Abstract
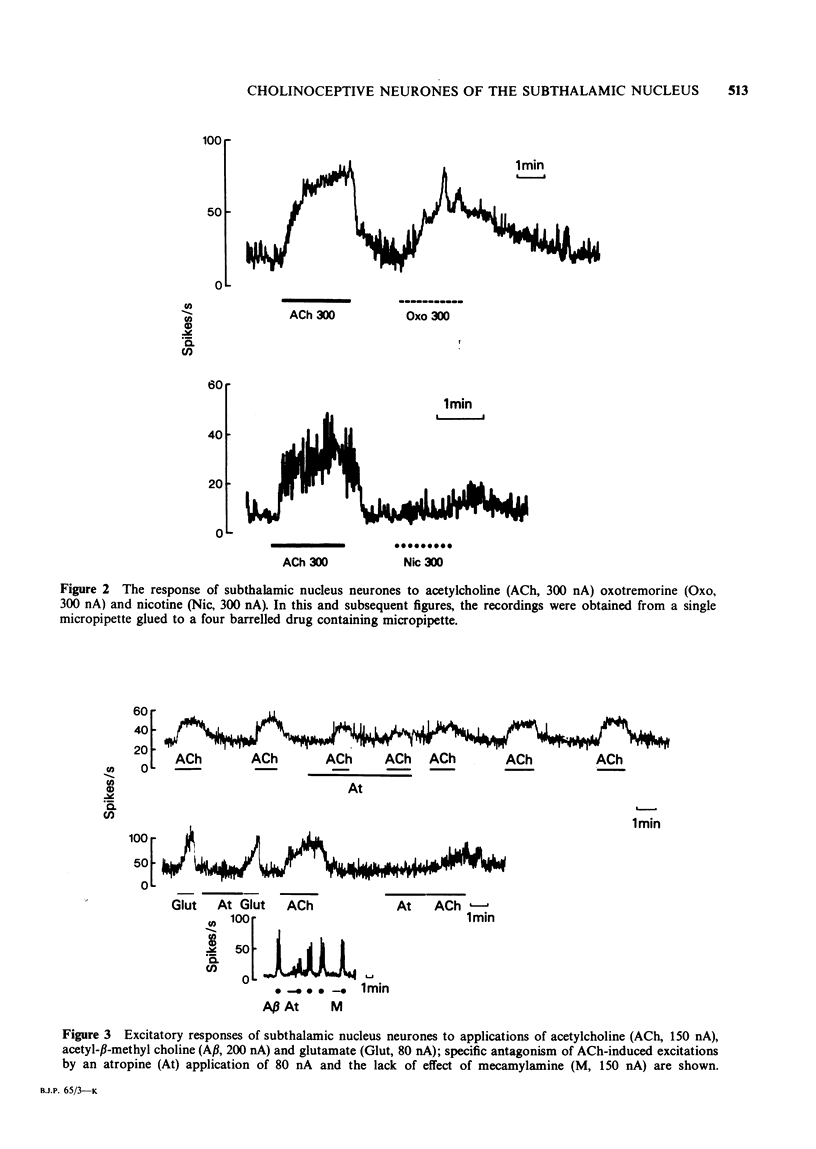
1. In 15 rats anaesthetized with ketamine, microiontophoretically applied acetylcholine (ACh) excited all 58 cells studied in the subthalamic nucleus (STN). 2. The ACh-evoked excitation was slow in onset and outlasted the ACh application. There was no sign of desensitization when the ACh application was prolonged or repeated. The excitation was prolonged by a concomitant application of physostigmine. 3. Acetyl-beta-methyl choline and oxotremorine were effective cholinomimetics. Nicotine had no effect. 4. The ACh excitation was antagonized by stropine and scopolamine but not by mecamylamine. 5. It was condluded that STN ACh receptors are muscarinic in character. 6. Since large microiontophoretic applications of Mg2+ did not suppress ACh-evoked excitation, it is suggested that ACh acts postsynaptically. 7. The excitatory response of STN cells to striatal or pallidal stimulation was unaffected by atropine administered either microiontophoretically to single cells or intravenously (3 mg/kg) to the whole animal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., CURTIS D. R. THE PHARMACOLOGY OF THE SYNAPTIC AND ACETYLCHOLINE-INDUCED EXCITATION OF VENTROBASAL THALAMIC NEURONES. Acta Physiol Scand. 1964 May-Jun;61:100–120. doi: 10.1111/j.1748-1716.1964.tb02946.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W. The action of procaine and atropine on spinal neurones. J Physiol. 1960 Aug;153:17–34. doi: 10.1113/jphysiol.1960.sp006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. B., Fraser R. A., Shriver J. E. The organization of pallidosubthalamic fibers in the monkey. Brain Res. 1968 Dec;11(3):522–559. doi: 10.1016/0006-8993(68)90145-5. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Strominger N. L. Efferent fibers of the subthalamic nucleus in the monkey. A comparison of the efferent projections of the subthalamic nucleus, substantia nigra and globus pallidus. Am J Anat. 1967 Jul;121(1):41–72. doi: 10.1002/aja.1001210105. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D. M., Palkovits M. Topographic atlas of catecholamine and acetylcholinesterase-containing neurons in the rat brain. I. Forebrain (telencephalon, diencephalon). J Comp Neurol. 1974 Sep 1;157(1):13–28. doi: 10.1002/cne.901570103. [DOI] [PubMed] [Google Scholar]
- Kobayashi R. M., Brownstein M., Saavedra J. M., Palkovits Choline acetyltransferase content in discrete regions of the rat brain stem. J Neurochem. 1975 Apr;24(4):637–640. [PubMed] [Google Scholar]
- Krnjević K., Phillis J. W. Pharmacological properties of acetylcholine-sensitive cells in the cerebral cortex. J Physiol. 1963 May;166(2):328–350. doi: 10.1113/jphysiol.1963.sp007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Hicks T. P. Pharmacological characterization of the excitatory cholinergic receptors of rat central neurones. Neuropharmacology. 1978 Jun;17(6):329–334. doi: 10.1016/0028-3908(78)90002-3. [DOI] [PubMed] [Google Scholar]
- Nauta H. J., Cole M. Efferent projections of the subthalamic nucleus: an autoradiographic study in monkey and cat. J Comp Neurol. 1978 Jul 1;180(1):1–16. doi: 10.1002/cne.901800102. [DOI] [PubMed] [Google Scholar]
- Ohye C., Le Gayader C., Feger J. Responses of subthalamic and pallidal neurons to striatal stimulation: an extracellular study on awake monkeys. Brain Res. 1976 Jul 30;111(2):241–252. doi: 10.1016/0006-8993(76)90769-1. [DOI] [PubMed] [Google Scholar]
- Olivier A., Parent A., Simard H., Poirier L. J. Cholinesterasic striatopallidal and striatonigral efferents in the cat and the monkey. Brain Res. 1970 Mar 3;18(2):273–282. doi: 10.1016/0006-8993(70)90328-8. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]


