Abstract
Transforming growth factor β (TGF-β) has been shown to participate in the pathophysiology of diabetic complications. As shown most recently, TGF-β stimulates the expression of a distinct serine/threonine kinase (hSGK) which had previously been cloned as an early gene transcriptionally regulated by cell volume alterations. The present study was performed to elucidate transcription and function of hSGK in diabetic nephropathy. As shown by Northern blotting, an increase of extracellular glucose concentration increased hSGK mRNA levels in cultured cells, an effect qualitatively mimicked by osmotic cell shrinkage or treatment with TGF-β (2 μg/liter), phorbol 12,13-didecanoate (1 μM), or the Ca2+ ionophore ionomycin (1 μM) and blunted by high concentrations of nifedipine (10 and 100 μM). In situ hybridization revealed that hSGK transcription was markedly enhanced in diabetic nephropathy, with particularly high expression in mesangial cells, interstitial cells, and cells in thick ascending limbs of Henle's loop and distal tubules. According to voltage clamp and tracer flux studies in Xenopus oocytes expressing the renal epithelial Na+ channel ENaC or the mouse thick ascending limb Na+,K+,2Cl− cotransporter BSC-1, coexpression with hSGK stimulated ENaC and BSC-1 11-fold and 6-fold, respectively, effects reversed by kinase inhibitors staurosporine (1 μM) and chelerythrine (1 μM) and not elicited by inactive hSGK. In conclusion, excessive extracellular glucose concentrations enhance hSGK transcription, which in turn stimulates renal tubular Na+ transport. These observations disclose an additional element in the pathophysiology of diabetic nephropathy.
Keywords: protein kinase C; endothelial cells; kidney; epithelial Na+ channel; Na+,K+,2Cl− cotransporter
Transforming growth factor β (TGF-β) is considered to be a crucial pathophysiological component in the generation of diabetic nephropathy (1–6). A sequence of events has been postulated, which causes the enhanced production of both TGF-β and TGF-β receptors in hyperglycemia (1, 7–12). Excessive extracellular glucose concentrations increase diacylglycerol synthesis (13, 14) and thus lead to activation of protein kinase C (PKC) (12–18). Among the PKC isoforms PKCα, PKCδ, and PKCɛ are up-regulated, whereas PKCβII is down-regulated (19). Activation of PKC subsequently stimulates the formation of TGF-β (20). The downstream targets of TGF-β mediating the pathophysiology of diabetic nephropathy remained largely elusive.
Recently, we identified a downstream target of TGF-β—i.e., a serine/threonine kinase (hSGK)—which is transcriptionally up-regulated by TGF-β in both U 937 macrophages and HepG2 liver cells (21). The human SGK has previously been cloned as cell-volume-sensitive gene (22). The rat SGK was originally identified as a serum- and glucocorticoid-regulated kinase cloned from rat mammary tumor cells (23) but was subsequently shown to be up-regulated by mineralocorticoids (24). As shown by Northern blot analysis, the human kinase is expressed in all human tissues studied, including pancreas, liver, heart, skeletal muscle, placenta, kidney, and brain (22). The present study has been performed to search for altered hSGK transcription in diabetic nephropathy and to explore the functional significance of this kinase.
Materials and Methods
Transcriptional Regulation of hSGK in Cultured Cells.
3T3 mouse fibroblasts were maintained in DMEM, supplemented with 10% (vol/vol) fetal calf serum (FCS) and 1% penicillin/streptomycin containing 5.5 mM glucose, and human endothelial cells (HMEC-1) were maintained in M199 containing 3.3 μM ribose and 5 mM glucose at 37°C and pH 7.4 (5% CO2/95% air atmosphere), 10% FCS, 10 ng/ml epidermal growth factor, 1 μg/ml hydrocortisone, and 0.5% gentamycin. Cells were grown to 90% confluency, deprived of FCS for 3 days before stimulation, and, after incubation, homogenized in TRIZOL (Life Technologies/BRL) (about 0.3 × 106 cells per sample). Total RNA was isolated as indicated in the protocol provided by the distributor. Northern blots were prepared with 10, 15, or 20 μg of total RNA that had been separated by electrophoresis through 10 g/liter agarose gels in the presence of 2.2 M formaldehyde. Vacuum blotting (Appligene Oncor Trans DNA Express Vacuum Blotter, Appligene, Heidelberg, Germany) was used for the transfer of the RNA on positively charged nylon membranes (Roche, Mannheim, Germany). The RNA was then cross-linked with the membrane under UV light (UV Stratalinker 2400, Stratagene, Heidelberg, Germany). Hybridization overnight was performed in DIG-Easy-Hyb (Roche, Mannheim) at a probe concentration of 25 μg/liter at 50°C. The digoxigenin (DIG)-labeled probe was generated by PCR as described in detail previously (22). The quantity of Northern blot signals was measured by scanning densitometry. Where indicated the experiments were performed in the presence of 30 μg/ml neutralizing anti-TGF-β-1 antibody (R & D Systems, Wiesbaden, Germany).
In Situ Hybridization.
Tissue specimens from intact kidneys (n = 7) and from kidneys with diabetic nephropathy (n = 7), taken from patients for diagnostic reasons, were fixed in 4% paraformaldehyde/0.1 M sodium phosphate buffer (pH 7.2) for 4 h and embedded in paraffin. Normal renal biopsies were obtained from intact parts of kidneys that had been surgically removed because of renal cancer. Diabetic renal biopsies were investigated from patients suffering diabetes type I with a disease duration of 5–12 years. All patients suffered from a persistent proteinuria, >300 mg/24 h. Tissue sections (4 μm) were deparaffined and hybridized basically as described (25–27). The mixture contained either the 35S-labeled RNA antisense or sense control hSGK probe (21) (500 ng/ml) in hybridization buffer [10 mM Tris⋅HCl, pH 7.4/50% (vol/vol) deionized formamide/600 mM NaCl/1 mM EDTA/0.02% polyvinylpyrrolidone/0.02% Ficoll/0.05% BSA/10% dextran sulfate/10 mM DTT containing denatured sonicated salmon sperm DNA at 200 μg/ml and rabbit liver tRNA at 100 μg/ml]. Hybridization with RNA probes proceeded at 42°C for 18 h. Slides were then washed as described (25–27), followed by 1 h at 55°C in 2× standard saline/citrate. Nonhybridized single-stranded RNA probes were digested by RNase A (20 μg/ml) in 10 mM Tris⋅HCl, pH 8.0/0.5 M NaCl for 30 min at 37°C. Tissue slide preparations were autoradiographed (27) and stained with hematoxylin/eosin.
Coexpression of hSGK with Rat Epithelial Na+ Channel (ENaC) or BSC-1.
Xenopus laevis females were purchased from the South African Xenopus facility (Knysna, Republic of South Africa). Oocytes (stages V and VI) were isolated by collagenase treatment as described (28) and allowed to recover overnight. Plasmid DNA of the mouse thick ascending limb Na+,K+,2Cl− cotransporter BSC-1 (29), hSGK (22), and the α, β, and γ subunits of ENaC (29, 30) were linearized with NotI and transcribed in vitro with T7 RNA polymerase in the presence of the cap analog m7G(5′)ppp(5′)G at a concentration of 1 mM. An hSGKK127R mutant was transcribed from a PCR product containing the T7 promoter. cRNA encoding the α and β subunits of ENaC were linearized with BglII and transcribed in vitro with SP6 polymerase. Template cDNA was removed by digestion with RNase-free DNase I. The complementary RNA (cRNA) was purified by extraction with phenol/chloroform followed by precipitation with 0.5 vol of 7.5 M ammonium acetate and 2.5 vol of ethanol to remove unincorporated nucleotides. The integrity of the transcript was checked by denaturing agarose gel electrophoresis.
Flux Measurements.
To study the effect of hSGK on BSC-1, oocytes were injected with 20 ng of cRNA of BSC-1 alone, 20 ng of BSC-1 + 20 ng of hSGK, or 20 ng of BSC-1 + 4 ng of hSGK[K127R] mutant per 50 nl water per oocyte; water-injected oocytes served as controls. To reduce the endogenous 22Na+ transport, uptake was determined in oocyte Ringer's solution containing reduced NaCl concentration (rNaOR2: 72.5 mM choline chloride/10 mM NaCl/2.5 mM KCl/1 mM CaCl2/1 mM MgCl2/1 mM Na2HPO4/5 mM Hepes, titrated with NaOH to pH 7.8). For each determination, groups of seven cRNA- or noninjected oocytes were washed twice with 4 ml of rNaOR2-buffer. They were then incubated at room temperature in a 5-ml polypropylene tube containing 100 μl of the same buffer containing 37 kBq of 22NaCl. Transport was stopped after 30 min by washing oocytes three times with 4 ml of ice-cold rNAOR2 buffer. Single oocytes were placed in scintillation vials, 3 ml of scintillation fluid was added, and the radioactivity was determined by liquid scintillation counting.
Two-Electrode Voltage Clamp and 22Na+-Flux Measurements.
For study of the effect of hSGK on ENaC, oocytes were injected with 5 ng of α,β,γ-ENaC cRNA alone or with 5 ng of α,β,γ-ENaC cRNA + 5 ng of hSGK cRNA per 50 nl of water per oocyte; water-injected oocytes served as controls. All experiments were performed at room temperature 1–2 days after injection. Two-electrode voltage-clamp recordings were performed at a holding potential of −80 mV. The data were filtered at 10 Hz and recorded with MacLab digital-to-analog converter and software for data acquisition and analysis (AD Instruments, Castle Hill, Australia) (31). In all experiments oocytes were superfused with ND96 solution (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.4). Amiloride-sensitive currents were measured with 30 μM amiloride for 2 min. Data are provided as means ± SEM; n represents the number of oocytes investigated. The magnitude of the induced currents varied 2- to 5-fold, depending on the time period after cRNA injection and on the batch of oocytes (from different animals). Therefore, throughout the paper we show experimental data obtained on the same day for each specific set of experiments. All experiments were repeated with at least two or three batches of oocytes; in all repetitions qualitatively similar data were obtained.
Statistical Analysis.
Data are expressed as arithmetic means ± SEM and n denotes the number of experiments. Statistical analysis has been made by Student t test or ANOVA, where applicable.
Results
Transcriptional Regulation of SGK in Cultured Cells.
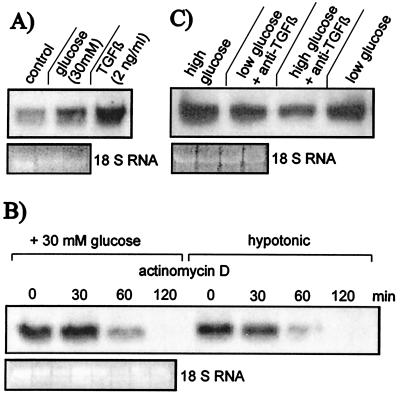
SGK transcription in 3T3 fibroblasts increased significantly (P < 0.05) by 22% ± 7% (n = 4) within 8 h after an increase of extracellular glucose concentration from 5.5 to 30 mM (Fig. 1A). TGF-β-1 (2 μg/liter) added at 5.5 mM glucose increased significantly (P < 0.02) SGK transcription in 3T3 fibroblasts within 8 h by 96% ± 20% (n = 4).
Figure 1.
Transcriptional regulation of the serine/threonine kinase hSGK in 3T3 fibroblasts. (A) Influence on hSGK mRNA of an 8-h increase of extracellular glucose concentration from 5.5 to 30 mM and of an 8-h exposure to 2 ng/ml TGF-β-1. (B) Decay of mRNA levels after addition of 5 μg/ml actinomycin D at 30 mM glucose and at 5.5 mM glucose in hypotonic extracellular fluid. Before the experimental period, the cells were exposed to 30 mM glucose. (C) Effect of an 8-h increase of extracellular glucose concentration from 5.5 to 30 mM in presence and absence of 30 μg/ml anti-TGF-β-1 antibody.
To test whether increased extracellular glucose concentrations delay the degradation rather than stimulate the formation of SGK mRNA, actinomycin D (5 μg/ml) was added to inhibit transcription. After a treatment for 6 h with 30 mM glucose to up-regulate SGK mRNA the cells were exposed to actinomycin D in the presence of either 30 mM glucose or in a medium of reduced extracellular osmolarity (190 mOsm) containing 5.5 mM glucose. Osmolarity was reduced because it is known to rapidly decrease hSGK mRNA (22). As shown in Fig. 1B, the decay of SGK mRNA was similar in the presence of 30 mM glucose and 5.5 mM glucose in hypotonic extracellular fluid. Thus, high extracellular glucose did not appreciably affect mRNA stability.
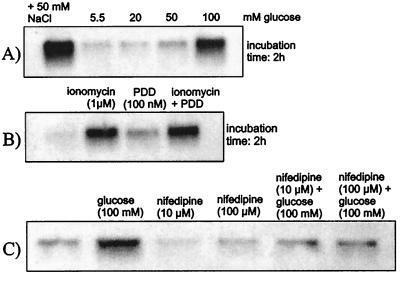
To test whether an increase of SGK transcription could be similarly elicited in other cells and at shorter exposure times, additional experiments were performed in human endothelial cells. An increase of extracellular glucose concentration from 5.5 mM to 20 mM, 50 mM, or 100 mM increased hSGK mRNA levels of human endothelial cells within 2 h by 35% ± 33% (n = 4), 77% ± 26% (P < 0.05, n = 4), and 299% ± 129% (P < 0.05, n = 16), respectively (Fig. 2A). After extended exposure to 100 mM glucose, the transcript levels increased further (not shown). Similar to the effect of glucose, osmotic shrinkage of endothelial cells with hypertonic raffinose (100 mM) or NaCl (50 mM) increased hSGK mRNA levels. The respective values approached within 2 h were +389% ± 168% (P < 0.05, n = 7) for raffinose and +256% ± 42% (P < 0.01, n = 8) for NaCl.
Figure 2.
Transcriptional regulation of hSGK in endothelial cells. (A) Effect of 20 mM, 50 mM, or 100 mM glucose within 2 h. (B) Effect of the Ca2+ ionophore ionomycin (1 μM), of phorbol 12,13-didecanoate (PDD; 1 μM) and of both ionomycin and PDD (incubation time 2 h). (C) Effect of glucose (100 mM), nifedipine (10 μM or 100 μM), and glucose (100 mM) in the presence of nifedipine (10 μM or 100 μM) (incubation time 2 h).
Because increased cytosolic Ca2+ concentrations have been invoked in the pathophysiology of hyperglycemia (32), we tested for a role of cytosolic Ca2+ in the transcriptional regulation of hSGK. Treatment of the endothelial cells with the Ca2+ ionophore ionomycin (1 μM) increased hSGK mRNA levels within 2 h by 536% ± 119% (P < 0.02, n = 4), indeed pointing to regulation of hSGK transcription by intracellular Ca2+ activity (Fig. 2B). Conversely, the Ca2+ channel blocker nifedipine decreased within 2 h hSGK transcription by 56% ± 10% (P < 0.02, n = 4) at 10 μM and by 45% ± 24% (n = 4) at 100 μM inhibitor (Fig. 2C). In the presence of nifedipine (10 μM or 100 μM) 100 mM glucose (2 h) was significantly (P < 0.05) less stimulatory for hSGK transcription than in the absence of the drug (58% ± 34%, n = 4, and 7% ± 29%, n = 4, respectively).
Phorbol 12,13-didecanoate (PDD) (1 μM) increased hSGK transcript levels by 281% ± 94% (P < 0.05, n = 4), pointing to the regulation of hSGK transcription by PKC (Fig. 2B). Addition of PDD (1 μM) on top of 1 μM ionomycin elicited no significant (P < 0.5) further increase in hSGK mRNA (+670% ± 138%, P < 0.02, n = 4).
To test whether the effect of high extracellular glucose involves TGF-β-1, 3T3 fibroblasts were exposed to 30 mM glucose for 8 h in the presence and absence of neutralizing TGF-β-1 antibody (30 μg/ml), which has been shown to bind endogenously formed TGF-β. As a result, neutralizing TGF-β-1 antibody significantly decreased SGK mRNA at 30 mM glucose (−36% ± 5%, P < 0.05, n = 3, Fig. 1C). Moreover, in the presence of the TGF-β-1 antibody, no significant increase was observed between 5.5 and 30 mM glucose concentration (−18% ± 10%, n = 3, Fig. 1C).
In Situ Hybridization.
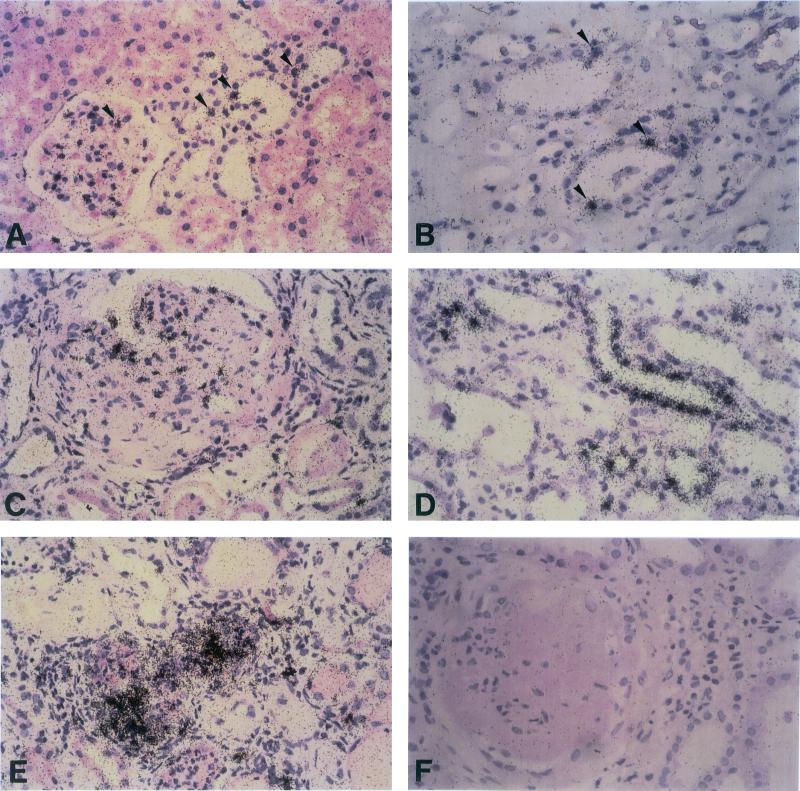
As shown in Fig. 3 A and B, expression of hSGK mRNA in intact kidneys was restricted to some mesangial cells of the glomeruli and a few epithelial cells in distal convoluted tubules and thick ascending limbs of Henle's loop. In contrast, in kidneys with diabetic nephropathy, transcription of hSGK was markedly enhanced in mesangial cells (Fig. 3C), distal tubules (not shown), and thick ascending limbs of Henle's loop (Fig. 3D). In addition, high levels of hSGK transcripts were observed in clusters of interstitial cells, most likely representing macrophages and fibroblasts as demonstrated in Fig. 3E. Unspecific labeling of kidney cells was excluded by hybridization of tissue sections with the 35S-labeled sense control RNA hSGK probe (Fig. 3F).
Figure 3.
In situ detection of hSGK mRNA in intact kidneys and diabetic nephropathy. In normal kidney significant transcription of hSGK is found in a few mesangial cells and single epithelial cells of the distal tubule (A) and thick ascending limbs of Henle's loop (B, arrowheads). In diabetic kidneys (C–F), however, high levels of hSGK transcripts are found in numerous glomerular cells (C) and epithelial cells from the thick ascending limb of Henle's loop (D) as well as in clusters of interstitial cells of fibrotic areas (E). No labeling of cells was observed after hybridization with the α-35S-labeled sense hSGK RNA probe (F). (×300.)
Stimulation of ENaC by hSGK.
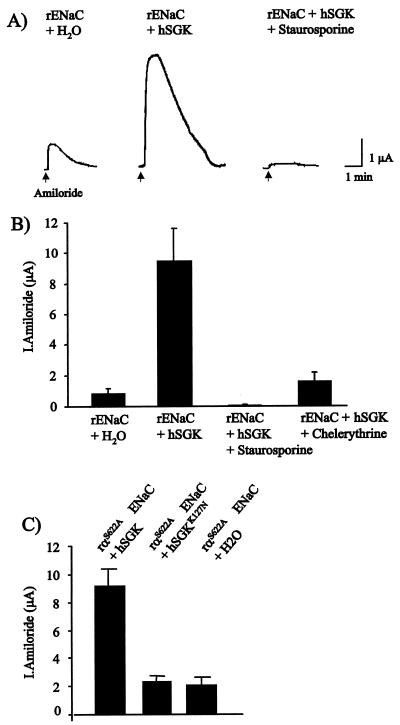
As shown in Fig. 4, coexpression of ENaC in Xenopus oocytes with hSGK resulted in an 11-fold stimulation of amiloride-sensitive (30 μM) currents. At the first day of expression, the measured current was 859 ± 302 nA (n = 5) in ENaC-expressing oocytes and 9,421 ± 2,188 nA (n = 5) in ENaC + hSGK oocytes, the difference being statistically significant (P < 0.02). The hSGK-induced stimulation of ENaC could be significantly (P < 0.02) reversed to 93 ± 60 nA and 1,753 ± 614 nA after incubation with the protein kinase inhibitors staurosporine (1 μM) or chelerythrine (1 μM), respectively for 10 h (n = 5, Fig. 4B). The consensus sequence for phosphorylation by hSGK has been reported to be RXRXX(S/T) (33), a sequence found in the α subunit (amino acids 617–622) but not the β and γ subunits of ENaC (29). To test whether hSGK acts through phosphorylation at this hSGK consensus site of the ENaC α subunit, we replaced the serine at position 622 with alanine. As shown in Fig. 4C, the S622A mutant of ENaC α subunit is still rigorously up-regulated by coexpression of hSGK.
Figure 4.
Stimulation of ENaC by hSGK. Amiloride-sensitive currents (30 μM amiloride) in Xenopus oocytes injected with the rat epithelial Na+ channel α,β,γ ENaC or the αS622A,β,γ ENaC mutant with or without hSGK, or with ENaC + hSGK in the presence of staurosporine (1 μM) or chelerythrine (1 μM). (A) Original traces. (B) Effect of hSGK on wild-type ENaC with and without protein kinase inhibitors (arithmetic means ± SEM). (C) Effect of hSGK and of inactive hSGKK127R on ENaC αS622A-mediated currents (arithmetic means ± SEM).
Stimulation of Na+,K+,2Cl− Cotransporter (BSC-1) by hSGK.
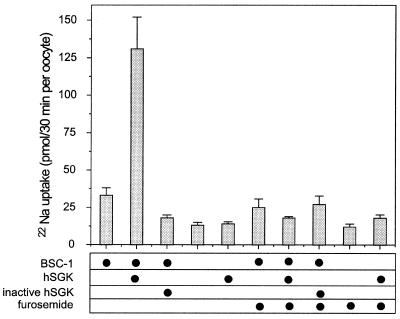
22Na+ flux measurements (Fig. 5) disclose a marked stimulatory effect of hSGK on BSC-1-mediated Na+ transport. Within 1 day of BSC-1 expression, 22Na+ flux was significantly (P < 0.01) higher (33 ± 5 pmol/30 min) than 22Na+ flux in water-injected oocytes (13 ± 2 pmol/30 min). In oocytes coinjected with BSC-1 and hSGK, the 22Na+ flux was 131 ± 21 pmol/30 min, which is 10-fold greater than 22Na+ flux in water-injected oocytes (P < 0.0001). Accordingly, BSC-1-mediated 22Na+ flux was stimulated some 6-fold by simultaneous expression of hSGK (P < 0.002). The effect was completely reversed in the presence of 100 μM furosemide (P < 0.005). Moreover, injection of the inactive kinase hSGKK127R significantly (P < 0.05) inhibited 22Na+ flux in BSC-1-injected oocytes (18 ± 2 pmol/30 min). Expression of hSGK alone had no significant effect on 22Na+ flux (Fig. 5).
Figure 5.
Stimulation of the thick ascending limb Na+,K+,2Cl− cotransporter BSC-1 by hSGK 22Na+ uptake in Xenopus oocytes injected with and without the thick ascending limb Na+,K+,2Cl− cotransporter BSC-1 and/or the active kinase hSGK or inactive kinase hSGKK127R. In some of the experiments furosemide (100 μM) was added to inhibit BSC-1. Arithmetic means ± SEM.
Discussion
As shown in this study, increased extracellular glucose concentrations increase the mRNA levels of hSGK, a cell-volume-regulated serine/threonine kinase. Because excessive extracellular glucose concentrations increase expression of both TGF-β and TGF-β receptor (1, 7–12), the enhanced hSGK transcription could have been due to autocrine stimulation by TGF-β. As a matter of fact, TGF-β-1 indeed exerts a similarly strong effect on hSGK transcription in fibroblasts (this study) as in U 937 macrophages (21) and HepG2 liver cells (21). Moreover, the effect of high extracellular glucose concentration is blunted in the presence of neutralizing TGF-β-1 antibody.
TGF-β leads to cell hypertrophy (34–36), which has similarly been observed in diabetes mellitus (37–39). On the other hand, intriguing evidence has been gathered for cell shrinkage in hyperosmolar diabetes mellitus, resulting in an increased cellular Ca2+ concentration and Ca2+-dependent cell damage (32, 40, 41). As the effect of excessive glucose concentrations is mimicked by osmotically equivalent increases of extracellular raffinose and NaCl concentrations, osmotic cell shrinkage could mediate at least part of the early mRNA increase.
Two observations suggest the involvement of cytosolic Ca2+ activity in the regulation of hSGK transcription. First, the effect of glucose on hSGK transcription was blunted by the Ca2+-channel blocker nifedipine and second, the Ca2+ ionophore ionomycin (1 μM) markedly increased hSGK transcription. Given the high inhibitor concentrations needed and the limited selectivity of nifedipine, the inhibitory effect of nifedipine on glucose-induced hSGK expression cannot be taken as evidence for an involvement of voltage-gated Ca2+ channels in glucose-induced hSGK transcription. Nevertheless, increases of cytosolic Ca2+ do stimulate hSGK transcription and could well contribute to the stimulatory effect of glucose.
Increased extracellular glucose concentrations (12–18) and TGF-β (42–46) lead to translocation and stimulation of PKC. Because phorbol esters stimulate hSGK transcription, PKC could similarly contribute to the effect of glucose on hSGK transcription. Because increased cytosolic Ca2+ activates PKC (47, 48) and activation of PKC could increase cytosolic Ca2+ concentration (32, 49), the two elements of cellular signal transduction are linked. Thus, both cellular mechanisms may converge to a common mechanism up-regulating hSGK transcription.
The present observations not only yield evidence for enhanced expression of hSGK in diabetic nephropathy but shed new light on the physiological function of this kinase—i.e., its ability to stimulate the renal epithelial Na+ channel ENaC and the thick ascending limb Na+,K+,2Cl− cotransporter BSC-1 (50). Coexpression of other transport proteins, such as basolateral Na+,K+,2Cl− cotransporter (unpublished observation) and K+ channel ROMK (24) did not prove sensitive to coexpression with hSGK. The stimulation of ENaC and BSC-1 would both be relevant for cell volume regulation and could contribute to TGF-β-induced cell hypertrophy (51). The stimulatory effect of hSGK on ENaC parallels a similar effect by mouse and Xenopus SGK (24, 52); the stimulation of Na+,K+,2Cl− cotransport is a previously undescribed function of hSGK. The effect of ENaC and BSC-1 requires the kinase function, as the inactive mutant does not stimulate the transport proteins. Only the α subunit contains a consensus site for phosphorylation by hSGK (33). Mutation of this site does, however, not eliminate the stimulatory effect of hSGK on ENaC. Along those lines, aldosterone does not stimulate phosphorylation of the α subunit (53). Taking these observations together, it is not likely that hSGK activates ENaC by direct phosphorylation. Instead, hSGK apparently stimulates trafficking of ENaC into the cell membrane (54).
Stimulation of Na+,K+,2Cl− cotransporter is expected to decrease the delivery of NaCl to the macula densa, to disinhibit tubuloglomerular feedback, and thus to enhance glomerular filtration rate (55). Hyperfiltration is in turn one of the early and deleterious derangements of renal function in diabetic nephropathy (56). As a matter of fact, reduced sensitivity of tubuloglomerular feedback has been shown to participate in the hyperfiltration of diabetic nephropathy (57, 58), and glomerular hyperfiltration could be reversed by inhibition of PKC (59). Even though other mechanisms may contribute to reduced tubuloglomerular feedback and hyperfiltration in diabetes mellitus (60, 61), stimulation of Na+,K+,2Cl− cotransporter in thick ascending limbs by hSGK is likely to participate in the generation of diabetic hyperfiltration. Beyond that, stimulation of ENaC and of BSC-1 is expected to induce Na+ retention and thus favor the development of hypertensive disease. As stimulation of hSGK transcription by high extracellular glucose concentrations and TGF-β is not restricted to renal epithelia, hSGK-mediated stimulation of cellular Na+ uptake may occur in a wide variety of cells and may contribute to increased Na+ and Ca2+ concentrations seen in diabetic patients (62). To the extent that altered intracellular electrolyte concentrations are relevant for vascular tone and development of hypertension (62), enhanced expression of hSGK could further increase blood pressure through an extrarenal mechanism.
In conclusion, we provide evidence that increased extracellular glucose concentrations stimulate the transcription of the cell-volume-regulated kinase hSGK and that hSGK transcription is enhanced in diabetic nephropathy. Because hSGK is a potent stimulator of the renal thick ascending limb Na+,K+,2Cl− cotransporter BSC-1 and the Na+ channel ENaC, enhanced transcription of this kinase could well contribute to deranged Na+ transport in diabetic nephropathy.
Acknowledgments
We acknowledge the technical assistance of A. Bröer, U. Hammacher, and B. Noll and the meticulous preparation of the manuscript by Tanja Loch. The constructs of ENaC were kindly supplied by B. Rossier. This study was supported by the Deutsche Forschungsgemeinschaft, La 315/4–3 and La 315/5–1, the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Center for Interdisciplinary Clinical Research) 01 KS 9602, the Fortüne Program of the University of Tübingen (no. 302), the Biomed Program of the European Community EG BMH4-CT96–0602, and the Baxter Extramural Grant Program.
Abbreviations
- TGF-β
transforming growth factor β
- PKC
protein kinase C
- ENaC
epithelial Na+ channel
Footnotes
See commentary on page 7667.
References
- 1.Cohen M P, Sharma K, Guo J, Eltayeb B O, Ziyadeh F N. Exp Nephrol. 1998;6:226–233. doi: 10.1159/000020527. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman B B, Sharma K, Zhu Y Q, Ziyadeh F N. Kidney Int. 1998;54:1107–1116. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma K, Ziyadeh F N, Alzahabi B, McGowan T A, Kapoor S, Kurnik B R, Kurnik P B, Weisberg L S. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 4.Ziyadeh F N, Han D C. Kidney Int. 1997;60:S7–S11. [PubMed] [Google Scholar]
- 5.Ziyadeh F N, Sharma K. Kidney Int. 1995;51:S34–S36. [PubMed] [Google Scholar]
- 6.Sharma K, Ziyadeh F N. Diabetes. 1995;44:1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer A, Schatz H. Exp Clin Endocrinol Diabetes. 1995;103:7–14. doi: 10.1055/s-0029-1211323. [DOI] [PubMed] [Google Scholar]
- 8.Rocco M V, Chen Y, Goldfarb S, Ziyadeh F N. Kidney Int. 1992;41:107–114. doi: 10.1038/ki.1992.14. [DOI] [PubMed] [Google Scholar]
- 9.Guh J Y, Yang M L, Chang C C, Chuang L Y. J Am Soc Nephrol. 1996;7:1207–1215. doi: 10.1681/ASN.V781207. [DOI] [PubMed] [Google Scholar]
- 10.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher E D. J Clin Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Templeton D M, Fan M Y. Metabolism. 1996;45:1136–1146. doi: 10.1016/s0026-0495(96)90014-1. [DOI] [PubMed] [Google Scholar]
- 12.Ishii H, Tada H, Isogai S. Diabetologia. 1998;41:362–364. doi: 10.1007/s001250050916. [DOI] [PubMed] [Google Scholar]
- 13.Koya D, Haneda M, Kikkawa R, King G L. Biofactors. 1998;7:69–76. doi: 10.1002/biof.5520070110. [DOI] [PubMed] [Google Scholar]
- 14.Keogh R J, Dunlop M E, Larkins R G. Metabolism. 1997;46:41–47. doi: 10.1016/s0026-0495(97)90165-7. [DOI] [PubMed] [Google Scholar]
- 15.Ayo S H, Radnik R, Garoni J A, Troyer D A, Kreisberg J I. Am J Physiol. 1991;261:F571–F577. doi: 10.1152/ajprenal.1991.261.4.F571. [DOI] [PubMed] [Google Scholar]
- 16.Draznin B, Leitner J W, Sussman K E, Sherman N A. Biochem Biophys Res Commun. 1988;156:570–575. doi: 10.1016/s0006-291x(88)80880-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee T S, Saltsman K A, Ohashi H, King G L. Proc Natl Acad Sci USA. 1989;86:5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 18.Ziyadeh F N, Fumo P, Rodenberger C H, Kuncio G S, Neilson E G. J Diabetes Complications. 1995;9:255–261. doi: 10.1016/1056-8727(95)80016-8. [DOI] [PubMed] [Google Scholar]
- 19.Babazono T, Kapor-Drezgic J, Dlugosz J-A, Whiteside C. Diabetes. 1998;47:668–676. doi: 10.2337/diabetes.47.4.668. [DOI] [PubMed] [Google Scholar]
- 20.Kasho M, Sakai M, Sasahara T, Anami Y, Matsumura T, Takemura T, Matsuda H, Kobori S, Shichiri M. Kidney Int. 1998;54:1083–1092. doi: 10.1046/j.1523-1755.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Waldegger S, Klingel K, Barth P, Sauter M, Lanzendörfer M, Kandolf R, Lang F. Gastroenterology. 1999;116:1081–1088. doi: 10.1016/s0016-5085(99)70011-9. [DOI] [PubMed] [Google Scholar]
- 22.Waldegger S, Barth P, Raber G, Lang F. Proc Natl Acad Sci USA. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster M K, Goya L, Ge Y, Maiyar A C, Firestone G L. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S Y, Bhargava A, Mastroberardino L, Meijer O C, Wang J, Buse P, Firestone G L, Verrey F, Pearce D. Proc Natl Acad Sci USA. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandolf R, Ameis D, Kirschner P, Canu A, Hofschneider P H. Proc Natl Acad Sci USA. 1987;84:6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohenadl C, Klingel K, Mertsching J, Hofschneider P H, Kandolf R. Mol Cell Probes. 1991;5:11–20. doi: 10.1016/0890-8508(91)90033-g. [DOI] [PubMed] [Google Scholar]
- 27.Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R. Proc Natl Acad Sci USA. 1992;89:314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bröer S, Bröer A, Hamprecht B. Biochim Biophys Acta. 1994;1192:95–100. doi: 10.1016/0005-2736(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 29.Canessa C M, Horisberger J D, Rossier B C. Nature (London) 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 30.Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 31.Busch A E, Suessbrich H, Kunzelmann K, Hipper A, Greger R, Waldegger S, Mutschler E, Lindemann B, Lang F. Pflügers Arch. 1996;432:760–766. doi: 10.1007/s004240050196. [DOI] [PubMed] [Google Scholar]
- 32.Demerdash T M, Seyrek N, Smogorzewski M, Marcinkowski W, Nassermoadelli S, Massry S G. Kidney Int. 1996;50:2032–2040. doi: 10.1038/ki.1996.526. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi T, Cohen P. Biochem J. 1999;339:319–328. [PMC free article] [PubMed] [Google Scholar]
- 34.Fine L G, Holley R W, Nasri H, Badie-Dezfooly B. Proc Natl Acad Sci USA. 1985;82:6163–6166. doi: 10.1073/pnas.82.18.6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling H, Vamvakas S, Busch G L, Daemmrich J, Schramm L, Lang F, Heidland A. Am J Physiol. 1995;269:F911–F917. doi: 10.1152/ajprenal.1995.269.6.F911. [DOI] [PubMed] [Google Scholar]
- 36.Sharma K, Jin Y, Guo J, Ziyadeh F N. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 37.Burg M B, Kador P F. J Clin Invest. 1988;81:635–640. doi: 10.1172/JCI113366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McManus M L, Churchwell K B, Strange K. New Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- 39.Morocutti A, Earle K A, Rodemann H P, Viberti G C. Diabetologia. 1997;40:244–246. doi: 10.1007/s001250050670. [DOI] [PubMed] [Google Scholar]
- 40.Krol E, Aqeel R, Banu S, Smogorzewski M, Kumar D, Massry S G. J Am Soc Nephrol. 1998;9:117A. [Google Scholar]
- 41.Seyrek N, Marcinkowski W, Smogorzewski M, Demerdash T M, Massry S G. Nephrol Dial Transplant. 1997;12:265–272. doi: 10.1093/ndt/12.2.265. [DOI] [PubMed] [Google Scholar]
- 42.Halstead J, Kemp K, Ignotz R A. J Biol Chem. 1995;270:13600–13603. doi: 10.1074/jbc.270.23.13600. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki M, Asplund T, Yamashita H, Heldin C H, Heldin P. Biochem J. 1995;307:817–821. doi: 10.1042/bj3070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss R H, Ramirez A. Nephrol Dial Transplant. 1998;13:2804–2813. doi: 10.1093/ndt/13.11.2804. [DOI] [PubMed] [Google Scholar]
- 45.Weiss R H, Yabes A P, Sinaee R. Kidney Int. 1995;48:738–744. doi: 10.1038/ki.1995.345. [DOI] [PubMed] [Google Scholar]
- 46.Wrenn R W, Raeuber C L, Herman L E, Walton W J, Rosenquist T H. In Vitro Cell Dev Biol. 1993;29A:73–78. doi: 10.1007/BF02634374. [DOI] [PubMed] [Google Scholar]
- 47.Sahai A, Fadda G Z, Massry S G. Endocrinology. 1992;131:1889–1894. doi: 10.1210/endo.131.4.1396333. [DOI] [PubMed] [Google Scholar]
- 48.Saitoh M, Salzman E W, Smith M, Ware J A. Blood. 1989;74:2001–2006. [PubMed] [Google Scholar]
- 49.Cirillo M, Canessa M, Quinn S, Conlin P R. Biochem Biophys Res Commun. 1998;245:466–471. doi: 10.1006/bbrc.1998.8458. [DOI] [PubMed] [Google Scholar]
- 50.Hebert S C, Gamba G, Kaplan M. Kidney Int. 1996;49:1638–1641. doi: 10.1038/ki.1996.238. [DOI] [PubMed] [Google Scholar]
- 51.Lang F, Busch G L, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 52.Naray-Fejes-Toth A, Canessa C, Cleaveland E S, Aldrich G, Fejes-Toth G. J Biol Chem. 1999;274:16973–16978. doi: 10.1074/jbc.274.24.16973. [DOI] [PubMed] [Google Scholar]
- 53.Shimkets R A, Lifton R, Canessa C M. Proc Natl Acad Sci USA. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De la Rosa D A, Zhang P, Náray-Fejes-Tóth A, Féjes-Tóth G, Canessa C M. J Biol Chem. 1999;274:37834–37839. doi: 10.1074/jbc.274.53.37834. [DOI] [PubMed] [Google Scholar]
- 55.Schnermann J, Briggs J. Kidney Int. 1982;22:82–89. [Google Scholar]
- 56.Hostetter T H, Troy J L, Brenner B M. Am J Med. 1981;72:375–380. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 57.Blantz R C, Peterson O W, Thompson S C. Am J Physiol. 1991;260:F749–F756. doi: 10.1152/ajprenal.1991.260.5.F749. [DOI] [PubMed] [Google Scholar]
- 58.Vallon V, Richter K. Kidney Blood Press Res. 1998;21:105. doi: 10.1159/000174151. [DOI] [PubMed] [Google Scholar]
- 59.Ishii H, Jirousek M R, Koya D, Takagi C, Xia P, Clermont A, Bursell S E, Kern T S, Ballas L M, Heath W F, et al. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 60.Jensen P K, Kristensen K S, Rasch R, Persson A. In: The Juxtaglomerular Apparatus. Persson A, Boberg B, editors. Amsterdam: Elsevier; 1988. pp. 333–338. [Google Scholar]
- 61.Vallon V, Blantz R C, Thomson S. Am J Physiol. 1995;269:F876–F883. doi: 10.1152/ajprenal.1995.269.6.F876. [DOI] [PubMed] [Google Scholar]
- 62.Resnick L M. Am J Med. 1992;93:11S–20S. doi: 10.1016/0002-9343(92)90290-r. [DOI] [PubMed] [Google Scholar]