Abstract
It has been accepted that xylem ray parenchyma cells (XRPCs) in hardwood species respond to subfreezing temperatures either by deep supercooling or by extracellular freezing. Present study by cryo-scanning electron microscopy examined the freezing responses of XRPCs in five boreal hardwoods: Salix sachalinensis Fr. Schmit, Populus sieboldii Miq., Betula platyphylla Sukat. var japonica Hara, Betula pubescens Ehrh., and red osier dogwood (Cornus sericea), in which XRPCs have been reported to respond by extracellular freezing. Cryo-scanning electron microscopy observations revealed that slow cooling of xylem to −80°C resulted in intracellular freezing in the majority of XRPCs in S. sachalinensis, an indication that these XRPCs had been deep supercooled. In contrast, in the majority of XRPCs in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, slow cooling to −80°C produced slight cytorrhysis without clear evidence of intracellular freezing, suggesting that these XRPCs might respond by extracellular freezing. In these XRPCs exhibited putative extracellular freezing; however, deep etching revealed the apparent formation of intracellular ice crystals in restricted local areas. To confirm the occurrence of intracellular freezing, we rewarmed these XRPCs after cooling and observed very large intracellular ice crystals as a result of the recrystallization. Thus, the XRPCs in all the boreal hardwoods that we examined responded by deep supercooling that was accompanied with incomplete desiccation. From these results, it seems possible that limitations to the deep-supercooling ability of XRPCs might be a limiting factor for adaptation of hardwoods to cold climates.
It is generally accepted that xylem ray parenchyma cells (XRPCs) in hardwood species adapt to freezing of apoplastic water by deep supercooling or by extracellular freezing (Burke et al., 1976; George et al., 1982; Sakai and Larcher, 1987; Ashworth, 1996; Fujikawa and Kuroda, 2000). Previous results, suggesting the presence of such contrasting freezing behaviors of XRPCs in hardwood species, were obtained mainly in studies by differential thermal analysis (DTA). DTA profiles of xylem under cooling conditions reveal a high temperature exotherm (HTE) generated by freezing of apoplastic water, generally at around −5°C, as a common freezing profile of almost all hardwood species. The HTE is gradually reduced by further cooling and generally disappears below −20°C (Quamme et al., 1973). In xylem containing XRPCs that undergo deep supercooling, a low temperature exotherm (LTE) is generally produced below −20°C. It has been suggested that the LTE is produced by intracellular freezing of XRPCs that have been supercooled (Hong and Sucoff, 1980). In contrast, in xylem that contains XRPCs that exhibit extracellular freezing, no LTE is produced because XRPCs become dehydrated during cooling and the lost water is frozen extracellularly as a part of the HTE (Burke et al., 1976). Thus, in DTA studies, deep supercooling or extracellular freezing of XRPCs has been distinguished by the presence or absence, respectively, of an LTE.
Supercooling is a freezing avoidance mechanism and allows the survival of XRPCs during supercooling. However, supercooling has physical limits and ice nucleation results in lethal intracellular freezing that leads to serious injury in all cells. The physical limit of supercooling of pure water is −38°C (Rasmussen and MacKenzie, 1972) and the limit of supercooling in XRPCs is similar, being approximately −40°C (Burke et al., 1976). The lethal injury in XRPCs leads to the death of the entire tree (George et al., 1982). Extracellular freezing, in contrast, has no such physical temperature limit. Plant cells, which undergo extracellular freezing, exhibit a broad range of freezing tolerance from complete sensitivity to survival in liquid nitrogen (Sakai and Larcher, 1987). Thus, the capacity for extracellular freezing is believed to be a prerequisite for the survival of the XRPCs of boreal hardwood species that are distributed in regions where minimum air temperatures fall far below −40°C (Burke et al., 1976; George et al., 1982).
The contrasting freeze responses of XRPCs, as revealed by DTA, correspond to the geographic distribution of hardwood species as it relates to the latitudinal minimum air temperatures (George et al., 1974). Hardwood species with XRPCs that undergo deep supercooling are distributed in warmer areas where minimum air temperatures never drop below −40°C, whereas species with XRPCs that undergo extracellular freezing can grow in colder regions where minimum air temperatures fall far below −40°C and even to −70°C (George et al., 1974; Burke et al., 1976; Quamme, 1985; Kuroda et al., 1997b). Only a small number of the most cold-hardy boreal hardwood species belonging to the genera Salix, Betula, Populus, and Cornus are distributed in cold regions where minimum air temperatures are near −70°C, and the XRPCs in these hardwood species have been reported to lack an LTE, which suggests the extracellular freezing of the XRPCs (Burke et al., 1976; Sakai and Larcher, 1987; Fujikawa and Kuroda, 2000).
It has been proposed that the adaptation of XRPCs to freezing by deep supercooling in many hardwood species is due to the presence of thick and rigid cell walls (Fujii et al., 1979; Fujikawa et al., 1999), which do not allow for cytorrhysis, the collapse of cell walls together with protoplasts, which is typical of extracellular freezing (Sakai and Larcher, 1987). Nonetheless, in the XRPCs of most cold-hardy boreal hardwood species that appear to undergo extracellular freezing, the XRPCs also have thick and rigid cell walls (Ristic and Ashworth, 1994; Fujikawa and Kuroda, 2000). Thus, it is unclear how XRPCs in boreal hardwood species might adapt to freezing temperatures by extracellular freezing. Electron microscopic observations have suggested that XRPCs of boreal hardwood species might not respond to freezing by typical extracellular freezing. Ristic and Ashworth (1994) examined the freezing behavior of XRPCs by modified freeze substitution electron microscopy and suggested that, in the XRPCs of red osier dogwood (Cornus sericea), which has been categorized as an extracellular-freezing species, freezing produces plasmolysis, with contraction of protoplasts that leaves rigid cell walls in the intact position. Cryo-scanning electron microscopy (SEM) studies of XRPCs under freezing conditions have, however, failed to conform the development of freezing-induced plasmolysis in the majority of XRPCs in red osier dogwood (Fujikawa et al., 1996; Kuroda et al., 1999), as well as in other extremely cold-hardy boreal hardwood species (Kuroda et al., 1999). Cryo-SEM observations showed that XRPCs in boreal hardwood species frozen to −50°C undergo typical extracellular freezing, with the exception that shrinkage of cells (cytorrhysis) is slight (Kuroda et al., 1999), as compared with the extracellular freezing of cells of other plant tissues (Fujikawa, 1994; Yamada et al., 2002).
Since XRPCs are a key element that limits the distribution of hardwood species in colder areas (Sakai and Larcher, 1987), we reexamined the freezing responses of XRPCs in boreal hardwood species in great detail. In this study, we examined freezing behavior, upon cooling to −80°C, of XRPCs of extremely cold-hardy boreal hardwood species that included Salix sachalinensis, Populus sieboldii, Betula platyphylla, Betula pubescens, and red osier dogwood, in which the XRPCs have been classified as responding by extracellular freezing (Quamme, 1985; Sakai and Larcher, 1987; Fujikawa and Kuroda, 2000). We also examined the behavior of XRPCs upon rewarming (to −20°C) of samples after freezing. We anticipated that rewarming of frozen samples would clearly reveal intracellular ice crystals if they had been produced during slow cooling, as a result of the enlargement of such crystals due to recrystallization. The present study provided novel evidence on freezing behavior of XRPCs in boreal hardwood species.
RESULTS
Typical Freezing Behavior of XRPCs as Observed by Cryo-SEM
Previous studies showed that the freezing behavior of XRPCs in hardwood species can be assessed accurately by cryo-SEM (Fujikawa and Kuroda, 2000). The XRPCs that had been cryofixed from room temperature, as reference samples, had well-preserved ultrastructure, and no intracellular ice was detected by cryo-SEM at a magnification of 10,000× (Fig. 1A). Cryofixation of the reference samples resulted in numerous, very small intracellular ice crystals, as a consequence of the cryofixation of intracellular water, with the even distribution of such crystals throughout entire fractured faces of protoplasts, when they were observed by freeze fracture replica electron microscopy (Kuroda et al., 1997a). The minimum diameter of the intracellular ice crystals produced by cryofixation in the reference samples of the boreal hardwood species examined in this study was 28 ± 4 nm (n = 25).
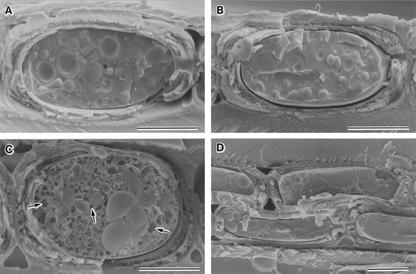
Figure 1.
Cryo-SEM photographs showing the various freezing behaviors of XRPCs in samples of boreal hardwood species that had been harvested in winter. A, Typical structure of an XRPC for reference. Xylem of S. sachalinensis was cryofixed at room temperature. B, Typical structure of an XRPC after deep supercooling. Xylem of S. sachalinensis was cooled slowly (10°C d−1) to −30°C and cryofixed. C, Typical structure of an XRPC after intracellular freezing. Xylem of S. sachalinensis was cooled slowly to −60°C and cryofixed. Arrows indicate intracellular ice crystals. D, Typical structure of XRPCs after putative extracellular freezing. The xylem of red osier dogwood was cooled slowly to −40°C and cryofixed. Bars = 5 μm.
Deep supercooling of XRPCs was judged, upon freezing of xylem, by the presence of well-preserved cellular ultrastructure and the absence of detectable intracellular ice crystals under the cryo-SEM (Fig. 1B), similar to that of reference samples. The minimum diameter of intracellular ice crystals produced by cryofixation in the supercooled XRPCs of the boreal hardwood species examined in this study was 26 ± 5 nm (n = 25), as determined by freeze fracture replica electron microscopy.
The occurrence of intracellular freezing in XRPCs during slow cooling (10°C d−1 = 0.007°C min−1) was easily recognized by cryo-SEM and was characterized by the presence of larger intracellular ice crystals that were dispersed evenly throughout entire fractured faces in the protoplasm (Fig. 1C). The intracellular ice crystals produced by slow cooling were more than 10 times larger than those produced by cryofixation (cooling rate of more than 1,000°C min−1), as we showed previously in the XRPCs from many hardwood species (Fujikawa and Kuroda, 2000) and as is also the case in other plant cells (Yamada et al., 2002). The minimum diameter of intracellular ice crystals produced by slow cooling in the XRPCs of the boreal hardwood species examined in this study was 620 ± 58 nm (n = 20).
Extracellular freezing of XRPCs was judged by the occurrence of cytorrhysis and the absence of typical intracellular ice crystals (both large ice crystals, formed as a result of intracellular freezing by slow cooling, and very small ice crystals, formed as a result of cryofixation) after cooling of xylem to lower temperatures, at least below −30°C (Fig. 1D).
Freezing Behavior of XRPCs in Boreal Hardwood Species
We examined the response to slow cooling (10°C d−1) from −40°C to −80°C of XRPCs in boreal hardwood species. Cryo-SEM revealed that the majority of XRPCs in these boreal hardwood species did not show evidence of intracellular freezing upon cooling to −40°C (Table I). However, almost all XRPCs in S. sachalinensis underwent typical intracellular freezing (Fig. 1C) upon slow cooling below −60°C (Table I). XRPCs in P. sieboldii also underwent typical intracellular freezing in about only one-quarter of the total cells upon cooling to −80°C (Table I). In XRPCs of B. platyphylla, B. pubescens, and red osier dogwood, the incidence of cells that underwent typical intracellular freezing was very low, bring only approximately 10% of the total cells, even after cooling to −80°C (Table I). The number of XRPCs with evidence of typical intracellular freezing in these species increased gradually in parallel with a decrease in cooling temperature from −60°C to −80°C (Table I).
Table I.
Percentage of XRPCs with evidence of intracellular ice crystals as observed at the frozen state
| Temperature | −40°C | −60°C | −80°C |
|---|---|---|---|
| S. sachalinensis | 0 ± 2 | 97 ± 2 | 100 ± 0 |
| P. sieboldii | 2 ± 2 | 19 ± 5 | 26 ± 17 |
| B. platyphylla | 4 ± 2 | 9 ± 1 | 13 ± 11 |
| B. pubescens | 0 ± 0 | 3 ± 2 | 8 ± 4 |
| Red osier dogwood | 1 ± 1 | 6 ± 3 | 10 ± 5 |
Data were obtained from Cryo-SEM observations. Percentages are means ± sd of results from three samples in each case. In each sample, more than 100 cells were observed.
The majority of XRPCs in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, which were exempted from typical intracellular freezing, exhibited freezing behavior suggestive of extracellular freezing. These cells underwent slight cytorrhysis although the extent of collapse was generally slight (Figs. 2A and 1D). In general, collapsed cells are located in adjacent to vessels in which the lumen was filled with ice crystals (Fig. 2A). In the XRPCs that underwent putative extracellular freezing, observations of fractured protoplasts in freeze fracture replicas at higher magnification also did not show clear evidence of the typical intracellular freezing produced by slow freezing or by cryofixation in samples cooled to −40°C (Fig. 2B). These results, obtained by observations in the frozen state, suggest that the majority of XRPCs in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood respond to subfreezing temperatures by extracellular freezing, confirming the results of a previous study (Fujikawa and Kuroda, 2000).
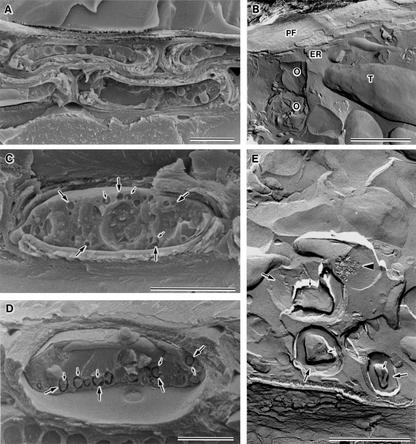
Figure 2.
Freezing behavior of XRPCs that had been cooled slowly (10°C d−1) to −80°C (except B, which shows a sample that was cooled slowly to −40°C) and cryofixed. A, Cryo-SEM photograph showing XRPCs in red osier dogwood. The sample was not deeply etched. B, Freeze fracture replica showing part of a fractured protoplast in B. platyphylla. The sample was not deeply etched. PF, Protoplasmic fracture face in the plasma membrane; O, oil droplet; T, tonoplast; and endoplasmic reticulum (ER) located just beneath the plasma membrane. C, Cryo-SEM photograph showing an XRPC in P. sieboldii. The sample was deeply etched. Many holes (large arrows), the result of removal of ice crystals by deep etching, were produced near the periphery of the plasma membrane, leaving shrunken materials (small arrows) in each hole. D, Cryo-SEM photograph showing an XRPC of B. platyphylla. The sample was deeply etched. Holes and shrunken materials are indicated by arrows and small arrows, respectively. E, Freeze fracture replica showing part of a fractured protoplast in B. platyphylla. The sample was deeply etched. Ice crystals or spaces produced by removal of ice crystals (large arrows) are seen at the periphery of oil droplets that left shrunken oil droplets (small arrows). Ice crystals were also produced occasionally in the lumen of vesicles (arrowhead). Note that the periphery of cell organelles other than oil droplets did not yield holes after deep etching. Bars in cryo-SEM photographs (A, C, and D) = 5 μm. Bars in freeze fracture replicas (B and E) = 1 μm.
Formation of Intracellular Ice Crystals with a Specific Distribution in XRPCs That Underwent Putative Extracellular Freezing
In the majority of XRPCs that underwent putative extracellular freezing in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, numerous small holes were produced in the fractured protoplasm when samples were observed in the frozen state after deep etching (Fig. 2, C and D). We postulated initially that these small holes might be concave fracture faces of vesicles produced by membrane fracturing, which left behind the exoplasmic fracture faces (EFs) of vesicle membranes. However, the presence of these holes was unclear before deep etching (Fig. 2, A and B), and deeper etching produced deeper holes. Therefore, we next postulated that the small holes produced by deep etching might be ice crystals with a specific distribution. The holes produced by deep etching were not detected upon freezing above −40°C, but showed a tendency to increase gradually in frequency upon a decrease in freezing temperature. After slow cooling to −80°C with deep etching, almost all XRPCs in the boreal hardwood species had holes in the fractured protoplasm.
Freeze fracture replicas developed from deeply etched samples revealed often that the bottom of each hole had a typically smooth surface that corresponded morphologically to the etched surface of an ice crystal (Fig. 2E). Furthermore, the surfaces of holes did not include structures that corresponded to exoplasmic fracture faces with membrane particles. Rather, the surfaces were smooth. The small holes produced by deep etching corresponded, in many cases, to the distribution of oil droplets (Fig. 2B), and included nonetchable shrunken materials in the center of holes (Figs. 2, C–E). The appearance of the shrunken materials corresponded to that of oil droplets, with multiplex fracture faces, which are typical of the appearance of oil droplets in reference samples (Fig. 2B).
Freeze fracture replicas also showed that, apart from the formation of etchable areas (possibly ice crystals) that surrounded oil droplets, small ice crystals were very occasionally produced within small vacuoles (Fig. 2E). Such small intracellular ice crystals within vesicles were difficult to detect by cryo-SEM, as well as on freeze-fracture replicas in the absence of deep etching.
Freezing Behavior of XRPCs as Judged by Results of Rewarming
We also examined the behavior of XRPCs after rewarming frozen samples. We postulated that the results of rewarming might provide clear evidence of intracellular freezing, if it had occurred during slow cooling, namely the enlargement of ice crystals upon recrystallization. In XRPCs of S. sachalinensis, which exhibited clear intracellular freezing during slow cooling below −60°C (Fig. 1C), the majority of XRPCs produced very large intracellular ice crystals upon rewarming to −20°C (Table II), and these crystals resembled those in Figure 3, A and B. After rewarming, only a small number of very large ice crystals of 3.2 ± 0.7 5 μm (minimum diameter; n = 20) were observed in fractured protoplasts.
Table II.
Percentage of XRPCs with evidence of intracellular freezing as a result of rewarming
| Temperature | −40°C | −60°C | −80°C |
|---|---|---|---|
| S. sachalinensis | 5 ± 2 | 76 ± 18 | 85 ± 10 |
| P. sieboldii | 2 ± 1 | 76 ± 15 | 85 ± 13 |
| B. platyphylla | 3 ± 2 | 75 ± 17 | 85 ± 7 |
| B. pubescens | 2 ± 1 | 33 ± 10 | 88 ± 4 |
| Red osier dogwood | 3 ± 2 | 68 ± 23 | 92 ± 3 |
Samples were frozen to indicated temperatures, rewarmed to −20°C, and cryofixed. Data were obtained from cryo-SEM observations. Percentages are means ± sd of results from three samples in each case. In each sample, more than 100 cells were observed.
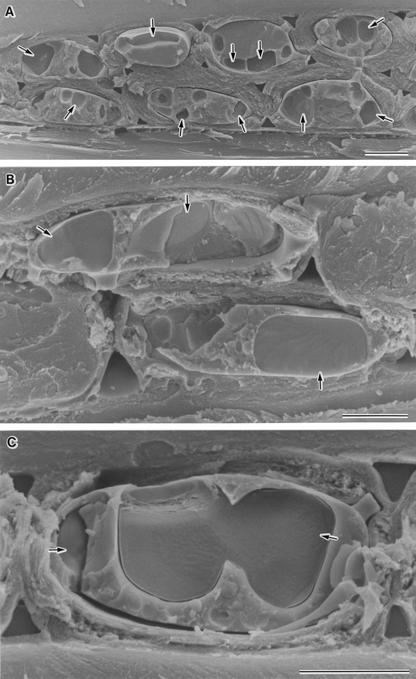
Figure 3.
Cryo-SEM photographs showing the behavior upon rewarming of XRPCs after cooling. A, XRPCs in B. platyphylla, after slow cooling to −60°C, rewarming to −20°C, and cryofixation. Arrows indicate very large intracellular ice crystals. B, XRPCs in red osier dogwood, after slow cooling to −80°C, rewarming to −20°C, and cryofixation. Arrows indicate very large ice crystals. C, An XRPC in P. sieboldii, after slow cooling to −60°C, rewarming to −20°C, and cryofixation. Arrows indicated very large ice crystals. All bars = 5 μm.
In other species, including P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, in which the majority of XRPCs apparently underwent extracellular freezing, with, perhaps, local development of small intracellular ice crystals, rewarming to −20°C after cooling below −40°C also produced small numbers of very large intracellular ice crystals of 3.8 ± 0.8 5 μm in minimum diameter (n = 50) in the majority of XRPCs (Fig. 3; Table II). The number of cells exhibiting local development of small intracellular ice crystals that were seen after deep etching (Fig. 2, C–E) and the number of cells exhibiting very large intracellular ice crystals after rewarming were roughly corresponded. Thus, rewarming provided clear evidence that almost all XRPCs in all the boreal hardwood species examined underwent intracellular freezing during slow cooling below −40°C, indicating that XRPCs in these species adapt to subfreezing temperatures by deep supercooling.
Some samples that had been slowly cooled to −60°C and −80°C were kept at these temperatures for an additional week, and some samples were kept at −20°C and −40°C for 1 week and then slowly cooled to −80°C. After such long-term preservation under freezing conditions, rewarming yielded the same results as routine slow cooling. These results indicate that, in nature, XRPCs of all the boreal hardwood species examined respond to the freezing of apoplastic water by deep supercooling between temperatures of −40°C and −80°C and do not respond by extracellular freezing.
DISCUSSION
Studies involving DTA, for the most part, have suggested that XRPCs in hardwood species exhibit contrasting freezing behaviors that are based on the presence or absence of an LTE. Previous studies by cryo-SEM confirmed that the development of an LTE corresponds to the intracellular freezing of XRPCs after the breakdown of deep supercooling (Fujikawa et al., 1994). In contrast, it has not been confirmed that lack of an LTE always corresponds to the extracellular freezing of XRPCs (Fujikawa et al., 1996). In XRPCs of tropical and subtropical hardwood species, in which DTA failed to reveal an LTE, cryo-SEM revealed the freezing response of XRPCs, namely deep supercooling, with a limit of around −10°C (Kuroda et al., 1997a). In this latter case, one of the reasons for the failure of DTA to detect an LTE was an overlap of the temperature range between the HTE and the LTE. Similarly, in XRPCs of a few moderately cold-hardy hardwood species, as well as those of many softwood species, although DTA failed to detect an LTE, cryo-SEM provided evidence of deep supercooling with around −20°C (Fujikawa et al., 1999; Fujikawa and Kuroda, 2000). In such cases, failure of DTA to reveal an LTE was probably due to shortage of LTE to be detected, due to a small number of XRPCs in these species. All these earlier results together suggested that the XRPCs of all hardwood species, with the exception of the most cold-hardy boreal hardwood species, respond to freezing by deep supercooling (Fujikawa and Kuroda, 2000).
The XRPCs in the most cold-hardy boreal hardwood species have been shown, in previous DTA studies, to lack an LTE throughout the entire year (Fujikawa et al., 1996). Previous cryo-SEM observations did, however, provide clear evidence of deep supercooling in these XRPCs, in samples harvested in summer exclusively, with a limit of around −10°C (Fujikawa et al., 1996). However, previous cryo-SEM observations did not provide evidence of deep supercooling in XRPCs in samples of boreal hardwood species harvested in winter. Thus, it remained possible that XRPCs in boreal hardwood species might change their freezing responses seasonally from deep supercooling in summer to extracellular freezing in winter (Fujikawa and Kuroda, 2000). However, in the present study, we found that XRPCs in boreal hardwood species respond, even in winter, to subfreezing temperatures by deep supercooling.
The evidence for deep supercooling of XRPCs in boreal hardwood species harvested in winter was difficult to detect clearly by cryo-SEM in our previous study when samples were observed in the frozen state at temperatures as low as −50°C (Kuroda et al., 1999). However, in the present study, when cryo-SEM observations were made to −80°C, even in the frozen state, XRPCs in S. sacchalinensis revealed the clear occurrence of intracellular freezing upon breakdown of deep supercooling below −60°C. Our previous cryo-SEM study showed that XRPCs in this species do not undergo intracellular freezing to −50°C (Kuroda et al., 1999). Thus, the limit of supercooling in XRPCs of S. sachalinensis is suggested to be close to −60°C. It is important to note again that, although cryo-SEM observations revealed clear evidence of intracellular freezing in XRPCs, DTA never revealed an LTE in S. sachalinensis. The failure of DTA to detect an LTE in this species might be due to shortage of LTE to be detected by the small numbers of XRPCs. Apart from S. sachalinensis, however, the majority of XRPCs in other boreal hardwood species did not show clear evidence of deep supercooling when they were observed by cryo-SEM, in the frozen state, even at −80°C (Fig. 2). The majority of XRPCs in these species, namely P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, exhibited cytorrhysis upon freezing, which was evidence of the dehydration that is characteristic of extracellular freezing, although the extent of collapse was slight. Other evidence for dehydration was also evident as the rearrangement of the ER just beneath the plasma membranes (Fig. 2B), as shown in the XRPCs of red osier dogwood during freezing in a previous study (Kuroda et al., 1997b). Fujikawa and Takabe (1994) showed that dehydration causes the rearrangement of ER in the cortical parenchyma cells of mulberry (Morus bombycis Koidz.). Thus, all these results support the hypothesis that XRPCs in most cold-hardy boreal hardwood species undergo extracellular freezing.
Careful cryo-SEM observations of XRPCs in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, after deep etching even in the frozen states, revealed the possibility that intracellular ice crystals might be produced in local areas of protoplasts in XRPCs upon cooling below −40°C. To confirm the intracellular freezing in these species, we rewarmed frozen samples, postulating that recrystallization of water upon rewarming might better reveal the presence of ice crystals if intracellular freezing had occurred during slow cooling. Observations of XRPCs upon rewarming clearly revealed that very large intracellular ice crystals were produced in the majority of XRPCs that had been cooled below −40°C (Fig. 3). Little intracellular ice crystals were produced upon rewarming in XRPCs that had been cooled above −40°C. It has also been shown that no intracellular ice crystals were produced upon rewarming of the cortical parenchyma cells of a hardwood that has undergone extracellular freezing to −80°C (Fujikawa, 1995). We confirmed similar enlargement of intracellular ice crystals by rewarming the XRPCs in S. sachalinensis, in which large and distinct intracellular ice crystals had been produced during slow cooling (Fig. 1C). Our results indicate clearly that the formation of very large intracellular ice crystals that occurs after rewarming is due to recrystallization of intracellular ice crystals and reflects the occurrence of intracellular freezing in XRPCs during slow cooling below −40°C. The formation of very large intracellular ice crystals after rewarming indicates that relatively large amounts of intracellular ice crystals were developed after slow cooling below −40°C. It is suggested that the etchable materials that surrounded oil droplets, observed in the frozen state (Fig. 2, C–E), might correspond to intracellular ice crystals, although the reasons for such local segregation of ice crystals, in particular around oil droplets, are unknown.
After rewarming of XRPCs in P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, the majority of XRPCs showed clear evidence of intracellular freezing below −60°C but a small number of XRPCs did not show any evidence of the formation of large ice crystals (Table II). This result might be interpreted to indicate that a small number of XRPCs adapt to freezing by extracellular freezing. However, in S. sachalinensis, although 97% to 100% of cells showed visible evidence of intracellular freezing in the frozen state below −60°C, only 76% to 85% cells contained very large intracellular ice crystals after rewarming. The reduction in the incidence of intracellularly frozen cells after rewarming in this case might originate from the reduced chance of a fracture passing through sparsely distributed, very large intracellular ice crystals. Similarly, the observation that, in XRPCs of P. sieboldii, B. platyphylla, B. pubescens, and red osier dogwood, the frequency of very large intracellular ice crystals after rewarming exceeded 85%, in terms of the total number of cells, might reflect the possibility that almost all cells underwent intracellular freezing.
Deep supercooling far below −40°C might occur as a result of the concentration of solutes in XRPCs during slow freezing (Rasmussen and MacKenzie, 1972). A DTA study by Gusta et al. (1983) showed depression of the LTE in XRPCs of scarlet oak (Quercus coccinea Muenchh.), riverbank grape (Vitis riparia Michx.), and American elm (Ulmus americana) due to dehydration of XRPCs during freezing. Clear evidence for dehydration in XRPCs of most cold-hardy boreal hardwood species during slow freezing is also provided by the occurrence of cytorrhysis, as well as by the dehydration-induced structural changes in the ER. However, it is important to stress that dehydration is incomplete. We showed that the XRPCs persisted in supercooling at the very slow cooling rate we used (10°C d−1) and even after more prolonged exposure to freezing. Thus, we can conclude that XRPCs in most cold-hardy boreal hardwood species, which were long believed to respond to subfreezing temperatures by extracellular freezing, actually respond by deep supercooling accompanied by incomplete desiccation, between −40°C and −80°C. The failure to detect an LTE in analyses of these boreal hardwood species might be due to shortage of LTE to be detected by the small numbers of XRPCs together with partial dehydration of these XRPCs during slow cooling.
One of the boreal hardwood species examined, B. pubescens, was a clone of trees that were originally grown at a forest boundary in Siberia where a minimum air temperature of −70°C had been recorded. Thus, the identification of the freezing behavior of XRPCs in this species as deep supercooling accompanied by incomplete desiccation might reflect a critical adaptive mechanism of XRPCs in hardwood species that grow in the coldest environments. Our present and previous studies provide evidence that the majority of XRPCs in all hardwood species respond to freezing of apoplastic water, for the most part, by deep supercooling. The adaptation to cold of XRPCs in boreal hardwood species by deep supercooling suggests that, in all hardwood species, limitations to the deep-supercooling ability of XRPCs might be a limiting factor for adaptation of hardwood species to cold climates in nature.
Freezing survival at liquid nitrogen temperature has been reported in twigs of boreal hardwood species belonging to genera Salix, Populus, and Betula (Sakai, 1965). However, all these results were obtained from twigs that were prefrozen and then immersed directly to cryogen. Such an experimental condition, rapid cooling by direct immersion to cryogen after partial dehydration by prefreezing, may produce vitrification of water in XRPCs and may result in survival of twigs by avoiding occurrence of intracellular freezing of XRPCs. The survival test of twigs in these species at slow cooling, which mimicked to natural condition, is now under examination in our laboratory.
MATERIALS AND METHODS
Plant Materials
Fresh twigs of approximately 4 years of age were obtained from Salix sachalinensis Fr. Schmit, Populus sieboldii Miq., Betula platyphylla Sukat. var japonica Hara, and red osier dogwood (Cornus sericea) that were growing on the campus of Hokkaido University (Sapporo) in winter (December to February). Similar twigs were also obtained in winter from a specimen of Betula pubescens Ehrh. that was a clone of trees that were originally growing at a forest boundary in Siberia, and was growing on the field in the University Forest in Hokkaido (University of Tokyo, Yamabe). Fresh twigs of these trees were kept at 0°C for 1 d after sampling. Small blocks (3 × 3 × 4 mm) were removed at 4°C from the xylem, including second and third annual rings, and used for experiments.
Freezing and Rewarming of Samples
A small block of xylem was put in a specimen holder and distilled water was added to surround the surfaces of the block located outside the holder to prevent drying during long-term cooling. The xylem blocks in specimen holders were placed in a freezer kept at −5°C, allowed to equilibrate for 1 h, and frozen by seeding the surrounding water with ice. Samples were cooled down by transferring them in a stepwise manner to refrigerators maintained at temperatures down to −80°C at intervals of 5°C. Samples were kept for 12 h at each temperature (approximate cooling rate: 10°C d−1). In some cases, cooling was performed linearly from −10°C at a rate of 0.007°C min−1 in a programmable freezer (ES-100P, Tajiri Co. Ltd., Sapporo, Japan). The linear cooling resulted in the same freezing responses of XRPCs as those obtained by step-wise freezing. Samples that had been cooled to a given temperature were cryofixed by direct immersion in cooled Freon 22 at −150°C. For rewarming, some samples that had been cooled to a given temperature were transferred directly to a refrigerator kept at −20°C for 4 h and then cryofixed. Reference samples were cryofixed at a room temperature. All cryofixed samples were stored in liquid nitrogen.
Cryo-SEM
Cryofixed samples were transferred to the cold stage and kept at −108°C, in a specimen preparation chamber of a cryo-SEM (840A-SEM, JEOL Co. Ltd., Tokyo). Samples were equilibrated and fractured with a cold knife to expose tangential faces of the earlywood xylem. Fracture faces were rotary-evaporated with platinum-carbon soon after fracturing or after etching for 5 min (deep etching). After evaporation, samples were transferred to the cold stage of an SEM column that was kept at −160°C. Secondary emission images were recorded at an accelerating voltage of 5 kV.
Freeze Fracture Replica Electron Microscopy
Freeze fracture replicas were produced with a freeze-etching apparatus (JFD-7000, JEOL Co. Ltd.) under fracture and etching conditions similar to those used for cryo-SEM. Fracture faces were shadowed unidirectionally with evaporation by platinum-carbon and followed by rotary evaporation with C. After removal of samples by immersion in concentrated sulfuric acid, commercial bleach, and acetone, replicas were observed with a transmission electron microscope (1200 EX, JEOL Co. Ltd.) at an accelerating voltage of 100 kV.
Measurement of Ice Crystal Size
Both cryo-SEM and freeze-fracture replica photographs were enlarged to more than 10,000×. The minimum diameter of individual ice crystals was measured by a scale directly on the photographs.
ACKNOWLEDGMENT
The authors thank Dr. S. Kamoda (University Forest in Hokkaido, University of Tokyo, Yamabe, Hokkaido) for the generous gift of B. pubescens twigs.
Footnotes
This work was supported by the Program for the Promotion of Basic Research Activities for Innovative Biosciences (grant), by the Ministry of Education, Science and Culture of Japan (grant), and by the Graduate School of Agriculture, Hokkaido University (current expenditure).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011601.
LITERATURE CITED
- Ashworth EN. Responses of bark and wood cells to freezing. Adv Low Temp Biol. 1996;3:65–106. [Google Scholar]
- Burke MJ, Gusta LV, Quamme HA, Weiser CJ, Li PH. Freezing and injury in plants. Annu Rev Plant Physiol. 1976;27:507–528. [Google Scholar]
- Fujii T, Harada H, Saiki H. The layered structure of ray parenchyma secondary wall in the wood of 49 Japanese angiosperm species. Mokuzai Gakkaishi. 1979;25:251–257. [Google Scholar]
- Fujikawa S. Seasonal ultrastructural alterations in the plasma membrane produced by slow freezing in cortical tissues of mulberry (Morus bombycis Koidz. cv. Goroji) Trees. 1994;8:288–296. [Google Scholar]
- Fujikawa S. A freeze-fracture study designed to clarify the mechanisms of freezing injury due to the freezing-induced close apposition of membranes in cortical parenchyma cells of mulberry. Cryobiology. 1995;32:444–454. [Google Scholar]
- Fujikawa S, Kuroda K. Cryo-scanning electron microscopic study on freezing behavior of xylem ray parenchyma cells in hardwood species. Micron. 2000;31:669–686. doi: 10.1016/s0968-4328(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Fujikawa S, Kuroda K, Fukazawa K. Ultrastructural study of deep supercooling of xylem ray parenchyma cells from Stylax obassia. Micron. 1994;25:241–252. [Google Scholar]
- Fujikawa S, Kuroda K, Jitsuyama Y, Sano Y, Ohtani J. Freezing behavior of xylem ray parenchyma cells in softwood species with differences in the organization of cell walls. Protoplasma. 1999;206:31–40. [Google Scholar]
- Fujikawa S, Kuroda K, Ohtani J. Seasonal changes in the low-temperature behaviour of xylem ray parenchyma cells in red osier dogwood (Cornus sericea L.) with respect to extracellular freezing and supercooling. Micron. 1996;27:181–191. [Google Scholar]
- Fujikawa S, Takabe K. Formation of multiplex lamellae by equilibrium slow freezing of cortical parenchyma cells of mulberry and its possible relationship to freezing tolerance. Protoplasma. 1996;190:189–203. [Google Scholar]
- George MF, Becwar MR, Burke MJ. Freezing avoidance by deep undercooling of tissue water in winter-hardy plants. Cryobiology. 1982;19:628–639. doi: 10.1016/0011-2240(82)90192-4. [DOI] [PubMed] [Google Scholar]
- George MF, Burke MJ, Pellet HM, Johnson AG. Low temperature exotherms and woody plant distribution. HortScience. 1974;9:519–522. [Google Scholar]
- Gusta LV, Tyler NJ, Chen THH. Deep undercooling in woody taxa growing north of the −40°C isotherm. Plant Physiol. 1983;72:122–128. doi: 10.1104/pp.72.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SG, Sucoff E. Units of freezing of deep supercooled water in woody xylem. Plant Physiol. 1980;66:40–45. doi: 10.1104/pp.66.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Ohtani J, Fujikawa S. Supercooling of xylem ray parenchyma cells in tropical and subtropical hardwood species. Trees. 1997a;12:97–106. [Google Scholar]
- Kuroda K, Ohtani J, Fujikawa S. Changes of low temperature behavior of xylem ray parenchyma cells of hardwood species. Cryobiol Cryotechnol. 1997b;43:99–111. [Google Scholar]
- Kuroda K, Ohtani J, Kubota M, Fujikawa S. Seasonal changes in the freezing behavior of xylem ray parenchyma cells in four boreal hardwood species. Cryobiology. 1999;38:81–88. doi: 10.1006/cryo.1998.2149. [DOI] [PubMed] [Google Scholar]
- Quamme HA. Avoidance of freezing injury in woody plants by deep supercooling. Acta Hortic. 1985;168:11–30. [Google Scholar]
- Quamme H, Weiser CJ, Stushnoff C. The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 1973;51:273–277. doi: 10.1104/pp.51.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DH, MacKenzie AP. Effects of solute on ice-solution interfacial free energy: calculation from measured homogeneous nucleation temperatures. In: Jellineck HHG, editor. Water Structure at the Water-Polymer Interface. New York: Plenum Press; 1972. pp. 126–145. [Google Scholar]
- Ristic Z, Ashworth EN. Response of xylem ray parenchyma cellscells of red osier dogwood (Cornus sericea L.) to freezing stress: microscopic evidence of protoplasm contraction. Plant Physiol. 1994;104:737–746. doi: 10.1104/pp.104.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A. Survival of plant tissue at super-low temperature: III. Relation between effective prefreezing temperatures and the degree of frost hardiness. Plant Physiol. 1965;40:882–887. doi: 10.1104/pp.40.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A, Larcher W, editors. Frost Survival of Plants: Response and Adaptation to Freezing Stress. Berlin: Springer; 1987. [Google Scholar]
- Yamada T, Kuroda K, Jitsuyama Y, Takezawa D, Arakawa K, Fujikawa S. Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta. 2002;215:770–778. doi: 10.1007/s00425-002-0814-5. [DOI] [PubMed] [Google Scholar]