Abstract
Phytochelatin (PC) plays an important role in heavy metal detoxification in plants and other living organisms. Therefore, we overexpressed an Arabidopsis PC synthase (AtPCS1) in transgenic Arabidopsis with the goal of increasing PC synthesis, metal accumulation, and metal tolerance in these plants. Transgenic Arabidopsis plants were selected, designated pcs lines, and analyzed for tolerance to cadmium (Cd). Transgenic pcs lines showed 12- to 25-fold higher accumulation of AtPCS1 mRNA, and production of PCs increased by 1.3- to 2.1-fold under 85 μm CdCl2 stress for 3 d when compared with wild-type plants. Cd tolerance was assessed by measuring root length of plants grown on agar medium containing 50 or 85 μm CdCl2. Pcs lines paradoxically showed hypersensitivity to Cd stress. This hypersensitivity was also observed for zinc (Zn) but not for copper (Cu). The overexpressed AtPCS1 protein itself was not responsible for Cd hypersensitivity as transgenic cad1-3 mutants overexpressing AtPCS1 to similar levels as those of pcs lines were not hypersensitive to Cd. Pcs lines were more sensitive to Cd than a PC-deficient Arabidopsis mutant, cad1-3, grown under low glutathione (GSH) levels. Cd hypersensitivity of pcs lines disappeared under increased GSH levels supplemented in the medium. Therefore, Cd hypersensitivity in pcs lines seems due to the toxicity of PCs as they existed at supraoptimal levels when compared with GSH levels.
Contamination of soils and waters with toxic heavy metals contributes to serious worldwide environmental and human health problems. These heavy metal pollutants have been discharged mainly by mining and combustion of fossil fuels during the period of global industrialization.
A promising new technology, referred to as phytoremediation, offers promise for clean up of polluted areas in a cost-effective and environment-friendly manner (Salt et al., 1995, 1998; Raskin et al., 1997). This technology involves removal of toxic heavy metals from contaminated soils and waters, or rendering them harmless by accumulating, chelating, or transforming these contaminants into biologically inactive forms through green plants. One possible approach for phytoremediation is to use “hyperaccumulators,” plant species that have evolved to accumulate high concentrations of heavy metals in their biomass. However, most known hyperaccumulators tend to grow slowly, and produce relatively low biomass (Cunningham et al., 1995). Due to these limitations, genetic and molecular investigations of plant defense mechanisms involved in heavy metal stress have been under way to improve the efficiency of phytoremediation.
Plants can produce Cys-rich peptides such as glutathione (GSH), phytochelatins (PCs), or metallothioneins (MTs) for detoxification or homeostasis of heavy metals (Rauser, 1999; Cobbett, 2000a, 2000b). PCs are a family of small enzymatically synthesized peptides having a general structure of (γ-Glu-Cys)n-Gly where n equals 2 to 11 (Rauser, 1990). These peptides are rapidly synthesized in response to toxic levels of heavy metals in all tested plants (Zenk, 1996; Cobbett, 1999). The role of PCs in heavy metal tolerance has been well characterized in Cd-sensitive mutants of Arabidopsis, cad1 and cad2 (Howden and Cobbett, 1992; Howden et al., 1995; Cobbett et al., 1998). These mutants are deficient in PC production due to mutations in γ-glutamyl-Cys synthetase in the case of cad2 mutants, or in PC synthase in the case of cad1 mutants. PCs form stable complexes with heavy metals in the cytosol, and these are subsequently sequestered into the vacuole (Grill et al., 1985; Steffens, 1990; Zenk, 1996; Cobbett, 2000a, 2000b). γ-Glu-Cys dipeptidyl transpeptidase (EC 2.3.2.15), named PC synthase, catalyzes the synthesis of PCs by transferring a γ-Glu-Cys moiety of GSH to GSH or to other PCs (Grill et al., 1989; Zenk, 1996). Genes encoding PC synthase have recently been cloned from Arabidopsis (AtPCS1), wheat (Triticum aestivum; TaPCS1), Schizosaccharomyces pombe (SpPCS1), and Caenorhabditis elegans (CePCS1; Clemens et al., 1999, 2001; Ha et al., 1999; Vatamaniuk et al., 1999, 2001).
There are several reports of transgenic plants showing higher accumulation and tolerance for Cd as a result of manipulating the expression of genes involved in PC synthesis. Overexpression of genes encoding O-acetyl-Ser(thiol) lyase (Domínguez-Solís et al., 2001), γ-glutamyl-Cys synthetase (Zhu et al., 1999b), and GSH synthetase (Zhu et al., 1999a) in transgenic plants have been reported to increase synthesis of PCs or GSH under Cd stress. Therefore, overexpression of PC synthase in plants may also be a promising approach for manipulating metal tolerance and accumulation in plants used for phytoremediation.
RESULTS
Selection of Transgenic Lines Overexpressing Arabidopsis PC Synthase (AtPCS1)
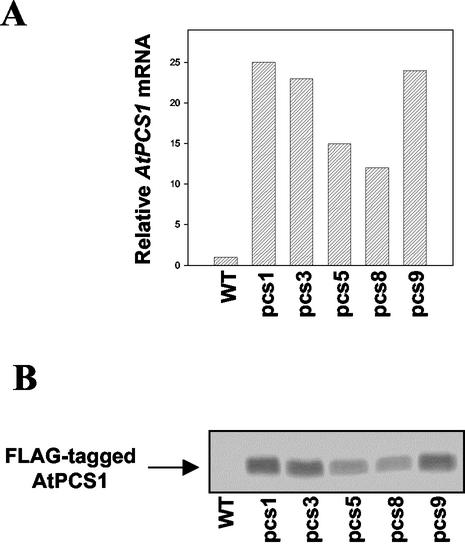
We have previously developed 16 independent transgenic Arabidopsis lines, designated pcs1 to pcs16, expressing a C-terminal FLAG (DYKDDDL)-tagged full-length AtPCS1 genomic DNA driven under the control of a 2.0-kb AtPCS1 promoter. Even though the AtPCS1 promoter is not as strong as the cauliflower mosaic virus 35S promoter, some transgenic lines have shown higher levels of ectopic expression of AtPCS1. Therefore, lines showing increased AtPCS1 mRNA levels by approximately 24-fold (pcs1, pcs3, and pcs9) or approximately 13-fold (pcs5 and pcs8), respectively, compared with wild-type plants have been selected (Fig. 1A). After western-blot analysis, pcs lines have exhibited production of FLAG-tagged AtPCS1 (Fig. 1B).
Figure 1.
Overexpression of the AtPCS1 gene in transgenic pcs lines compared with wild-type (WT) Arabidopsis plants. A, Total RNA was extracted from 10-d-old seedlings, and 10 μg of total RNA was used for RNA-blot analysis. The blot was hybridized with the AtPCS1 cDNA probe, and was subsequently probed with a β-tubulin cDNA to confirm equal RNA loading of the gel. The numbers along the y axis correspond to the relative intensity of the signal after it has been corrected for the β-tubulin signal. B, Western-blot analysis of the same transgenic lines and wild-type (WT) examined in A. Equal amounts of the total protein (5 μg) was used in each lane, separated on a 10% (w/v) SDS-PAGE gel, blotted, and probed with an anti-FLAG M2 antibody to detect the FLAG-tagged AtPCS1.
Overexpression of AtPCS1 Leads to Increased Production of Nonprotein Thiol (NPT) and PC under Cd Stress
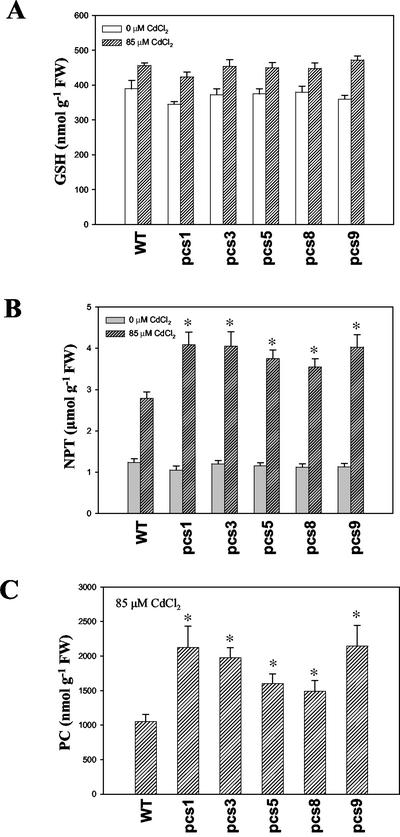
To investigate effects of AtPCS1 overexpression on production of heavy metal-binding peptides, total contents of GSH, NPT, and PC were analyzed in pcs lines and wild-type plants grown with or without Cd stress. Under control conditions (0 μm Cd), GSH and NPT contents in pcs lines were not significantly different from those in wild-type Arabidopsis (Fig. 2, A and B). When plants were subjected to 85 μm CdCl2 treatment for 3 d, a slight increase in GSH content was observed in pcs lines and wild-type Arabidopsis compared with untreated plants (Fig. 2A). Also, pcs lines and wild-type plants showed increased levels of NPT under Cd stress than without Cd; moreover, NPT levels in pcs lines were substantially higher than those detected in wild-type plants (Fig. 2B). To further investigate whether increased NPT contents in Arabidopsis subjected to Cd stress actually reflected increased PC contents, total PCs were measured in pcs lines and wild-type plants. Pcs lines showed approximately 1.3- to approximately 2.1-fold increase in total PCs production under Cd stress compared with wild-type plants (Fig. 2C), whereas no PC was detected in pcs lines or wild-type plants without Cd stress (data not shown).
Figure 2.
Effects of AtPCS1 overexpression on GSH, NPT, and PC contents with or without Cd treatment. Plants grown on Murashige and Skoog agar medium for 10 d were transferred to a fresh medium containing 0 or 85 μm CdCl2, and were incubated for another 3 d. Whole seedlings of transgenic pcs lines and wild-type (WT) were used for analysis of total GSH (A), NPT (B), and PC (C). Values correspond to means ± se of four samples. An asterisk indicates significantly different (P < 0.05) from WT of the same treatment.
Cd Hypersensitivity Was Observed in pcs Lines Overexpressing Higher Levels of the AtPCS1 Gene
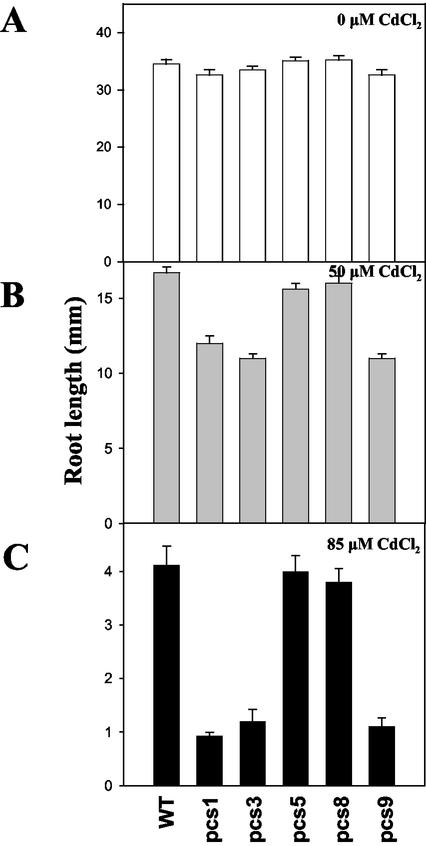
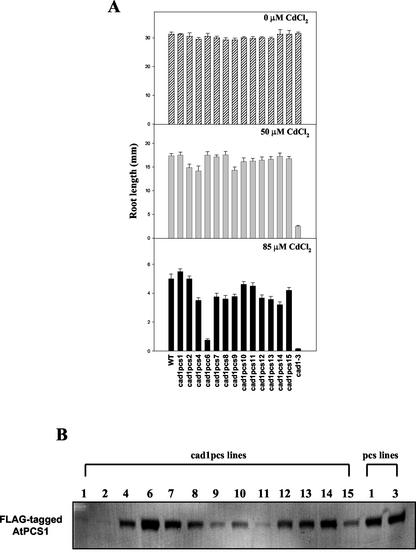
In a preliminary experiment, higher differences in plant growth were observed between wild-type Arabidopsis and Cd-sensitive mutant, cad1-3, when grown under various concentrations of Cd (ranging between 50 and 85 μm). Thus, the Cd concentrations of 50 and 85 μm were selected to evaluate Cd tolerance in transgenic pcs lines and wild-type plants. When seeds were grown under control condition (0 μm Cd), all seedlings of pcs lines grew and developed normally, and overall root length in these lines was not significantly different from that of wild-type seedlings (Fig. 3A). When seeds were subjected to 50 μm Cd, root growth of all seedlings was affected; however, root length of several pcs lines (pcs1, pcs3, and pcs9) was significantly inhibited compared with wild-type seedlings (Fig. 3B). When Cd concentration was increased to 85 μm, a similar pattern of root growth inhibition was observed (Fig. 3C). Thus, higher levels of ectopic expression of PC synthase paradoxically resulted in increased sensitivity to Cd.
Figure 3.
Effect of AtPCS1 overexpression on root elongation of plants grown under Cd stress. Seeds were germinated on Murashige and Skoog agar medium containing 0 μm (A), 50 μm (B), or 85 μm (C) CdCl2, and petri dishes were placed in a vertical orientation upon onset of growth. After 10 d of growth, root lengths of pcs lines and WT were measured. Values correspond to means ± se of 10 plants.
Overexpression of AtPCS1 Also Leads to Increased Sensitivity to Zn But Not to Cu
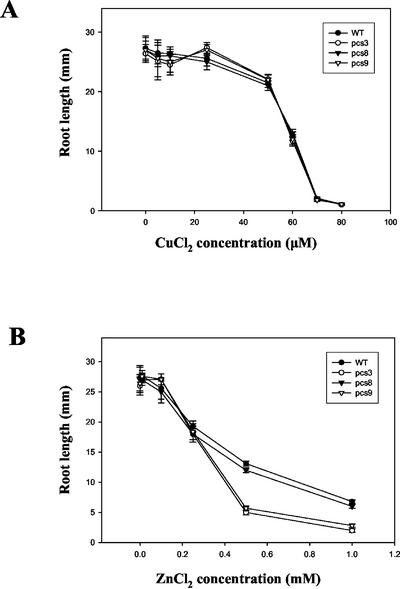
To explore whether Cd hypersensitivity shown in several pcs lines also applied to other metals such as Cu and Zn, the effects of these metals on growth of pcs3, pcs8, pcs9, and wild-type Arabidopsis was investigated. pcs lines and wild-type seedlings did not show significant differences in root growth at various levels of CuCl2 (Fig. 4A). However, pcs3 and pcs9 showed Zn hypersensitivity at 0.5 and 1.0 mm ZnCl2, whereas pcs8 did not (Fig. 4B). Under these experimental conditions, pcs1 also showed Zn hypersensitivity similar to that observed for pcs3, but neither pcs5 nor pcs8 did (data not shown).
Figure 4.
Comparative sensitivity of transgenic pcs lines and wild-type (WT) for Cu and Zn. Seeds were germinated and grown in a vertical orientation for 8 d on Murashige and Skoog agar medium containing various concentrations of CuCl2 (A) or ZnCl2 (B), and root lengths were then measured. Values correspond to means ± se of 10 plants.
Overexpressed AtPCS1 Protein Is Not Toxic to Plants Grown under Cd Stress
To investigate whether Cd hypersensitivity observed in several pcs lines was due to toxicity of the overexpressed FLAG-tagged AtPCS1 protein itself, a functional complementation test was conducted using a cad1-3 Arabidopsis mutant defective in PC synthase. Thirteen independent T2 transgenic cad1-3 lines, designated cad1pcs, were developed after transformation of cad1-3 plants with the AtPCS1 construct. Seedlings of all cad1pcs lines showed normal phenotype, and their root growth was not significantly different from that of cad1-3 and wild-type seedlings (Fig. 5A). When cad1pcs lines were subjected to 50 or 85 μm Cd, no significant differences in Cd sensitivity were observed compared with wild type, except for cad1pcs6 (Fig. 5A). Line cad1pcs6 showed Cd hypersensitivity at 85 μm Cd, but not at 50 μm Cd, even though it was less sensitive to Cd than untransformed cad1-3 seedlings.
Figure 5.
Functional complementation of cad1-3 by AtPCS1construct. A, Cad1-3 mutants were transformed with C-terminal FLAG-tagged genomic AtPCS1 under the control of a 2.0-kb AtPCS1 promoter. After screening of transgenic (cad1pcs1) lines on kanamycin, selected T2 seeds were used for testing the recovery of Cd hypersensitivity in cad1-3 plant. Approximately 20 seeds were germinated and grown in a vertical orientation for 10 d on Murashige and Skoog agar medium containing 0 μm (top), 50 μm (middle), or 85 μm (bottom) CdCl2, and root lengths were then measured. Values correspond to means ± se. B, Western-blot analysis was performed on various cad1pcs lines to evaluate the amount of the expressed C-terminal FLAG-tagged AtPCS1. Proteins were extracted from 10-d-old seedlings and were analyzed as described in Figure 1. The pcs1 and pcs3 lines were used as standards.
The AtPCS1 transgene was able to complement the cad1 mutation in most of the analyzed transgenic lines, thus indicating that this construct was functional and not defective. The Cd hypersensitivity of cad1pcs6 at 85 μm Cd suggested that this line was expressing a higher level of FLAG-tagged AtPCS1 than pcs lines. To verify this hypothesis, western-blot analysis was conducted for all cad1pcs lines, and pcs1 and pcs3 lines were used as standards for comparison. Variability in levels of FLAG-tagged AtPCS1 expression was observed in cad1pcs lines (Fig. 5B). Lines cad1pcs7 and cad1pcs14 showed similar levels of expression to those of pcs1 and pcs3 lines. Among all cad1pcs lines, cad1pcs6 showed the highest level of FLAG-tagged AtPCS1 expression, and this was even higher than was detected in wild-type pcs lines.
Thus, overexpression of FLAG-tagged AtPCS1 levels observed in pcs lines does not contribute to Cd hypersensitivity, as some cad1pcs lines expressing the same levels of FLAG-tagged AtPCS1 as those detected in pcs lines are not hypersensitive to Cd.
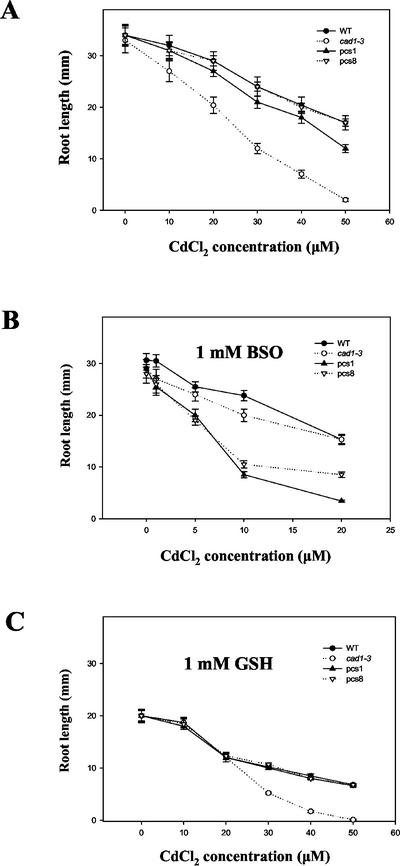
Pcs Lines Were More Sensitive to Cd Than Cad1-3 under Conditions of Low Intracellular GSH Levels, Whereas Cd Hypersensitivity of pcs Lines Disappeared When Medium Was Supplemented with GSH
The Cd hypersensitivity of pcs lines seemed to be due to toxicity of PC at supraoptimal concentrations. To investigate this hypothesis, we compared Cd sensitivity between PC-deficient cad1-3 mutant and pcs lines under conditions of low intracellular GSH concentrations. Cad1-3 was more sensitive to Cd than all of pcs lines grown under various concentrations of Cd (Fig. 6A). However, pcs lines were more sensitive to Cd than cad1-3 under reduced intracellular GSH levels after treatment with 1 mm buthionine sulfoximine (BSO), an inhibitor of γ-glutamyl-Cys synthetase (Griffith, 1982; Fig. 6B). In this experiment, pcs3 and pcs9 showed similar responses to that of pcs1, whereas pcs5 showed similar response to that of pcs8. Adding 1 mm BSO to the medium slightly inhibited plant growth of wild-type and pcs lines. Moreover, this treatment reduced total GSH content of wild-type and pcs lines to approximately 42% of those observed in nontreated plants. Adding 1 mm BSO plus 20 μm CdCl2 resulted in approximately 35% reduction in GSH levels compared with nontreated plants (data not shown). The Cd hypersensitivity of several pcs lines (e.g. pcs1 and pcs8) disappeared after treatment of plants with 1 mm GSH, whereas cad1-3 continued to show higher sensitivity to Cd (20–50 μm) compared with wild-type Arabidopsis (Fig. 6C). Moreover, total GSH contents of pcs lines and wild-type plants increased by approximately 20% after treatment with 1 mm GSH, and by approximately 60% after treatment with 1 mm GSH plus 50 μm CdCl2 compared with nontreated plants (data not shown).
Figure 6.
Effect of BSO and GSH on Cd sensitivity of Arabidopsis plants. Seeds were germinated and grown for 10 d on Murashige and Skoog agar medium containing various concentrations of CdCl2 (A) with 1 mm BSO (B) or 1 mm GSH (C). Petri dishes were placed in a vertical orientation at the onset of seed germination. Root length was measured after 10 d of growth. Values correspond to means ± se of 10 plants.
DISCUSSION
The Arabidopsis PC synthase gene, AtPCS1, was manipulated to increase PC production, and thereby conferring enhanced Cd accumulation and tolerance to Arabidopsis plants. In fact, PC production increased in transformed plants overexpressing the AtPCS1 gene; however, there was no correlation between the level of PC production and the level of the AtPCS1 gene transcript. It was assumed that the normal level of Arabidopsis PC synthase expression was sufficient to synthesize the required PCs in response to supplemented levels of Cd. Therefore, the dramatic increase of the PC synthase transcript (or protein) did not comparably affect the synthesis of PCs. Thus, the level of the true substrate, GSH-Cd, for PC synthase might be the limiting factor determining the rate of PCs synthesis (Vatamaniuk et al., 2000).
The results obtained in this study are in contrast with those previously reported by others (Zhu et al., 1999a, 1999b). It has been observed that increased Cd tolerance is related to elevated PC synthesis; however, this study shows that the increased capacity of PC synthesis does not lead to Cd tolerance, but paradoxically leads to Cd hypersensitivity. In this study, the hypersensitivity of several pcs lines has also been noted for Zn, but not for Cu. In yet an unpublished study, we have observed that a Cd-tolerant transgenic Arabidopsis overexpressing O-acetyl-Ser(thiol) lyase (Domínguez-Solís et al., 2001) is not tolerant to Cu stress, whereas the Cd-sensitive PC-deficient Arabidopsis mutant cad2-1 (Cobbett et al., 1998) is resistant to Cu stress when compared with wild-type plants. Even though Cu stress can induce PC synthesis (Grill et al., 1987; De Vos et al., 1992; Hartley-Whitaker et al., 2001) and produces a stable Cu-PC complex (Scarano and Morelli, 1998), there is no conclusive evidence that PC functions in Cu tolerance because wild-type and cad1 mutants have very similar/identical responses to Cu. Therefore, this study also supports that PC is not a major factor in Cu tolerance in plants.
The plant gene expression construct used for transformation of wild-type Arabidopsis to overexpress PC synthase can functionally complement the cad1-3 mutant. This finding suggests that the construct itself is properly designed, and is functioning appropriately. Furthermore, the increased PC synthase protein levels found in transgenic cad1pcs lines, similar to those detected in pcs1 and pcs3 lines, do not result in Cd hypersensitivity. This suggests that the observed Cd hypersensitivity in pcs lines is not due to increased levels of the PC synthase protein itself, but is likely due to yet another factor.
The phytotoxicity of Cd is generally ascribed to its reactivity with ligands containing oxygen, nitrogen, and sulfur (Van Assche and Clijsters, 1990). This often results in oxidative stress, possibly by generating reactive oxygen species (Toppi and Gabbrielli, 1999). It is also possible that the antioxidative system in plants might have been affected by overexpression of AtPCS1, and this may have resulted in the observed Cd hypersensitivity in pcs lines. However, growth of pcs lines does not significantly differ from that of wild-type plants when these are subjected to stress using the strong oxidant hydrogen peroxide (data not shown). Thus, overexpression of AtPCS1 does not affect the antioxidative system in plants, but this cannot be completely excluded with regard to Cd stress.
The toxicity of PCs components including Gly, Cys, Glu, γ-Glu-Cys, γ-Glu-Gly, Cys-Gly, and GSH on plant growth was evaluated by treating plants used in this study with each of these components (supplied at 1 mm). Except for Gly, all other components inhibited plant growth, and Cys was more toxic than Glu (data not shown). This indicated that the observed Cd hypersensitivity in pcs lines might be related to the toxicity of PCs, as this compound has a high Cys content.
It is being proposed that PC is toxic to plants when present at supraoptimal concentrations as in cases of Cys and GSH. Even though the toxicity of Cys and GSH on plant growth at these supraoptimal concentrations is not well understood, the toxicity of PC may be similar to that of Cys and GSH. Furthermore, the toxicity of PC may depend on the GSH concentration, as low GSH levels resulted in higher observed sensitivity of pcs lines to Cd than the PC-deficient cad1-3 mutant. Moreover, Cd hypersensitivity in pcs lines is eliminated when plants are supplemented with GSH, whereas cad1-3 maintains its Cd hypersensitivity.
The FLAG-tagged AtPCS1 protein is capable of directly binding to heavy metals (Vatamaniuk et al., 1999). Therefore, the observed Cd hypersensitivity in pcs lines is possibly due to the metal binding activity of overexpressed PCS, as this may interfere with metal homeostasis. Moreover, nonspecific protein-protein interactions caused by the modification of the AtPCS1 C-terminal region by the FLAG may also contribute to the observed Cd hypersensitivity in pcs lines.
In another aspect relevant to phytoremediation, we have observed that an increase in the capacity of PC production alone is not enough to result in an increase in Cd tolerance in plants. It is likely that the increased capacity of sequestering PC-metal complexes into the vacuole is also required due to the toxicity of PCs in plants.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Treatments
Plants (Arabidopsis ecotype Columbia) were grown on one-half-strength Murashige and Skoog (1962) agar medium (pH 5.8) and 2% (w/v) Suc in 100 × 100 × 15-mm square plates. These plates were maintained in a growth chamber at 23°C with a 12-h photoperiod provided by cool-white fluorescent tubes at a light intensity of approximately 80 μm−2 s−1. The cad1-3 mutant has been previously described by Howden et al. (1995). Arabidopsis seeds were germinated on Murashige and Skoog agar medium containing heavy metals or other chemicals, and plates were placed vertically on shelves to facilitate comparison of root growth rate.
Cad1-3 Complementation
Plants of cad1-3 mutant were grown in a growth chamber for 7 weeks in 10-cm2 pots containing soil mixture (Sunshine Mix no. 1; Sun Gro Horticulture, Bellevue, WA), as described above. These were used for transformation by the floral-dip method (Clough and Bent, 1998) using Agrobacterium tumefaciens GV3101 (pMP90) harboring a plant gene expression construct (P1::gDNA::FLAG). In this construct, a 2.0-kb AtPCS1 promoter is fused with a C-terminal FLAG (DYKDDDDL)-tagged AtPCS1 genomic DNA, as previously described (Lee et al., 2002). Transformed seeds were selected on Murashige and Skoog agar medium containing 50 mg L−1 kanamycin, and T2 seeds were used for complementation experiments.
RNA-Blot Analysis
For gene expression analysis, RNA was extracted from axenically grown 10-d-old Arabidopsis seedlings using an RNeasy Plant Mini-kit (Qiagen, Valencia, CA). Ten micrograms of total RNA from each sample was separated on a formaldehyde gel, blotted onto a Zeta-Probe membrane (Bio-Rad, Hercules, CA), and immobilized to the membrane by UV crosslinking. The 32P-labeled DNA probes were made using a random primer labeling kit (Invitrogen, Carlsbad, CA). Probes corresponded to a full-length 1.5-kb AtPCS1 cDNA and an expressed sequence tag clone, B64XP (T04000; provided by the Arabidopsis Biological Resource Center, Ohio State University, Columbus), encoding for β-tubulin. The relative intensity of the RNA signal was measured using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Western-Blot Analysis
Total proteins were extracted from axenically grown 10-d-old transgenic and wild-type Arabidopsis seedlings. Plants were ground into a powder in 50 mm Tris-HCl (pH 7.5) extraction buffer using a mortar and pestle. The protein concentration was measured using the Bradford protein assay (Bradford, 1976). Total protein (5 μg) was separated on a 10% (w/v) SDS-PAGE, transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA), and consecutively probed with an anti-FLAG M2 monoclonal antibody (Sigma, St. Louis) and an alkaline phosphatase-linked anti-mouse antibody (Sigma). A chemiluminescence reagent (Renaissance kit; NEN, Boston, MA) was used to detect the immune complex.
GSH, NPT, and PC Analysis
Transgenic and wild-type Arabidopsis seedlings were grown on Murashige and Skoog medium for 10 d, were then transferred to fresh Murashige and Skoog medium containing 0 or 85 μm CdCl2, and were grown for another 3 d. Seedlings were harvested and used for analysis for total GSH and total NPT using a spectrophotometer (Shimadzu, Tokyo), and for total PC using HPLC. Samples of 100 mg were frozen in liquid nitrogen and ground with a mortar and pestle. Three hundred microliters of a solution containing 1 m NaOH and 1 mg L−1 NaBH4 was then added. These homogenates were centrifuged for 5 min at 13,000g at 4°C, and a 300-μL supernatant was acidified by adding 50 μL of 37% (w/v) HCl. This solution was used for spectrophotometric measurement of GSH and NPT. For analysis of total NPT content, a 10 μL solution was added to 500 μL of 6 mm 5,5′-dithiobis(2-nitrobenzoic acid), Ellman's reagent (Ellman, 1959) in stock buffer (143 mm sodium phosphate and 6.3 mm Na2-EDTA, pH 7.5), and was incubated at 30°C for 2 min. The A412 was then measured. Analysis of total GSH content was conducted using the glutathione reductase recycling assay as described by Anderson (1985). For PCs measurement, 200 mg of frozen tissue was ground to a powder, and then 200 μL of 0.5 m HCl was added. The mixture was kept on ice for 10 min and was vortexed three times for 30 s each during this time. After centrifugation for 10 min in a microcentrifuge at 4°C, the supernatant was removed. A total of 50 μL of 2 mm N-acetyl Cys was added to 200 μL of the supernatant. This was then analyzed by reverse phase-HPLC with postcolumn derivatization of thiol compounds as described by Gupta and Goldsbrough (1991).
ACKNOWLEDGMENTS
We thank Dr. Christopher S. Cobbett (University of Melbourne, Victoria, Australia) for the generous gift of Arabidopsis mutant seeds and cad1-3, and the Arabidopsis Biological Resource Center (Columbus, OH) for providing us with the expressed sequence tag clone.
Footnotes
This work was supported by the Illinois Department of Natural Resources.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.014118.
LITERATURE CITED
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder JI, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. Eur J Biochem. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. A family of phytochelatin synthase genes from plant, fungal and animal species. Trends Plant Sci. 1999;4:335–337. doi: 10.1016/s1360-1385(99)01465-x. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000a;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol. 2000b;3:211–216. [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutants, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. Plant J. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Cunningham SD, Betri WR, Huang JW. Phytoremediation of contaminated soils. Trends Biotechnol. 1995;13:393–397. [Google Scholar]
- De Vos CHRD, Vonk MJ, Vooijs R, Schat H. Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol. 1992;98:853–858. doi: 10.1104/pp.98.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Solís JR, Gutiérrez-Alcalá G, Romero LC, Gotor C. The cytosolic O-acetylserine(thiol) lyase gene is required by heavy metals and can function in cadmium tolerance. J Biol Chem. 2001;276:9297–9302. doi: 10.1074/jbc.M009574200. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Mechanism of action, metabolism and toxicity of buthione sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982;107:1067–1073. [PubMed] [Google Scholar]
- Grill E, Loffler S, Winnacker E-L, Zenk MH. Phytochelatins, the heavy metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proc Natl Acad Sci USA. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy metal-binding peptides from plants are functionally analogous to metallothioneins. Proc Natl Acad Sci USA. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SC, Goldsbrough PB. Phytochelatin accumulation and cadmium tolerance in selected tomato cell lines. Plant Physiol. 1991;97:306–312. doi: 10.1104/pp.97.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O'Conell MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast, Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1164. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley-Whitaker J, Ainsworth G, Meharg AA. Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001;24:713–722. [Google Scholar]
- Howden R, Cobbett CS. Cadmium sensitive mutants of Arabidopsis thaliana. Plant Physiol. 1992;99:100–107. doi: 10.1104/pp.100.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Goldsbrough PB, Anderson CR, Cobbett CS. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiol. 1995;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Moon JS, Domier LL, Korban SS. Molecular characterization of phytochelatin synthase expression in transgenic Arabidopsis. Plant Physiol Biochem. 2002;40:727–733. [Google Scholar]
- Murashige T, Skoog T. A revised medium for growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479. [Google Scholar]
- Raskin I, Smith RD, Salt DE. Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol. 1997;8:221–226. doi: 10.1016/s0958-1669(97)80106-1. [DOI] [PubMed] [Google Scholar]
- Rauser WE. Phytochelatins. Annu Rev Biochem. 1990;59:61–86. doi: 10.1146/annurev.bi.59.070190.000425. [DOI] [PubMed] [Google Scholar]
- Rauser WE. Structure and function of metal chelators produced by plants: the case for organic acids, amino acids, phytin and metallothioneins. Cell Biochem Biophysics. 1999;32:19–48. doi: 10.1007/BF02738153. [DOI] [PubMed] [Google Scholar]
- Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Scarano G, Morelli E. Polarographic behavior of metal phytochelatin complexes. Electroanalysis. 1998;10:39–43. [Google Scholar]
- Steffens JC. The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Mol Biol. 1990;41:553–575. [Google Scholar]
- Toppi LS, Gabbrielli R. Response to cadmium in higher plants. Environ Exp Bot. 1999;41:105–130. [Google Scholar]
- Van Assche F, Clijsters H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990;13:195–206. [Google Scholar]
- Vatamaniuk OK, Bucher E, Ward JT, Rea PA. A new pathway for heavy metal detoxification in animals. J Biol Chem. 2001;276:20817–20820. doi: 10.1074/jbc.C100152200. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitution. Proc Natl Acad Sci USA. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu Y-P, Rea PA. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase. J Biol Chem. 2000;275:31451–31459. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- Zenk MH. Heavy metal detoxification in higher plants: a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smiths EAH, Tarun AS, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999a;119:73–79. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smiths EAH, Tarun AS, Weber SU, Jouanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999b;121:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]