Abstract
Exposure of the hyperaccumulator Alyssum lesbiacum to nickel (Ni) is known to result in a dose-dependent increase in xylem sap concentrations of Ni and the chelator free histidine (His). Addition of equimolar concentrations of exogenous l-His to an Ni-amended hydroponic rooting medium enhances Ni flux into the xylem in the nonaccumulator Alyssum montanum, and, as reported here, in Brassica juncea L. cv Vitasso. In B. juncea, reducing the entry of l-His into the root by supplying d-His instead of l-His, or l-His in the presence of a 10-fold excess of l-alanine, did not affect root Ni uptake, but reduced Ni release into the xylem. Compared with B. juncea, root His concentrations were constitutively about 4.4-fold higher in A. lesbiacum, and did not increase within 9 h of exposure to Ni. Cycloheximide did not affect root His or Ni concentrations, but strongly decreased the release of His and Ni from the root into the xylem of A. lesbiacum, whereas xylem sap concentrations of Ca and Mg remained unaffected. Near-quantitative chelation of Ni with nitrilotriacetate in the rooting medium did not enhance Ni flux into the xylem of A. lesbiacum and B. juncea, suggesting the absence of a significant apoplastic pathway for Ni entry into the xylem. The data suggest that in B. juncea roots, Ni2+ uptake is independent of simultaneous uptake of His. In both species, enhanced release of Ni into the xylem is associated with concurrent release of His from an increased root free His pool.
Transition metals are usually present in the environment as trace elements. As a consequence of worldwide industrialization, the release of potentially toxic metals into the biosphere has been accelerating over the past 150 years (Nriagu and Pacyna, 1988). Biological systems require a number of metal ions as micronutrients, but numerous enzymatic and cellular functions can be disrupted in the presence of excess essential or nonessential metal ions (Maroney, 1999; Clemens, 2001). The potentially harmful effects of metal ions are a consequence of their high binding affinities for proteins, membranes, and organic metabolites, as well as of the ability of some metals to undergo and catalyze redox reactions.
Naturally occurring hyperaccumulation of metals like nickel (Ni), zinc, cobalt, manganese, or cadmium thus far has been reported in more than 400 plant species (Baker et al., 1999). Metal hyperaccumulation has been proposed to serve as defense against herbivory as well as against fungal and bacterial pathogens (Boyd, 1998), or confer high levels of metal tolerance (Boyd et al., 2000). To date, field specimen from 48 different taxa in the genus Alyssum have been found to contain hyperaccumulator levels of between 1,000 μg g−1 and 30,000 μg g−1 Ni in leaf dry biomass (Baker and Brooks, 1989).
The potential use of wild-type or engineered plants for extracting contaminant metals from polluted soils has received much attention in recent years (Chaney, 1983; Salt et al., 1998; Meagher, 2000). The success of phytoremediation, however, will require an improved understanding of the biological processes involved in metal acquisition, movement inside the plant, shoot accumulation, and metal detoxification in both metal hyperaccumulator and nonaccumulator plants. Recently, an association has been reported between elevated steady-state transcript levels of metal transporter genes of the Zn-regulated transporter (ZRT) Fe-regulated transporter (IRT)-like protein (ZIP) and cation diffusion facilitator protein (CDF) families and metal hyperaccumulation (Pence et al., 2000; Lombi et al., 2002) or metal tolerance (Van der Zaal et al., 1999; Assuncao et al., 2001; Persans et al., 2001). Metal chelation by specific low-Mr ligands is another major process determining metal tolerance of a plant. All plants are able to produce phytochelatins, which can bind and detoxify Cd, As, Ag, and possibly Cu and Hg (Ha et al., 1999). In plants that hyperaccumulate Ni, the low-Mr chelators His (Krämer et al., 1996) and citrate (Lee et al., 1977, 1978; Kersten et al., 1980; Sagner et al., 1998) have been implicated in metal detoxification. Tolerance of yeast (Saccharomyces cerevisiae) to Ni2+ and other metal ions has been reported to correlate with high cellular His levels (Joho et al., 1992; Pearce and Sherman, 1999). Metal chelators like phytosiderophores (Marschner, 1995; Von Wiren et al., 1996; Curie et al., 2001) or organic acids (Ma et al., 2001; Ryan et al., 2001) also play an important role in regulating the availability of transportable substrates for metal uptake by plant cells. Phytosiderophores have been detected in the xylem sap of barley (Hordeum vulgare) plants (Shah et al., 2001).
Knowledge about the processes that mediate and control the partitioning of metal and nutrient ions between roots and shoots of plants is still sketchy. Long-distance transport of metal ions in the xylem is of high importance for plant nutrition (Tiffin, 1971), for the entry of toxic metals into the food chain (Senden et al., 1995), in metal hyperaccumulation (Krämer et al., 1996), and in phytoremediation (Salt et al., 1995, 1998). Recently, the putative plasma membrane transporters PHO1 and SOS1 have been reported to control xylem loading of phosphate and sodium ions, respectively (Hamburger et al., 2002; Shi et al., 2002). Several authors have proposed an involvement of organic or amino acid chelation in enhancing the rate of root-to-shoot transport of transition metal ions (Lee et al., 1977; White et al., 1981; Senden and Wolterbeek, 1992; Krämer et al., 1996; Liao et al., 2000). A correlation was found between xylem sap concentrations of copper and nicotianamine (Pich et al., 1994; Liao et al., 2000) and copper and His (Liao et al., 2000). The authors proposed an involvement of both amino acids in the chelation of copper ions in the xylem sap. His supplied from exogenous sources increased Ni flux into the xylem in the nonaccumulator Alyssum montanum (Krämer et al., 1996). Here, we show that this effect is not restricted to A. montanum (Krämer et al., 1996), but can also be observed in the nonaccumulator plant Brassica juncea L. cv Vitasso. Growth rate and ample seed availability make B. juncea a feasible model to study the basis of this effect using biochemical methods. B. juncea has previously been successfully subjected to Agrobacterium tumefaciens-mediated transformation (Zhu et al., 1999a, 1999b) and grown in phytoremediation field trials (Blaylock et al., 1997; Ebbs et al., 1997).
Here we attempt to clarify the role of His in root Ni uptake and translocation of Ni from the root to the shoot in the hyperaccumulator Alyssum lesbiacum and the nonaccumulator B. juncea. For this, xylem sap was collected as root pressure exudates from excised root systems of A. lesbiacum and B. juncea exposed to various combinations of Ni, His, amino acids, chelators, and inhibitors in a hydroponic culture solution (Krämer et al., 1996; Schurr, 1998). Concentrations of Ni and His were determined in the xylem sap and in the root systems at the end of the xylem exudate collection period.
RESULTS
Early Time Course of the His Response in A. lesbiacum
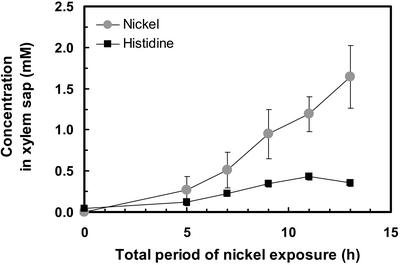
To investigate the early time course of the His response, Ni and His concentrations were measured in root pressure exudates of plants transferred to a solution supplemented with 300 μm NiSO4 for between 0 and 8 h before shoot excision and collection of root pressure exudates for a further 5 h (Fig. 1). Including the 5-h xylem sap collection period, a significant increase in xylem Ni and His concentrations was observed as early as 5 h after the addition of Ni2+ to the root medium (P < 0.05). Both Ni and His concentrations displayed a continuous increase over time. The timing of the responses was identical when calculating xylem Ni and His fluxes (in μmol h−1) instead of evaluating Ni and His concentrations in the xylem sap. In all subsequent experiments, NiSO4 (with or without additional compounds) was added to the hydroponic root medium 4 h before shoot excision, followed by a 5-h period of root pressure exudate collection, corresponding to Ni exposure for 9 h in total.
Figure 1.
Time course of the effect of Ni exposure on Ni and His concentrations in the xylem sap of A. lesbiacum. Values are arithmetic means ± sd of n = 3 replicates. The hydroponic root medium of 8-week-old plants was supplemented with 300 μm Ni for between 0 and 8 h. Subsequently, the shoots were cut off to collect xylem sap as root pressure exudates for 5 h, while Ni exposure continued. Each replicate value corresponds to pooled xylem sap from three plants in one culture vessel. Data are from one experiment representative of a total of two independent experiments, with three replicate culture vessels per treatment in each experiment.
Is There an Ni-Induced Increase in Root Free His Concentration in A. lesbiacum?
To investigate whether the Ni-induced increase in xylem free His concentrations is a consequence of an Ni-induced increase in root free His concentrations we determined His concentrations in the roots of A. lesbiacum. In the absence of added Ni in the root medium, final root His concentrations were about 4.4-fold higher in the hyperaccumulator plants (0.53 ± 0.19 μmol g−1 fresh biomass, mean ± sd, Table I) than in the nonaccumulator Brassicaceae species B. juncea (0.12 ± 0.03 μmol g−1 fresh biomass). Thus, compared with B. juncea, His concentrations were constitutively elevated in the roots of the hyperaccumulator A. lesbiacum. Exposure to Ni for 9 h did not result in a significant change in His concentrations in the roots of A. lesbiacum (0.47 ± 0.15 μmol g−1 fresh biomass). The export of His into the xylem amounted to 0.08 ± 0.03 μmol g−1 fresh biomass during the 5-h xylem sap collection period, and can be extrapolated to a total of approximately 0.12 μmol g−1 fresh biomass during the entire period of exposure to Ni (Table I).
Table I.
Concentrations in root tissues and total export into the xylem of Ni and l-His in A. lesbiacum and B. juncea
| Treatment |
A. lesbiacum
|
B. juncea
|
|||
|---|---|---|---|---|---|
| Control | Ni | Control | Ni | Ni + l-His | |
| μmol g−1 fresh wt | |||||
| Concentration in root | |||||
| Ni | n.d. | 0.56a | n.d. | 0.66a | 0.15b |
| l-His | 0.53b | 0.47b | 0.12c | 0.11c | 5.17a |
| Export into xylem(in 5 h) | |||||
| Ni | n.d. | 0.10a | n.d. | 0.04b | 0.20a |
| l-His | 0.01c | 0.08b | 0.02c | 0.01c | 1.32a |
Plants were transferred into a hydroponic medium supplemented with Ni and chelators as indicated (control: no additions) 4 h before the initiation of a 5-h root pressure exudate collection period. Total amounts of Ni and His were determined in roots and root pressure exudates and related to root fresh biomass (fresh wt). Values are arithmetic means of n = 6 replicates. Each replicate value corresponds to pooled samples from three plants per culture vessel. Two independent experiments were performed with three replicate culture vessels per treatment in each experiment. In each row, different letters indicate that mean values are significantly different at P < 0.05 (n.d., not detectable).
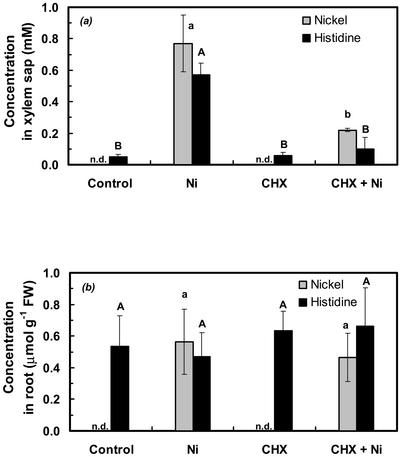
To further investigate the transport of Ni into the xylem of A. lesbiacum, the root medium was supplemented with 5 μm cycloheximide (CHX), a translational inhibitor (Zinck et al., 1995), 1 h before further addition of 300 μm Ni2+. CHX treatment resulted in a reduction in xylem volume flux in Ni-exposed plants by approximately 32% (34.2 ± 5.7 and 23.9 ± 3.3 μL h−1 g−1 root fresh biomass in − CHX and + CHX plants, respectively). CHX had no effect on Mg or Ca concentrations in the xylem sap (control, 2.82 ± 0.91 mm Mg and 2.91 ± 0.53 mm Ca; and CHX treated, 3.02 ± 0.87 mm Mg and 2.53 ± 0.16 mm Ca). In contrast, CHX treatment resulted in a significant reduction in Ni and His concentrations in the xylem sap by about 70% and 82%, respectively (Fig. 2a). CHX treatment did not result in a change in root uptake of Ni or in root His concentrations (Fig. 2b). This conclusion was also valid when corrections were made for the estimated amounts of His and Ni exported from the root via the xylem (see above, Table I).
Figure 2.
Effect of CHX on Ni and His concentrations in xylem sap (a) and in root fresh biomass (b; fresh weight) of A. lesbiacum. Where indicated, the hydroponic root medium of 8-week-old plants was supplemented with 5 μm CHX for 1 h before addition of 300 μm Ni (controls correspond to no additions). Values are arithmetic means ± sd of n = 6 replicates. Each replicate value corresponds to pooled samples from three plants in one culture vessel. For each compound analyzed, different characters indicate that mean values are significantly different at P < 0.05 (uppercase for His and lowercase for Ni). Data are from two independent experiments with three replicate culture vessels per treatment in each experiment. n.d., Not detectable.
The Effect of Addition of Exogenous Free l-His on Ni Flux into the Xylem in B. juncea
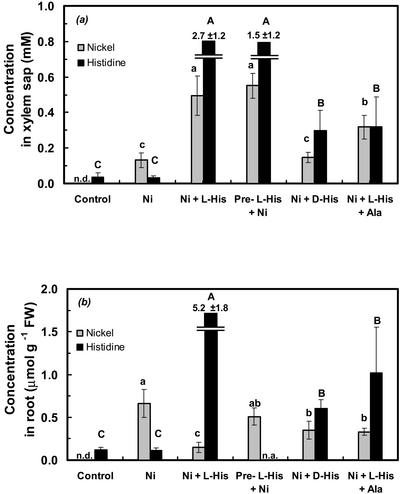
Xylem sap and root systems of B. juncea were sampled from plants provided with a hydroponic solution supplemented with 300 μm Ni2+, or 300 μm Ni and 300 μm l-His. According to computer modeling of the hydroponic media, the addition of His resulted in the presence of approximately 63.8 μm free aqueous Ni2+ (Ni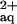 ), 224.6 μm Ni-His as a 1:1 complex, and 8.5 μm Ni in the form of a 1:2 complex with His (Parker et al., 1995). Exposure of B. juncea plants to 300 μm Ni2+ alone caused only a minor increase in Ni concentrations in the xylem sap, which amounted to about 17% of the Ni concentrations observed in the xylem sap of A. lesbiacum under equivalent conditions (Figs. 1 and 3a; Table I). Exposure of B. juncea to Ni2+ did not result in a change in xylem His concentrations (Fig. 3a). When B. juncea was supplied with a combination of 300 μm l-His and 300 μm Ni, both xylem sap Ni and His concentrations increased substantially (Fig. 3a).
), 224.6 μm Ni-His as a 1:1 complex, and 8.5 μm Ni in the form of a 1:2 complex with His (Parker et al., 1995). Exposure of B. juncea plants to 300 μm Ni2+ alone caused only a minor increase in Ni concentrations in the xylem sap, which amounted to about 17% of the Ni concentrations observed in the xylem sap of A. lesbiacum under equivalent conditions (Figs. 1 and 3a; Table I). Exposure of B. juncea to Ni2+ did not result in a change in xylem His concentrations (Fig. 3a). When B. juncea was supplied with a combination of 300 μm l-His and 300 μm Ni, both xylem sap Ni and His concentrations increased substantially (Fig. 3a).
Figure 3.
Ni and His concentrations in xylem sap (a) and in root fresh biomass (b; fresh weight) of B. juncea. The hydroponic root medium of 6-week-old plants was supplemented with various solutes, i.e. none (control), 300 μm Ni, 300 μm Ni and 300 μm l-His, 300 μm Ni preceded by a pre-exposure to 1 mm l-His for 4 h (Pre-l-His + Ni), 300 μm Ni and 300 μm d-His, or 300 μm Ni and 300 μm l-His with an excess of 3 mm Ala. Values are arithmetic means ± sd of n = 6 replicates, and means ± sd of n = 3 replicates for the Ni + l-His + Ala and the Ni + d-His treatments. Each replicate value corresponds to pooled samples from three plants in one culture vessel. For each compound analyzed, different characters indicate that mean values are significantly different at P < 0.05 (uppercase for His and lowercase for Ni). Two independent experiments were performed with three replicate culture vessels per treatment in each experiment, except for the Ni + l-His + Ala treatment, which was not repeated in an independent experiment. n.a., Not analyzed; n.d., not detectable.
Is Ni Taken up by the Root as the Free Divalent Cation or as an Ni-His Chelate?
To test whether Ni uptake by the roots of B. juncea is dependent on the simultaneous presence of both Ni and free His in the root medium, the medium of B. juncea was supplemented with 1 mm His for a pre-incubation period of 4 h. Subsequent to loading of the roots with His, the root medium was replaced with fresh culture solution containing 300 μm Ni2+ for 4 h, followed by root pressure exudate collection. In this treatment, xylem Ni concentrations were equivalent to the Ni concentrations found in the xylem sap of B. juncea plants exposed to 300 μm Ni and 300 μm l-His simultaneously (Fig. 3a). This suggested that the presence of elevated His concentrations inside the root symplasm may be sufficient for enhanced Ni flux into the xylem.
An attempt was made to identify compounds that inhibit the uptake of His by the roots of B. juncea without affecting the overall speciation of Ni(II) in the rooting medium. Because there is free rotation of the imidazole ring around the Cβ carbon of the His molecule, the Ni complexes of the d- and l-stereoisomer are expected to display identical stability constants. Upon supplementing the root medium with a combination of 300 μm Ni and 300 μm d-His, total root His concentrations were reduced by approximately 88% compared with the roots provided with Ni and the stereoisomer, l-His, instead (Fig. 3b). Similarly, the addition of a 10-fold excess of 3 mm Ala to a hydroponic solution supplemented with 300 μm Ni and 300 μm l-His resulted in a reduction of root His concentrations by approximately 80% (Fig. 3b). Under the experimental conditions used, the stability of the Ni-Ala complex is substantially lower (pKapp ≅ 1.1; Dawson et al., 1986) than that of an Ni-His complex (pKapp ≅ 5.5; Dawson et al., 1986). The addition of Ala was predicted to reduce the concentration of the Ni-His complex by no more than 4.1% (Parker et al., 1995). We next investigated the effects of these treatments on Ni concentrations in the xylem sap. When roots of B. juncea were supplied with Ni2+ or a combination of d-His and Ni, xylem sap Ni concentrations were approximately 87% and 70% lower, respectively, compared with supply of a combination of Ni and l-His (Fig. 3a). Similarly, when the root medium was supplemented with 300 μm Ni and 300 μm l-His, further addition of an excess of 3 mm Ala reduced xylem Ni concentrations by about 36% (Fig. 3a). The total uptake of Ni into the roots was similar in all three treatments, when taking into account the elevated rate of export of Ni into the xylem in the combined Ni and l-His treatment (Fig. 3b; Table I).
Symplastic or Apoplastic Pathway of Ni Entry into the Xylem?
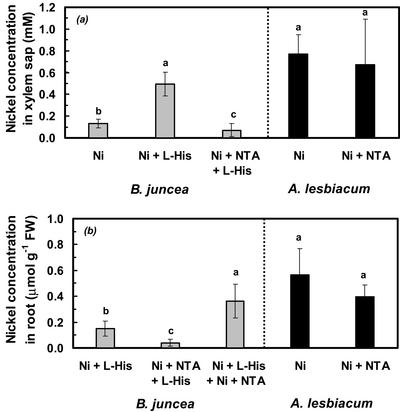
If His chelation in the root medium promoted the entry of Ni into the xylem by enhancing Ni flux through a predominantly apoplastic pathway, other high-affinity Ni chelators, which are distinct from His, should also enhance Ni flux into the xylem of B. juncea. To test this, we investigated the effect of addition of nitrilotriacetate (NTA), which chelates Ni at an approximately 50-fold higher apparent complex stability (pKapp ≅ 7.2) than His (see above), on Ni concentrations in the xylem sap of B. juncea. According to computer modeling (Geochem-PC, Parker et al., 1995), the rooting medium supplemented with 300 μm Ni, 300 μm NTA, and 300 μm l-His contained approximately 289.3 μm Ni complexed with NTA, 10.2 μm Ni complexed with His, and 0.48 μm free Ni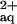 . Under these conditions, Ni concentrations in the xylem sap of B. juncea were lower than in the 300 μm Ni2+ treatment (Fig. 4a, left). The presence of 300 μm Ni and 300 μm NTA in the root medium of A. lesbiacum increased the total concentration of Ni present in chelated form more than 100-fold when compared with a root medium supplemented with 300 μm Ni2+. Ni concentrations in the xylem sap of A. lesbiacum were not significantly affected by NTA (Fig. 4a, right).
. Under these conditions, Ni concentrations in the xylem sap of B. juncea were lower than in the 300 μm Ni2+ treatment (Fig. 4a, left). The presence of 300 μm Ni and 300 μm NTA in the root medium of A. lesbiacum increased the total concentration of Ni present in chelated form more than 100-fold when compared with a root medium supplemented with 300 μm Ni2+. Ni concentrations in the xylem sap of A. lesbiacum were not significantly affected by NTA (Fig. 4a, right).
Figure 4.
Effect of addition of NTA to the hydroponic root medium on Ni concentration in xylem sap (a) and in root fresh biomass (b; fresh weight) of B. juncea (left) and A. lesbiacum (right). The hydroponic medium was supplemented with 300 μm Ni, 300 μm Ni + 300 μm l-His, 300 μm Ni + 300 μm NTA, 600 μm Ni + 300 μm l-His + 300 μm NTA (Ni + L-His + Ni + NTA), or 300 μm Ni + 300 μm NTA + 300 μm l-His (Ni + NTA + L-His). Values are arithmetic means ± sd of n = 3 replicates for B. juncea and n = 6 replicates for A. lesbiacum. Each replicate value corresponds to pooled samples from three plants per culture vessel. Different characters indicate means that are significantly different at P < 0.05. Data are from two independent experiments for A. lesbiacum and from one for B. juncea, with three replicate culture vessels per treatment in each experiment. n.a., Not analyzed; n.d., not detectable.
Compared with the combined 300 μm Ni and 300 μm l-His treatment, the roots of B. juncea accumulated substantially lower concentrations of Ni in the presence of an equimolar amount of NTA (Fig. 4b, left). In a control experiment, B. juncea plants were supplied with 600 μm Ni, 300 μm NTA, and 300 μm His, resulting in the presence of predicted concentrations of approximately 300 μm Ni-NTA and 233.5 μm Ni-His and 63.9 μm free Ni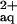 in the root medium. Root Ni concentrations were restored to 0.36 ± 0.13 μmol g−1 fresh weight (Fig. 4b). The addition of 300 μm NTA to a rooting medium amended with 300 μm Ni reduced the concentration of free Ni
in the root medium. Root Ni concentrations were restored to 0.36 ± 0.13 μmol g−1 fresh weight (Fig. 4b). The addition of 300 μm NTA to a rooting medium amended with 300 μm Ni reduced the concentration of free Ni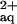 to 2.3 μm (Parker et al., 1995), but had no effect on Ni concentrations in either roots or xylem sap of A. lesbiacum (see above; Fig. 4b, right).
to 2.3 μm (Parker et al., 1995), but had no effect on Ni concentrations in either roots or xylem sap of A. lesbiacum (see above; Fig. 4b, right).
DISCUSSION
Within 0 to 13 h of exposure of A. lesbiacum root systems to Ni2+, significant increases in xylem Ni and endogenous free His concentrations were detected as early as 5 h after initiation of exposure. Subsequently, increases were observed over time in the concentrations of free His and Ni in the xylem sap. A “His response” was reported previously in xylem sap collected after an 8-d period of exposure of A. lesbiacum to Ni (Fig. 1; Krämer et al., 1996). In all xylem sap samples obtained, Ni concentrations were significantly higher than His concentrations, with average ratios of Ni versus His concentrations between 1.3 (Fig. 2A, at 9 h) and 4.6 (Fig. 1, at 13 h). After 9 h of Ni exposure, final root free His concentrations were 0.47 μmol g−1 fresh weight, and thus slightly, but not significantly, lower than in control plants (free His: 0.53 μmol g−1 fresh weight). This suggested that in response to Ni exposure, the approximately 8-fold increase in xylem sap free His concentrations was not accompanied by a major increase in root free His concentrations (Table I). In contrast, root free His concentrations were constitutively about 4.4-fold higher in A. lesbiacum than in the nonaccumulator B. juncea (0.12 μmol g−1 fresh weight; Table I). Within 9 h after the onset of Ni exposure, the estimated total amount of His released into the xylem of A. lesbiacum (0.12 μmol g−1 fresh weight) corresponded to approximately 22% of the preexisting pool of His in the root symplasm (Table I). Thus, there may not be a need for a major up-regulation of His biosynthesis as a primary response to Ni exposure before efficient xylem loading of Ni can begin. This idea is consistent with the finding that within 9 h of exposure to 300 μm Ni2+, the presence of 75 μm of the His biosynthesis inhibitor IRL1803 (Mori et al., 1995) had no significant effect on either xylem His or Ni concentrations (data not shown). Persans et al. (1999) reported elevated free His concentrations in roots of the Ni hyperaccumulator Thlaspi goesingense (0.74 μmol g−1 fresh biomass) compared with Thlaspi arvense (0.04 μmol g−1 fresh biomass). In response to 24 and 48 h of exposure of the Ni hyperaccumulator T. goesingense to Ni, Persans et al. (1999) could not detect an increase in gene expression of three genes encoding enzymes of the His biosynthesis pathway. Presumably, the steady-state pool of free His in the roots of A. lesbiacum is continuously replenished because similar, elevated His concentrations were detected in the xylem sap of plants exposed to Ni for 8 d (Krämer et al., 1996) and 1, 3, 5, and 22 d (U. Krämer, A.J.M. Baker, and J.A.C. Smith, unpublished data). In the presence of CHX, which did not significantly affect the total amount of root-derived free His in A. lesbiacum, the release of His and Ni, but not of Ca and Mg, into the xylem was dramatically and selectively decreased (Fig. 2, a and b). This suggests that once Ni is inside the root of A. lesbiacum, the presence of elevated levels of His inside the root is not sufficient for the release of major amounts of Ni and His into the xylem, but that there is also a need for continued translation in A. lesbiacum. This may be required for the synthesis of Ni-induced proteins or for the maintenance of adequate levels of high-turnover proteins involved in the translocation of Ni and His across the root toward the xylem or from the root symplasm into the xylem.
The addition of exogenous His to the root medium of the nonaccumulator B. juncea resulted in an increase in Ni and His concentrations in the xylem sap (Fig. 3, a and b), as observed earlier in A. montanum (Krämer et al., 1996). This shows that the enhancing effect of His on Ni flux into the xylem is not restricted to A. montanum. In a few other instances, the addition of naturally occurring low-Mr metal chelators has been reported to enhance the rate of root-to-shoot translocation of metals. The supply of exogenous phytosiderophores, but not EDTA, was shown to increase Cu, Fe, Mn, Zn, and phytosiderophore concentrations in the xylem sap of barley (Shah et al., 2001). Preloading of tomato (Lycopersicon esculentum) root systems with citrate before Cd exposure enhances the rate of root-to-shoot transport of Cd approximately 5-fold, without affecting Cd accumulation in the roots (Senden et al., 1995).
Three possible models were tested of how His may act to increase Ni flux into the xylem. The first model is the action of His in the root apoplast/rhizosphere, analogous to the role of phytosiderophores in iron acquisition in graminaceous plants (Marschner, 1995). In this model, His released from the roots acts as a chelator to mobilize soil Ni, which is subsequently taken up by the roots as an Ni-chelate. According to this model, as a consequence of increased Ni uptake into the root symplasm, Ni concentrations are elevated throughout the plant, including the xylem. If this model were to be accurate, reducing the rate of entry of His into the root should reduce the entry of Ni into the root. Two experimental conditions were established to reduce the entry of His into the root of B. juncea. First, l-His was replaced by d-His, the stereoisomer that is less efficiently taken into root cells, as previously reported for rat (Rattus norvegicus) brain microvascular endothelial cells (Yamakami et al., 1998). Second, a 10-fold excess was added of the amino acid l-Ala, which reduced His uptake, presumably by competitive inhibition (Fig. 3, a and b; Fischer et al., 1995). Neither of these treatments changed overall chelation of Ni in the root medium, compared with the supply of equimolar amounts of Ni and l-His. Ni uptake by the roots was equivalent in all three treatments. These results suggested that roots of B. juncea took up Ni2+ predominantly as the free aqueous cation, and not as an Ni-His complex. This was supported by other findings. Similar and higher Ni concentrations were observed in roots of B. juncea plants supplied with Ni2+ in the absence of simultaneous supply of any chelator (Table I; Fig. 3b). In agreement with this, the high rate of Zn2+ uptake by the roots of the Zn hyperaccumulator Thlaspi caerulescens was associated with high expression levels of the Zn2+ transporter gene ZNT1 (Pence et al., 2000). To our knowledge, no specific plant Ni2+ transporters have been reported or characterized so far (Li et al., 2001).
In the second possible model, the action of His is confined to the apoplast. His released from the roots may increase the apoplastic mobility of Ni by reducing the binding to root cell walls, thereby reducing the retention of Ni in the apoplast of the roots (Senden and Wolterbeek, 1992). In this model, chelation increases the rate of entry of Ni into the xylem via a purely apoplastic pathway. This pathway has been proposed to exist in young regions of the root, where the endodermis is not fully developed to form a barrier for the entry of solutes into the xylem, and at sites where lateral roots penetrate the endodermis (White, 2001). If this model were to be accurate, chelation of Ni with another high-affinity ligand of a comparable molecular size should have a similar effect on movement of Ni into the xylem, as does His. Chelation of Ni with NTA in the rooting medium did not support Ni flux into the xylem of B. juncea (Fig. 4). Thus, the data indicated that the contribution of a purely apoplastic pathway for the entry of Ni-His into the xylem of B. juncea is insignificant under our experimental conditions. This conclusion is supported by the results obtained in the d-His and l-Ala treatments (Fig. 3): Ni flux into the xylem was substantially reduced when the His molecules that were chelating Ni in the rooting medium could not be as effectively taken up by the roots. Furthermore, the fact that supply of a major proportion of Ni as an NTA complex did not increase the concentration of Ni in the xylem sap, when compared with a root medium supplemented merely with Ni2+, provided evidence that, also in A. lesbiacum, a purely apoplastic route for Ni entry into the xylem is of minor significance (Fig. 4). In T. caerulescens, Ernst et al. (2002) proposed a similar conclusion based primarily on the observed metal selectivity of long-term metal accumulation in the leaves.
A third possible model for the role of His is to increase Ni mobility for movement toward and into the xylem once Ni is inside the root symplasm. In this model, Ni2+ is taken up by root cells as the free hydrated cation, independent of the uptake of His. Once inside the root symplasm, Ni2+ ions would depend on the presence of, and probably chelation by, His for further movement toward the xylem or possibly for release into the xylem. Our data unequivocally support this model for both the hyperaccumulator A. lesbiacum and the nonaccumulator B. juncea. In the xylem of A. lesbiacum, Ni and His concentrations were correlated (Fig. 1), as shown previously (Krämer et al., 1996). When root-to-xylem export of His was reduced in CHX-treated A. lesbiacum plants, the entry of Ni into the xylem was also reduced (Fig. 2). When the entry of His from the root medium into the root of B. juncea was reduced (Fig. 3), the entry of His and Ni into the xylem was also substantially reduced. Under these conditions, entry of Ni into the roots of B. juncea was unaffected. This suggests that the His-associated flux of Ni from the root symplasm into the xylem of B. juncea is dependent on the transport of His from the root symplasm into the xylem (Fig. 3). Thus, an association between His and Ni release into the xylem was found in both A. lesbiacum and B. juncea. At the molecular level, this may involve export from the root symplasm into the xylem of an Ni-His complex through a membrane transport system. Alternatively, chelation of Ni by His in the root symplasm might increase symplastic mobility and, thus, the net rate of movement of Ni across the root toward and into the stele. This may result in an increased net flux of Ni and free His into the xylem. Chelation of metals by His has been demonstrated in frozen root tissues of the Ni hyperaccumulator A. lesbiacum (Krämer et al., 1996) and the Zn hyperaccumulator T. caerulescens J & C Presl (Salt et al., 1999) using extended x-ray absorption fine-structure analysis.
In B. juncea, supply of exogenous l-His was sufficient to increase the flux of Ni into the xylem. At root His concentrations of 1.02 ± 0.54 μmol g−1 fresh biomass (and an average of 0.132 ± 0.03 μmol His g−1 root fresh weight exported into the xylem in 5 h), as observed in the combined Ni and His treatment with an excess of Ala, Ni flux into the xylem of B. juncea was comparable with that observed in Ni2+-exposed A. lesbiacum (Fig. 3; Table I). In Ni2+-exposed A. lesbiacum, average His concentrations were 0.47 ± 0.15 μmol g−1 fresh weight in roots and 0.08 ± 0.04 μmol g−1 root fresh weight exported into the xylem sap in 5 h, respectively (Table I; Fig. 3). Our results are consistent with the hypothesis that B. juncea and A. lesbiacum share a common mechanism of His-associated release of Ni into the xylem.
CONCLUSIONS
The results obtained in this study suggest that Ni is taken up as the free aqueous cation by the roots of B. juncea, and that root Ni uptake is independent of the uptake of free His. Within hours of the onset of Ni exposure, the two main factors that determine enhanced root-to-shoot transport of Ni in the hyperaccumulator A. lesbiacum are constitutively elevated levels of His in the roots and an interdependent release of Ni and His into the xylem, which is inhibited by CHX. In B. juncea, increasing root steady-state His concentrations through supply of exogenous l-His triggers root-to-shoot mobility of Ni. Ni loading into the xylem is tightly associated with His export from the roots into the xylem in B. juncea and A. lesbiacum. Our results suggest that increasing steady-state concentrations of His in a transgenic approach may be sufficient to increase shoot Ni accumulation in model Brassicaceae plants. In addition, to optimize Ni uptake rates by the roots it may be desirable to also engineer the overexpression of a high-affinity Ni2+ uptake system in the roots.
MATERIALS AND METHODS
Plant Growth
Seeds of Alyssum lesbiacum were a gift from Alan Baker (School of Botany, The University of Melbourne, Parkville, Australia). Seeds of Brassica juncea L. cv Vitasso were a gift from Thomas Rausch (Institute for Plant Sciences, University of Heidelberg). Seeds were placed onto a layer of 300 μL of solidified 0.75% (w/v) agarose (Seakem LE, Biowhittaker Molecular Applications, Rockland, ME) in black 0.5-mL polypropylene microtubes (Trefflab Easifit, Treff AG, Degersheim, Switzerland), with the bottom 10 mm clipped off. Forty tubes were placed into holes in a polyethylene lid floating on 4 L of 1 mm CaCl2. Unless indicated otherwise, all chemicals were of analytical grade or purer (Merck KgaA, Darmstadt, Germany). After 2 weeks, uniform seedlings of A. lesbiacum and B. juncea were selected and transferred into black plastic polystyrene vessels (three plants per vessel), each supplied with 400 mL of a modified one-tenth-strength Hoagland solution number 1 (Hoagland and Arnon, 1950) containing 0.1 mm KH2PO4, 0.6 mm KNO3, 0.28 mm Ca(NO3)2, 0.2 mm MgSO4, 5 μm of a complex of Fe(III) and N,N′-di-(2-hydroxybenzoyl)-ethylenediamine-N,N′-diacetate (Strem Chemicals, Inc., Newburyport, MA) prepared according to Chaney (1988), 4.6 μm H3BO3, 0.5 μm MnCl2, 0.08 μm ZnSO4, 0.03 μm CuSO4, and 0.01 μm Na2MoO4, buffered at pH 5.5 with 1 mm MES (Sigma, St. Louis). The growth medium was continuously aerated using aquarium pumps, and nutrient solutions were exchanged once a week. Seedlings and plants were grown in a greenhouse with supplementary light provided by sodium vapor lamps at a photon flux density of 350 μmol m−2 s−1 during the day, a photoperiod of 16 h of light and 8 h of dark, day and night temperatures of 25°C and 18°C, respectively, and 60% constant relative humidity.
Experimental Treatments
Plants of B. juncea and A. lesbiacum grown for 4 and 6 weeks in modified Hoagland solution, respectively, were used for xylem sap collection. The effect of Ni exposure and other compounds on xylem sap and root composition was investigated by replacing the nutrient solution with a fresh solution containing the respective added compounds 4 h before the onset of xylem sap collection, except when specified otherwise. The indicated total concentrations of Ni were supplied as NiSO4. NTA (Fluka Chemie AG, Buchs, Switzerland) was supplied adjusted to pH 5.5 with KOH. CHX (Sigma) treatment (5 μm) was initiated 1 h before further addition of Ni. In this experiment, exudate collection was initiated 4 h after the onset of Ni exposure. The amino acids l-His (Sigma) and d-His (Fluka Chemie AG) were supplied as hydrochlorides and l-Ala (Sigma) was supplied as the free amino acid. After shoot excision, xylem sap was collected as root pressure exudate for 5 h from 10 to 15 h after the onset of the light period (Krämer et al., 1996). At the end of the collection period, xylem sap samples collected from the three plants in each culture vessel were pooled to give one pool per container, and frozen at −20°C until further analysis. Subsequently, roots were harvested, washed in 1 mm EDTA for 5 min, rinsed in deionized water, blotted dry with tissue paper, weighed, frozen in liquid nitrogen, and stored at −80°C until analysis.
Amino Acid Extraction and Analysis
Frozen roots were ground to a fine powder in liquid nitrogen. A subsample of approximately 200 mg fresh biomass was mixed with a solution of 0.4 mL of 80% (v/v) ethanol and 2.5 mm HEPES (pH 7.5; Scheible et al., 1997), and the suspension shaken at 1,000 rpm at 80°C for 20 min in a heating block (Thermomixer Comfort, Eppendorf GmbH, Hamburg, Germany). After centrifugation at 14,000 rpm for 10 min, the supernatant was collected and the pellet re-extracted with 0.4 mL of 50% (v/v) ethanol and 2.5 mm HEPES (pH 7.5) as described above. After centrifugation as described above, the pellet was suspended in 0.2 mL of 80% (v/v) ethanol with shaking at 1,000 rpm at 80°C for 20 min, and the supernatant collected after centrifugation. For each sample, all three supernatants were pooled and stored at −20°C.
For HPLC, amino acids were derivatized using dabsyl chloride (Sigma) according to Sudhop and Habermann (1995) with some modifications. These were necessary because it was observed that Ni2+ specifically inhibits the derivatization of His in the absence of EDTA (data not shown). Twenty-five microliters of root extract or xylem sap was mixed with 5 nmol internal standard nor-Val and 250 nmol EDTA in a total volume of 75 μL. The mixture was dried under vacuum at 65°C, and then dissolved in 80 μL of 1 g L−1 dabsyl chloride in acetonitrile and 40 μL of 16.7 mm NaHCO3 (pH 8.1). After incubation at 70°C with constant shaking at 1,350 rpm for 12 min in a heating block, the final volume was adjusted to 250 μL using a buffer containing 11.46 g L−1 Na2HPO4 × 2H2O, 4.91 g L−1 NaH2PO4 × water, and 50% (v/v) ethanol. Amino acids were separated by HPLC (Dionex, Sunnyvale, CA) on a 250- × 4.6-mm, 5-μm Spherisorb ODS-II column (Dionex) using a gradient of 65 mm Na-acetate with 7% (v/v) DMF at pH 6.5 and acetonitrile at a constant flow of 1.2 mL min−1 (Table II). Detection was carried out at 436 nm with a UV/VIS detector (PDA 100, Dionex).
Table II.
The HPLC gradient used to separate amino acids
| Time | Solvent B |
|---|---|
| min | % |
| 0 | 15 |
| 6.2 | 24 |
| 8.5 | 25 |
| 9.6 | 27 |
| 20 | 40 |
| 25 | 51 |
| 27.5 | 52 |
| 30 | 52 |
| 32 | 70 |
| 34 | 70 |
| 36 | 15 |
Solvent A was 65 mm sodium acetate and 7% (v/v) DMF in water at pH 6.5. Solvent B was acetonitrile.
Element Analysis
Subsamples of root material were oven dried at 60°C for 48 h and, after determination of dry biomass, dry ashed at 500°C for 19 h. The ash was dissolved in 10 mL of 2% (w/v) nitric acid (Suprapur, Merck). For xylem sap samples, a subsample of 100 μL was mixed with 5 mL of 2% (w/v) nitric acid. Total concentrations of Ni, Mg, Ca, and K were determined by inductively coupled plasma atomic emission spectroscopy (Optima 3000, PerkinElmer Instruments, Norwalk, CT).
Experimental Design, Computational Data Analysis, and Computer Modeling
Samples were pooled from three plants per culture vessel, with three replicate culture vessels per treatment in each experiment. Unless indicated otherwise, the presented data include both of two independent replicate experiments carried out for all treatments. Data were analyzed by ANOVA, followed by Duncan's new-multiple range test. Differences between means were considered significant at a confidence level of P < 0.05. All statistical analyses were done on logarithmically transformed data using the software Statgraphics Plus (StatPoint, LLC, Englewood Cliffs, NJ). Computer modeling of the hydroponic rooting media was carried out using the program Geochem-PC (Parker et al., 1995), including as input the pH and the total concentrations of all solutes added to the hydroponic treatment solutions.
ACKNOWLEDGMENTS
We thank Prof. Alan J. M. Baker for the gift of A. lesbiacum seeds, Prof. Thomas Rausch for the donation of B. juncea seeds, and Dr. Markus Klein for providing a sample of IRL1803. We are grateful to Susanne Köppen for technical assistance and to Dr. Karin Köhl and the Institute's gardeners for assistance in plant cultivation.
Footnotes
This work was supported by the German Federal Ministry of Education and Research (Biofuture Grant no. 031877 to U.K.) and by the Max Planck Institute of Molecular Plant Physiology (to L.K.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp102.010686.
LITERATURE CITED
- Assuncao AGL, Martins Da Costa P, De Folter S, Vooijs R, Schat H, Aarts MGM. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2001;24:217–226. [Google Scholar]
- Baker AJM, Brooks RR. Terrestrial higher plant which hyperaccumulate metallic elements: a review of their distribution, ecology and phytochemistry. Biorecovery. 1989;1:81–126. [Google Scholar]
- Baker AJM, McGrath SP, Reeves RD, Smith JAC. A review of the biological resource for possible exploitation in the phytoremediation of metal-polluted soils. In: Terry N, Bañuelos GS, editors. Phytoremediation of Contaminated Soil and Water. Boca Raton, FL: CRC Press LLC; 1999. pp. 85–107. [Google Scholar]
- Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of lead in Indian Mustard by soil-applied chelating agents. Environ Sci Technol. 1997;31:860–865. [Google Scholar]
- Boyd RS. Hyperaccumulation as a plant defensive strategy. In: Brooks RR, editor. Plants that Hyperaccumulate Heavy Metals. New York: CAB International; 1998. pp. 181–201. [Google Scholar]
- Boyd RS, Wall MA, Watkins JE. Correspondence between Ni tolerance and hyperaccumulation in Streptanthus (Brassicaceae) Madrono. 2000;47:97–105. [Google Scholar]
- Chaney RL. Plant uptake of inorganic waste. In: Parr JE, Marsh PB, Kla JM, editors. Land Treatment of Hazardous Wastes. Noyes Data Corp., IL: Park Ridge; 1983. pp. 50–76. [Google Scholar]
- Chaney RL. Plants can utilize iron from Fe-N,N′-DI-(2-hydroxybenzoyl)-ethylenediamine-N,N′-diacetic acid, a ferric chelate with 10(6) greater formation constant than Fe-EDDHA. J Plant Nutr. 1988;11:1033–1050. [Google Scholar]
- Clemens S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta. 2001;212:475–486. doi: 10.1007/s004250000458. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe 1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Dawson RMC, Elliott DC, Elliott WH, Jones KM. Data for Biochemical Research. Ed 3. Oxford: Clarendon Press; 1986. [Google Scholar]
- Ebbs SD, Lasat MM, Brady DJ, Cornish J, Gordon R, Kochian LV. Phytoextraction of cadmium and zinc from a contaminated soil. J Environ Qual. 1997;26:1424–1430. [Google Scholar]
- Ernst WHO, Assunção AGL, Verkleij JAC, Schat H. How important is apoplastic zinc xylem loading in Thlaspi caerulescens? New Phytol. 2002;155:4–5. doi: 10.1046/j.1469-8137.2002.00449_2.x. [DOI] [PubMed] [Google Scholar]
- Fischer WN, Kwart M, Hummel S, Frommer WB. Substrate specificity and expression profile of amino acid transporters (AAPs) in Arabidopsis. J Biol Chem. 1995;270:16315–16320. doi: 10.1074/jbc.270.27.16315. [DOI] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O'Connell MJ, Goldsbrough PB, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. Plant Cell. 1999;11:1153–1163. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell. 2002;14:889–902. doi: 10.1105/tpc.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Sta Circ. 1950;347:1–32. [Google Scholar]
- Joho M, Ishikawa Y, Kunikane M, Inouhe M, Tohoyama H, Murayama T. The subcellular distribution of nickel in Ni-sensitive and Ni-resistant strains of Saccharomyces cerevisiae. Microbios. 1992;71:149–159. [PubMed] [Google Scholar]
- Kersten WJ, Brooks RR, Reeves RD, Jaffré T. Nature of nickel complexes in Psychotria douarrei and other nickel-accumulating plants. Phytochemistry. 1980;19:1963–1965. [Google Scholar]
- Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC. Free histidine as a metal chelator in plants that accumulate nickel. Nature. 1996;379:635–638. [Google Scholar]
- Lee J, Reeves RD, Brooks RR, Jaffré T. Isolation and identification of a citrato-complex of nickel from nickel-accumulating plants. Phytochemistry. 1977;16:1503–1505. [Google Scholar]
- Lee J, Reeves RD, Brooks RR, Jaffré T. The relation between nickel and citric acid in some nickel-accumulating plants. Phytochemistry. 1978;17:1033–1035. [Google Scholar]
- Li L, Tutone AF, Drummond RSM, Gardner RC, Luan S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell. 2001;13:2761–2775. doi: 10.1105/tpc.010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao MT, Hedley MJ, Woolley DJ, Brooks RR, Nichols MA. Copper uptake and translocation in chicory (Cichorium intybus L. cv Grasslands Puna) and tomato (Lycopersicon esculentum Mill. Cv Rondy) plants grown in NFT system: II. The role of nicotianamine and histidine in xylem sap copper transport. Plant Soil. 2000;223:243–252. [Google Scholar]
- Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2002;128:1359–1367. doi: 10.1104/pp.010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6:273–278. doi: 10.1016/s1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Maroney MJ. Structure/function relationship in nickel metallobiochemistry. Curr Opin Chem Biol. 1999;3:188–199. doi: 10.1016/S1367-5931(99)80032-5. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition in higher plants. Ed 2. London: Academic Press Ltd.; 1995. [Google Scholar]
- Meagher RB. Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol. 2000;3:153–162. doi: 10.1016/s1369-5266(99)00054-0. [DOI] [PubMed] [Google Scholar]
- Mori I, Fonne-Pfister R, Matsunaga SI, Tada S, Kimura Y, Iwasaki G, Mano JI, Hatano M, Nakano T et al. A novel class of herbicides. Plant Physiol. 1995;107:719–723. doi: 10.1104/pp.107.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air water and soils by trace metals. Nature. 1988;333:134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. Geochem-PC: a chemical speciation program for IBM and compatible personal computers. In: Loeppert RH, Schwab AP, Goldberg S, editors. Chemical Equilibrium and Reaction Models (Special Publication No. 42). Madison, WI: Soil Science Society of America; 1995. pp. 253–269. [Google Scholar]
- Pearce DA, Sherman F. Toxicity of copper, cobalt, and nickel salts is dependent on histidine metabolism in the yeast Saccharomyces cerevisiae. J Bacteriol. 1999;181:4774–4779. doi: 10.1128/jb.181.16.4774-4779.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc Natl Acad Sci USA. 2000;97:4956–4960. doi: 10.1073/pnas.97.9.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persans MW, Niemann K, Salt DE. Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc Natl Acad Sci USA. 2001;98:9995–10000. doi: 10.1073/pnas.171039798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persans MW, Yan X, Patnoe JM, Kramer U, Salt DE. Molecular dissection of the role of histidine in nickel hyperaccumulation in Thlaspi goesingense (Halacsy) Plant Physiol. 1999;121:1117–1126. doi: 10.1104/pp.121.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich A, Scholz G, Stephan UW. Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant Soil. 1994;165:189–196. [Google Scholar]
- Ryan P, Delhaize E, Jones D. Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:527–560. doi: 10.1146/annurev.arplant.52.1.527. [DOI] [PubMed] [Google Scholar]
- Sagner S, Kneer R, Wanner G, Cosson JP, Deus-Neumann B, Zenk MH. Hyperaccumulation, complexation and distribution of nickel in Sebertia acuminata. Phytochemistry. 1998;47:339–347. doi: 10.1016/s0031-9422(97)00593-1. [DOI] [PubMed] [Google Scholar]
- Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Salt DE, Prince RC, Baker AJM, Raskin I, Pickering IJ. Zinc ligands in the metal hyperaccumulator Thlaspi caerulescens as determined using x-ray absorption spectroscopy. Environ Sci Technol. 1999;33:713–717. [Google Scholar]
- Salt DE, Smith RD, Raskin I. Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Scheible WR, Gonzalez-Fontes A, Morcuende R, Lauerer M, Geiger M, Glaab J, Gojon A, Schulze ED, Stitt M. Tobacco mutants with a decreased number of functional nia genes compensate by modifying the diurnal regulation of transcription, post-translational modification and turnover of nitrate reductase. Planta. 1997;203:304–319. doi: 10.1007/s004250050196. [DOI] [PubMed] [Google Scholar]
- Schurr U. Xylem sap sampling: new approaches to an old topic. Trends Plant Sci. 1998;3:293–298. [Google Scholar]
- Senden MHMN, Van Der Meer AJGM, Verburg TG, Wolterbeek HT. Citric acid in tomato plant roots and its effect on cadmium uptake and distribution. Plant Soil. 1995;171:333–339. [Google Scholar]
- Senden MHMN, Wolterbeek HT. Effect of citric acid on the transport of cadmium through xylem vessels of excised tomato stem-leaf systems. Acta Bot Neerl. 1992;39:297–303. [Google Scholar]
- Shah A, Kamei S, Kawai S. Metal micronutrients in xylem sap of iron-deficient barley as affected by plant-borne, microbial and synthetic metal chelators. Soil Sci Plant Nutr. 2001;47:149–156. [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhop B, Habermann M. Vorsäulenderivatisierung für die routinebestimmung von primären und sekundären aminosäuren im femtomolbereich. GIT Spezial Chromatographie. 1995;1/95:13–17. [Google Scholar]
- Tiffin LO. Translocation of nickel in xylem exudate of plants. Plant Physiol. 1971;48:273–277. doi: 10.1104/pp.48.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zaal BJ, Neuteboom LW, Pinas JE, Chardonnens AN, Schat H, Verkleij JA, Hooykaas PJ. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 1999;119:1047–1055. doi: 10.1104/pp.119.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Wiren N, Marschner H, Römheld V. Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc. Plant Physiol. 1996;111:1119–1125. doi: 10.1104/pp.111.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. The pathways of calcium movement to the xylem. J Exp Bot. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- White MC, Baker FD, Chaney RL, Decker AM. Metal complexation in xylem fluid: I. Chemical composition of tomato and soybean stem exudates. Plant Physiol. 1981;67:292–300. doi: 10.1104/pp.67.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakami J, Sakurai E, Sakurada T, Maeda K, Hikichi N. Stereoselective blood-brain barrier transport of histidine in rats. Brain Res. 1998;812:105–112. doi: 10.1016/s0006-8993(98)00958-5. [DOI] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N. Overexpression of glutathione synthetase in Indian mustard enhances cadmium accumulation and tolerance. Plant Physiol. 1999a;119:73–79. doi: 10.1104/pp.119.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N. Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1999b;121:1169–1177. doi: 10.1104/pp.121.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck R, Cahill MA, Kracht M, Sachsenmaier C, Hipskind RA, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]