Abstract
We report the isolation and characterization of a new Medicago truncatula hyper-nodulation mutant, designated sunn (super numeric nodules). Similar to the previously described ethylene-insensitive mutant sickle, sunn exhibits a 10-fold increase in the number of nodules within the primary nodulation zone. Despite this general similarity, these two mutants are readily distinguished based on anatomical, genetic, physiological, and molecular criteria. In contrast to sickle, where insensitivity to ethylene is thought to be causal to the hyper-nodulation phenotype (R.V. Penmetsa, D.R. Cook [1997] Science 275: 527–530), nodulation in sunn is normally sensitive to ethylene. Nevertheless, sunn exhibits seedling root growth that is insensitive to ethylene, although other aspects of the ethylene triple response are normal; these observations suggest that hormonal responses might condition the sunn phenotype in a manner distinct from sickle. The two mutants also differ in the anatomy of the nodulation zone: Successful infection and nodule development in sunn occur predominantly opposite xylem poles, similar to wild type. In sickle, however, both infection and nodulation occur randomly throughout the circumference of the developing root. Genetic analysis indicates that sunn and sickle correspond to separate and unlinked loci, whereas the sunn/skl double mutant exhibits a novel and additive super-nodulation phenotype. Taken together, these results suggest a working hypothesis wherein sunn and sickle define distinct genetic pathways, with skl regulating the number and distribution of successful infection events, and sunn regulating nodule organogenesis.
Legumes form a novel organ on their roots in response to lipooligosaccharide signals, the “Nod factors,” delivered by specific soil bacteria called rhizobia. Nodules are colonized by the inciting rhizobia, and ultimately provide a physiological context necessary for symbiotic nitrogen fixation by the bacterium (Crawford et al., 2000). Key aspects of symbiotic metabolism include the supply of energy in the form of carbon, from the plant to the bacterium, and the return of reduced nitrogen in the form of ammonia, from the bacterium to the plant. The benefits of this cross-kingdom collaboration extend beyond the exchange of carbon and nitrogen between the symbiotic partners, to the ecosystem level where the increased abundance of biologically available nitrogen impacts coresident species across several trophic levels. Understanding the nodulation process represents an important objective for plant biologists, with significant implications for both agricultural and natural ecosystems.
Numerous genetic studies establish that the initiation of symbiotic development depends on the perception of Nod factor signals by the plant host. A description of the molecular mechanisms underlying this process is beginning to emerge primarily from studies involving two model legume species, Medicago truncatula and Lotus japonicus. Numerous non-nodulating mutants have been identified in these model legume systems, and in M. truncatula, several such mutants are implicated in transduction of the bacterial lipooligosaccharide signal (Catoira et al., 2000, 2001; Wais et al., 2000). Recently, Endre et al. (2002) demonstrated that the dmi2 (doesn't make infections) locus of M. truncatula is a class I protein kinase. Orthologs of dmi2 appear to regulate symbiotic development in alfalfa (Medicago sativa), pea (Pisum sativum; Endre et al., 2002), and L. japonicus (Stracke et al., 2002), providing the first functional proof of a common ancestry for nodulation in legumes. The likely complexity of Nod factor signaling is indicated by the ability of various ligand structures to incite common responses with varying efficiencies; a recent demonstration of such Nod factor structure-specific responses is the negative feedback of wild–type Nod factor perception involving the dmi3 locus of M. truncatula (Oldroyd et al., 2002).
In addition to the perception of exogenous signals, it is now apparent that the perception of endogenous signals, particularly plant hormones, is important for proper symbiotic development. The best evidence to date involves the role of the plant hormone ethylene. In M. truncatula, genetic insensitivity to ethylene is correlated with an increase in the number of successful infections and differentiated nodules (Penmetsa and Cook, 1997). One model suggests that ethylene acts to negatively regulate the persistence of infection by Sinorhizobium meliloti. Thus, the numbers of successful bacterial infections are substantially increased in the sickle mutant, and chemicals that antagonize or promote ethylene synthesis (e.g. aminoethoxyvinyl-Gly [AVG] and 1-aminocyclopropane carboxylic acid [ACC]) enhance or decrease infection by S. meliloti, respectively. Consistent with this model, Heidstra et al. (1997) determined that the distribution of transcripts for ACC oxidase, a committed step in ethylene synthesis, was negatively correlated with the spatial distribution of nodule development. Recently, Oldroyd et al. (2001) demonstrated that the sensitivity of root hair cells to Nod factor is significantly increased in the sickle mutant, and that modulation of ethylene synthesis in wild type had comparable effects on the sensitivity of Nod factor perception. It appears, therefore, that the symbiotic phenotypes of altered ethylene perception may arise from an interaction between pathways for perception of Nod factor and ethylene.
There is accumulating evidence that other plant hormones may also have important roles in the process of symbiotic development. In particular, the induction of nodule organogenesis in root cortical cells may be mediated by local changes in the auxin/cytokinin ratio (for review, see Hirsch and Fang, 1994). A role for auxin in nodule organogenesis was first suggested by Thimann (1936), and subsequently supported by physiological rather than genetic studies. For example, the localized application of auxin transport inhibitors to legume roots, thought to disrupt auxin transport and thereby modulate the endogenous auxin to cytokinin balance, induces nodule-like structures in several legumes (Allen et al., 1953; Hirsch et al., 1989; van de Weil et al., 1990). More recently Boot et al. (1999) obtained evidence for a reduction in endogenous auxin transport in response to bacterial Nod factors. Consistent with this finding, Mathesius et al. (1998) observed transient down regulation of the auxin-responsive GH3:GUS reporter gene fusion in white clover (Trifolium repens) roots acropetal to the site of rhizobial inoculation. Evidence suggestive of a role for cytokinin in nodule organogenesis includes the finding that cytokinin treatment induces ENOD2 expression in Sesbania rostrata (Dehio and deBruijn, 1992) and cortical cell divisions and ENOD12 induction in alfalfa (Bauer et al., 1996). Moreover, transformation of Nod factor-deficient S. meliloti strains with a gene for cytokinin synthesis (i.e. the trans-zeatin secretion gene) confers the ability to induce nodule-like structures on host alfalfa plants (Cooper and Long, 1994). Taken together, these studies suggest that a low ratio of auxin to cytokinin in legume roots may be sufficient to signal the induction of cortical cell divisions, with additional cues being required for the coordination of these cell division foci into organized nodule meristems.
In this study, we describe the analysis of a new hyper-nodulating mutant of M. truncatula, designated sunn. Genetic, physiological, anatomical, and molecular analyses distinguish sunn from the previously described hyper-nodulating mutant sickle. Although the nodulation phenotype of sickle is associated with altered ethylene perception, nodulation in sunn remains normally sensitive to ethylene. Instead, sunn exhibits root growth that is insensitive to ethylene. Similar to super-nodulation mutants described in soybean (Glycine max) and L. japonicus (Carroll et al., 1985; Wopereis et al., 2000), grafting experiments demonstrate that the sunn phenotype is determined by the genotype of the shoot, implicating a mobile signal in conditioning nodule number. We suggest a model for the genetic control of nodule number in M. truncatula, wherein rhizobial infection and nodule organogenesis are mediated by distinct genetic pathways involving skl and sunn, respectively.
RESULTS
Isolation of a Novel Super-Nodulation Mutant
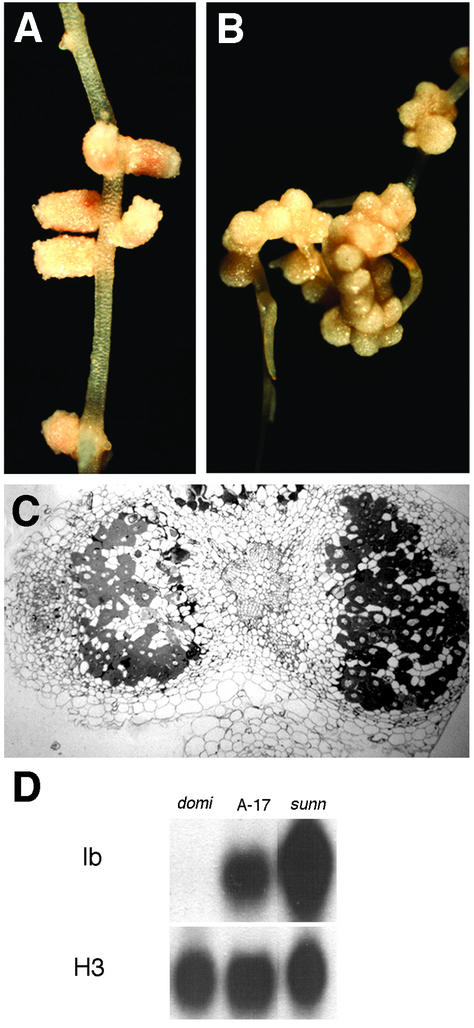
Based on a visual screen for altered nodule development, we identified a mutant displaying roughly 70 nodules within the primary nodulation zone (Fig. 1, A and B). This compares with the parental genotype A17, which develops approximately eight nodules per root in response to inoculation with S. meliloti ABS7 (Penmetsa and Cook, 1997). We have designated this mutant “sunn ” (super numeric nodules). Similar to wild-type plants, the nodulation response in sunn was transient, with an absence of bacterial infection and nodule development in the region of the root that develops subsequent to initial infection by S. meliloti. We used several criteria to assess the status of symbiotic development in sunn. In wild-type plants, the occurrence of leghemoglobin transcript serves as a molecular marker for the transition from nodule development to nodule function. As shown in Figure 1D, leghemoglobin transcript was readily detected in 23-d-old sunn nodules, although transcript levels were not increased in proportion to the increased mass of nodule tissue. Nevertheless, cytological analysis of sunn nodules indicated full differentiation of both symbionts. In particular, bacteriods, which represent the fully differentiated state of the bacterium, were abundant in the majority of cells within the nodule central tissue, surrounded by uninfected host cells as is typical of wild-type nodules (Fig. 1C). As an alternate measure of the differentiation of both partners, we compared nitrogenase activity in sunn and wild type based on the ability of excised nodulation zones to reduce acetylene to ethylene. On a per root basis, sunn possessed similar nitrogenase activity as observed in wild type (Table I). The absence of a correspondence between nitrogenase activity and nodule number in sunn is consistent with previously described hyper-nodulating mutants in both M. truncatula (Penmetsa and Cook, 1997) and in other legumes (Carroll et al., 1985; Wopereis et al., 2000).
Figure 1.
Infection and nodulation phenotypes of wild-type M. truncatula and mutant sunn. Light micrographs of primary nodulation zones from wild-type (A) and sunn (B) roots. C, Bright-field micrograph of a transverse section of 21-d-old sunn nodules from the primary nodulation zone. X, Root xylem tissue; C, nodule central tissue; E, nodule endodermis; M, nodule meristem. D, Leghemoglobin expression in wild type and mutant sunn. Lanes contain total RNA from roots 23 d after inoculation with S. meliloti. Comparable root tissues were assayed for nitrogenase activity (Table I) and tissue differentiation (C). lb, Leghemoglobin; H3, histone expression.
Table I.
Nitrogenase activity in select M. truncatula genotypes determined by the acetylene reduction assay
| Genotype | Acetylene Reduction, Mean + se |
|---|---|
| pmol ethylene min−1 root−1 | |
| A17 (wild type) | 233 ± 54 |
| sunn | 374 ± 145 |
| skl | 200 ± 87 |
| Double mutant | 265 ± 32 |
In both the soybean Nts mutant (Mathews et al., 1990) and the L. japonicus har1 mutant (Wopereis et al., 2000), the super-nodulation phenotype is controlled by the genotype of the shoot. To determine if the sunn hyper-nodulation phenotype might also be shoot genotype controlled, we used a simple grafting protocol for M. truncatula to construct chimeric plants with opposing root and shoot genotypes. As shown in Table II, the nodulation phenotype of the shoot was dominant, with sunn shoots conferring excessive nodulation on wild-type root systems, and wild-type shoots conferring reduced nodule numbers on sunn root systems. Despite the similarity of increased nodule number and shoot control over nodulation that are common to sunn and the Har1 mutant of L. japonicus, the phenotype of sunn is distinct from that of Har1. Although both mutants exhibit retarded root growth in the absence of rhizobia, the short root of the sunn mutant is not accompanied by increased lateral root number that has been observed in Har1. Moreover, whereas the Har1 mutant exhibits a pronounced retardation of root growth after rhizobial inoculation, root growth in sunn continues largely unabated after inoculation (J.A. Frugoli, unpublished data).
Table II.
Nodulation in grafted plants of sunn and wild type, 12 to 17 d postinoculation
| Shoot Genotype | Root Genotype | No. of Plants | No. of Nodules |
|---|---|---|---|
| A17 (wt) | A17 | 6 | 18 ± 5 |
| A17 | sunn | 11 | 18 ± 7 |
| sunn | A17 | 5 | 45 ± 11 |
| sunn | sunn | 7 | 50 ± 12 |
Genetic Analysis of sunn and Comparison with the Previously Described Nodulation Mutant Sickle
To determine the genetic nature of the sunn mutation, pollen from wild-type Jemalong (A17) was used in crosses with homozygous sunn individuals. F2 seedlings (288) were scored for nodule number at 7 to 10 d subsequent to inoculation with S. meliloti, revealing 77 plants that contained an estimated >70 nodules/plant. The remaining 211 individuals were either wild type or had intermediate numbers of nodules; thus, whereas wild-type plants consistently produce a range of three to 12 nodules per plant, with an average of 7.6 ± 1.5, the F2 population displayed an average of 11.4 ± 5.9 nodules (excluding the strong sunn phenotype of >50 nodules per plant) with a range of three to 28 nodules. The phenotypic ratio of 77:211 is consistent with a single gene (χ2 = 0.463, P > 0.475), whereas the observations that nodule number is significantly increased in a portion of F2 individuals and in F1 heterozygotes derived from the sunn mutant (data not shown), are suggestive of a semidominant mutation at the sunn locus.
To test if the sunn mutation was allelic to the previously described hyper-nodulation mutant sickle, we crossed the two mutants and examined phenotypes in F1 and F2 populations. F1 individuals possessed an intermediate number of nodules similar to the inferred sunn F2 heterozygotes described above, suggesting that these mutants were not allelic. This conclusion was supported by the occurrence of wild-type and both hyper-nodulation phenotypes in F2 populations, by extensive test cross analysis (see below), and by genetic mapping of the two mutants to different linkage groups (H.K. Choi and D.R. Cook, unpublished data).
Nodule Position Is Different between sunn and sickle
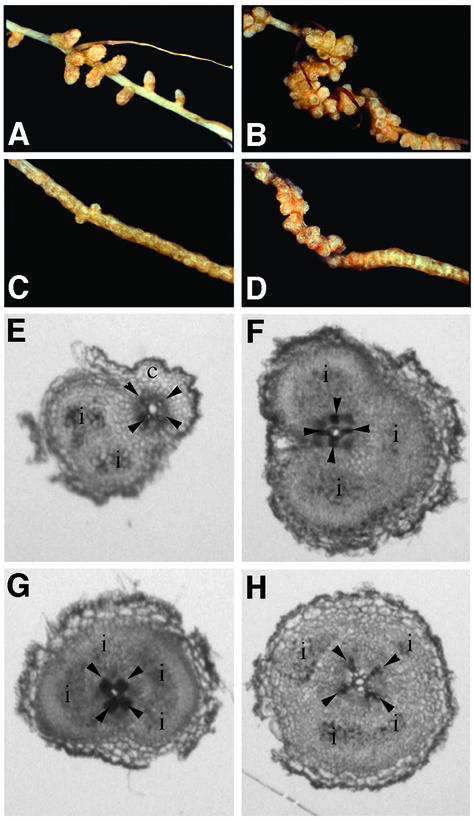
In pea, nodule organogenesis typically initiates in the root cortex across the protoxylem poles (Libbenga et al., 1973; Heidstra et al., 1997). Based on visual inspection, nodules in sunn appear to be arranged in parallel files along the length of the root (Fig. 1B). This phenotype contrasts with the sickle mutant, where the nodulated root zone expands radially and discrete nodule foci are not evident until several weeks after infection. To further characterize the spatial pattern of nodulation in these two mutants, we prepared transverse sections of entire nodulation zones from seedlings 4 to 6 d after rhizobial inoculation and examined the position of nodule development relative to subtending protoxylem poles and phloem tissue. Scoring of early nodule development was aided by using an S. meliloti ABS7 strain that expresses lacZ and, therefore, stains blue upon treatment with the X-Gal substrate. In both the wild-type and sunn genotypes, the majority of nodules (approximately 80%) developed within a 30° arc centered on the protoxylem poles (Table III). In contrast, this spatial restriction was absent in sickle, where nodules were developed with approximately equal frequencies throughout all portions of the root (Table III). In fact, in many cases, scoring nodule position in sickle was not possible because single infection events often ramified throughout the entire circumference of the root cortex. Thus, the two super-nodulating mutants exhibit distinct nodule zone anatomies, with nodule position in sunn, but not sickle, spatially restricted to sites across protoxylem poles.
Table III.
Frequency distribution of nodule development in relation to the root vascular tissues in wild type, sunn, sickle, and the sunn/skl double mutant
| Genotype | No. of Nodules Analyzed | Nodules across Pole of
|
% of Nodules across Xylem Pole | |
|---|---|---|---|---|
| Xylem | Phloem | |||
| A17 (wild type) | 32 | 26 | 6 | 81.25 |
| sunn | 56 | 43 | 13 | 76.79 |
| sickle | 58 | 31 | 27 | 56.34 |
| Double mutant | 80 | 45 | 35 | 56.25 |
Also see Figure 5.
Marker Gene Expression Distinguishes Super-Nodulators from Wild Type, and sunn from sickle
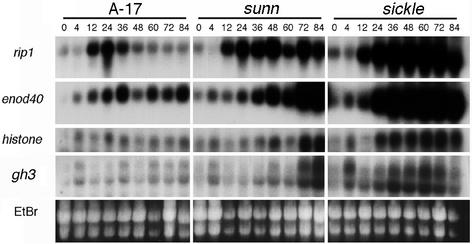
In an effort to determine whether the hyper-nodulating mutants (i.e. sunn and sickle) could be distinguished from one another and either mutant from wild type on the basis of their molecular phenotypes, we examined the expression of four genes in S. meliloti-inoculated roots of sunn, sickle, and wild type. Of the two nodulin genes we examined, expression of the S. meliloti-induced peroxidase homolog RIP1 is correlated with early responses in the root epidermis and at sites of infection (Cook et al., 1995; Peng et al., 1996), whereas the expression of ENOD40 is correlated with nodule organogenesis (Yang et al., 1993; Charon et al., 1997). As shown in Figure 2, there was close correspondence in the onset of RIP1 induction between wild type and both hyper-nodulation mutants, with increases in transcript first evident at 12 h. However, the transience of RIP1 expression, which is characteristic of wild-type plants (see Fig. 2; Cook et al., 1995), was absent from either mutant. In the case of ENOD40, increased transcript levels were first evident between 12 and 24 h for each of the genotypes, although in contrast to wild type, the ENOD40 transcript continued to increase in both mutants. The expression of these early nodulin genes was most enhanced in the sickle background, with transcript levels increasing earlier and generally to a higher level. The observation that sickle plants display a significant increase in the extent of the root inner cortex involved in symbiotic development (see above) compared with both wild type and sunn may explain the increased abundance of nodulation transcripts.
Figure 2.
Symbiotic gene expression in wild-type, sunn, and sickle genotypes. Gel blots were prepared from total RNA isolated from S. meliloti-inoculated roots at the indicated times (hours postinoculation). Blots were hybridized sequentially with radiolabeled rip1, enod40, histone H3, and gh3 probes. EtBr, Image of ethidium bromide-stained gel of the RNA samples before transfer to membrane and hybridization.
Nodule organogenesis involves the reactivation of the cell cycle in the root cortex, including both active cell division in inner cortical cells and S-phase activation without cell division in outer cortical cells. As a measure of the timing and extent of cell cycle activation, we analyzed the abundance of histone H3 transcript. In wild-type roots, the level of histone H3 transcript appeared to be slightly elevated after inoculation with S. meliloti, but in general, expression was low and varied little over the time course of the experiment (Fig. 2). We assume that the inability to detect significant increases in histone H3 expression in wild-type inoculated roots is a consequence of dilution by the much larger portion of the root not involved in symbiotic development. In contrast, both sunn and sickle exhibited increased levels of H3 transcript between 24 and 72 h after inoculation with S. meliloti, with significantly earlier induction evident in sickle compared with sunn.
Mathesius et al. (1998, 2000b) used the auxin-responsive GH3:GUS fusion from soybean (Gee et al., 1991; Hagen et al., 1991) to infer changes in auxin activity during nodulation of white clover by Rhizobium leguminosarum. In particular, their results suggest that GH3 expression is a useful marker for cortical cell activation during the formation of nodule primordia. To investigate whether the expression GH3 was also correlated with nodule development in M. truncatula, we examined the expression of a GH3 sequence homolog in M. truncatula in inoculated roots of wild-type, sunn, and sickle genotypes. The Institute for Genomic Research gene index for M. truncatula (http://www.tigr.org/tdb/mtgi) suggests that GH3 genes comprise a small gene family in M. truncatula; thus, RNA blots were hybridized and washed at moderate stringency to allow detection of multiple transcripts with similarity to GmGH3. As shown in Figure 2, this strategy revealed two distinct messages with differing electrophoretic mobilities. In wild-type roots, transcript levels were low and only slightly increased after inoculation with S. meliloti. In contrast, significant increases in GH3 transcripts were observed in both sunn and sickle, with a time course essentially identical to that observed with histone H3. These results are consistent with the results of Mathesius et al. (1998, 2000b), indicating a temporal relationship between induction of GH3 and cell cycle activation.
Responsiveness to Ethylene Differs between sunn and Wild Type
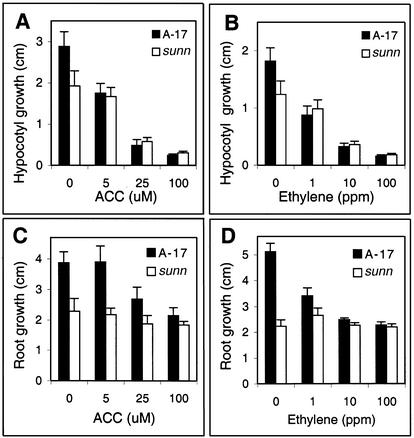
Previous analyses suggest that the plant hormone ethylene plays a central role in the regulation of the number of successful infections in M. truncatula (Penmetsa and Cook, 1997; Oldroyd et al., 2001). To determine whether the sunn mutant might be perturbed in ethylene responses, hypocotyl and root length of wild-type and sunn seedlings were measured in the presence of various concentrations of ACC or pure ethylene. ACC is a biosynthetic precursor that is readily converted to ethylene by endogenous ACC oxidase activity. In the presence of ACC or ethylene, sunn plants displayed most aspects of the “triple response” in a dose-dependent manner. For example, a reduction of hypocotyl length in both wild type and sunn was correlated with increasing doses of ACC and ethylene (Fig. 3, A and B, respectively). In contrast, sunn root growth was significantly less sensitive than wild-type roots to ACC or ethylene (Fig. 3, C and D). Other components of the ethylene response syndrome in sunn were similar to wild type, including increased root diameter and increased root hair density (data not shown), suggesting that with the exception of elongation, roots of sunn are not generally insensitive to ethylene.
Figure 3.
Sensitivity of wild-type and sunn seedlings to exogenous ACC and ethylene gas. Hypocotyl growth response of wild-type (solid bars) and sunn (white bars) seedlings to exogenous ACC (A) and to ethylene gas (B). Root growth response of wild-type (solid bars) and sunn (white bars) seedlings to exogenous ACC (C) and to ethylene gas (D).
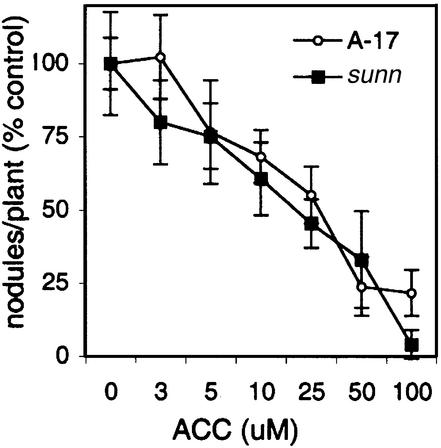
To determine whether the root growth insensitivity to the ethylene phenotype of sunn might have a direct role in the increased nodule number phenotype, we analyzed the nodulation response of sunn in the presence of increasing concentrations of ACC. As shown in Figure 4, ACC treatment reduced nodulation in a dose-dependent manner, with similar sensitivities in both sunn and wild type (Fig. 4). Thus, the nodulation response of sunn to exogenous ACC is indistinguishable from that in wild type. Taken together, these results suggest that the altered ethylene response of sunn roots is not causal to the hyper-nodulation phenotype, and serve to further distinguish sunn from the sickle mutant, where ethylene insensitivity is pleiotropic for many aspects of plant development, including the nodulation response (Penmetsa and Cook, 1997).
Figure 4.
Effect of ACC on nodulation in wild-type and sunn genotypes of M. truncatula. Seedlings were grown in growth pouches and ACC applied at 48 h after inoculation with S. meliloti, a time previously determined to provide maximal inhibition. Nodule numbers at different concentrations of ACC are presented as percentage of response relative to untreated plants. Nodule numbers of pouch grown seedlings in the absence of ACC were 7.6 ± 0.7 in wild type and 23.6 ± 4.2 in sunn.
Genetic Interaction between sunn and sickle
To test if the sunn and sickle genes function as part of a single genetic pathway or act in independent genetic pathways, the genetic interaction between these loci was examined by crossing sickle into sunn. The nodulation phenotypes of F1 individuals were similar to previously characterized sunn heterozygotes, suggesting that these mutants were not allelic. This inference was further verified by the segregation of wild-type nodulation in the F2 generation and the corresponding F3 progeny. Four phenotypic classes were scored in F2 populations: wild type (including putative sunn heterozygotes), sunn, sickle, and a novel super-nodulation phenotype corresponding to the presumptive double mutant (see below), in a ratio of 186:53:47:12. Putative sunn heterozygotes were pooled with the “wild-type” class, because the range of the number of nodules on presumptive heterozygotes significantly overlaps with bona fide wild-type individuals (see above), complicating precise phenotypic assignment. The observed ratio of F2 phenotypes most closely approximates 9:3:3:1 (χ2 = 5.9, P > 0.1), suggesting interaction between the two homozygous loci. Testing F2 phenotypic ratios for gene action models of either sunn epistatic to sickle or sickle epistatic to sunn (i.e. 9:3:4) led to a rejection of both hypotheses (P ≪ 0.01; data not shown).
Progeny testing and test cross analysis of selected individuals representing F2 phenotypes allowed us to subsequently confirm the inferred F2 genotypes. For example, of 23 independent F3 populations derived from F2s with the sunn nodulation phenotype, approximately two-thirds (16 of 23) of the F3 populations segregated for the sunn and the double-mutant phenotype. The remaining one-third (seven of 23) contained only sunn homozygous phenotypes. Chi-square analysis of the proportions of lines segregating for sunn and double mutant phenotypes (16 of 23 lines) or containing only sunn phenotypes (seven of 23 lines) was consistent with the expected 2:1 ratio (χ2 = 0.087, P > 0.750). Similarly, F3 populations derived from multiple F2 individuals with the novel super-nodulation phenotype bred true, yielding exclusively individuals with the double-mutant phenotype in the F3. To directly verify the genotype of the inferred double mutant, we performed test crosses to homozygotes of the parental sunn and sickle super-nodulation loci. To eliminate confounding effects produced by seed set from self-pollination in these crosses, we took advantage of a M. truncatula male-sterile, female-fertile floral homeotic mutant tap, which in itself does not affect nodulation (Penmetsa and Cook, 2000). The tap mutation was moved into both sunn and sickle backgrounds to obtain male-sterile, female-fertile individuals to serve as pollen recipients. All F1 progeny obtained using pollen from the double mutant in test crosses to either tap/sunn or tap/skl pollen recipients exhibited phenotypes of the corresponding single super-nodulation locus, thereby confirming the genotype of the double mutant. Taken together, these genetic data unambiguously establish that the novel super-nodulation phenotype corresponds to that of the sunn/skl double mutant.
As shown in Figure 5, the phenotype of the double mutant (Fig. 5D) was a novel super-nodulation phenotype with aspects of phenotypes observed in the single super-nodulation mutants (Fig. 5, B and C). In particular, nodules at the proximal end (toward the hypocotyl) of the nodulation zone were well developed and discrete, similar to nodules of sunn homozygotes at a comparable age, whereas nodule development at the distal end (toward the root tip) was characterized by radial expansion of the root without discrete nodule foci, reminiscent of nodule development observed in similarly aged sickle plants. In contrast to either of the single mutants or wild type, root growth was arrested subsequent to nodulation in the double mutant. We scored the position of rhizobial infection and inner cortical cell division in the double mutant and determined that, like sickle, nodule foci occurred throughout the circumference of the root (Fig. 5, G and H; Table II). Moreover, nitrogenase activity in nodules of the double mutants, as measured by the acetylene reduction assay, was similar to that observed in wild type and the single parental mutants (Table I).
Figure 5.
Distribution of rhizobial infections and nodules in wild type, sunn, sickle, and the sunn/skl double mutant. Typical macroscopic nodulation zones of wild type (A), sunn (B), sickle (C), and the sunn/skl double mutant (D), and transverse sections through the nodulation zone of wild type (E), sunn (F), sickle (G), and the sunn/skl double mutant (H) are shown. c, Root cortex; i, rhizobial infection. Arrowheads mark position of xylem poles.
DISCUSSION
Based on a visual screen for nodulation mutants in M. truncatula, we identified a novel hyper-nodulation mutant, designated sunn. Similar to the previously reported hyper-nodulation mutant sickle, sunn is characterized by an approximately 10-fold increase in nodule number. Despite this similarity, sunn and sickle exhibit distinct symbiotic, genetic, anatomical, molecular, and physiological properties that readily distinguish them from one another. In addition to the genetic separation of the mutant alleles based on complementation analysis and genetic mapping (data not shown), the sunn/skl double mutant exhibits a novel hyper-nodulation phenotype. The additive nature of the double mutant phenotype suggests that the sickle and sunn genes may act in separate genetic pathways, underscoring the likely complexity of host control of symbiotic establishment.
Using a set of marker genes as probes in northern-blot analysis, we profiled gene expression in S. meliloti-inoculated roots of sunn, sickle, and wild type. In the case of the early nodulin genes, RIP1 and ENOD40, transcript levels were significantly elevated in both mutants, with the highest transcript levels observed in sickle, although the timing of the onset of gene expression was not changed. Histone transcripts increase specifically during S phase of the eukaryotic cell cycle (e.g. Meskiene et al., 1995) and have been used previously as a specific marker of S phase during early nodulation (Yang et al., 1994). In the current study, we observed that histone H3 transcript was induced to high levels earlier in sickle than in sunn (see Fig. 2). This result suggests that S phase is activated precociously in sickle. S-phase markers have been correlated with infection thread formation during nodulation, where cells enter the cell cycle but arrest in G2 (Yang et al., 1994), and should also be expressed in association with actively dividing cells in the nodule primordium and nodule meristem. Both bacterial infection and inner cortical cell division occur in near coincidence throughout the circumference of the root in sickle; thus, it is likely that both contribute to the observed increase in histone H3 transcript. Increased infection and inner cortical cell division in sickle compared with sunn may also explain the generally higher levels of RIP1 and ENOD40 transcription in sickle. In contrast to sickle, histone H3 transcription was not substantially elevated in sunn until 48 to 72 h postinoculation. This timing is consistent with the appearance of abundant infection and the onset of inner cortical divisions in wild-type plants, suggesting that the timing of symbiotic development in sunn is largely similar to wild type. In the case of wild-type plants, the relatively small amount of root involved in symbiotic development (compared with the hyper-nodulating mutants) is likely to obscure our ability to observe increased histone H3 transcript, especially in RNA prepared from whole roots as was the case here.
Altered levels of ENOD40 have been correlated positively with changes in nodule number in M. truncatula; in particular, accelerated nodulation was observed in lines with elevated levels of ENOD40 and decreased nodulation was associated with lowered ENOD40 transcript abundance (Charon et al., 1997, 1999). Genetic mapping of sunn and sickle relative to ENOD40 demonstrates that ENOD40 is genetically distinct from either mutant locus (H.K. Choi and D.R. Cook, unpublished data). Moreover, although we cannot formally rule out the possibility that increased expression of ENOD40 is causal to the hyper-nodulation phenotypes of either mutant, the similar increases in ENOD40 and RIP1 expression suggests that both genes are downstream of the causal events.
In pea, nodule organogenesis typically initiates in the root cortex across the protoxylem poles (Libbenga et al., 1973). Two lines of evidence suggest that ethylene might provide a spatial cue to limit the occurrence of infection across phloem poles in the pea system (Heidstra et al., 1997). First, transcripts for ACC oxidase are highly localized to cells opposite phloem poles in pea roots, with the resulting production of the poorly diffusible ethylene gas presumed to establish a gradient of ethylene opposite phloem poles. Second, treatment of pea roots with either Ag+ or AVG, inhibitors of ethylene perception and synthesis, respectively, was shown to substantially increase the occurrence of nodulation across phloem poles. Based on these results in pea, we analyzed the position of nodule development in wild-type M. truncatula, and in the sickle and sunn mutants. We determined that although nodulation occurred preferentially opposite xylem poles in wild type, there was a complete loss of spatial control in the sickle mutant. The fact that sickle is a strong ethylene-insensitive mutant suggests that pea and M. truncatula possess similar mechanisms to regulate nodule position, and that both require ethylene perception. In contrast to sickle, we observed that nodulation in sunn was restricted primarily to locations opposite xylem poles. In fact, nodules in sunn were often evident as parallel files along the long axis of the root, and spatial constraints due to nodule expansion are presumed to cause the characteristic distortion-induced twist in the nodulation zone of sunn (see Fig. 1).
The conclusion that ethylene insensitivity is causal to the hyper-nodulation phenotype of sickle (Penmetsa and Cook, 1997) provided the rationale to examine ethylene responses in sunn. We determined that, unlike sickle, nodulation in sunn was efficiently suppressed by ACC, with a dose sensitivity indistinguishable from wild-type plants (Fig. 4). Most aspects of seedling responses to ethylene were normal in sunn, including stem responses such as exaggeration of the apical hook and reduced hypocotyl growth, and root responses such as radial enlargement and ectopic root hair production. These data suggest that hyper-nodulation in sunn is unlikely to be mediated by a defect in ethylene perception, which is consistent with the observation that spatial control of nodulation is not affected in sunn (as described above). However, sunn root growth was insensitive to concentrations of ACC or ethylene that were inhibitory to root growth in wild-type plants.
The Arabidopsis eir1/agr1 mutants, which affect the putative auxin efflux carrier PIN2, also display an ethylene-insensitive root phenotype (Luschnig et al., 1998; see also Chen et al., 1998). Several physiological studies have suggested that lowering the ratio of auxin to cytokinin in the root serves as a cue for the activation of nodule organogenesis (Hirsch and Fang, 1994). Experimental manipulations that lower this ratio either by increasing the level of cytokinin (Cooper and Long, 1994) or decreasing auxin concentrations from the application of auxin transport inhibitors (Hirsch et al., 1989) result in the development of nodule-like structures on legume roots. Whether sunn might exhibit defects in sensitivity to effectors of auxin action and transport, or be altered in auxin transport directly, is a topic under investigation. However, our determination that the sunn super-nodulation phenotype is influenced by the genotype of the shoot suggests that a mobile shoot-derived signal (potentially auxin?) is altered in the sunn genotype.
Indirect evidence that a reduction in root auxin transport precedes nodulation in white clover was provided by studies of an auxin-responsive GH3:GUS gene fusion (Hagen et al., 1984; Mathesius et al., 1998; Boot et al., 1999; Li et al., 1999). Inoculation with R. leguminosarum was correlated with an initial down-regulation of GH3:GUS expression in the root zone acropetal to the point of inoculation, with a subsequent up-regulation at the point of inoculation during formation of nodule primordia (Mathesius et al., 1998). Application of auxin transport inhibitors resulted in a similar down-regulation of the GH3:GUS reporter gene as obtained with R. leguminosarum, suggesting that the changes in gene expression might be mediated by R. leguminosarum-induced alteration of auxin transport. Flavonoids have been suggested to act as negative regulators of auxin transport in planta (Jacobs and Rubery, 1988; Brown et al., 2001; Peer et al., 2001), and rhizobial inoculation is associated with increased gene expression and flux through the phenyl-propanoid biosynthetic pathway (Recourt et al., 1992; Lawson et al., 1994, 1996), providing a potential mechanism to integrate perception of the rhizobial partner with nodule organogenesis. In the current study, we observed increased expression, in both sickle and sunn, of transcripts with homology to the soybean GH3 gene. Altered expression was correlated with changes in histone H3, with earlier induction in sickle than sunn, presumably reflecting cell cycle activation during the nodulation response. Mathesius et al. (1998) observed increased GH3:GUS expression in nascent nodule primordia in white clover, establishing a spatial and temporal correlation between GH3 expression and the initial activation of the cell cycle. Moreover, auxin and cytokinin are implicated (probably in concert) in regulating the G1 to S transition (den Boer and Murray, 2000). Thus, the finding that increases in GH3 transcripts are tightly correlated with increases in histone H3 transcripts is not surprising, although our current results are inadequate to distinguish whether GH3 induction preceded histone H3 accumulation. One might speculate that a rapid auxin response in sickle is causal to the apparently precocious entry into S phase (indicated by histone H3 expression).
The recent positional cloning of genes underlying the super-nodulating Nts mutant of soybean (Searle et al., 2002), and its candidate orthologous mutants Har1 of L. japonicus and Sym29 of pea (Krusell et al., 2002; Nishimura et al., 2002), suggest commonalities between the regulation of apical meristem proliferation and the regulation of nodule number in legumes. In particular, the Nts, Har1, and Sym29 genes encode receptor kinases most similar to the Clavata1 receptor kinase of Arabidopsis, providing additional evidence of an overlap between symbiotic functions and the control of plant growth and development (Szczyglowski and Amyot, 2003). If sunn is orthologous to the Nts family, then the phenotypic differences between sunn and Har1 may reflect differences in the species context or correspond to allele-specific effects; alternatively, sunn could encode an unrelated protein.
In this study, we describe a new M. truncatula super-nodulation mutant, designated sunn. Although root growth in sunn is insensitive to exogenous ethylene or ACC, nodulation in sunn remains sensitive to these treatments, suggesting that altered ethylene perception is not causal to the sunn phenotype. Together with the ethylene-insensitive mutant sickle, this study suggests that the control of nodule number in M. truncatula occurs by two genetic pathways, with the sickle pathway mediated by perception of the pant hormone ethylene (Penmetsa and Cook, 1997). Whether sunn might have altered auxin phenotypes similar to certain ethylene-insensitive root mutants in Arabidopsis (Luschnig et al., 1998) is a topic under active investigation in our laboratories. The discovery that these mutants display both symbiotic and nonsymbiotic phenotypes provides a valuable opportunity to dissect the overlap between symbiotic development and hormonal mechanisms regulating general plant growth and development.
MATERIALS AND METHODS
Mutant Screen
M2 generation plants derived from two 0.15% (v/v) ethyl methanesulfonate-treated seed bulks of Medicago truncatula genotype A17 (bulks C and B; Penmetsa and Cook, 2000) were screened for nodulation mutants. Procedures for plant growth and germination were as previously reported (Cook et al., 1995; Penmetsa and Cook, 2000). In brief, seedlings were grown in aeroponic chambers for 5 d before inoculation with 5 mL of washed inoculum from an overnight culture of compatible Sinorhizobium meliloti strain ABS7M or ABS7M containing pXLGD4 (Leong et al., 1985). Seven to 10 d subsequent to inoculation, at which time nitrogen-fixing nodules could be observed on wild-type control plants, plantlets were visually assessed for gross changes in nodule number or morphology. Individuals scored as having altered nodulation properties were further examined with a dissecting microscope (model SZH10, Olympus, Tokyo) to provide a more precise description of the corresponding phenotype. Heritability of M2 phenotypes was initially determined by examining nodulation phenotypes in multiple M3 siblings for each putative mutant. Lines containing heritable phenotypes were used as pollen donors in crosses to a male-sterile mutant of M. truncatula genotype A17, previously described as a tool for efficient genetic analysis (Penmetsa and Cook, 2000).
Histochemical Staining for Analysis of S. meliloti Infections
Histochemical staining for lacZ activity was performed essentially as reported by Boivin et al. (1990). In brief, whole roots were vacuum infiltrated with 2.5% (v/v) glutaraldehyde in 0.1 m PIPES (pH 7.2) buffer and incubated for 1 h at room temperature before rinsing twice (15 min each rinse) in 0.1 m PIPES (pH 7.2). To visualize bacterial strains expressing lacZ, samples were incubated in staining buffer (50 mm potassium ferricyanide, 50 mm potassium ferrocyanide, and 0.08% [w/v] X-Gal in 0.1 m PIPES [pH 7.2] buffer) for 16 h in the dark at room temperature, rinsed twice in PIPES buffer, and stored at 4°C until further analysis. For microscopy, specimens were rinsed in deionized water, mounted on slides with coverslips, and viewed under an Olympus SZH10 dissection microscope and/or an Axioskop compound microscope (Zeiss, Jena, Germany). Transverse sections of the nodulation zone were obtained by embedding 0.5- to 1.0-cm root segments in 3.5% (w/v) washed agar dissolved in 0.1 m PIPES (pH 7.2). Embedded agar blocks were trimmed by hand and sections of 75 to 100 μm were obtained with a microslicer (Ted Pella, Inc., Redding, CA).
Light Micrographs of Nodules
Nodulation zones were dissected from aeroponically grown seedlings and dehydrated by immersion for 10 min each in a 0% to 100% (v/v) water:ethanol gradient, with a 10% increase in ethanol concentration per step followed by two cycles in 100% ethanol. Ethanol was replaced with LR White acrylic resin by immersion for 1 h each in 2:1, 1:1, and 1:2 (v/v) mixtures of ethanol:resin, followed by immersion in pure resin. Resin was replaced several times to obtain a total infiltration time in pure resin of more than 24 h, before polymerization at 55°C for 24 h. Transverse sections of nodules were obtained by semithin (1 μm) sectioning using an ultracut microtome (Leica Microsystems, Wetzlar, Germany). Semithin sections were stained with basic fuchsin and observed by bright-field optics.
Acetylene Reduction Assay
Nitrogenase activity was assayed on entire nodulation zones excised from seedlings at 16 d postinoculation. Excised tissue was blotted on filter paper and the nodulation zones from three seedlings were sealed in a 1.8-mL serum-stoppered glass vial. One hundred microliters of air was replaced with 100 μL of pure acetylene and after 60 to 120 min of incubation, the conversion of acetylene to ethylene was measured by gas chromatography using a Photovac 10S (Photovac Inc., Markham, ON, Canada) equipped with a flame-ionizing detector. Ethylene was quantified by comparison to a 5-ppm ethylene standard, and the values were normalized for injection volumes and incubation times. Ethylene production in the absence of acetylene was typically at or below the detection limit of this assay.
Northern-Blot Analysis
Northern-blot analysis was performed on RNA extracted from whole roots essentially as described by Cook et al. (1995), with the exception that total RNA was extracted using the RNeasy Plant kits (Qiagen USA, Valencia, CA) according to the manufacturer's instructions. General nucleic acid manipulations, including gel electrophoresis and transfer of RNA to nylon membranes, were performed as described by Sambrook and Russell (2001). DNA probes were obtained either as cloned plasmid DNAs, or by PCR amplification from genomic templates using gene-specific primers designed from characterized genes. The accession numbers for the respective sequences are as follows: RIP1 (U1627), ENOD40 (X80262). Radioactive probes were prepared with α-32P-dCTP using the procedure of Feinberg and Vogelstein (1984). Hybridization was conducted at 60°C in a solution of 7% (w/v) SDS, 0.25 m NaH2PO4 (pH 7.0), and 0.1 mm EDTA. After hybridization, filters were washed successively with solutions of 0.1% (w/v) SDS and 2× SSC (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate; 21°C for 15 min), 0.1% (w/v) SDS and 0.1× SSC (21°C for 15 min), and 0.1% (w/v) SDS and 0.1× SSC (65°C for 1 h).
Phytohormone Assays of Seedlings
Assays for ethylene sensitivity were performed in magenta boxes containing 50 mL of 1.5% (w/v) washed agar in water medium, poured at a slant, and supplemented with specified concentrations of phytohormones. Seedlings were germinated overnight at room temperature in the dark, transferred to agar-containing magenta boxes, and maintained at room temperature in the dark for 3 d before growth measurements. For assays employing ethylene gas, seedlings were grown on phytohormone-free washed agar medium. Magenta boxes were placed in a 3-L airtight plastic jar with a lid containing a serum septum, through which specified amounts of pure ethylene were injected.
The effect of exogenous ACC on nodulation was assessed using seedlings grown in growth pouches (Vaughn's Seed Company, Downers Grove, IL) containing 10 mL of nutrient solution devoid of nitrogen (Cook et al., 1995). Each pouch contained five to six pregerminated 1-d-old seedlings with roots approximately 1 cm in length. Seedlings were grown at 21°C in the dark for 36 to 48 h before being moved to light, with the root zone shielded from light by means of an aluminum foil covering. Pouches were weighed at 1-d intervals and sterile water was used to bring the weight of each pouch to the initial medium-filled pouch weight. One to 2 days after transfer to light, pouches were inoculated with 10 μL of washed inoculum from a late log phase culture of S. meliloti. ACC solutions in sterile water were added at 48 h after inoculation.
Root Graft Experiments
Root grafts were prepared essentially according to the protocol described by Journet et al. (2001). Details of the refined EMBO protocol can be obtained from http://www.isv.cnrs-gif.fr/embo01/manuels/index.html. In the present study, seedlings were germinated overnight in the dark at room temperature and transferred directly to 24-× 24-cm2 sterile plastic trays containing buffered Nod medium (Erhardt et al., 1992) supplemented with 1 μm AVG medium in 1.5% (w/v) agar. Plantlets were grown for an additional 2 to 3 d before preparation of grafts. Seedlings with similar hypocotyl diameters were selected and cut transversely within the lower, chlorophyll-containing hypocotyl zone using a fresh razor blade. Roots were exchanged between plants, and the graft junction was sealed by application of 1.5% (w/v) purified water agar (Sigma, St. Louis). The agar trays were sealed with Parafilm and transferred in a near-vertical position to a growth chamber maintained at 24°C with a 16-h photoperiod (100 μE m−2 s−1). Grafted plants were allowed to recover for at least 1 week before inoculation with S. meliloti strain 1021.
Footnotes
This work was supported by the Samuel Roberts Noble Foundation (Ardmore, OK; grant), by the Human Frontiers Science Program (grant no. RG–0327), by the U.S. National Science Foundation (grant no. IBN 9507535 to D.R.C.), and by the College of Agriculture and Life Sciences, Texas A&M University (Tom Slick Graduate Fellowship to R.V.P.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.015677.
LITERATURE CITED
- Allen EK, Allen ON, Newman AS. Pseudonodulation of leguminous plants induced by 1-bromo-3,5-dichlorobenzoic acid. Am J Bot. 1953;40:429–435. [Google Scholar]
- Bauer P, Ratet P, Crespi MD, Schultze M, Kondorosi A. Nod-factors and cytokinins induce similar cortical cell divisions, amyloplast deposition and MsENOD12A expression patterns in alfalfa roots. Plant J. 1996;10:91–105. [Google Scholar]
- Boivin C, Camut S, Malpica CA, Truchet G, Rosenberg C. Rhizobium meliloti genes encoding catabolism of trigonelline are induced under symbiotic conditions. Plant Cell. 1990;2:1157–1170. doi: 10.1105/tpc.2.12.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW. Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Mol Plant-Microbe Interact. 1999;12:839–844. [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly M, Tague BW, Peer WA, Taiz L, Muday GK. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean (Glycine max (L.) Merr.) mutants that nodulate in the presence of high nitrate concentrations. Proc Natl Acad Sci USA. 1985;82:4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R, Antonius C, Timmers J, Maillet F, Galera C, Penmetsa RV, Cook D, Dénarié J, Gough C. The hcl gene of Medicago truncatula controls Rhizobium induced root hair curling. Development. 2001;128:1507–1518. doi: 10.1242/dev.128.9.1507. [DOI] [PubMed] [Google Scholar]
- Catoira R, Galera C, de Billy F, Penmetsa V, Journet EP, Maillet F, Rosenberg C, Cook D, Gough C, Dénarié J. Four genes of Medicago truncatula controlling components of a Nod factor transduction pathway. Plant Cell. 2000;12:1647–1665. doi: 10.1105/tpc.12.9.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon C, Johansson C, Kondorosi E, Kondorosi A, Crespi M. ENOD40 induces dedifferentiation and division of root cortical cells in legumes. Proc Natl Acad Sci USA. 1997;94:8901–8906. doi: 10.1073/pnas.94.16.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon C, Sousa C, Crespi M, Kondorosi A. Alteration of ENOD40 expression modifies Medicago truncatula root nodule development induced by Sinorhizobium meliloti. Plant Cell. 1999;11:1953–1966. doi: 10.1105/tpc.11.10.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson P. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Dreyer D, Bonnet D, Howell M, Nony E, VandenBosch K. Transient induction of a peroxidase gene in Medicago truncatula precedes infection by Rhizobium meliloti. Plant Cell. 1995;7:43–55. doi: 10.1105/tpc.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JB, Long SR. Morphogenetic rescue of Rhizobium meliloti nodulation mutants by trans-zeatin secretion. Plant Cell. 1994;6:215–225. doi: 10.1105/tpc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Kahn ML, Leustek T, Long SR. Nitrogen and sulfur. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Association of Plant Biologists; 2000. pp. 787–849. [Google Scholar]
- Dehio C, deBruijn FJ. The early nodulin gene SrEnod2 from Sesbania rostrata is induced by cytokinin. Plant J. 1992;2:117–128. doi: 10.1046/j.1365-313x.1992.t01-51-00999.x. [DOI] [PubMed] [Google Scholar]
- den Boer BGW, Murray JAH. Triggering the cell cycle in plants. Trends Cell Biol. 2000;10:245–250. doi: 10.1016/s0962-8924(00)01765-7. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Endre G, Kereszt A, Kevei Z, Mihacea S, Kaló P, Kiss GB. A receptor kinase gene regulating symbiotic nodule development. Nature. 2002;417:962–966. doi: 10.1038/nature00842. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1984;137:226–237. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gee MA, Hagen G, Guilfoyle TJ. Tissue-specific and organ-specific expression of soybean auxin responsive transcripts GH3 and SAURs. Plant Cell. 1991;3:419–430. doi: 10.1105/tpc.3.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Kleinschmidt A, Guilfoyle T. Auxin regulated gene expression in intact soybean hypocotyls and excised hypocotyl sections. Planta. 1984;162:147–153. doi: 10.1007/BF00410211. [DOI] [PubMed] [Google Scholar]
- Hagen G, Martin G, Li Y, Guilfoyle TJ. Auxin-induced expression of the soybean GH3 promoter in transgenic tobacco plants. Plant Mol Biol. 1991;17:567–579. doi: 10.1007/BF00040658. [DOI] [PubMed] [Google Scholar]
- Heidstra R, Yang WC, Yalcin Y, Peck S, Emons A, Van Kammen A, Bisseling T. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interactions. Development. 1997;124:1781–1787. doi: 10.1242/dev.124.9.1781. [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bhuvaneswari TV, Torrey JG, Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci USA. 1989;86:1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM, Fang Y. Plant hormones and nodulation: What's the connection? Plant Mol Biol. 1994;26:5–9. doi: 10.1007/BF00039514. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Journet E, Barker D, Harrison M, Kondorosi E (2001) Module 1: M. truncatula as biological material. EMBO practical course on the new plant Model System Medicago truncatula (http://www.isv.cnrs-gif.fr/embo01/manuels/index.html)
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- Lawson CGR, Djordjevic MA, Weinman JJ, Rolfe BG. Rhizobium inoculation and physical wounding result in the rapid induction of the same chalcone synthase copy in Trifolium subterraneum. Mol Plant-Microbe Interact. 1994;7:498–507. doi: 10.1094/mpmi-7-0498. [DOI] [PubMed] [Google Scholar]
- Lawson CGR, Rolfe BG, Djordjevic MA. Rhizobium inoculation induces condition dependent changes in the flavonoid composition of root exudates from Trifolium subterraneum. Aust J Plant Physiol. 1996;23:93–101. [Google Scholar]
- Leong SA, Williams PH, Ditta GS. Analysis of the 5′ regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res. 1985;13:5965–5976. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wu YH, Hagen G, Guilfoyle T. Expression of the auxin-inducible GH3 promoter/GUS fusion gene as a useful molecular marker for auxin physiology. Plant Cell Physiol. 1999;40:675–682. [Google Scholar]
- Libbenga KR, Van Iren F, Bogers RJ, Schraag-Lammers MF. The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativa L. Planta. 1973;114:29–39. doi: 10.1007/BF00390282. [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required fro gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HR, Spaink H, Sautter C, Rolfe B, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavanoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- Mathesius U, Weinman JJ, Rolfe BJ, Djordjevic MA. Rhizobia can induce nodules in white clover by “hijacking” mature cortical cells activated during lateral root development. Mol Plant-Microbe Interact. 2000b;13:170–182. doi: 10.1094/MPMI.2000.13.2.170. [DOI] [PubMed] [Google Scholar]
- Mathews A, Carroll BJ, Gresshoff PM. The genetic interaction between non-nodulation and supernodulation in soybean: an example of developmental epistasis. Theor Appl Genet. 1990;79:125–130. doi: 10.1007/BF00223798. [DOI] [PubMed] [Google Scholar]
- Meskiene I, Bogre L, Dahl M, Pirck M, Ha DT, Swoboda I, Heberle-Bors E, Ammerer G, Hirt H. cycMs3, a novel B-type alfalfa cyclin gene, is induced in the G0-to-G1 transition of the cell cycle. Plant Cell. 1995;7:759–771. doi: 10.1105/tpc.7.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu G, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Engstrom EM, Long SR. Ethylene Inhibits the nod factor signal transduction pathway of Medicago truncatula. Plant Cell. 2001;13:1835–1849. doi: 10.1105/TPC.010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd GED, Mitra RM, Wais RJ, Long SR. Evidence for structurally specific negative feedback in the Nod factor signal transduction pathway. Plant J. 2002;28:191–199. doi: 10.1046/j.1365-313x.2001.01149.x. [DOI] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol. 2001;126:536–548. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-M, Dreyer DA, VandenBosch KA, Cook D. Gene structure and differential regulation of the Rhizobium-induced peroxidase gene RIP1. Plant Physiol. 1996;11:1437–1446. doi: 10.1104/pp.112.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. Isolation and characterization of diverse developmental mutants in Medicago truncatula. Plant Physiol. 2000;123:1387–1397. doi: 10.1104/pp.123.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K, van Tunen AJ, van Brussel AA, Lugtenberg BJ, Kijne JW. Activation of flavonoid biosynthesis in roots of Vicia sativa subsp. nigra plants by inoculation with Rhizobium leguminosarum biovar viciae. Plant Mol Biol. 1992;19:411–420. doi: 10.1007/BF00023389. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Ed 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2002;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, Parniske M. A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature. 2002;417:959–962. doi: 10.1038/nature00841. [DOI] [PubMed] [Google Scholar]
- Szczyglowski K, Amyot L. Symbiosis, inventiveness by recruitment? Plant Physiol. 2003;131:935–940. doi: 10.1104/pp.017186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV. On the physiology of the formation of nodules on legume roots. Proc Natl Acad Sci USA. 1936;22:511–514. doi: 10.1073/pnas.22.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weil C, Norris JH, Bochenek B, Dickstein R, Bisseling T, Hirsch AM. Nodulin gene expression and ENOD2 localization in effective, fixing and ineffective, bacteria free nodules of alfalfa. Plant Cell. 1990;2:1009–1017. doi: 10.1105/tpc.2.10.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais RJ, Galera C, Oldroyd G, Catoira R, Penmetsa RV, Cook D, Gough C, Long SR. Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc Natl Acad Sci USA. 2000;97:13407–13412. doi: 10.1073/pnas.230439797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wopereis J, Pajuelo E, Dazzo FB, Jiang D, Gresshoff PM, de Bruijn FJ, Stougaard J, Szczyglowski K. Short root mutant of Lotus japonicus with a dramatically altered symbiotic phenotype. Plant J. 2000;23:97–114. doi: 10.1046/j.1365-313x.2000.00799.x. [DOI] [PubMed] [Google Scholar]
- Yang WC, de Blank C, Meskiene I, Hirt M, Bakker J, van Kammen A, Franssen H, Bisseling T. Rhizobium Nod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell. 1994;6:1415–1426. doi: 10.1105/tpc.6.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, Katinakis P, Hendriks P, Smolders A, de Vries F, Spee J, van Kammen A, Bisseling T, Franssen H. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 1993;3:573–585. doi: 10.1046/j.1365-313x.1993.03040573.x. [DOI] [PubMed] [Google Scholar]