Simpson and Bonnett, 1993; Marx et al., 1997a, 1997b; De Roover et al., 1999), the final 1-FEH w1 and w2 preparations showed negligible invertase activity (Table II). In severe contrast to plant FEHs, which are only capable of degrading fructans and not Suc, β-fructo(furano)sidases (EC 3.2.1.80) can degrade Suc as well as fructans. Therefore, plant FEHs are clearly different from β-fructo(furano)sidases and cannot be classified under EC 3.2.1.80. In our opinion, a new EC number should be appointed to be able to properly classify this kind of enzyme.
The apparent molecular mass of 70 kD as estimated by SDS-PAGE is in the same range of 1-FEH I from chicory (72 kD; Claessens et al., 1990), 1-FEH II from chicory (64 kD; De Roover et al., 1999), a 1-FEH from Jerusalem artichoke (75 kD; Marx et al., 1997a), a 6-FEH from ryegrass (69 kD; Marx et al., 1997b) and a 1-FEH from barley (62.5 kD; Henson, 1989). However, considerably lower molecular masses were reported for a purified FEH from barley (33 kD; Henson and Livingston, 1998), a 6-FEH from oat (43 kD; Henson and Livingston, 1996), and a 6-FEH from Dactylis glomerata (57 kD; Yamatoto and Mino, 1989). It is not clear whether the partially purified preparation of wheat stem 1-FEH by Jeong (1991) is identical to one of our purified enzymes because they estimated the molecular mass at 63.7 kD. In contrast to the generally heterodimeric fructan biosynthetic enzymes, which are believed to have evolved from vacuolar-type invertases (Van den Ende et al., 2002b), all reported plant FEHs are monomeric enzymes.
It is widely accepted that cereal fructan metabolic enzymes, including FEHs, are located within the vacuole (Wagner and Wiemken, 1986). Like FEHs in other grasses (Simpson and Bonnett, 1993; Marx et al., 1997b), wheat 1-FEH has an acidic pH optimum supporting vacuolar localization. Glycosylation of 1-FEH w1 and w2, as proven by tryptic analysis (Table II) and binding on Con A, is also consistent with a vacuolar localization. Also chicory 1-FEHs were suggested to be vacuolar because it was impossible to find them in apoplastic fluid at levels higher than can be explained by cellular leakage (Van den Ende et al., 2000, 2001).
Plant FEHs Are Derived from Cell Wall-Type Invertases
So far, no monocot FEH enzymes had been cloned. On the basis of sequences conserved in cell wall invertases and dicot 1-FEHs, two highly identical 1-FEH cDNAs were cloned from wheat stems. The tryptic peptides retrieved from both purified 1-FEHs covered 43% (1-FEH w1) and 21% (1-FEH w2) of the cDNA-derived sequence information with an identity of 100% (Tables III and IV; Fig. 5), convincingly demonstrating that the cDNAs code for the corresponding enzymes.
Like the genes encoding dicot 1-FEHs from chicory (Van den Ende et al., 2002a), wheat 1-FEH w1 and w2 are most similar to cell wall invertases. They cluster together in a group (IV in Fig. 6) harboring fructosidases with low pIs. Therefore, most likely all plant FEHs, in dicots as well as in monocots, have probably evolved from genes of cell wall-type invertases that obtained a low pI and a vacuolar targeting signal. It has now become clear that enzymes with a totally different functionality (FEH versus invertase) and localization (cell wall versus vacuole) can cluster together. Because only a minority of the genes in Figure 6 have been identified by other means than homology, it is precocious to call them invertases. Therefore to prevent further confusion, it is important that future clones be investigated more thoroughly before being classified simply as “cell wall invertase.” In this respect, it would be tempting to determine the functionality of the Arabidopsis clone that groups together with 1-FEHs in group IV (Fig. 6).
Putative Functions of FEHs
Co-expression of 1-SST and 1-FEH has been found in primary leaves of barley (Wagner and Wiemken, 1989) and in mature wheat stems (Bancal and Triboï, 1993). If both enzymes are vacuolar, both fructan biosynthetic and breakdown enzymes would operate simultaneously in cereal grasses. In dicots, co-expression of fructan biosynthetic and breakdown enzymes has never been reported (Van Laere and Van den Ende, 2002).
Suc and illumination drastically increases the 1-SST to 1-FEH ratio and fructan content (Wagner and Wiemken, 1989), and Suc stimulates 6-SFT expression in barley (Nagaraj et al., 2001) and down-regulates the 1-FEH genes. Suc export and fructan degradation by newly synthesized 6-FEH enzymes was demonstrated in D. glomerata (Yamatoto and Mino, 1985). Apart from the putative control of Suc on gene expression, Suc directly inhibits wheat 1-FEH w1 and w2, chicory 1-FEH II (De Roover et al., 1999), and most—but not all—monocot FEHs in vitro (Simpson and Bonnett, 1993; Marx et al., 1997b). Therefore, FEH activity in vivo is probably controlled and/or modulated by the Suc concentration.
Defoliation induces expression of 1-FEH II in chicory (Van den Ende et al., 2001) but very low 1-FEH to 1-SST ratios were found in control plants throughout the period of fructan biosynthesis (Van den Ende and Van Laere, 1996b). In temperate grasses, a much higher 1-FEH to 1-SST ratio can be derived from data in the literature (Wagner and Wiemken, 1989; Prud'homme et al., 1992; Bancal and Triboï, 1993). It can be speculated that temperate grass plants (at least in certain parts like e.g. the stubble) might profit from maintaining relatively high FEH activities that are ready to use but largely inhibited under normal circumstances by the high Suc concentrations. Increased Suc export, such as induced by grazing and defoliation (temperate fodder grasses) or during grain filling, would almost immediately activate these FEH enzymes. A later (slower) increase of FEH activities could then be accomplished by up-regulating the genes.
Figure 1 clearly demonstrates the high basal level of 1-FEH activity throughout the period of fructan biosynthesis in mature wheat stems. Starting from about 1 week after anthesis, 1-FEH activities rise and probably both 1-FEH and 6-FEH-type enzymes contribute to the complete fructan breakdown in the stem during grain filling. The considerable 1-FEH activity in young, fructan biosynthesizing stems is not attributable to minor activities of invertase because a specific 1-FEH w2 could be purified from this stage (Figs. 2 and 3). Therefore, our results support the idea that 1-FEHs might be involved as β-(2,1)-trimmers during graminan biosynthesis. In this way, they might prevent formation of inulin-type fructans by 1-SST and 1-FFT or further β-(2,1)-elongation of branched graminans by 1-FFT. No inulin oligomers above DP 5 can be detected in wheat. Moreover, it is striking that FEH enzymes that preferentially degrade β-(2,1)-linkages were only reported from species that accumulate predominantly low-DP β-(2,6)-rich fructans (wheat, barley, and L. rigidum). Our results, however, do not support the view (Bancal et al., 1992) that small branched graminans can be selectively trimmed by 1-FEH because branched molecules apparently are poor substrates for 1-FEH w1 and w2 (Fig. 5). Therefore, it seems unlikely that 6-kestose formation in wheat stems might originate from breakdown of bifurcose. The inability of the 1-FEHs to efficiently hydrolyze small branched graminans might explain why these types of fructan accumulate in wheat.
6-FEH activity is also measurable in young wheat stems. Purification and characterization of this enzyme is still a subject of ongoing research. The presence of both 1-FEH and 6-FEH does not indicate a general turnover of fructan. Winzeler et al. (1990) convincingly demonstrated that there is no fructan turnover in fructan-biosynthesizing wheat stems. The lack of fructan turnover is consistent with a long-term reserve function, and this is in severe contrast with findings in gramineous leaves where fructans turnover very rapidly (Farrar and Farrar, 1985). Preliminary experiments on partially purified 6-FEH showed even a stronger inhibition by Suc compared with 1-FEH w1 and w2, and therefore the activity of this enzyme might be nearly completely inhibited in vivo by the high Suc concentrations during the period of active fructan biosynthesis.
We propose that the species-specific fructan patterns in cereal grasses, and in wheat in particular, are the result both of the relative abundances and the specificities of fructan biosynthetic enzymes (1-SST, 1-FFT, 6-SFT, and/or other) and of fructan exohydrolases acting as trimmers (1-FEH and 6-FEH). A third important factor is most probably the Suc concentration at the site of fructan biosynthesis, acting as an activator/substrate (1-SST and 6-SFT) or inhibitor (fructan exohydrolases) at the DNA and protein level. In support of this idea, it was demonstrated (Bancal and Triboï, 1993) that a much larger proportion of β-(2,1)-linked fructans accumulates in chilled leaf blade seedlings of wheat (Suc content, 35 mg g−1 fresh weight) than in mature wheat culms (Suc content, 8 mg g−1 fresh weight). The effect of silencing 1-FEH genes on DP and relative abundance of β-(2,1)-linkages in wheat graminans might further elucidate this point.
Different Substrate Specificities for 1-FEH w1 and w2
Despite the 98% identity at the amino acid level between 1-FEH w1 and w2, we were able to demonstrate a different specificity toward endogenous, branched wheat stem fructans (Fig. 5). Bancal et al. (1993) found that the partially purified 1-FEH from barley (Henson, 1989) also preferred inulin-type fructans over branched fructans such as bifurcose. The steric hindrance by adjacent β-(2,6)-linked Fru, as suggested by Bancal et al. (1993), is apparently more important for 1-FEH w1 than for 1-FEH w2 (Fig. 4). Moreover, 6-kestose-based branched carbohydrates like 6;1&6 kestopentaose and/or 6;6,1 kestopentaose are substrates for 1-FEH w1 but not for 1-FEH w2 (Fig. 4). Further confirmation by incubation with pure substrates is needed but is hampered by the fact that these products are not commercially available, occur in only low concentrations in plant tissues, and are very difficult to separate (e.g. 6;1&6 kestopentaose and/or 6;6, 1 kestopentaose). Nevertheless, it would be very interesting to compare both enzymes by site-directed mutagenesis.
CONCLUSIONS
Two highly identical 1-FEHs (1-FEH w1 and w2) from wheat stems were characterized at the protein and DNA level. Besides their function during fructan breakdown, results indicate that at least 1-FEH w2 might be involved as a β-(2,1)-trimmer during graminan biosynthesis. Therefore, 1-FEHs might play a crucial role in determining the fructan pattern and final DP in cereals. Despite being highly identical, both isoenzymes have different activity toward endogenous branched-type fructans. This first-time cloning of 1-FEHs from a monocot species should aid in cloning of other FEHs (e.g. 6-FEHs) from cereals and temperate fodder grasses and should contribute to our understanding of FEHs regulation. As a future goal, control of 1-FEHs in wheat grains or leaves of temperate fodder grasses by genetic engineering might enhance the levels of graminan-type fructans in food and feed. These soluble fibers might have even better beneficial effects than inulin (Roberfroid et al., 1998) as a prebiotic throughout the colon.
MATERIALS AND METHODS
Plant Material
Wheat (Triticum aestivum cv Pajero) was sown and grown in local fields with sandy loam soil during the growing seasons in 1999, 2000, and 2001. Field-grown stems (first 10 cm below the ear) were collected two to three times per week covering the periods of heading (Feekes scales 10.1–10.5), flowering (Feekes scales 10.51–10.54), and grain ripening. The first sample was taken when the first ears became just visible (Feekes scale 10.1). The whole sampling period covered about 9 weeks. The samples were used for carbohydrate analyses, for enzyme activity determinations, and for enzyme purification purposes.
Purification of 1-FEH w1 and w2
Extraction
Wheat stems were sampled at anthesis (fructan biosynthesis in stems) and 24 d after anthesis (ripening grain and fructan breakdown in stems) and cut in very small pieces. One kilogram was immersed in liquid nitrogen and was subsequently homogenized dry with a Waring blender. Thereafter, a second dry homogenization was performed in a smaller Waring blender until a fine powder was obtained. Finally, this powder was dissolved in 1.5 L of 50 mm sodium-acetate buffer, pH 5, containing 1 mm EDTA, 10 mm NaHSO3, 1 mm mercaptoethanol, and 0.1% (w/v) Polyclar AT (Serva, Heidelberg), and a final homogenization step was performed. The homogenate was squeezed through cheesecloth.
Purification and Electrophoresis
Ammonium sulfate was added to a saturation of 30% and gently stirred on ice for 30 min. After centrifugation for 20 min at 40,000g and 4°C, precipitated protein was discarded. Again ammonium sulfate was added to the supernatant to a final saturation of 80%. After a second centrifugation (20 min at 40,000g and 4°C), the precipitate was collected and redissolved in 150 mL of 50 mm sodium-acetate buffer, pH 5.0. Undissolved material was spun down for 15 min at 40,000g and 4°C. The supernatant was applied to a Con A Sepharose column (25 × 100 mm) and eluted as described (Van den Ende et al., 1996). Active fractions were pooled, adjusted to pH 7.0 with concentrated Tris, and applied on Mono Q1 (HR 5/5, Pharmacia AB, Uppsala) equilibrated with 20 mm Tris-HCl buffer, pH 7.0. Proteins were eluted as described (Van den Ende et al., 1996). 1-FEH w1 (fraction 15) and 1-FEH w2 (fractions 18–20) were diluted five times with 20 mm His-HCl buffer, pH 6.0, and subsequently loaded again on Mono Q2 (1-FEH w1) and Mono Q3 (1-FEH w2), equilibrated with 20 mm His-HCl buffer, pH 6.0. Finally, the active fractions 17 and 18 from Mono Q3 were diluted five times in 50 mm sodium-acetate buffer, pH 5.2, and loaded on Mono Q4 equilibrated with 50 mm sodium-acetate buffer, pH 5.2. Whenever possible, enzymes were kept on ice throughout the whole purification. Sodium-azide (0.02%, w/v) was added to all buffers to prevent microbial growth. SDS-PAGE was performed on 12.5% (w/v) polyacrylamide gels and stained with Coomassie Brilliant Blue-R250 as described (Van den Ende et al., 1996).
Carbohydrate Analyses and Enzyme Activity Determinations
Protein extracts of field-grown wheat stems were precipitated with ammonium sulfate, redissolved, and desalted to remove endogenous substrates as described (Van den Ende and Van Laere, 1996b). To measure the activities of 1-FEH, 6-FEH, and invertase, aliquots were incubated with 3% (w/v) commercial chicory root inulin (Sigma-Aldrich, St. Louis), 10 mm levan, and 100 mm Suc in 50 mm sodium-acetate buffer, pH 5.0, for different time intervals at 30°C. Sodium-azide (0.02%, w/v) was added to all buffers to prevent microbial growth. Fru formation was determined by AEC-PAD, and proteins were determined as described (Van den Ende and Van Laere, 1996b). Throughout enzyme purification, aliquots were taken for activity determination. Enzymatic activity is expressed in units, defined as the amount of enzyme that formed 1 μmol Fru min−1.
Fructan Substrates and Structure Determination
Wheat stem low-DP fructan (anthesis) was obtained as follows. Boiling water extracts were prepared. After centrifugation (20 min at 40,000g and 4°C), yeast α-glucosidase was added to the supernatant (1.5 units mL−1) and incubated overnight at 37°C to specifically degrade Suc. The sample was subsequently loaded and eluted from a Ca-Dowex column as described by Timmermans et al. (2001). Hexose-free fractions were pooled and used as a substrate for wheat 1-FEH enzymes. 1-Kestose and 1,1-nystose were prepared from Neosugar P (Beghin-Meiji Industries, Paris) by preparative reversed-phase HPLC (Nucleosil 7 C18, 250 × 12.7 mm) with water as solvent and a flow rate of 2 mL min−1. Manually collected fractions were pooled and lyophilized. Low-Mr levan and 6-kestose were generous gifts from Dr. M. Iizuka (Iizuka et al., 1993). Neokestose was kindly provided by Dr. N.J. Chatterton. Levanbiose (F2) was purified as described by Timmermans et al. (2001). Structures of branched wheat fructans were derived from the work of Bancal et al. (1993), but reconfirmation occurred by manual collection of AEC-PAD peaks and enzymatic hydrolysis by the purified wheat 1-FEHs.
Total fructan content throughout wheat stem development was estimated by total Fru liberated after mild acid hydrolysis in 0.06 n HCl at 75°C for 1.5 h. Fru and Suc present before hydrolysis were subtracted.
Q-TOF Analyses on Tryptic Fragments
The SDS-PAGE protein bands of 1-FEH w1 and w2 (both 70 kD) exhibiting 1-FEH activity were subjected to MS identification. The Coomassie Brilliant Blue-stained protein bands were excised, trypsinized, extracted, desalted, and analyzed on Q-TOF as previously described (Van den Ende et al., 2001). Sequence information was derived from the MS/MS spectra with the aid of the MaxEnt 3 (deconvoluting and deisotoping of data) and PepSeq software from the Micromass BioLynx software package. To assist MS/MS-based glycosylation characterization, we have developed a software application named Sweet Substitute (S. Clerens, W. Van den Ende, P.D. Verhaert, L. Geenen, F. Vandesande, and L. Arckens, unpublished data).
RNA Isolation, RT-PCR, and Cloning
Total RNA was isolated from wheat stems (24 d after anthesis) by using the RNeasy Plant Mini kit (Qiagen USA, Valencia, CA). The conserved amino acid sequences HFQP (N-terminal), AFNN, and VFNN (C-terminal) were used to make the degenerated primers HFQP (5′-GSWTWYCAYTTYCARCC-3′), CTERMA (5′-GTCNCCR-TTRTTRAANGC-3′), and CTERMV (5′-GTCNCCRTTRTTRAANAC-3′) occurring in dicot 1-FEHs and in plant cell wall invertases. One-step RT-PCR was performed (Access RT-PCR System, Promega, Madison, WI) with HFQP-CTERMA and HFQP-CTERMV. RT reaction was at 48°C. For PCR, the following conditions were used: 94°C, 3 min; followed by 35 cycles: 94°C, 40 s; 48°C, 40 s; and 72°C, 2 min. Final extension was at 72°C, 10 min (PCR Access kit, Promega). Only in the HFQP-CTERMA condition, a very weak band of about 1,500 bp was visible. Therefore, seminested PCR was attempted on this PCR product with primers ECPD (derived from the conserved amino acid sequence WECPD: 5′-GAATGTGGGARTGYCCNGA-3′) and CTERMA. The 960-bp band was ligated in the TOPO-TA vector and transformed to Escherichia coli (TOPO-TA cloning kit, Invitrogen, Groningen, The Netherlands). Plasmid was extracted using Wizard Plus SV Minipreps (Promega). Partial sequencing yielded two types of clones with high similarity to cell wall-type invertases and dicot 1-FEHs (named 12.16 and 12.18). A mixture of PCR products derived from these clones was subsequently used as hybridization probes to screen a winter wheat cDNA library by plaque hybridization (described by Kawakami and Yoshida [2002]). Sequences were determined and analyzed as described (Kawakami and Yoshida, 2002).
One of the positive clones (named B62) resembled 12.16 but was still a partial cDNA (5′ part missing). Therefore, the cDNA library was subsequently rescreened using this partial cDNA as probe, and then a full-length sequence clone (named C42) corresponding to the 12.16 sequence of the original probe was obtained. Later, protein purification and molecular mass determination of tryptic fragments of the purified proteins revealed that the protein 1-FEH w2 corresponds to clone C42 and 1-FEH w1 corresponds to clone B62. For unknown reasons, efforts to obtain full-length B62 by screening the wheat cDNA library remained unsuccessful. Therefore, the missing 5′-sequence part of 1-FEH w1 was cloned by two-step RT-PCR and the use of Pfu polymerase as previously described (Van den Ende et al., 2001). A degenerate sense primer (5′-GCCGCCATGGCNCARGC-3′) was chosen based on the start codon region in 1-FEH w2. This was combined with a 1-FEH w1-specific antisense primer (5′-AACAACAACTGCTTGCCATT-3′). Sequence analysis revealed a perfect match with the B62 partial sequence. Sequences were deposited in the EMBL sequence library (accession no. AJ516025 for 1-FEH w1 and accession no. AJ508387 for 1-FEH w2).
Statistics
Values in the graphs represent means of three replicates on one enzyme preparation. The corresponding se is indicated. Experiments were repeated on different enzyme preparations with consistent results.
Supplementary Material
ACKNOWLEDGMENTS
We thank Edgard Nackaerts for his technical assistance. The help offered by Pierre Bancal for fructan structure determination on AEC-PAD is highly appreciated.
Footnotes
This work was supported by the Fund for Scientific Research Flanders. W.V.d.E. is a Postdoc supported by the Fund for Scientific Research Flanders.
The online version of this article contains Web-only data. The supplemental material is available at www.plantphysiol.org.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.015305.
LITERATURE CITED
- Bancal P, Carpita NC, Gaudillère JP. Differences in fructan accumulated in induced and field-grown wheat plants: an elongation-trimming pathway for their synthesis. New Phytol. 1992;120:313–321. [Google Scholar]
- Bancal P, Gibeaut DM, Carpita NC. Analytical methods for the determination of fructan structure and biosynthesis. In: Suzuki M, Chatterton NJ, editors. Science and Technology of Fructans. Boca Raton, FL: CRC Press; 1993. pp. 83–118. [Google Scholar]
- Bancal P, Triboï E. Temperature effect on fructan oligomer contents and fructan-related enzyme activities in stems of wheat. New Phytol. 1993;123:247–253. [Google Scholar]
- Bonnett GD, Simpson RJ. Fructan-hydrolyzing activities from Lolium rigidum Gaudin. New Phytol. 1993;123:443–451. doi: 10.1111/j.1469-8137.1993.tb03756.x. [DOI] [PubMed] [Google Scholar]
- Chatterton NJ, Harrison PA. Fructan oligomers in Poa ampla. New Phytol. 1997;136:3–10. [Google Scholar]
- Claessens G, Van Laere A, De Proft M. Purification and properties of an inulinase from chicory roots (Cichorium intybus L.) J Plant Physiol. 1990;136:35–39. [Google Scholar]
- De Roover J, De Winter M, Van Laere A, Timmermans JW, Van den Ende W. Purification and properties of a second fructan exohydrolase from the roots of Cichorium intybus L. Physiol Plant. 1999;106:28–34. [Google Scholar]
- Edelman J, Jefford TG. The mechanism of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol. 1968;67:517–531. [Google Scholar]
- Farrar SC, Farrar JF. Carbon fluxes in leaf blades of barley. New Phytol. 1985;100:271–283. [Google Scholar]
- Hellwege EM, Czapla S, Jahnke A, Willmitzer L, Heyer AG. Transgenic potato (Solanum tuberosum) tubers synthesize the full spectrum of inulin molecules naturally occurring in globe artichoke (Cynara scolymus) roots. Proc Natl Acad Sci USA. 2000;97:8699–8704. doi: 10.1073/pnas.150043797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry G. Evolutionary origins and natural functions of fructans: a climatological, biogeographic and mechanistic appraisal. New Phytol. 1993;123:3–14. [Google Scholar]
- Henson CA. Purification and properties of barley stem fructan exohydrolase. J Plant Physiol. 1989;134:186–191. [Google Scholar]
- Henson CA, Livingston DP. Purification and characterization of an oat fructan exohydrolase that preferentially hydrolyzes β-2,6 fructans. Plant Physiol. 1996;110:639–644. doi: 10.1104/pp.110.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson CA, Livingston DP. Characterization of a fructan exohydrolase purified from barley stems that hydrolyzes multiple fructofuranosidic linkages. Plant Physiol Biochem Paris. 1998;36:715–720. [Google Scholar]
- Hincha DK, Zuther E, Hellwege EM, Heyer AG. Specific effects of fructo-and gluco-oligosaccharides in the preservation of liposomes during drying. Glycobiology. 2002;12:103–110. doi: 10.1093/glycob/12.2.103. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Yamaguchi H, Ono S, Minamiura N. Production and isolation of levan by use of levansucrase immobilized on the ceramic support SM-10. Biosci Biotechnol Biochem. 1993;57:322–324. doi: 10.1271/bbb.57.322. [DOI] [PubMed] [Google Scholar]
- Jeong B-R. Fructan metabolism in wheat (Triticum aestivum L.). PhD thesis. West Lafayette, IN: Perdue University; 1991. [Google Scholar]
- Jeong B-R, Housley TL. Purification and characterization of wheat β (2,1) fructan fructan fructosyl transferase activity. Plant Physiol. 1992;100:199–204. doi: 10.1104/pp.100.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Yoshida M. Molecular characterization of sucrose:sucrose 1-fructosyltransferase and sucrose:fructan 6-fructosyltransferase associated with fructan accumulation in winter wheat during cold hardening. Biosci Biotechnol Biochem. 2002;66:2297–2305. doi: 10.1271/bbb.66.2297. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Yoshida M, Terami F. Molecular cloning of fructan:fructan fructosyltransferase from wheat. Plant Cell Physiol Suppl S. 2002;43:S95–S95. [Google Scholar]
- Livingston DP, Henson CA. Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol. 1998;116:403–408. [Google Scholar]
- Mann M, Wilm M. Error-tolerant identification of peptides in sequence databases by peptide sequence tags. Anal Chem. 1994;66:4390–4399. doi: 10.1021/ac00096a002. [DOI] [PubMed] [Google Scholar]
- Marx SP, Nösberger J, Frehner M. Seasonal variation of fructan-β-fructosidase (FEH) activity and characterization of a β-(2,1)-linkage specific FEH. New Phytol. 1997a;135:267–277. [Google Scholar]
- Marx SP, Nösberger J, Frehner M. Hydrolysis of fructan in grasses: a β-(2,6)-linkage specific fructan-β-fructosidase from stubble of Lolium perenne. New Phytol. 1997b;135:279–290. [Google Scholar]
- Nagaraj VJ, Riedl R, Boller T, Wiemken A, Meyer AD. Light and sugar regulation of the barley sucrose:fructan 6-fructosyltransferase promoter. J Plant Physiol. 2001;158:1601–1607. [Google Scholar]
- Pavis N, Boucaud J, Prud'homme MP. Fructans and fructan-metabolizing enzymes in leaves of Lolium perenne. New Phytol. 2001a;150:97–109. [Google Scholar]
- Pavis N, Chatterton NJ, Harrison PA, Baumgartner S, Praznik W, Boucaud J, Prud'homme MP. Structure of fructans in roots and leaf tissues of Lolium perenne. New Phytol. 2001b;150:83–95. [Google Scholar]
- Penson SP, Cairns AJ. Fructan biosynthesis in excised leaves of wheat (Triticum aestivum L.): a comparison of de novo synthesis in vivo and in vitro. New Phytol. 1994;128:395–402. doi: 10.1111/j.1469-8137.1994.tb02985.x. [DOI] [PubMed] [Google Scholar]
- Pollock CJ, Cairns AJ. Fructan metabolism in grasses and cereals. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:77–101. [Google Scholar]
- Prud'homme MP, Gonzalez B, Billard JP, Boucaud J. Carbohydrate content, fructan and sucrose enzyme-activities in roots, stubble and leaves of ryegrass (Lolium perenne L.) as affected by source sink modification after cutting. J Plant Physiol. 1992;140:282–291. [Google Scholar]
- Roberfroid MB, Van Loo JAE, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr. 1998;128:11–19. doi: 10.1093/jn/128.1.11. [DOI] [PubMed] [Google Scholar]
- Schnyder H. The role of carbohydrate storage and redistribution in the source-sink relations of wheat and barley during grain filling: a review. New Phytol. 1996;123:233–245. [Google Scholar]
- Simpson RJ, Bonnett GD. Fructan exohydrolase from grasses. New Phytol. 1993;123:453–469. doi: 10.1111/j.1469-8137.1993.tb03757.x. [DOI] [PubMed] [Google Scholar]
- Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A. Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA. 1995;92:11652–11656. doi: 10.1073/pnas.92.25.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans JW, Slaghek T, Iizuka M, De Roover J, Van Laere A, Van den Ende W. Isolation and structural analysis of new fructans produced by chicory. J Carbohydr Chem. 2001;20:375–395. [Google Scholar]
- Van den Ende W, Michiels A, De Roover J, Van Laere A. Fructan biosynthetic and breakdown enzymes in dicots evolved from different invertases: expression of fructan genes throughout chicory development. Sci World J. 2002a;2:1273–1287. doi: 10.1100/tsw.2002.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, Michiels A, De Roover J, Van Laere A. Cloning of chicory (Cichorium intybus L.) vacuolar invertase. Physiol Plant. 2002b;115:504–512. doi: 10.1034/j.1399-3054.2002.1150404.x. [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Michiels A, De Roover J, Verhaert P, Van Laere A. Cloning and functional analysis of chicory root fructan 1-exohydrolase I (1-FEH I): a vacuolar enzyme derived from a cell-wall invertase ancestor? Mass fingerprint of the 1-FEH I enzyme. Plant J. 2000;24:447–456. doi: 10.1046/j.1365-313x.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- Van den Ende W, Michiels A, Van Wonterghem D, Clerens S, De Roover J, Van Laere A. Defoliation induces 1-FEH II (fructan 1-exohydrolase II) in witloof chicory roots: cloning and purification of two isoforms (1-FEH IIa and 1-FEH IIb). Mass fingerprint of the 1-FEH II enzymes. Plant Physiol. 2001;126:1186–1195. doi: 10.1104/pp.126.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, Van Laere A. De novo synthesis of fructans from sucrose in vitro by a combination of two purified enzymes (SST and FFT) from chicory roots (Cichorium intybus L.) Planta. 1996a;200:335–342. [Google Scholar]
- Van den Ende W, Van Laere A. Fructan synthesizing and degrading activities in chicory roots (Cichorium intybus L.) during growth, storage and forcing. J Plant Physiol. 1996b;149:43–50. [Google Scholar]
- Van den Ende W, Van Wonterghem D, Verhaert P, Dewil E, Van Laere A. Purification and characterization of fructan:fructan fructosyl transferase from chicory roots (Cichorium intybus L.) Planta. 1996;199:493–502. [Google Scholar]
- van der Meer IM, Koops AJ, Hakkert JC, Van Tunen AJ. Cloning of the fructan biosynthesis pathway of Jerusalem artichoke. Plant J. 1998;15:489–500. doi: 10.1046/j.1365-313x.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- Van Laere A, Van den Ende W. Inulin metabolism in dicots: chicory as a model system. Plant Cell Environ. 2002;25:803–815. [Google Scholar]
- Vergauwen R, Van den Ende W, Van Laere A. The role of fructans in flowering of Campanula rapunculoides. J Exp Bot. 2000;51:1261–1266. [PubMed] [Google Scholar]
- Vijn I, Smeekens SCM. Fructan: more than a reserve carbohydrate? Plant Physiol. 1999;120:351–359. doi: 10.1104/pp.120.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Wiemken A. Properties and subcellular localization of fructan hydrolase in the leaves of barley (Hordeum vulgare L. cv. Gerbel) J Plant Physiol. 1986;123:429–439. [Google Scholar]
- Wagner W, Wiemken A. Fructan metabolism in expanded primary leaves of barley (Hordeum vulgare L. cv. Gerbel): change upon aging and spatial organization along the leaf blade. J Plant Physiol. 1989;134:237–242. [Google Scholar]
- Wei JZ, Chatterton NJ, Harrison PA, Wang RRC, Larson SR. Characterization of fructan biosynthesis in big bluegrass (Poa secunda) J Plant Physiol. 2002;159:705–715. [Google Scholar]
- Wiemken A, Frehner M, Keller F, Wagner W. Fructan metabolism, enzymology and compartmentation. Curr Top Plant Biochem Physiol. 1986;5:17–37. [Google Scholar]
- Winzeler M, Dubois D, Nösberger J. Absence of fructan degradation during fructan accumulation in wheat stems. J Plant Physiol. 1990;136:324–329. [Google Scholar]
- Yamatoto S, Mino Y. Mechanism of phleinase induction in the stem base of orchard grass after defoliation. J Plant Physiol. 1989;134:258–260. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.