Abstract
Exogenously supplied auxin (1-naphthaleneacetic acid) inhibited light-induced activity increase of polyamine oxidase (PAO), a hydrogen peroxide-producing enzyme, in the outer tissues of maize (Zea mays) mesocotyl. The same phenomenon operates at PAO protein and mRNA accumulation levels. The wall-bound to extractable PAO activity ratio was unaffected by auxin treatment, either in the dark or after light exposure. Ethylene treatment did not affect PAO activity, thus excluding an effect of auxin via increased ethylene biosynthesis. The auxin polar transport inhibitors N1-naphthylphthalamic acid or 2,3,5-triiodobenzoic acid caused a further increase of PAO expression in outer tissues after light treatment. The small increase of PAO expression, normally occurring in the mesocotyl epidermis during plant development in the dark, was also inhibited by auxin, although to a lesser extent with respect to light-exposed tissue, and was stimulated by N1-naphthylphthalamic acid or 2,3,5-triiodobenzoic acid, thus suggesting a complex regulation of PAO expression. Immunogold ultrastructural analysis in epidermal cells revealed the association of PAO with the secretory pathway and the cell walls. The presence of the enzyme in the cell walls of this tissue greatly increased in response to light treatment. Consistent with auxin effects on light-induced PAO expression, the hormone treatment inhibited the increase in immunogold staining both intraprotoplasmically and in the cell wall. These results suggest that both light and auxin finely tune PAO expression during the light-induced differentiation of the cell wall in the maize mesocotyl epidermal tissues.
Dark-light transitions dramatically affect organ architecture and growth rate during the first stages of plant development. In particular, for a young seedling buried under the soil surface, rapid extension growth of hypogean organs occurs in the dark to reach sunlight. The mesocotyl is devoted to accomplish this important function in maize (Zea mays) and other Gramineae, the growth of this organ being strongly stimulated in the dark, whereas it is inhibited by light as soon as the coleoptile sprouts from the soil surface. This complex photomorphogenic event, mediated by different classes of photoreceptors (Vanderhoef and Briggs, 1978), is thought to be linked to the reduction of indole-3-acetic acid (IAA) supply from the coleoptile to the mesocotyl (Iino, 1982), particularly in its epidermis (Barker-Bridgers et al., 1998). This process causes a tension increase in the tissue, which constrains the growth of the whole organ, the greatest tensile force loading on the outer walls of epidermal cells (Masuda and Yamamoto, 1972; Kutschera and Briggs, 1987; Bret-Harte et al., 1991; Kutschera, 1992).
Cell wall yielding properties depend on a finely regulated balance between wall-loosening and -stiffening events. Wall loosening is thought to be mediated by either enzymatic (Cosgrove, 2000; Darley et al., 2001) or chemical agents (Miller, 1986; Fry, 1998; Schopfer, 2001). To this respect, it has been hypothesized recently that the hydroxyl radical (.OH), may represent a wall-loosening agent causally involved in auxin-induced growth (Chen and Schopfer, 1999; Schopfer et al., 2002). .OH can be produced by peroxidase in the presence of superoxide anion (O2−.) and hydrogen peroxide (H2O2; Chen and Schopfer, 1999).
On the other hand, peroxidase activity is likewise responsible for H2O2-dependent wall stiffening that causes cell wall mechanical fortification and confers extension irreversibility during cell growth (Fry, 1986; Hohl et al., 1995; Schopfer, 1996).
Taken together, available data suggest that the relative levels of H2O2 and O2−. in the apoplast (Musel et al., 1997; Chen and Schopfer, 1999) and in the secretory pathway (Fry et al., 2000) may play a pivotal role in the modulation of both cell wall expansion and maturation after cessation of growth. Moreover, these reactive oxygen intermediates are known to play a key role in defense against plant pathogens as well (for review, see Grant and Loake, 2000).
A main goal in the understanding of molecular events underlying cell wall expansion and maturation is the analysis of the specific contribution of the molecular machineries synthesizing H2O2, O2−., and their cognate reaction product .OH, in the apoplast and in the secretion pathway. O2−. production in the apoplast has been thoroughly studied and it can be ascribed to several enzyme activities, depending on the particular physiological context or plant species (Bolwell, 1999). O2−. synthesis in the apoplast seems to be redundant because it can be accomplished by either the NAD(P)H oxidizing activity of wall peroxidases (Elstner and Heupel, 1976; Bolwell et al., 1998; Frahry and Schopfer, 2001) or by a plasma membrane NAD(P)H oxidase similar to the inducible NADPH oxidase complex of the mammalian phagocytes (Wojtaszek, 1997, and refs. therein). H2O2 synthesis in the cell wall is likewise redundant because it can originate either from O2−. dismutation (either spontaneous or catalyzed by cell wall superoxide dismutases), or from the oxidative cycle of peroxidases in the presence of a reductant (Elstner and Heupel, 1976; Bolwell, 1999). Amine oxidases (Angelini and Federico, 1989; Allan and Fluhr, 1997; Møller and McPherson, 1998; Laurenzi et al., 2001) or oxalate oxidases (Lane, 1994) can also be involved in H2O2 production in the apoplast. Circumstantial evidence fosters the view that copper amine oxidases and flavin-containing polyamine oxidases (PAOs) play a key role in H2O2 production in the cell wall during ontogenesis as well as in response to wounding or pathogen invasion in different species (Angelini et al., 1990, 1993; Rea et al., 1998; Laurenzi et al., 1999; Wisniewski et al., 2000; Asthir et al., 2002; Rea et al., 2002; Cooley and Walters, 2002).
Plant PAOs are FAD-containing glycoproteins responsible for the terminal catabolism of polyamines containing a secondary amino group (Šebela et al., 2001). These enzymes oxidize spermine and spermidine to the corresponding aminoaldehydes and 1,3-diaminopropane, releasing H2O2 upon reoxidation of the reduced enzyme (Federico et al., 1990; Tavladoraki et al., 1998). PAO is especially abundant in the primary and secondary cell walls of xylem, xylem parenchyma, endodermis, and epidermis of maize seedlings where it has been localized by means of biochemical, histochemical, and immunocytochemical methods (Kaur-Sawhney et al., 1981; Angelini and Federico, 1989; Slocum and Furey, 1991; Angelini et al., 1995; Laurenzi et al., 1999). Furthermore, in maize mesocotyl epidermis PAO expression has been shown recently to be stimulated by light, this phenomenon being mediated by phytochrome (Laurenzi et al., 1999). In particular, PAO mRNA and protein levels, as well as enzyme activity, increase in epidermal tissues of maize mesocotyl in response to de-etiolation, both in the elongating and the mature zone. The time course of light-induced increase of PAO activity in the outer tissues of apical growing zone of the mesocotyl is tightly correlated to the inhibition of extension growth (Laurenzi et al., 1999). Moreover, H2O2 production in maize mesocotyl segments is inhibited by guazatine, a powerful PAO inhibitor (Laurenzi et al., 1999). Characterization of promoter sequences in two genes encoding PAO (MPAO1 and MPAO2; Cervelli et al., 2000) revealed the presence of putative cis-acting motifs responsive to light or auxin, thus suggesting the possibility of a transcriptional control of PAO expression by the hormone and light.
The principal aim of the present study was to investigate whether light-induced reduction of diffusible IAA in the maize mesocotyl could regulate PAO gene expression during cell differentiation after cessation of mesocotyl growth. For this purpose, we have studied the effect of exogenous auxin supply on PAO expression levels in the outer tissues of nongrowing zone of the maize mesocotyl either in the dark or after light pulses. A further scope of this study was to understand how the variation of endogenous auxin levels, obtained by using auxin polar transport inhibitors (ATIs), could affect light-induced expression of the PAO gene. Moreover, immunoelectron microscopic analysis was performed to investigate possible effects of auxin and light on PAO subcellular localization in the epidermal cells. The physiological significance of the modulation of PAO gene expression in the maize mesocotyl by light, auxin, or ATI and changes in subcellular distribution of the enzyme are discussed in relation to cell wall stiffening and differentiation.
RESULTS
Time Course of Light-Dependent Induction of PAO Expression Revealed an Early Enzyme Inactivation
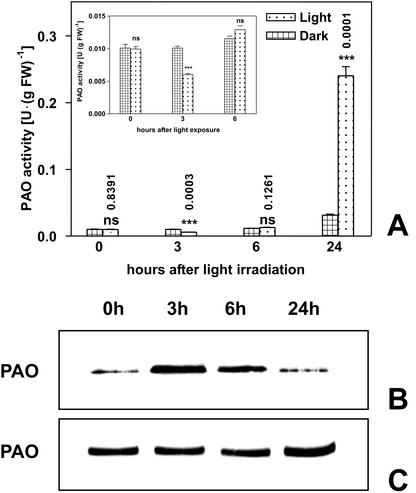
A previous study showed the induction of PAO expression in the outer tissues (i.e. cortical and epidermal tissues) of the nongrowing zone of maize mesocotyl in the days after the onset of white light exposure (Laurenzi et al., 1999). The present study, besides confirming previous results on a long-term basis, reports a time course analysis revealing an early light-induced inhibition of PAO activity. In particular, PAO activity levels expressed on a fresh weight (Fig. 1A) or protein basis (data not shown) was 40% decreased in the mesocotyl outer tissues 3 h after a 10-min white light exposure (18,000 lux), as compared with dark controls, whereas an 8-fold increase of PAO activity levels was observed at 24 h. Western-immunoblotting analysis performed after SDS-PAGE loaded on the basis of equal enzyme activity demonstrated a higher PAO protein level in de-etiolated tissues with respect to dark control at 3 and 6 h (Fig. 1B). In contrast, when SDS-PAGE gels were loaded on the basis of total protein level, no differences in PAO protein level were observed up to 6 h, whereas a more intense band was evident at 24 h (Fig. 1C). This result suggests early enzyme inactivation in light-exposed tissues, while confirming the increase of PAO activity levels in the next hours. It was demonstrated previously that light had a stimulatory effect on the accumulation level of PAO mRNA a few hours after the onset of light treatment (Laurenzi et al., 1999). White light exposure up to 3 h of crude extracts from the outer tissues of etiolated mesocotyl caused an enzyme activity decrease (this study; 44% as compared dark control value) similar to that detected after irradiating the whole plant, whereas a lower inhibition (23%) was observed in homogenates obtained from green tissues. Because catalytic activity of purified PAO was unaffected by light exposure, a direct damaging effect of light on PAO protein or FAD cofactor could be excluded. Moreover, the lower light-induced inhibition of PAO activity detected in crude homogenates from green tissues suggests the presence of some protecting substances in tissues previously exposed to light. During light exposure of dark-grown plants or crude homogenates from etiolated tissues, the formation of unknown compounds affecting PAO protein occurred. In fact, after SDS-PAGE and western-immunoblotting analyses of irradiated crude extracts obtained from etiolated shoots, additional bands probably related to PAO protein degradation were detectable (this study; data not shown). Enzyme inactivation was irreversible because PAO immunoprecipitation and dialysis failed to restore enzyme activity, suggesting that inactivation was not caused by a soluble inhibitor (this study; data not shown). Based on these observations, we concluded that the early, light-induced inhibition of PAO activity was probably caused by unknown compounds produced during sudden exposure of dark-grown plants or crude homogenates to high-intensity white light. The inhibitory effect was transient (Fig. 1), and completely reversed thereafter. As a consequence of this phenomenon, effect exerted by auxin on light induced-PAO expression was evaluated 24 h after plant irradiation.
Figure 1.
Effect of white light on PAO enzyme activity and protein levels in the outer tissues of the maize mesocotyl. Etiolated plants (108 h after soaking in the dark) were irradiated under white light (18,000 lux) for 10 min and extractable PAO activity and protein levels determined in the outer tissues of the mature zone of the maize mesocotyl at the times indicated. A, PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis in dark-grown and light-irradiated plants (inset: PAO activity levels plotted on a lower scale graph). P values have been calculated comparing PAO activity levels in control and irradiated plants for each time. ns, Not significant; *, * *, and * * *, P values ≤ 0.05, 0.01, and 0.001, respectively (the nos. above the bars represent actual P values). B and C, Western immunoblotting carried out after SDS-PAGE loaded on the basis of the equal enzyme activity (B) or total protein content (C).
Auxin Treatment Strongly Inhibited Light-Induced Increase of PAO Expression in Outer Tissues of the Maize Mesocotyl
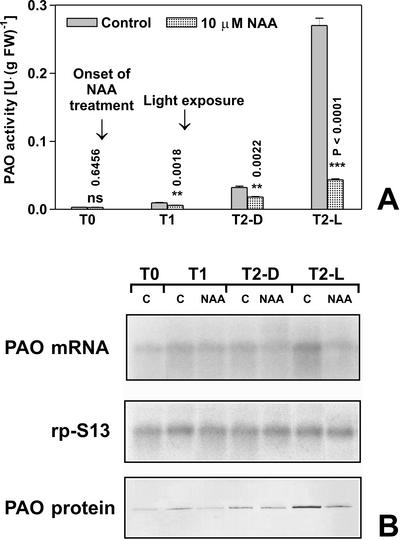
To ascertain the possible involvement of auxin in regulating PAO gene expression, we analyzed the effect of exogenously supplied synthetic auxin 1-naphthaleneacetic acid (NAA) in the outer tissues of the nongrowing zone of the maize mesocotyl, either in the dark or after a short light treatment. Four-day-old maize seedlings (96 h after soaking [T0]) were irradiated with white light 12 h after T0 (T1) as described in “Materials and Methods.” As shown in Figure 2A, 10 μm NAA treatment at 0, 12, and 24 h after T0 almost totally inhibited the light-stimulated increase of PAO activity expressed on a fresh weight basis occurring 24 h after the 10-min white light exposure at T1 (T2-L). The small PAO activity increase in the dark (T1-D and T2-D), normally occurring during seedling development, was also inhibited by NAA treatment, although to a lesser extent. Very similar results were obtained when activity was expressed on total protein basis (data not shown). At 10 μm auxin concentration of the treatment solution, no changes in plant morphology were observed. At concentration lower than 10 μm, the hormone was less effective, and in plants treated with 1 μm NAA, inhibition of light-induced PAO activity increase was hardly detected (data not shown). In the same samples, PAO mRNA and protein accumulation was examined. Total RNA purified from outer tissues of maize mesocotyl of the corresponding samples indicated in Figure 1A was analyzed by northern blot using maize PAO cDNA (EMBL Database accession no. AJ002204) as a probe (Tavladoraki et al., 1998) or maize RP-S13 (GenBank accession no. AF067732) as loading control. The analysis revealed a unique hybridizing band of ≅1,900 nucleotides accumulating in a fashion that exactly paralleled the PAO activity values measured for the untreated and NAA-treated samples (Fig. 2B). In fact, the T2-L control sample showed about a 10-fold increase in PAO mRNA amount with respect to T2-D control, indicating a light-induced increase in PAO transcript accumulation level. On the contrary, T2-L NAA sample only showed a 2-fold increase in PAO mRNA level, thus confirming the role of NAA in inhibiting the light-stimulated expression of PAO genes. Furthermore, a western-blot analysis of the crude extract of these samples revealed that the intensity of each protein band reflected the accumulation of the corresponding transcript and paralleled the relative PAO activity values (Fig. 2B).
Figure 2.
Effects of auxin and light on PAO activity, protein levels and transcript accumulation in the outer tissues of the mature zone of the maize mesocotyl. Etiolated plants were sprayed with 10 μm NAA every 12 h starting from T0 (96 h after soaking). Light irradiation was performed for 10 min under white light (18,000 lux) immediately after T1. T1, Twelve hours of dark; T2-D, 36 h of dark; T2-L, 12 h of dark +10-min light irradiation + 23 h 50 min of dark. A, Extractable PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis in control and 10 μm NAA-treated plants. P values have been calculated comparing PAO activity levels in control and 10 μm NAA-treated plants for each time and for each light condition. ns, Not significant; *, * *, and * * *, P values ≤ 0.05, 0.01, and 0.001, respectively (the nos. above the bars represent actual P values). B, Northern- and western-blot analyses (samples as in A). Total RNA was fractionated by agarose/formaldehyde gel electrophoresis, blotted onto a nylon membrane, and hybridized with 32P-labeled maize PAO cDNA probe (upper insert). As a loading control, samples were also hybridized with the cDNA of the S13 ribosomal protein (middle insert). Western immunoblotting performed after SDS-PAGE loaded on the basis of the total protein content in control (C) and 10 μm NAA-treated plants (NAA; lower insert).
Wall-bound to extractable PAO activity ratio in outer tissues of the mesocotyl showed no significant differences in auxin-treated and control plants, either in the dark or after light treatment. However, we could observe that wall-bound PAO units per gram fresh weight increased from 6% (T0) to approximately 30% of total PAO units (T2 samples) as cells mature (Table I).
Table I.
Wall-bound PAO activity expressed as the percentage of total PAO activity (wall-bound plus extractable) in the outer tissues of the mature zone of the maize mesocotyl
| Time/Light Treatment | Wall-Bound PAO Activity (% of Total PAO Activity Values)
|
|
|---|---|---|
| Control | NAA-treated plants | |
| T0 (96 h after soaking) | 6% (± 0.39) | – |
| T1 (12 h after T0 in the dark) | 23% (±1.9) | 18% (± 1.35) |
| T2-D (36 h after T0 in the dark) | 32% (±2.75) | 31% (±2.13) |
| T2-L (12 h after T0 in the dark + 10-min light irradiation + 23 h 50 min in the dark) | 30% (±2.42) | 33% (±2.91) |
Etiolated plants were sprayed with 10 μM NAA every 12 h starting from T0 (96 h after soaking). Light irradiation was performed 12 h after T0 (T1) for 10 min (18,000 lux) in a growth chamber. T1, Twelve hours after T0 in the dark; T2-D, 36 h after T0 in the dark; T2-L, 12 h after T0 in the dark + 10-min light irradiation + 23 h 50 min in the dark. Mean values ± sd.
Regulation of PAO Gene Expression by Auxin Is Not Mediated by Ethylene
With the aim to investigate whether the effects of auxin in the regulation of PAO gene expression could be ascribed to auxin-induced ethylene synthesis, we measured enzyme activity levels in outer tissues from dark-grown or light-exposed maize mesocotyls treated, or not, with the soluble ethylene-releasing compound 2-chloroethylphosphonic acid (Ethephon). Exogenously supplied ethylene at 0, 12, and 24 h after T0 (Ethephon concentrations ranging from 0.1 μm to 1 mm), did not affect PAO activity levels either in the dark or after light exposure (data not shown).
Exogenous Supply of ATIs Amplified the Light-Induced Increase of PAO Expression
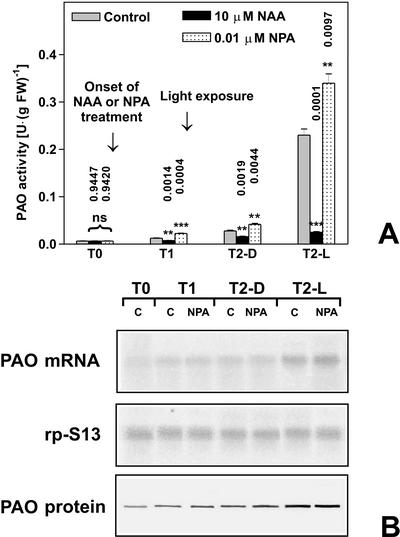
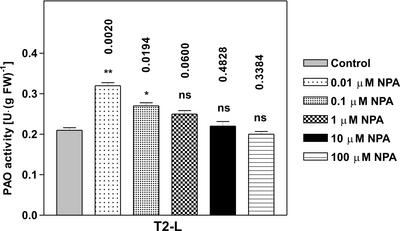
The role played by the light-induced reduction of diffusible auxin in the mesocotyl (Iino, 1982; Barker-Bridgers et al., 1998) in regulating PAO gene expression was further analyzed utilizing two different ATIs. Because auxin synthesis mainly occurs in the coleoptile (Iino and Carr, 1982), plants treated with ATIs were expected to contain lower levels of the hormone in the mesocotyl. Based on the inhibitory effect exerted by auxin on light-induced PAO gene expression, ATIs were supposed to amplify light-induced PAO expression. In line with this hypothesis, exogenous supply of the phytotropinic ATI N1-naphthylphthalamic acid (NPA) at a concentration of 0.01 μm caused a further increase of PAO activity levels after light exposure with respect to untreated plants. In fact, the T2-L NPA sample showed an approximately 30% increase in PAO activity with respect to the T2-L control sample (Fig. 3A). On the contrary, the increase of PAO activity in the dark was stimulated by NPA to a lesser extent, as observed in T2-D NPA and T1-D NPA samples, when compared with T2-D control and T1-D control samples, respectively (Fig. 3A). Moreover, simultaneous supply of NPA and NAA resulted in an inhibition of light-induced increase in PAO activity comparable with that obtained with NAA alone (data not shown), consistent with the well-known evidence that auxin does not compete with NPA for its binding site (Sussman and Goldsmith, 1981). Also in this case, PAO mRNA and protein accumulation were examined in parallel to study PAO gene expression in response to NPA treatment under light or dark conditions. Northern-blot analysis revealed that PAO mRNA accumulates in a fashion that again parallels PAO activity levels measured in NPA-treated samples (Fig. 3B). Western-blot analysis of the corresponding protein extracts essentially reflects the accumulation of the corresponding transcript and enzyme activity values (Fig. 3B). NPA supply at concentrations higher than 0.01 μm caused a lower effect in amplifying the PAO activity increase in T2-L samples, with 10 μm NPA being totally ineffective (Fig. 4).
Figure 3.
Effects of NPA and light on PAO activity, protein levels, and transcript accumulation in the outer tissues of the mature zone of the maize mesocotyl. Etiolated plants were sprayed with 0.01 μm NPA or 10 μm NAA every 12 h starting from T0 (96 h after soaking). Light irradiation was performed for 10 min under white light (18,000 lux) immediately after T1. T1, Twelve hours of dark; T2-D, 36 h of dark; T2-L, 12 h of dark +10-min light irradiation + 23 h 50 min dark. A, Extractable PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis, in control, 0.01 μm NPA-, or 10 μm NAA-treated plants. P values have been calculated comparing PAO activity levels in NPA and NAA treated plants with respect to controls for each time and for each light condition. ns, Not significant; *, * *, and * * *, P values ≤ 0.05, 0.01, and 0.001, respectively (the nos. above the bars represent actual P values). B, Northern- and western-blot analysis. Total RNA was fractionated by agarose/formaldehyde gel electrophoresis, blotted onto a nylon membrane, and hybridized with 32P-labeled maize PAO cDNA probe (upper insert). As a loading control, samples were also hybridized with the cDNA of the S13 ribosomal protein (middle insert). Western immunoblotting performed after SDS-PAGE loaded on the basis of the total protein content in control (C) and 0.01 μm NPA-treated plants (NPA; lower insert).
Figure 4.
Effects of NPA and light on PAO activity levels in the outer tissues of the mature zone of the maize mesocotyl. Extractable PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis after treatments with NPA at different concentrations. Plants were sampled at T2. P values have been calculated comparing PAO activity levels in NPA- and NAA-treated plants with respect to controls. ns, Not significant; *, * *, and * * *, P values ≤ 0.05, 0.01, and 0.001, respectively (the nos. above the bars represent actual P values).
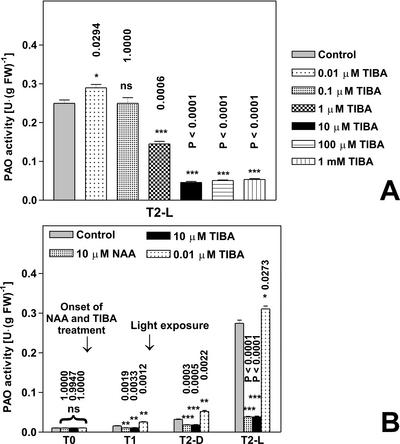
As shown in Figure 5A, exogenous supply of the non-phytotropinic ATI 2,3,5-triiodobenzoic acid (TIBA) resulted in opposite effects depending on ATI concentration. This result is consistent with what was described previously about the auxin activity of TIBA (Thomson et al., 1973). In particular, 0.01 μm TIBA slightly increased PAO activity level in light-exposed plants with respect to control plants (T2-L control and T2-L TIBA at 0.01 μm). On the contrary, 10 μm TIBA acted in an auxin-like fashion by inhibiting light-induced increase of PAO activity levels to the same extent of 10 μm NAA (Fig. 5, A and B).
Figure 5.
Effects of TIBA and light on PAO activity levels in the outer tissues of the mature zone of the maize mesocotyl. Etiolated plants were sprayed with TIBA (ranging from 0.0l μm to 1 mm) every 12 h starting from T0 (96 h after soaking). Light irradiation was performed for 10 min under white light (18,000 lux) immediately after T1 time. P values have been calculated comparing PAO activity in control and TIBA-treated plants for each time and for each light condition. ns, Not significant; *, * *, and * * *, P values ≤ 0.05, 0.01, and 0.001, respectively (the nos. above the bars represent actual P values). A, Extractable PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis after treatments with TIBA at different concentrations (plants were sampled at T2). B, PAO activity levels (mean values ± sd; n = 3) expressed on a fresh weight basis in control, 10 μm TIBA-, 0.01 μm TIBA-, and 10 μm NAA-treated plants. T1, Twelve hours of dark; T2-D, 36 h of dark; T2-L, 12 h of dark +10-min light irradiation + 23 h 50 min of dark.
PAO Ultrastructural Localization in the Epidermis of Light-Exposed Maize Mesocotyl. Effect of Auxin
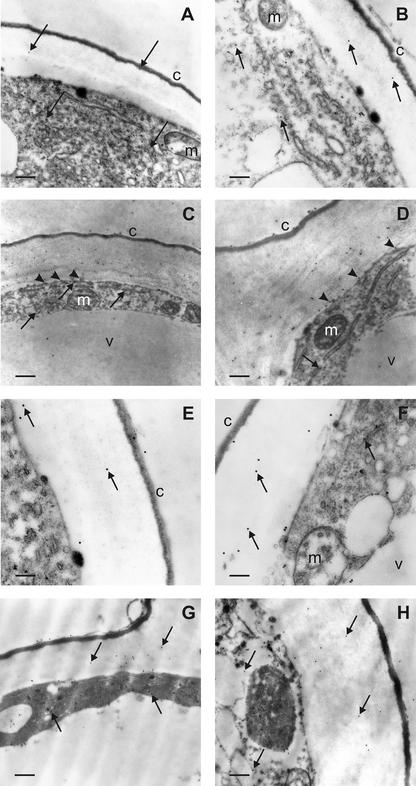
With the aim of studying whether light or auxin treatment influenced subcellular localization of PAO in epidermal cells, a transmission electron microscopic immunogold analysis was performed in the epidermal tissues of dark-grown or light-exposed maize mesocotyls treated, or not, with 10 μm NAA. A rabbit polyclonal anti-PAO antiserum, fractionated by affinity chromatography through a Sepharose 4B column coupled to bromelain to eliminate anti-glycan antibodies, was utilized as the primary probe as described in “Materials and Methods.” Ultrastructural analysis revealed fair preservation of tissue morphology, together with good retention of PAO antigenic properties (Fig. 6). Background labeling was practically absent from the anti-PAO immunoreacted sections and control grids (where the primary antibody was substituted for pre-immune rabbit serum or anti-PAO antiserum pre-adsorbed on PAO-Sepharose, as described in “Materials and Methods”). Immunoelectron microscopy demonstrated a specific PAO labeling in the tissue studied, although different positivity degrees depending on the light conditions were observed. In etiolated plants, faint PAO immunoreactivity in the cytoplasm and wall of epidermal cells was detected (Fig. 6, A and B). After light exposure, a remarkably increased PAO immunolabeling in the wall and, to a lesser extent, in the cytoplasm of epidermal cells was detected (Fig. 6, C and D). Concerning the distribution of gold particles in the cell wall, these showed preferential localization in the inner portion of this structure, and often were observed at the interface with the plasma membrane (Fig. 6, C and D). In the cytoplasm, particles sometimes appeared associated to specific intracellular compartments, such as endoplasmic reticulum cisternae and vesicles (Fig. 6C). The external cuticle, the mitochondria, and the large internal vacuole were consistently unlabeled.
Figure 6.
PAO immunoelectron microscopy of epidermal cells from maize mesocotyls: effect of light exposure and auxin treatment. A and B, Portions of epidermal cells from etiolated mesocotyls showing scattered gold particles in the cytoplasm and the outer cell wall (arrows). C and D, Portions of epidermal cells from light-exposed maize mesocotyls with numerous gold particles in the cytoplasm and the outer cell wall. Note the preferential localization of labeling in the inner half of the cell wall and the presence of some grains in close proximity to the plasma membrane (arrowheads). In the cytoplasm, immunoparticles are sometimes found inside endoplasmic reticulum cisternae and vesicles (arrows). The cuticle, vacuole, and mitochondria are negative. E and F, Portions of epidermal cells from etiolated, NAA-treated maize mesocotyls. Few gold particles are found in the cytoplasm and the outer cell wall (arrows). The cuticle, vacuole, and mitochondria are unlabeled. G and H, Portions of epidermal cells from light-exposed, NAA-treated maize mesocotyl showing a moderate number of gold particles in the cytoplasm and the outer cell wall (arrows). Magnification: A, ×14,400, bar = 0.4 μm; B, ×21,600, bar = 0.25 μm; C, ×8,600, bar = 0.6 μm; D, ×21,600, bar = 0.25 μm; E, ×36,000, bar = 0.15 μm; F, ×36,000, bar = 0.15 μm; G, ×21,600, bar = 0.25 μm; H, ×21,600, bar = 0.25 μm. c, Cuticle; v, vacuole; m, mitochondrion. Micrographs shown are representative fields of sections obtained from three independent experiments.
The effect of the auxin analog NAA on PAO subcellular localization in epidermal cells was also studied at the ultrastructural level by immunogold cytochemistry. In etiolated, NAA-treated plants, PAO immunoreactivity was similar to that found in etiolated untreated plants, except in the cell wall where the amount of gold particles was slightly higher (Fig. 6, E and F). Light-exposed, NAA-treated plants showed a mild increase in PAO labeling in the cell wall of epidermal cells with respect to either untreated or NAA-treated etiolated samples (Fig. 6, G and H). In the cell wall, gold particles were often seen in the inner part of this structure (Fig. 6, G and H).
DISCUSSION
In this work, we report that light-mediated induction of PAO expression in the outer tissues of maize mesocotyl is negatively regulated by auxin. Exogenously supplied auxin totally reversed the increase of PAO activity induced by light. In this organ, high auxin levels supplied from the coleoptile in the dark (Iino and Carr, 1982) are supposed to keep walls in a loosening state, possibly by triggering O2−. production and, as a consequence, increasing .OH levels, thus promoting fast extension growth (Schopfer, 2001). De-etiolation results in a strong reduction in the level of diffusible auxin, especially in the epidermis (Iino, 1982; Barker-Bridgers et al., 1998). It can be argued that the expression of H2O2-delivering systems should be kept at low levels in the dark (the only H2O2 source available being O2−. dismutation), while being strongly induced after light exposure. In this scenario, it is conceivable that auxin down-regulates the expression or activity of these systems. The inhibition of light-induced increase in both PAO mRNA and protein levels by auxin treatment, together with the occurrence of auxin-responsive cis-acting motifs in promoter regions of MPAO1 and MPAO2 (Cervelli et al., 2000), suggests that the regulation of PAO expression is mainly accomplished at the transcriptional level. The presence of several potential auxin and light-responsive elements (LREs) in the 5′-flanking regions of MPAO1 and MPAO2 genes revealed a rather complex multipartite promoter motif arrangement. This promoter organization suggests a common regulation operating on the cis-acting elements, an AuxRE box and a G box, located in the proximal region (Cervelli et al., 2000). It is noteworthy that in the distal promoter regions of MPAO1 and MPAO2 genes, other putative LREs have been described (Cervelli et al., 2000). Although posttranscriptional regulation of PAO expression cannot be ruled out, the different promoter architecture shown by MPAO1 and MPAO2 genes, likewise the activity/presence of cognate trans-acting factors, could play a role in the modulation and/or fine control of PAO expression in different organs and tissues of the maize seedling. PAO expression in mesocotyl stelar tissues is independent of light conditions under which plants are grown (Laurenzi et al., 1999).
In this scenario, auxin should thus act as a negative regulatory factor in the modulation of PAO gene expression in the mesocotyl epidermis, the decrease in the hormone level occurring after plant exposure to light being a necessary condition for light-induced stimulus on this event. On the other hand, dark-grown plants treated with NPA or TIBA, in which endogenous auxin levels have been decreased in the mesocotyl by inhibiting auxin polar transport, failed to show a strong increase in PAO expression, thus excluding that the latter be solely modulated by the variation in diffusible auxin. As a consequence, it can be argued that light-induced decrease in auxin levels in the mesocotyl epidermis is not sufficient to stimulate an increase in PAO expression. It is likely that light-induced increase of PAO expression in the maize mesocotyl during de-etiolation is the result of a complex regulation. Two or more different transcription factors, namely light-inducible activators and auxin-dependent repressors, could modulate PAO gene transcription. To this respect, light could play both a direct and an indirect role in the stimulation of PAO gene expression. Light-mediated reduction of auxin supply from the coleoptile into the mesocotyl could disable putative auxin-responsive repressors. A helpful comparative model could be ARF transcription factors first described in Arabidopsis (Ulmasov et al., 1997). These are either transcriptional activators or repressors, bound to TGTCTC (or the degenerate version TGTCCCAT) AuxRE composite elements that dimerize with AUX/IAA proteins at low IAA level, thus being inactivated. At high IAA levels, AUX/IAA proteins dissociate from ARF and are degraded by the proteasome complex, thus enabling ARF to exert their activity (in this case it could be repression of PAO gene transcription; Tiwari et al., 2001). The functional significance of the TGTCTC AuxRE-like elements C(G/A)TCCCAT present in the proximal region of MPAO1 and MPAO2 promoters (Cervelli et al., 2000) remains to be assessed. On the other hand, light could directly stimulate light-responsive trans-activators as a result of light signal transduction by phytochrome, in a way reminiscent of that described in Arabidopsis for light-mediated regulation of gene expression exerted by HY5 (Oyama et al., 1997) and associated photomorphogenic repressor COP1 (Deng et al., 1991). When exogenous auxin is supplied to light-exposed plants, the auxin-responsive repressor would be kept in an active form, thus repressing PAO gene transcription. However, it cannot be excluded that light-responsive activators could be inactivated, degraded, or disabled to bind LREs on PAO promoter as a result of auxin treatment after light exposure.
An additional factor possibly influencing the cell wall-loosening/-stiffening equilibrium is the formation of intermolecular cross bridges between hemicelluloses or structural proteins, which is compartment specific and cell age dependent. These events have a remarkable physiological significance during elongation growth and cell wall maturation in late developmental stages. In particular, the formation of diferuloyl bridges in arabinoxylans in young maize cells or tissues may occur early in the secretion pathway, resulting in the formation of cross-linked arabinoxylan coagula in Golgi vesicles (Fry et al., 2000). It is expected that these coagula will show poor capacity of hydrogen bond formation with cellulose microfibrils after being secreted, thus helping in maintaining high cell wall extensibility. As the cell matures, cross-linking activity occurs mostly in the apoplast concomitant with cellulose microfibril binding, thus having a wall-stiffening effect (Fry et al., 2000). Concerning this, immunoelectron microscopic localization of PAO, independent of the treatment, showed localization of the enzyme in both the cytoplasm and wall of epidermal cells, suggesting a role in the synthesis of H2O2 both intraprotoplasmically and wall localized.
Although PAO labeling was scarce in etiolated epidermal tissues, a substantial increase in PAO immunolabeling was observed in the mesocotyl epidermis after exposing plants to light. Interestingly, immunoparticles were often observed inside secretory cytoplasmic organelles, such as endoplasmic reticulum and vesicles, thus suggesting enhanced neosynthesis and exocytosis of the enzyme. The intense labeling found in the inner part of the cell walls, often at the boundary with the plasma membrane, further supports this hypothesis. This event can be functional to the need for a wall-localized increased production of H2O2 to sustain in muro di-ferulate cross bridge formation and lignification (Musel et al., 1997; Fry et al., 2000). According to results obtained on the inhibitory effect of auxin on light-induced PAO gene expression, treatment of maize plants with auxin resulted in a visible attenuation of PAO immunolabeling. Nevertheless, a modest augmentation of immunolabeling in the epidermal walls was observed in either etiolated or light-exposed NAA-treated plants as compared with etiolated untreated samples, this phenomenon probably being due to a stimulation of cell secretory activity by exogenously added auxin (for review, see Napier and Venis, 1995).
CONCLUSION
Recent progress in the understanding of the engagement of reactive oxygen intermediates in the biogenesis and modification of cell wall structure, as well as the present results concerning the regulation of PAO gene expression by light and auxin, allow envisioning a new scenario in the molecular events regulating cell wall extension and differentiation.
Fast extension growth of maize mesocotyl in the dark is known to be sustained by auxin, mostly transported from the coleoptile (Iino and Carr, 1982). High auxin levels will result in the down-regulation of PAO gene expression as well, possibly through auxin-dependent transcriptional repressors acting on AuxREs present in promoter regions. The cytoplasmic localization of PAO in this condition may account for the need of intraprotoplasmic production of H2O2 for polymer cross-linking in the secretory pathway (Fry et al., 2000). Exposure of maize seedlings to light results in the induction of PAO expression in epidermal tissues throughout the mesocotyl, with a great increase of PAO abundance in the cell walls. This event, at least in part mediated by phytochrome (Laurenzi et al., 1999), is probably linked to the physiological requirement of higher production of H2O2 in the apoplast to drive peroxidase-catalyzed cross-linking and lignification to complete cell wall stiffening and differentiation. Although a light-mediated posttranslational modification of PAO protein stimulating the enzyme activity cannot be excluded, light-induced PAO gene expression in the epidermis of maize mesocotyl is most probably mediated by phytochrome-dependent transcriptional activators acting at the LREs. The negative regulation of auxin-dependent repressors on PAO gene expression in the mesocotyl epidermal tissues would be counteracted by the light-induced decrease of diffusible auxin from the coleoptile after light exposure (Iino, 1982; Barker-Bridgers et al., 1998).
MATERIALS AND METHODS
Plant Material
Maize (Zea mays L. cv DK 300; Dekalb-Monsanto, Mestre, Italy) seeds were soaked for 12 h in running tap water and germinated on paper under 1 cm of loam at 20°C in a growth chamber in the dark. Some plants were sprayed with NAA, Ethephon, or TIBA (Sigma-Aldrich, Milan), or NPA (Duchefa Biochemie, Haarlem, The Netherlands) aqueous solutions at the indicated concentrations every 12 h starting from 96 h after soaking (T0). A wetting agent (Etravon, Novartis, Origgio, Italy) was added to all solutions at 0.1% (v/v). Control plants were sprayed with 0.1% (v/v) Etravon aqueous solution. No apparent morphological modifications were observed after treatments. Light irradiation was given for 10 min, 12 h after T0 (T1), at 20°C in a growth chamber equipped with HCI high pressure discharge lamps (Osram, Milan; 18,000 lux at plant level). The following abbreviation will be used to summarize light treatment and times: T1, 12 h after T0 in the dark; T2-D, 36 h after T0 in the dark; and T2-L, 12 h after T0 in the dark + 10-min light irradiation + 23 h 50 min in the dark. Cortical plus epidermal tissues were obtained by drawing out the stele from 2-cm-long segments excised from the nonelongating zone of the mesocotyl after eliminating the 1-cm-long subnodal segment.
Plant material was ground with mortar and pestle at 4°C in 0.2 m sodium phosphate buffer (pH 6.5; tissue to buffer ratio 1:5 [w/v]). Homogenates were centrifuged at 12,000g for 20 min at 4°C. Supernatants were used for the determination of protein concentration and extractable PAO activity. Western-blot analysis was also performed on crude extract supernatant. For determination of wall-bound PAO activity, pellets obtained after centrifugation of crude homogenates were resuspended in the appropriate volume of 0.2 m sodium phosphate buffer (pH 6.5) containing 0.01% (w/v) Triton X-100 and centrifuged at 15,000g at 4°C for 5 min. This step was repeated three times to remove traces of extractable enzyme. The washed pellets were resuspended in 0.2 m sodium phosphate buffer (pH 6.5; 1 mL · g fresh weight−1) and the suspension used for the polarographic determination of wall-bound PAO activity.
Reported activity values are the mean of three independent experiments, each performed with three replicates. P values have been calculated with Student's t test analysis, comparing PAO activity in control and treated plants, for each time and for each light condition.
Enzyme Assays, Protein Determination, and Western-Blot Analysis
Extractable PAO activity was measured spectrophotometrically by following the formation of a pink adduct (ε515 = 2.6 × 104 m−1 cm−1) as a result of the oxidation and following condensation of 4-aminoantipyrine and 3,5-dichloro-2-hydroxybenzene sulfonic acid (Sigma-Aldrich) catalyzed by peroxidase (Smith and Barker, 1988). The assays were performed in 0.2 m sodium phosphate buffer (pH 6.5) containing 0.06 mg horseradish peroxidase (Sigma-Aldrich), with 2 mm spermidine as the substrate in 1 mL total volume. Wall-bound PAO activities were determined at 30°C measuring oxygen consumption in an oxygraph (Hansatech, Norfolk, UK) equipped with a Clark electrode, as described by Augeri et al. (1990). Copper amine oxidase activity, determined by using the same extracts and assays with putrescine as the substrate, was undetectable in all samples. Thus, spermidine oxidation could be entirely ascribed to PAO activity.
Enzyme activities were expressed in International Units (1 unit is the amount of enzyme that catalyzes the oxidation of 1 μmol substrate per min). Protein content was estimated by the method of Bradford (1976) with bovine serum albumin as a standard. SDS-PAGE was performed according to the method of Laemmli (1970). Western-blot analysis was performed after protein deglycosylation (Woodward et al., 1985) according to Towbin et al. (1979). Analyses were performed on 20 μg of total soluble proteins of each extract. After electroblotting, nitrocellulose membranes were tested for equal loading by staining with Ponceau S (Sigma-Aldrich; 0.1% [w/v] Ponceau S in 5% [v/v] acetic acid; data not shown). A 1,000-fold diluted maize-PAO rabbit polyclonal antibody and a 5,000-fold diluted peroxidase-conjugated goat anti-rabbit IgG (Sigma-Aldrich) were employed to detect PAO protein accumulation using 4-chloro-1-naphthol (Sigma-Aldrich) and H2O2 as substrates, according to the manufacturer's instructions. Experiments were performed independently at least five times, yielding reproducible results. Single representative experiments are shown in the figures. Immunoprecipitation was performed with rabbit polyclonal anti-maize-PAO antiserum using Protein A Sepharose CL-4B (Amersham Pharmacia, Uppsala) according to the manufacturer's instructions.
RNA-Blot Analysis
Total RNA was isolated using the TRIzol reagent (Life Technologies/Gibco-BRL, Milan), following the manufacturer's instructions. Twenty micrograms of total RNA was fractionated on a 1.2% (w/v) agarose/formaldehyde gel and transferred to a Hybond N+ nylon membrane (Amersham Pharmacia). Hybridization was carried out according to Sambrook et al. (1989) with the QuickHyb (Stratagene, La Jolla, CA), following the manufacturer's instructions, and using P32-labeled maize PAO cDNA as probe. As a control, samples were also hybridized with an internal portion of the cDNA specific for the ribosomal protein S13 (RP-S13, GenBank accession no. AF067732).
Electron Microscopic Immunolocalization
Subapical mesocotyl segments obtained either from etiolated or light-exposed maize seedlings, treated or not with auxin as described above (T2-D, T2-L, T2-D NAA, and T2-L NAA) were used for this study. One-millimeter-long fragments were fixed by immersion, under vacuum aspiration, in 4% (w/v) paraformaldehyde and 0.5% (w/v) glutaraldehyde in 50 mm sodium cacodylate buffer (CB; pH 7.4) for 2 h at room temperature (RT), and for 2 h at 4°C. Specimens were treated with 0.25% (w/v) tannic acid in CB for 1 h at 4°C, with 50 mm ammonium chloride for 30 min at 4°C and with 2% (w/v) uranyl acetate for 1 h. The above incubations were separated by extensive washings in CB and the whole process was performed at 4°C. Samples were partially dehydrated in graded ethanol (to 70% [w/v]) at 4°C, immersed in 90% (w/v) ethanol for 45 min at −20°C, then gradually infiltrated with London Resin Gold (Agar Scientific Ltd., Stansted, UK) at −20°C. After immersion in pure resin overnight at −20°C, polymerization was carried out using fresh resin and 0.5% (w/v) benzoin methyl ether as UV catalyst at −20°C under UV light for 48 h. Ultrathin sections were obtained by a Reichert Ultracut S ultramicrotome (Leica Microsystems, Milan) from randomly chosen embedding blocks from plants grown and treated as above described. Sections were collected on collodium-coated nickel grids and then processed for immunogold labeling, using a rabbit polyclonal anti-PAO antiserum, fractionated by affinity chromatography through a Sepharose 4B column (Amersham Pharmacia) coupled to bromelain (Sigma-Aldrich), according to the manufacturer's instructions, to eliminate anti-glycan antibodies (Satoh, 1990). Grids were incubated in the primary antibody diluted 1:50 (v/v) in phosphate-buffered saline containing 1% (w/v) bovine serum albumin (medium A) for 1 h at RT. After washings in medium A containing 0.01% (v/v) Tween 20 (Merck, Darmstadt, Germany), sections were incubated in goat anti-rabbit IgG conjugated to 15-nm colloidal gold particles (British BioCell Int., Cardiff, UK) diluted 1:100 (w/v) in medium A containing 2% (w/v) fish gelatin for 1 h at RT. Anti-PAO antiserum pre-adsorbed onto a column of Sepharose 4B conjugated to pure maize PAO was used as primary antibody control. Some sections were incubated with colloidal gold-conjugated goat anti-rabbit IgG as secondary antibody control. Grids were thoroughly rinsed in distilled water and briefly contrasted with uranyl acetate and lead citrate. Immunoreacted sections were observed in a 120 CM electron microscope (Philips, Monza, Italy) and images were electronically captured.
ACKNOWLEDGMENTS
We wish to thank Paraskevi Tavladoraki (Biology Department, University “Roma Tre,” Italy) for critical reading of the manuscript and Daniela Cesare (Biology Department, University “Roma Tre”) for her invaluable help and enthusiasm. We also gratefully acknowledge Annarosa Luzzatto (Biology Department, University “Roma Tre”) and Paola Bonfante (Plant Biology Department, Turin University, Italy) for many helpful suggestions.
Footnotes
This work was supported by the Italian Ministry for University and Research.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.011379.
LITERATURE CITED
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini R, Bragaloni M, Federico R, Infantino A, Porta-Puglia A. Involvement of polyamines, diamine oxidase and peroxidase in resistance of chickpea to Ascochyta rabiei. J Plant Physiol. 1993;142:704–709. [Google Scholar]
- Angelini R, Federico R. Histochemical evidence of polyamine oxidation and hydrogen peroxide generation in the cell wall. J Plant Physiol. 1989;135:212–217. [Google Scholar]
- Angelini R, Federico R, Bonfante P. Maize polyamine oxidase: antibody production and ultrastructural localization. J Plant Physiol. 1995;145:686–692. [Google Scholar]
- Angelini R, Manes F, Federico R. Spatial and functional correlation between diamine oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chickpea stem. Planta. 1990;182:89–96. doi: 10.1007/BF00239989. [DOI] [PubMed] [Google Scholar]
- Asthir B, Duffus CM, Smith RC, Spoor W. Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. J Exp Bot. 2002;53:677–682. doi: 10.1093/jexbot/53.369.677. [DOI] [PubMed] [Google Scholar]
- Augeri M, Angelini R, Federico R. Sub-cellular localization and tissue distribution of polyamine oxidase in maize (Zea mays L.) seedlings. J Plant Physiol. 1990;136:690–695. [Google Scholar]
- Barker-Bridgers M, Ribnicky DM, Cohen JD, Jones AM. Red-light-regulated growth. Changes in the abundance of indole acetic acid in the maize (Zea mays L.) mesocotyl. Planta. 1998;204:207–211. [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defence responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanism. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bret-Harte M, Baskin TI, Green PB. Auxin stimulates both deposition and breakdown of material in the pea outer epidermal cell wall, as measured interferometrically. Planta. 1991;185:462–471. doi: 10.1007/BF00202954. [DOI] [PubMed] [Google Scholar]
- Cervelli M, Tavladoraki P, Di Agostino S, Angelini R, Federico R, Mariottini P. Isolation and characterization of three polyamine oxidase genes from Zea mays. Plant Physiol Biochem. 2000;38:667–677. [Google Scholar]
- Chen S, Schopfer P. Hydroxyl-radical production in physiological reactions, a novel function of peroxidase. Eur J Biochem. 1999;260:726–735. doi: 10.1046/j.1432-1327.1999.00199.x. [DOI] [PubMed] [Google Scholar]
- Cooley T, Walters DR. Polyamine metabolism in barley reacting hypersensitively to the powdery mildew fungus Blumeria graminis f. sp. Hordei. Plant Cell Environ. 2002;25:461–468. [Google Scholar]
- Cosgrove DJ. Expansive growth of plant cell wall. Plant Physiol Biochem. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Darley P, Forrester AM, McQueen-Mason SJ. The molecular basis of plant cell wall extension. Plant Mol Biol. 2001;47:179–195. [PubMed] [Google Scholar]
- Deng XW, Caspar T, Quail PH. cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Elstner EF, Heupel A. Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib.) Planta. 1976;130:175–180. doi: 10.1007/BF00384416. [DOI] [PubMed] [Google Scholar]
- Federico R, Cona A, Angelini A, Schininà ME, Giartosio A. Characterization of maize polyamine oxidase. Phytochemistry. 1990;29:2411–2414. doi: 10.1016/0031-9422(90)85157-b. [DOI] [PubMed] [Google Scholar]
- Frahry G, Schopfer P. NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta. 2001;212:175–183. doi: 10.1007/s004250000376. [DOI] [PubMed] [Google Scholar]
- Fry SC. Cross-linking of matrix polymers in the growing cell walls of angiosperms. Annu Rev Plant Physiol. 1986;37:165–186. [Google Scholar]
- Fry SC. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J. 1998;332:507–515. doi: 10.1042/bj3320507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Willis SC, Paterson AEJ. Intraprotoplasmic and wall-localised formation of arabinoxylan-bound diferulates and larger ferulate coupling-products in maize cell-suspension cultures. Planta. 2000;211:679–692. doi: 10.1007/s004250000330. [DOI] [PubMed] [Google Scholar]
- Grant JJ, Loake GJ. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000;124:21–29. doi: 10.1104/pp.124.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Greiner H, Schopfer P. The cryptic growth response of maize coleoptiles and its relationship to H2O2-dependent cell-wall stiffening. Physiol Plant. 1995;94:491–498. [Google Scholar]
- Iino M. Inhibitory action of red light on the growth of the maize mesocotyl: evaluation of the auxin hypothesis. Planta. 1982;156:388–395. doi: 10.1007/BF00393308. [DOI] [PubMed] [Google Scholar]
- Iino M, Carr DJ. Sources of free IAA in the mesocotyl of etiolated maize seedlings. Plant Physiol. 1982;69:1109–1112. doi: 10.1104/pp.69.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R, Flores HE, Galston AW. Polyamine oxidase in oat leaves, a cell-wall localized enzyme. Plant Physiol. 1981;68:494–498. doi: 10.1104/pp.68.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. The role of the epidermis in the control of elongation growth in stems and coleoptiles. Bot Acta. 1992;105:246–252. [Google Scholar]
- Kutschera U, Briggs WR. Differential effect of auxin on in vivo extensibility of cortical cylinder and epidermis in pea internodes. Plant Physiol. 1987;84:1361–1366. doi: 10.1104/pp.84.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane BG. Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 1994;8:294–301. doi: 10.1096/fasebj.8.3.8143935. [DOI] [PubMed] [Google Scholar]
- Laurenzi M, Rea G, Federico R, Tavladoraki P, Angelini R. De-etiolation causes a phytochrome-mediated increase of polyamine oxidase expression in outer tissues of the maize mesocotyl: a role in the photomodulation of growth and cell wall differentiation. Planta. 1999;208:146–154. [Google Scholar]
- Laurenzi M, Tipping AJ, Marcus SE, Knox JP, Federico R, Angelini R, McPherson MJ. Analysis of the distribution of copper amine oxidase in cell walls of legume seedlings. Planta. 2001;214:37–45. doi: 10.1007/s004250100600. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Yamamoto R. Control of auxin-induced stem elongation by the epidermis (peas) Physiol Plant. 1972;27:109–115. [Google Scholar]
- Miller AR. Oxidation of cell wall polysaccharides by hydrogen peroxide: a potential mechanism for cell wall breakdown in plants. Biochem Biophys Res Commun. 1986;141:238–244. doi: 10.1016/s0006-291x(86)80359-x. [DOI] [PubMed] [Google Scholar]
- Møller SG, McPherson MJ. Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 1998;13:781–791. doi: 10.1046/j.1365-313x.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Musel G, Schindler T, Bergfeld R, Ruel K, Jacquet G, Lapierre C, Speth V, Schopfer P. Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological probes. Planta. 1997;201:146–159. [Google Scholar]
- Napier RM, Venis MA. Auxin action and auxin binding proteins. New Phytol. 1995;129:167–201. doi: 10.1111/j.1469-8137.1995.tb04291.x. [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyls. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea G, Laurenzi M, Tranquilli E, D'Ovidio R, Federico R, Angelini R. Developmentally and wound regulated expression of the gene encoding a cell wall copper amine oxidase in chickpea seedlings. FEBS Lett. 1998;437:177–182. doi: 10.1016/s0014-5793(98)01219-8. [DOI] [PubMed] [Google Scholar]
- Rea G, Metoui O, Infantino A, Federico R, Angelini R. Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 2002;128:865–875. doi: 10.1104/pp.010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Satoh S. Improvement of the specificity of an antiserum raised against a carrot glycoprotein by eliminating the anti-glycan antibody. Agric Biol Chem. 1990;54:3201–3204. [Google Scholar]
- Schopfer P. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta. 1996;199:43–49. [Google Scholar]
- Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–828. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- Šebela M, Radovà A, Angelini R, Tavladoraki P, Frébort I, Pêc P. Fad-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci. 2001;160:197–207. doi: 10.1016/s0168-9452(00)00380-0. [DOI] [PubMed] [Google Scholar]
- Slocum RD, Furey MJ., III Electron-microscopic cytochemical localization of diamine oxidase and polyamine oxidase in pea and maize tissue. Planta. 1991;183:443–450. doi: 10.1007/BF00197744. [DOI] [PubMed] [Google Scholar]
- Smith TA, Barker JHA. The di- and polyamine oxidase of plants. In: Zappia V, Pegg AE, editors. Progress in Polyamine Research. Novel Biochemical, Pharmacological and Clinical Aspects. New York: Plenum Press; 1988. pp. 573–587. [Google Scholar]
- Sussman MR, Goldsmith MHM. The action of specific inhibitors of auxin transport on uptake of auxin and binding of N-1-naphthylphthalamic acid to a membrane site in maize coleoptiles. Planta. 1981;152:13–18. doi: 10.1007/BF00384978. [DOI] [PubMed] [Google Scholar]
- Tavladoraki P, Schinina ME, Cecconi F, Di Agostino S, Manera F, Rea G, Federico R, Mariottini P, Angelini R. Maize polyamine oxidase: primary structure from protein and cDNA sequencing. FEBS Lett. 1998;426:62–66. doi: 10.1016/s0014-5793(98)00311-1. [DOI] [PubMed] [Google Scholar]
- Thomson KS, Hertel R, Muller S, Tavares JE. 1-N-Naphthylphthalamic acid and 2,3,5-triiodobenzoic acid: in vivo binding to particulate fractions and action on auxin transport in corn coleoptiles. Planta. 1973;109:337–352. doi: 10.1007/BF00387102. [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Wang X, Hagen G, Guilfoyle TJ. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell. 2001;13:2809–2822. doi: 10.1105/tpc.010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. ARF1, a transcription factor that binds auxin response elements. Science. 1997;276:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- Vanderhoef LN, Briggs WR. Red light-inhibited mesocotyl elongation in maize seedlings: I. The auxin hypothesis. Plant Physiol. 1978;61:534–537. doi: 10.1104/pp.61.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JP, Rathbun EA, Knox JP, Brewin NJ. Involvement of diamine oxidase and peroxidase in insolubilization of the extracellular matrix: implications for pea nodule initiation by Rhizobium leguminosarum. Mol Plant-Microbe Interact. 2000;13:413–420. doi: 10.1094/MPMI.2000.13.4.413. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward MP, Young WW, Bloodgood RA. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]