Abstract
1 The effects of prostaglandin (PGE1), following local administration during different phases of developing sponge-induced granulomata, were studied in normal and essential fatty acid deficient (EFAD) rats.
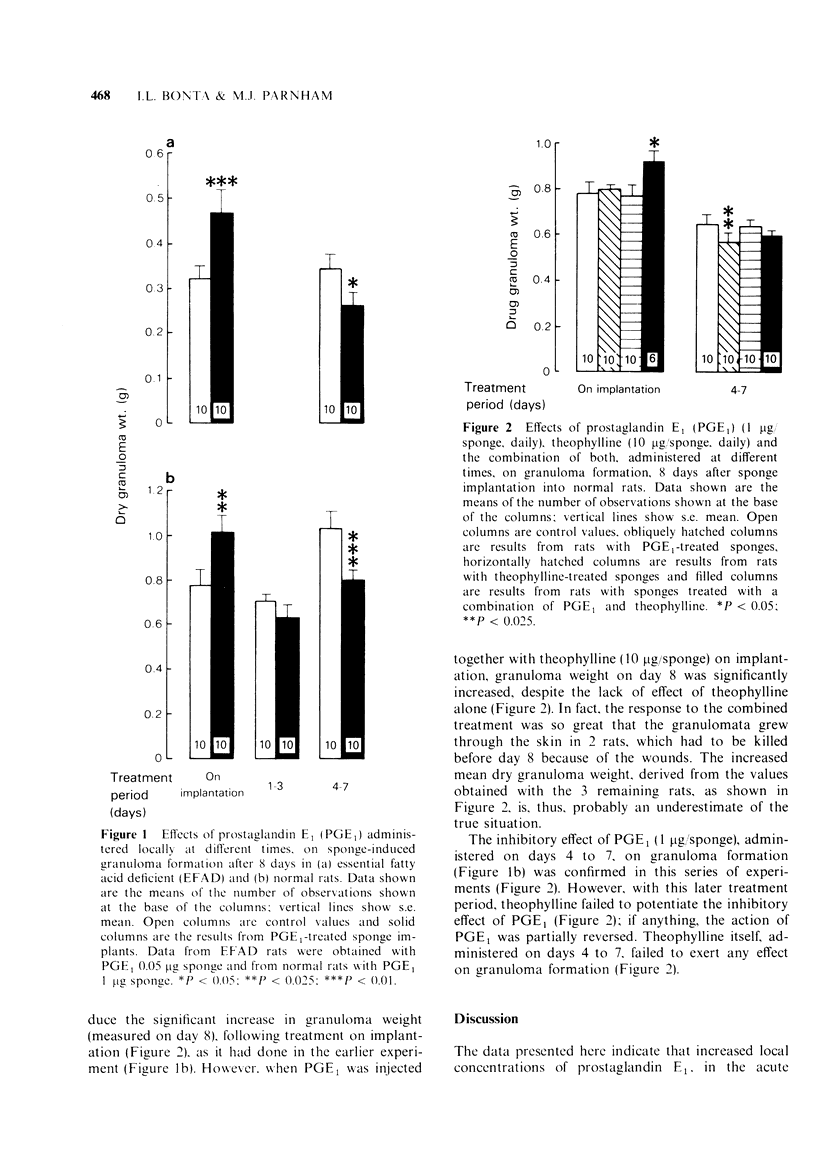
2 In normal rats, a single dose of 1 μg PGE1 on implantation (day 1) increased exudate production without altering total leucocyte counts after 6 h and stimulated granulomatous tissue formation after 8 days.
3 Repeated daily administration of the same dose of PGE1 on days 1 to 3 had no effect, while administration on days 4 to 7 (i.e. when tissue growth is already in progress) inhibited granuloma formation.
4 In EFAD rats, which are known to produce only very small amounts of endogenous prostaglandins, acute (6 h) exudate formation was unaffected by 0.05 μg PGE1. However, early stimulatory and later inhibitory effects of 0.05 μg PGE1 per day were obtained on the granulomatous tissue, similar to those obtained with the 20 fold higher dose in normal rats.
5 The early stimulatory action of PGE1 on granulomatous tissue formation was enhanced, in normal rats, by concomitant administration of 10 μg theophylline. This latter compound did not influence the later inhibitory effect of PGE1.
6 These results indicate that PGE1 exerts either pro- or anti-inflammatory actions on the proliferative (tissue) component of the inflammatory process, depending on the time of administration. While the stimulatory effect following early administration may have been secondary to an initial cyclic adenosine 3′,5′-monophosphate-mediated, vascular response, such a mechanism is unlikely to have been responsible for the later anti-inflammatory action of PGE1.
7 The implications of these results are discussed in relation to the postulated negative-feedback role of endogenous PGE in chronic inflammation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Sondergaard J. Effect of prostaglandins E 1 and F 1 on biosynthesis of collagen. Nat New Biol. 1972 Oct 25;239(95):246–246. doi: 10.1038/newbio239246a0. [DOI] [PubMed] [Google Scholar]
- Bonta I. L., Chrispijn H., Noordhoek J., Vincent J. E. Reduction of prostaglandin-phase in hind-paw inflammation and partial failure of indomethacin to exert anti-inflammatory effect in rats on essential fatty acid deficient diet. Prostaglandins. 1974 Mar 10;5(5):495–503. doi: 10.1016/s0090-6980(74)80023-7. [DOI] [PubMed] [Google Scholar]
- Bonta I. L., Parnham M. J. Prostaglandins and chronic inflammation. Biochem Pharmacol. 1978;27(12):1611–1623. doi: 10.1016/0006-2952(78)90169-7. [DOI] [PubMed] [Google Scholar]
- Bonta I. L., Parnham M. J. Time-dependent pro- and anti- inflammatory effects of prostaglandin (PG)E1 on experimental granulomata rats [proceedings]. Br J Pharmacol. 1978 Mar;62(3):417P–418P. [PMC free article] [PubMed] [Google Scholar]
- Bonta I. L., Parnham M. J., Van Vliet L. Combination of theophylline and prostaglandin E1 as inhibitors of the adjuvant-induced arthritis syndrome of rats. Ann Rheum Dis. 1978 Jun;37(3):212–217. doi: 10.1136/ard.37.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E. Effects of prostaglandins on adenosine 3',5'-monophosphate levels in fat and other tissues. J Biol Chem. 1968 Apr 25;243(8):1713–1717. [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. VII. Evidence supporting a role for prostaglandins and cyclic nucleotides. J Lab Clin Med. 1975 Mar;85(3):392–404. [PubMed] [Google Scholar]
- Chang W. C., Murota S. I., Tsurufuji S. Role of prostaglandin E in carrageenin-induced inflammation in rats. Biochem Pharmacol. 1976 Sep 15;25(18):2045–2050. doi: 10.1016/0006-2952(76)90428-7. [DOI] [PubMed] [Google Scholar]
- Chang W. C., Tsurufiji S. Differences in the mode of exucative reaction between early phase and late phase of carrageenin-induced inflammation in rats. Eur J Pharmacol. 1976 Mar;36(1):7–14. doi: 10.1016/0014-2999(76)90250-8. [DOI] [PubMed] [Google Scholar]
- Denko C. W. Effect of prostaglandins in urate crystal inflammation. Pharmacology. 1974;12(6):331–339. doi: 10.1159/000136556. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Sawyer B. D. Synthesis of collagen by chondrocytes in suspension culture: modulation by calcium, 3':5'-cyclic AMP, and prostaglandins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3864–3868. doi: 10.1073/pnas.74.9.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPasquale G., Rassaert C., Richter R., Welaj P., Tripp L. Influence of prostaglandins (Pg) E2 and F2 on the inflammatory process. Prostaglandins. 1973 Jun;3(6):741–757. doi: 10.1016/0090-6980(73)90001-4. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Harvey E. A., Ferreira S. H., Vane J. R. The effects of antiinflammatory drugs on the production of prostaglandins in vivo. Adv Prostaglandin Thromboxane Res. 1976;1:105–110. [PubMed] [Google Scholar]
- Higgs G. A., McCall E., Youlten L. J. A chemotactic role for prostaglandins released from polymorphonuclear leucocytes during phagocytosis. Br J Pharmacol. 1975 Apr;53(4):539–546. doi: 10.1111/j.1476-5381.1975.tb07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Change in growth and morphology of fibroblasts by prostaglandins. J Natl Cancer Inst. 1971 Dec;47(6):1357–1364. [PubMed] [Google Scholar]
- Kahn A., Brachet E. Effect of some mediators of inflammation on cyclic AMP concentrations in the incubated rat mesentery. Arch Int Physiol Biochim. 1976;84(3):553–555. [PubMed] [Google Scholar]
- Ko S. D., Page R. C., Narayanan A. S. Fibroblast heterogeneity and prostaglandin regulation of subpopulations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3429–3432. doi: 10.1073/pnas.74.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall E., Youlten L. J. Proceedings: The effect of indomethacin and depletion of complement on cell migration and prostaglandin levels in carrageenin-induced air bleb inflammation. Br J Pharmacol. 1974 Nov;52(3):452P–452P. [PMC free article] [PubMed] [Google Scholar]
- McClatchey W., Snyderman R. Prostaglandins and inflammation: enhancement of monocyte chemotactic responsiveness by prostaglandin E2. Prostaglandins. 1976 Sep;12(3):415–426. doi: 10.1016/0090-6980(76)90022-8. [DOI] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Interaction between anti-inflammatory drugs and inflammatory mediators. A reference to products of arachidonic acid metabolism. Agents Actions Suppl. 1977;(3):141–148. doi: 10.1007/978-3-0348-7290-4_14. [DOI] [PubMed] [Google Scholar]
- Ohuchi K., Sato H., Tsurufuji S. The content of prostaglandin E and prostaglandin F2alpha in the exudate of carrageenin granuloma of rats. Biochim Biophys Acta. 1976 Mar 26;424(3):439–448. doi: 10.1016/0005-2760(76)90033-3. [DOI] [PubMed] [Google Scholar]
- Parnham M. J., Adolfs M. J., Bonta I. L. The effect of metyrapone on granuloma induced by carrageenan-impregnated sponges in normal and essential fatty acid deficient rats. J Pharm Pharmacol. 1977 Nov;29(11):670–673. doi: 10.1111/j.2042-7158.1977.tb11432.x. [DOI] [PubMed] [Google Scholar]
- Parnham M. J., Shoshan S., Bonta I. L., Neiman-Wollner S. Increased collagen metabolism in granulomata induced in rats deficient in endogenous prostaglandin precursors. Prostaglandins. 1977 Oct;14(4):709–714. doi: 10.1016/0090-6980(77)90198-8. [DOI] [PubMed] [Google Scholar]
- Peters H. D., Peskar B. A., Schönhöfer P. S. Influence of prostaglandins on connective tissue cell growth and function. Naunyn Schmiedebergs Arch Pharmacol. 1977;297 (Suppl 1):S89–S93. doi: 10.1007/BF00587790. [DOI] [PubMed] [Google Scholar]
- Raisz L. G., Koolemans-Beynen A. R. Inhibition of bone collagen synthesis by prostaglandin E2 in organ culture. Prostaglandins. 1974 Dec 10;8(5):377–385. doi: 10.1016/0090-6980(74)90113-0. [DOI] [PubMed] [Google Scholar]
- Shibuya E., Masuda K., Izawa Y. Effects of prostaglandins on leukocyte migration. Prostaglandins. 1976 Aug;12(2):165–174. doi: 10.1016/0090-6980(76)90110-6. [DOI] [PubMed] [Google Scholar]
- Smith R. J. Modulation of phagocytosis by and lysosomal enzyme secretion from guinea-pig neutrophils: effect of nonsteroid anti-inflammatory agents and prostaglindins. J Pharmacol Exp Ther. 1977 Mar;200(3):647–657. [PubMed] [Google Scholar]
- Swingle K. F., Shideman F. E. Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain anti-inflammatory agents. J Pharmacol Exp Ther. 1972 Oct;183(1):226–234. [PubMed] [Google Scholar]
- Vincent J. E., Zijlstra F. J., Bonta I. L. The effect of non-steroid anti-inflammatory drugs, dibutyryl cyclic 3',5'-adenosine monophosphate and phosphodiesterase inhibitors on platelet aggregation and the platelet release reaction in normal and essential fatty acid deficient rats. Prostaglandins. 1975 Nov;10(5):899–911. doi: 10.1016/0090-6980(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Smith M. J., Ford-Hutchinson A. W. Prostaglandins and leucotaxis. J Pharm Pharmacol. 1976 Oct;28(10):745–747. doi: 10.1111/j.2042-7158.1976.tb04039.x. [DOI] [PubMed] [Google Scholar]
- Weinryb I., Chasin M., Free C. A., Harris D. N., Goldenberg H., Michel I. M., Paik V. S., Phillips M., Samaniego S., Hess S. M. Effects of therapeutic agents on cyclic AMP metabolism in vitro. J Pharm Sci. 1972 Oct;61(10):1556–1567. doi: 10.1002/jps.2600611003. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Willis A. L. Parallel assay of prostaglandin-like activity in rat inflammatory exudate by means of cascade superfusion. J Pharm Pharmacol. 1969 Feb;21(2):126–128. doi: 10.1111/j.2042-7158.1969.tb08213.x. [DOI] [PubMed] [Google Scholar]
- Yu J. H., Wells H., Ryan W. J., Lloyd W. S. Effects of prostaglandins and other drugs on the cyclic AMP content of cultured bone cells. Prostaglandins. 1976 Oct;12(4):501–513. doi: 10.1016/0090-6980(76)90031-9. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Ballas M. Prostaglandin E 1 (PGE 1 ) suppression of adjuvant arthritis. Histopathology. Arthritis Rheum. 1973 Mar-Apr;16(2):251–257. doi: 10.1002/art.1780160218. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Suppression of acute and chronic inflammation in adrenalectomized rats by pharmacologic amounts of prostaglandins. Arthritis Rheum. 1973 Sep-Oct;16(5):606–618. doi: 10.1002/art.1780160505. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Quagliata F. Effect of prostaglandin E 1 on adjuvant arthritis. Nature. 1971 Dec 3;234(5327):304–305. doi: 10.1038/234304a0. [DOI] [PubMed] [Google Scholar]


