Abstract
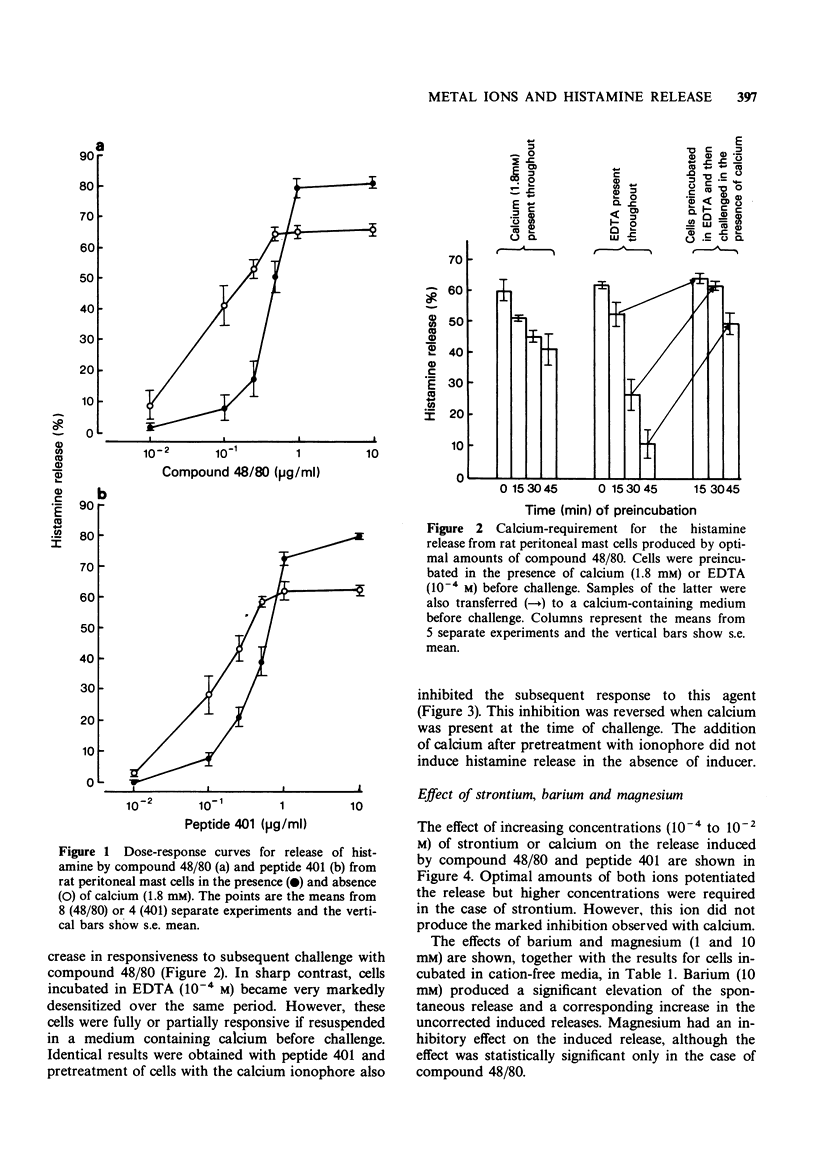
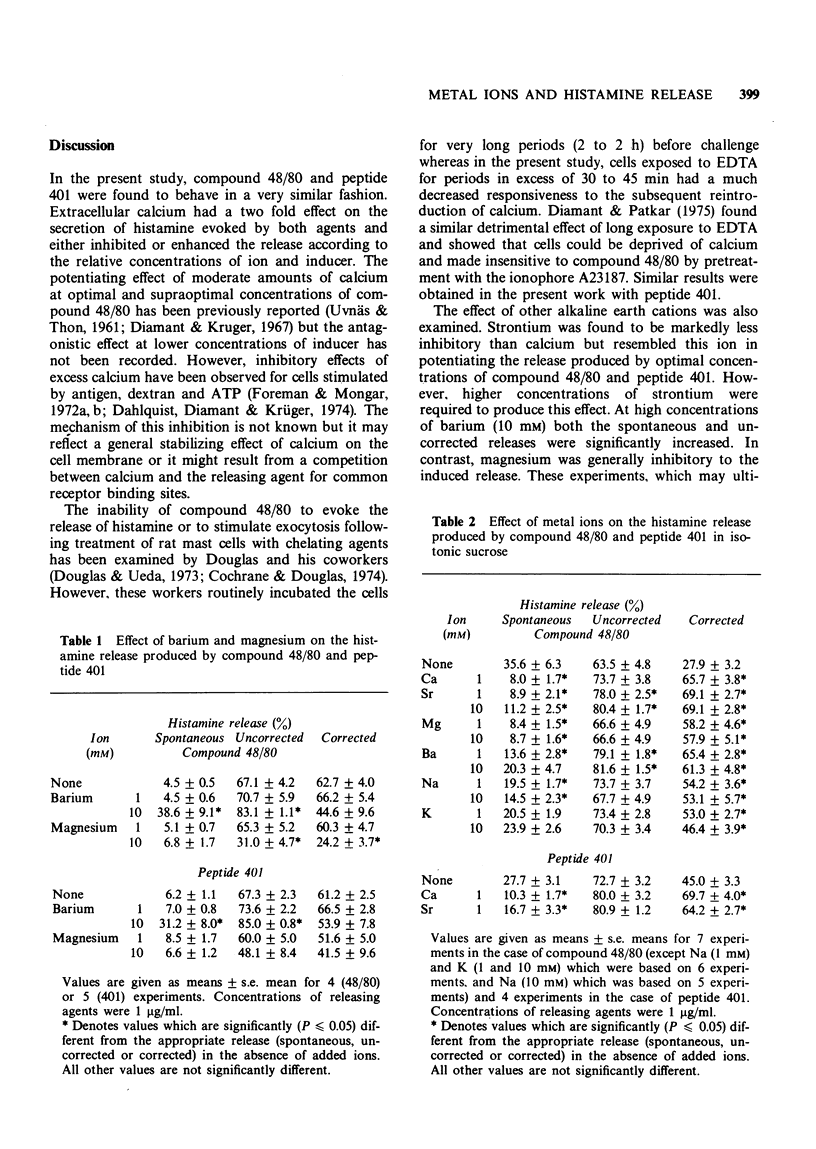
1 Extracellular calcium ions have a dual effect on the release of histamine from rat peritoneal mast cells treated with compound 48/80 and peptide 401. The release is either potentiated or inhibited according to the relative concentrations of ion and inducer.
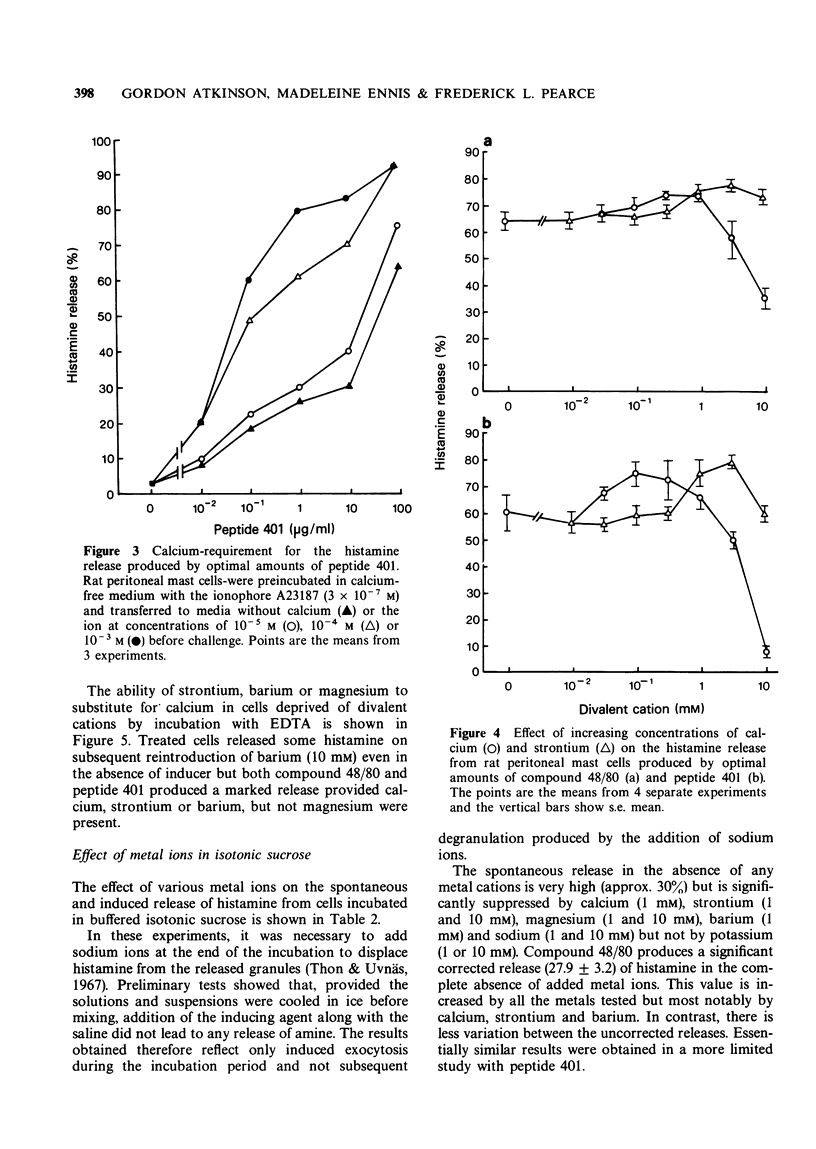
2 Strontium similarly potentiates the release produced by optimal concentrations of inducer but higher concentrations are required than in the case of calcium. Strontium is markedly less inhibitory than calcium.
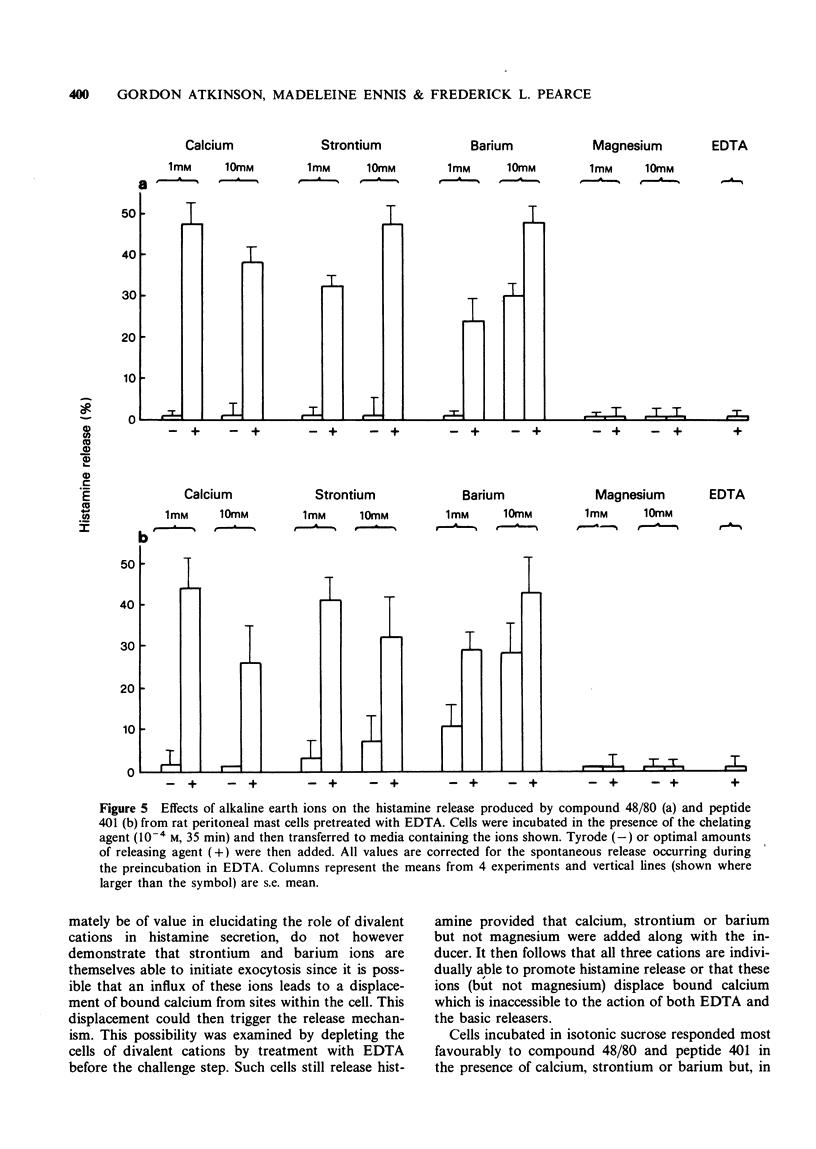
3 Mast cells may be depleted of intracellular calcium by incubation for short periods with the chelating agent, ethylenediamine tetraacetic acid (EDTA). They thereby become unresponsive to compound 48/80 and peptide 401 unless calcium is reintroduced into the incubation medium. Strontium and barium, but not magnesium, will substitute for calcium in this system. Barium additionally produces a marked release of histamine even in the absence of inducer. Pretreatment with the ionophore A23187 similarly inhibits the subsequent response to peptide 401 in divalent cation-free medium. This inhibition is reversed on the reintroduction of calcium.
4 Compound 48/80 and peptide 401 release histamine from mast cells incubated in isotonic sucrose in the complete absence of added metal ions. However, the corrected release under these conditions is potentiated by both mono and divalent cations.
5 On the basis of these results, the possible mechanism of action of the basic releasing agents and their usefulness as models for studying histamine secretion is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assem E. S., Atkinson G. Histamine release by MCDP (401), a peptide from the venom of the honey bee. Br J Pharmacol. 1973 Jun;48(2):337P–338P. [PMC free article] [PubMed] [Google Scholar]
- Breithaupt H., Habermann E. Mastzelldegranulierendes Peptid (MCD-Pepid) aus Bienengift: Isolierung, biochemische und pharmakologische Eigenschaften. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(3):252–270. [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Histamine release by exocytosis from rat mast cells on reduction of extracellular sodium: a secretory response inhibited by calcium, strontium, barium or magnesium. J Physiol. 1976 May;257(2):433–448. doi: 10.1113/jphysiol.1976.sp011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist R., Diamant B., Krüger P. G. Increased permeability of the rat mast cell membrane to sodium and potassium caused by extracellular ATP and its relationship to histamine release. Int Arch Allergy Appl Immunol. 1974;46(5):655–675. doi: 10.1159/000231167. [DOI] [PubMed] [Google Scholar]
- Dahlquist R. Relationship of uptake of sodium and 45calcium to ATP-induced histamine release from rat mast cells. Acta Pharmacol Toxicol (Copenh) 1974 Jul;35(1):11–22. doi: 10.1111/j.1600-0773.1974.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Diamant B., Krüger P. G. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5'-triphosphate. Acta Physiol Scand. 1967 Dec;71(4):291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Diamant B., Patkar S. A. Stimulation and inhibition of histamine release from isolated rat mast cells. Dual effects of the ionophore A23187. Int Arch Allergy Appl Immunol. 1975;49(1-2):183–207. doi: 10.1159/000231394. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Ueda Y. Proceedings: Mast cell secretion (histamine release) induced by 48-80: calcium-dependent exocytosis inhibited strongly by cytochalasin only when glycolysis is rate-limiting. J Physiol. 1973 Oct;234(2):97P–98P. [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Movement of strontium ions into mast cells and its relationship to the secretory response. J Physiol. 1977 Sep;271(1):233–251. doi: 10.1113/jphysiol.1977.sp011998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Site of action of the antiallergic drugs cromoglycate and doxantrazole [proceedings]. Br J Pharmacol. 1977 Mar;59(3):473P–474P. [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. Effect of calcium on dextran-induced histamine release from isolated mast cells. Br J Pharmacol. 1972 Dec;46(4):767–769. doi: 10.1111/j.1476-5381.1972.tb06902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland L. G., Mongar J. L. Differential histamine release by dextran and the ionophore A23187: the actions of inhibitors. Int Arch Allergy Appl Immunol. 1976;50(1):27–42. doi: 10.1159/000231477. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Hanson J. M., Rumjanek F. D., Shipolini R. A., Vernon C. A. The peptide components of bee venom. Eur J Biochem. 1976 Jan 15;61(2):369–376. doi: 10.1111/j.1432-1033.1976.tb10030.x. [DOI] [PubMed] [Google Scholar]
- Loeffler L. J., Lovenberg W., Sjoerdsma A. Effects of dibutyryl-3',5'-cyclic adenosine monophosphage, phosphodiesterase inhibitors and prostaglandin E1 on compound 48-80-induced histamine release from rat peritoneal mast cells in vitro. Biochem Pharmacol. 1971 Sep;20(9):2287–2297. doi: 10.1016/0006-2952(71)90228-0. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Thon I. L., Uvnäs B. Degranulation and histamine release, two consecutive steps in the response of rat mast cells to compound 48-80. Acta Physiol Scand. 1967 Dec;71(4):303–315. doi: 10.1111/j.1748-1716.1967.tb03737.x. [DOI] [PubMed] [Google Scholar]
- UVNAS B., THON I. L. Evidence for enzymatic histamine release from isolated rat mast cells. Exp Cell Res. 1961 Feb;23:45–57. doi: 10.1016/0014-4827(61)90062-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Sugiyama K. Histamine release from isolated rat mast cells in metal ion-free medium. Jpn J Pharmacol. 1972 Jun;22(3):325–337. doi: 10.1254/jjp.22.325. [DOI] [PubMed] [Google Scholar]



