Abstract
Background
Young adults who use stimulants (e.g., cocaine, amphetamines) are at particular risk of transitioning to dependence. Previously, we demonstrated increased risk-taking in young adults who had used stimulants (Leland and Paulus, 2005). Since outcome uncertainty is a critical element of risk, we investigated whether such individuals have different neural responses to uncertainty than their stimulant-naïve peers.
Method
Eleven young adults (age 18–25) who had used stimulants were compared with 11 age- and education-matched stimulant-naïve controls using functional magnetic resonance imaging and a card prediction task with relatively certain/uncertain outcome conditions.
Results
The caudate, an area involved in processing salient events, was among those regions more active in users than controls in response to uncertainty. Personality measures revealed that users were more impulsive than controls, and that neural response to uncertainty in a number of areas including the thalamus/caudate was positively correlated with impulsivity.
Conclusions
These results are consistent with the idea that young adults who have used stimulant find uncertainty particularly salient, due in part to preexisting differences in impulsivity, and may be subject to more “action pressure” when making decisions under uncertainty. This neural and personality profile may constitute a marker for increased risk of stimulant use.
Keywords: fMRI, stimulants, uncertainty, striatum, impulsivity, salience
Introduction
Drug abuse is one of the most pressing public health problems in the U.S. The use of stimulants among young adults is of particular concern, given the high risk of dependence. The risk of transitioning from cocaine use to dependence peaks between ages 23 and 25, with most cases occurring within 3 years of initial use (Wagner and Anthony, 2002). In 2003, 18–25 year-olds were the age group with the highest lifetime prevalence of nonmedical use of illicit or prescription amphetamine-type stimulants (10.8 %), and shared with 12–17 year-olds the highest prevalence of past-year dependence on or abuse of these substances (0.4 %) (Substance Abuse and Mental Health Services Administration, 2004). Previous research has shown that young adults who have used stimulants exhibit increased risk-taking decision-making in the laboratory, relative to their stimulant-naïve peers, and that such risk-taking is related to impulsivity and sensation-seeking traits (Leland and Paulus, 2005). However, it is unclear what neural factors contribute to this behavioral difference.
Risk comprises the notions of hazard, probability, consequence, and potential adversity or threat (Slovic, 1987). In risk-taking situations, options vary along a number of dimensions, including the probability and magnitude of positive and negative outcomes (Leigh, 1999). Uncertainty about outcomes is key in risk-taking because, without an element of chance, a decision does not entail risk but, instead, is an expression of preference among guaranteed options. Therefore, clarifying the role of uncertainty in decision-making situations has important implications for understanding risky behavior.
There is evidence to suggest that overlapping brain systems subserve uncertainty processing and reinforcement. Uncertainty about rewards increases sustained dopamine neuron activity in monkeys and may itself be reinforcing (Fiorillo et al., 2003). Dopamine neurons also respond to salient nonreward events (e.g. unexpected or intense stimuli). Salient stimuli are those that, by virtue of their intensity or importance, increase attention, arousal, and mobilization of behavioral resources, thus contributing to action (Horvitz, 2000; Redgrave et al., 1999). Recent neuroimaging work has demonstrated in humans that the striatum, a key reinforcement-related dopaminergic target structure (Robbins and Everitt, 1996), is also responsive to reward and nonreward salience (Zink et al., 2003; Zink et al., 2004). Aron et al. (2004), meanwhile, have found that individuals’ response uncertainty in a nonreward category learning task predicts midbrain activation, which, in turn, predicts ventral striatal and medial frontal response to negative feedback. This led us to consider whether young adult stimulant users find uncertainty more salient than stimulant-naïve controls when making decisions, as reflected by an increased response in the striatum, even when the uncertainty is not about rewards per se. If so, this would suggest that the uncertainty inherent in risky decision-making situations may create pressure to act in these individuals, contributing to their stimulant use and risk for stimulant dependence. To investigate this, we used functional magnetic resonance imaging (fMRI) to compare users’ and controls’ brain activation as they performed a card task requiring prediction of outcomes under conditions of relative certainty and uncertainty.
Materials and Methods
Subjects
Eleven young adults (age 21.0 +/− 2.2) who had used stimulants (users) were compared with 11 age- and education-matched stimulant-naïve controls (controls) (see Table 1 for group demographic details). All participants provided written informed consent, were right-handed, and were free of medical and psychiatric disorders as determined by a physical exam and structured clinical interview for DSM-IV (American Psychiatric Association, 1994) diagnoses. Users reported having used cocaine, amphetamines, and/or other stimulant drugs when not prescribed 21 ± 19 times (range 2 – 50) but were neither treatment-seeking nor stimulant-dependent. Subjects were instructed to abstain from illicit substance use for 48 hours prior to the experimental session.
Table 1.
Sociodemographics, personality, and drug use compared between stimulant-using and stimulant-naive subjects.
| Stimulant Users | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | p | |
| Age (yrs) | 21.00 | 2.24 | 18–25 | 22.18 | 3.84 | 18–28 | 0.90 | .39 |
| Education (yrs) | 14.64 | 1.21 | 13–16 | 14.64 | 1.80 | 13–18 | 0.00 | 1.00 |
| BIS total | 68.10 | 6.19 | 60–81 | 60.13 | 5.69 | 49–67 | 2.81 | .01 |
| SSS total | 22.94 | 5.03 | 15–30 | 20.93 | 6.57 | 9–32 | 0.74 | .47 |
| N | % | N | % | |||||
| Gender | ||||||||
| Female | 10 | 91% | 9 | 82% | ||||
| Male | 1 | 9% | 2 | 18% | ||||
| Ethnicity | ||||||||
| Caucasian | 7 | 64% | 5 | 45% | ||||
| Hispanic | 2 | 18% | 3 | 27% | ||||
| Filipino | 1 | 9% | 2 | 18% | ||||
| Asian | 0 | 0% | 1 | 9% | ||||
| Mixed | 1 | 9% | 0 | 0% | ||||
| Ever Used | ||||||||
| Stimulants | 11 | 100% | 0 | 0% | ||||
| Tobacco | 5 | 45% | 0 | 0% | ||||
| Marijuana | 11 | 100% | 5 | 45% | ||||
| Ecstasy | 3 | 27% | 0 | 0% | ||||
| Opiates | 2 | 18% | 0 | 0% | ||||
| Sedatives | 1 | 9% | 0 | 0% | ||||
| Hallucinogens | 1 | 9% | 0 | 0% | ||||
Drug use and personality measures
All subjects completed a standard drug and alcohol questionnaire (Brown et al., 1998), indicating whether they had ever used each of the following nonmedically: stimulants, tobacco, marijuana, ecstasy, opiates, sedatives, hallucinogens. Ten of the 11 users and 8 of the 11 controls also completed the Sensation Seeking Scale (SSS) (Zuckerman et al., 1978) and the Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995).
Behavioral task
The card paradigm is a modified version of that used by Critchley and colleagues (2001). Subjects viewed playing cards numbered 2–10 in a series of trials, each consisting of an initial “cue card” and a subsequent “feedback card.” After seeing each cue card, individuals predicted whether the feedback card would be lower or higher in value. Subjects were told that cards were drawn from a virtual deck that was regular and randomly shuffled except that 1) there were no “face cards” or aces, and 2) there would be no “ties” between the pairs of cards on a trial (i.e. the feedback card was always either lower or higher than the cue card). The goal was to make as many correct predictions as possible. On each trial, the cue card was presented first alone for 2000 ms, after which the words “Lower” and “Higher” were added below, prompting the subject to respond with a lower/higher prediction by pressing the corresponding button. After the response, all stimuli were removed for 3500 ms, followed by the feedback card as well as a sound and message (“You Win” and a chime for a correct prediction, “You Lose” and a buzz for an incorrect prediction). To minimize the confounding effects of overt reward, correct predictions did not earn subjects’ real or virtual dollars or points. The feedback card remained onscreen for 1500–3500 ms (depending on the subject’s response latency). After each trial, a baseline fixation cross was presented for a variable length of time (randomized between 4000 and 6000 ms). Each trial ranged from 13–15 seconds in length. The frequency of feedback cards being lower or higher than the preceding cue cards approximated the true probabilities for a randomly shuffled deck of cards. “Uncertain” decision trials were operationally defined as those on which the cue card was numbered 5, 6, or 7, because in those cases it was less predictable (50% – 62.5%) whether the feedback card would be lower or higher. “Certain” decision trials were those on which the cue card was numbered 2, 3, 4, 8, 9, or 10, with more predictable (75% – 100%) feedback card outcomes. To provide a more balanced number of certain and uncertain decision trials, the latter were oversampled to provide 16 of the 36 trials, with 8 trials using a “6” cue card and 4 trials each using “5” and “7” cue cards.
Behavioral data analysis
Three 2 x 2 ANOVAs were used to analyze task data, all using group (user vs. control) as the between-subjects factor and trial certainty (uncertain vs. certain) as the within-subject factor. The first compared reaction times, the second compared the proportion of incorrect predictions, and the third compared response entropy. Entropy is a value, derived from information theory, that quantifies the degree of uncertainty associated with an event as determined by the formula h = −(p × log2 p + (1 − p) log2 (1 − p)) (Shannon and Weaver, 1949). In this context, p represents the proportion of “lower” prediction responses. Uncertainty is greatest, i.e. h=1, if subjects respond “lower” 50% of the time and ”higher” 50% of the time (p = 0.5). Entropy decreases as p approaches 0 or 1, when subjects always respond “higher” or always respond “lower.” At those extremes, entropy is defined such that h = 0, since there is no uncertainty associated with responses. One concern when assessing decision-making in a task with repeated presentation of similar trials is that individuals may acquire a strategy early in the task and subsequently engage in automated response selection without significant cognitive involvement. If this were the case, one would expect to see a marked decrease in response latencies over the course of the session as response selection becomes automated. To test for this, a regression analysis was performed looking at RT as a function of trial.
fMRI procedure
Stimuli were presented via back-projection onto a screen near the feet, which could be seen via a mirror attached to the head coil (visual angle ~4°) as subjects lay prone in the scanner. One functional imaging run sensitive to blood oxygenation level-dependent (BOLD) contrast was collected for each subject during task performance using a 1.5-Tesla Siemens (Erlangen, Germany) scanner (T2*-weighted echo-planar imaging, TR = 2000 ms, TE = 40 ms, 64 × 64 matrix, 20 4-mm axial slices, 256 scans), providing coverage of the majority of the cortex. During the same session, a T1-weighted image (MPRAGE, TR = 11.4 ms, TE = 4.4 ms, flip angle = 10°, FOV = 256 x 256, 1 mm3 voxels) was obtained for anatomical reference.
fMRI data analysis
All data were preprocessed, normalized to Talairach coordinates (Talairach and Tournoux, 1988) and analyzed with the AFNI software package (Cox, 1996). For preprocessing, voxel time series data were interpolated to correct for non-simultaneous slice acquisition within each volume and corrected for three-dimensional motion. Motion-corrected voxel time series data were visually inspected to remove large movement artifacts. Preprocessed time series data for each subject were analyzed using a multiple regression model consisting of nine regressors. Three regressors were used to model residual motion (in the roll, pitch, and yaw directions). Two regressors, modeling a baseline and a linear trend, were used to factor out slow signal drifts. Two regressors were used to model positive and negative outcomes (i.e. following correct and incorrect predictions), and were used to account for those factors in the model. The remaining two regressors modeled action selection during uncertain trials and certain trials, respectively. These latter two regressors of interest were created based on each subject's individual response patterns, representing the 2000 ms between initial presentation of the cue card and the addition of the “Lower” and “Higher” response prompt, plus the response latency from that point (i.e. 2000 ms + RT). Both regressors were convolved with a modified gamma variate function modeling a prototypical hemodynamic response prior to inclusion in the regression model. The AFNI program 3dDeconvolve (Cox, 1996) was used to calculate the estimated voxel-wise impulse response function for each regressor and to generate an Uncertain – Certain action selection contrast. A Gaussian filter with FWHM 6 mm was applied to voxel-wise percent signal change data to account for individual variations of anatomical landmarks. Voxel-wise t-tests were used to identify the brain areas in which the percent signal change associated with the Uncertain – Certain contrast was significantly different between groups. A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation (Forman et al., 1995). Based on these simulations, it was determined that a voxel-wise a-priori probability of 0.05 would result in a corrected cluster-wise activation probability of 0.05 if a minimum cluster volume of 1024 μl and a connectivity radius of 4.0 mm were considered. All clusters surviving these thresholds are reported in Table 2. The listed cortical areas are based on Talairach Daemon software (Lancaster et al., 2000). The average BOLD signal within each cluster of activation for the between-groups contrast was extracted for each subject. These were later correlated with personality measures.
Table 2.
Areas of greater activation for uncertain-certain action selection in stimulant users than in controls.
| Center of Mass | BIS | SSS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | Brain Regions | BA | L/R | x | y | z | Volume | t | r | r |
| 1 | Caudate | L | −7 | 18 | 6 | 3584 | 4.64 | 0.33 | 0.10 | |
| 2 | Superior Temporal Gyrus, Insula | 13, 22, 41 | L | −48 | −31 | 13 | 2432 | 4.33 | 0.57* | 0.06 |
| 3 | Thalamus (L/R), Caudate (L) | −3 | −2 | 10 | 1984 | 3.70 | 0.58* | 0.07 | ||
| 4 | Precuneus | 19 | L | −27 | −68 | 31 | 1792 | 3.40 | 0.62** | 0.09 |
| 5 | Precuneus | 7, 19 | R | 29 | −69 | 35 | 1792 | 3.67 | 0.62** | 0.28 |
| 6 | Medial Frontal Gyrus | 10 | R | 5 | 56 | 7 | 1600 | 3.92 | 0.39 | 0.01 |
| 7 | Superior Temporal Gyrus | 39 | L | −45 | −47 | 16 | 1344 | 3.16 | 0.53* | 0.21 |
Coordinates indicate center of mass in Talairach space. L/R indicates left/right hemisphere. t-values are for group comparisons (p < .05) while r-values are for correlations between impulsivity (BIS) and sensation-seeking (SSS) and activation (* p < .05, ** p < .01).
Results
Drug use and personality
Table 1 summarizes group demographic, drug use, and personality comparisons. In addition to stimulants, subjects in the user group had all used marijuana, and nearly half had used tobacco. A few had also used ecstasy, opiates, sedatives, and/or hallucinogens. Nearly half of controls had used marijuana, but none reported use of other assessed substances. The user group scored higher on impulsivity than the control group but was not significantly different in sensation-seeking score.
Behavioral performance
On uncertain trials as compared to certain ones, reaction times were longer (F(1,20) = 8.51, p =.009, Figure 1a) and there were more incorrect predictions (F(1,20) = 136.29, p < .001, Figure 1b). There was no main or interaction effect of group for either measure (F < 1 in all cases). On 98% of certain trials, subjects predicted that the feedback card would be higher given a cue card of 2, 3, or 4, or lower if the cue card was an 8, 9, or 10. Lower/higher selections varied more on uncertain trials, when the cue card was a 5 (28%/72%), 6 (63%/37%), or 7 (86%/14%). Figure 1c shows the entropy associated with each card value for both groups. Entropy was significantly higher for uncertain trials (F(1,20) = 81.03, p < .001). Entropy was also higher for selections in general made by users than controls (F(1,20) = 4.75, p = .041), but there was no interaction effect, i.e. the groups did not differ in the relative entropy of their uncertain versus certain responses. Post-hoc correlations revealed that impulsivity scores were positively associated with response entropy (r = .65, p = .004, uncorrected), but not with percent correct predictions or response latency (p-values > .10, uncorrected). The response latency slope, as a function of trial, was not significantly different from zero, i.e. there was no significant downward trend in RT over the course of the session (F(1,34) = 2.14, p = .152).
Figure 1.
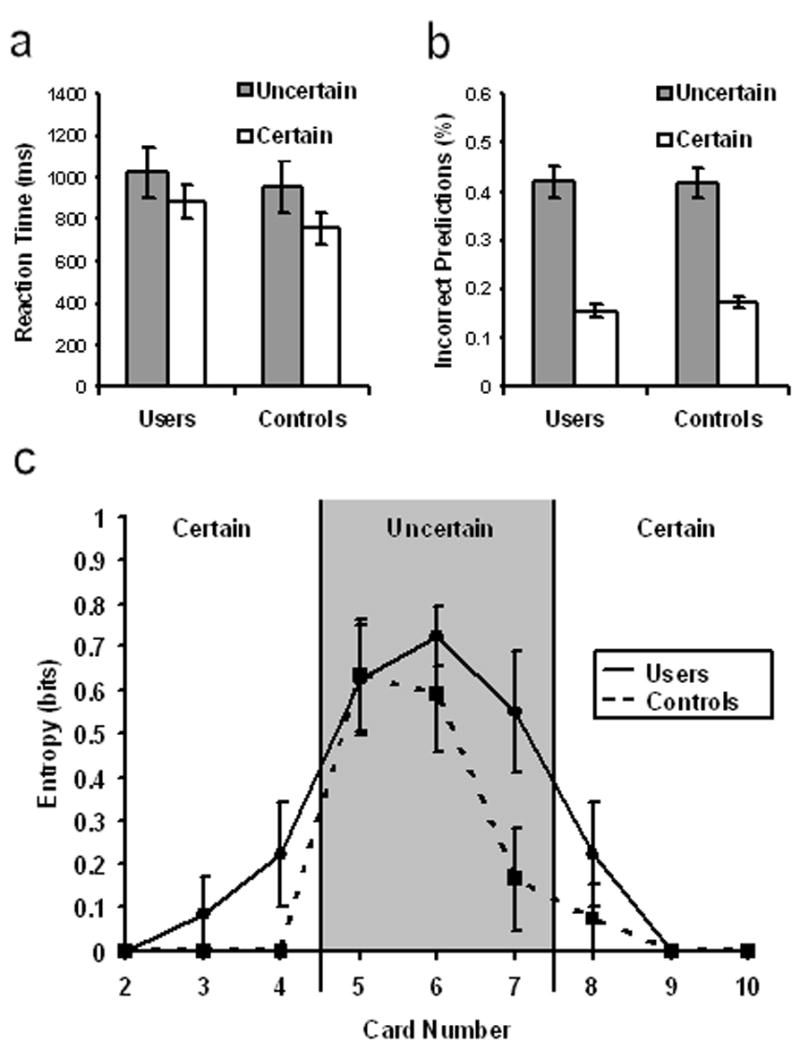
Behavioral performance on the card prediction task. (a,b) Reaction times were slower and more incorrect predictions made on uncertain trials than certain ones, but stimulant users performed similarly to controls in both conditions. (c) There was more response uncertainty as reflected by entropy calculations (see Methods and Materials) on uncertain trials than certain ones. Stimulant users had higher response entropy than controls overall but not as a function of trial certainty. Error bars indicate SE.
Functional activation
There were 7 clusters of activity in which the Uncertain-Certain action selection contrast revealed significant group differences, covering parts of the left caudate, thalamus, left superior temporal gyrus, left insula, left and right precuneus, and right medial frontal gyrus (see Table 2). In all such areas, the user group showed a larger percent signal change difference. In 5 of the 7 clusters, there were significant correlations between contrast activations and BIS scores, such that individuals with higher impulsivity ratings had increased activation in these areas. In contrast, none of the activations related to the contrast between uncertain and certain trials correlated with sensation-seeking scores. Figure 2 illustrates the group difference and correlation with BIS scores for the cluster covering part of the left caudate and thalamus.
Figure 2.
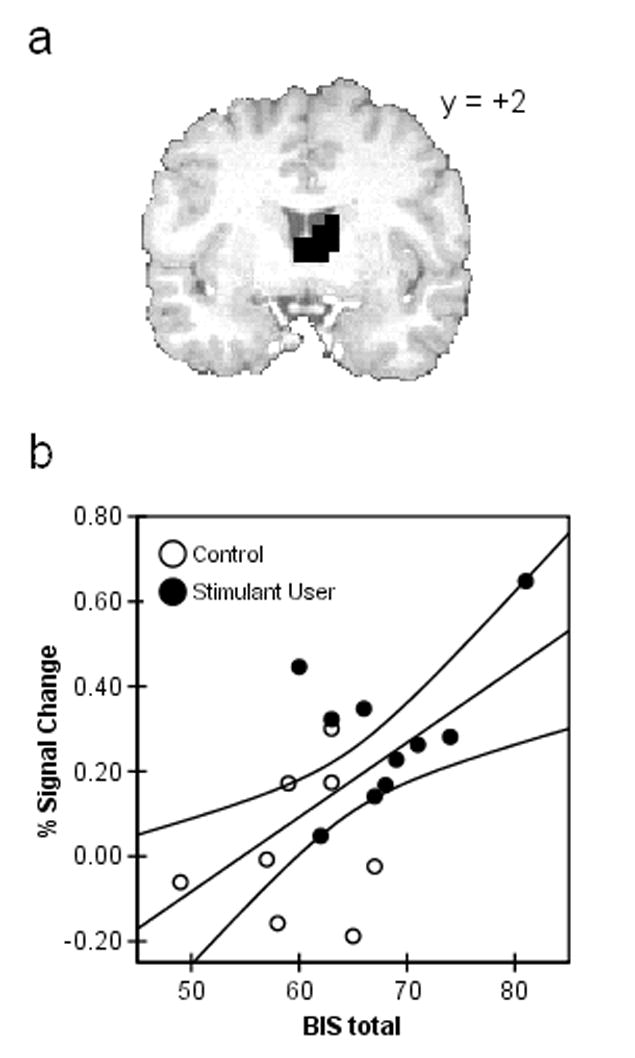
Activation related to uncertain-certain trials in stimulant users and controls. (a) Activation of the bilateral thalamus/left caudate in stimulant users > controls during action selection in the uncertain-certain contrast (p < 0.05, corrected cluster-wise). (b) Percent signal change for the activation in a was positively correlated with BIS-11 impulsivity scores in the 10 users and 8 controls for whom personality data were available.
Discussion
These findings demonstrate an enhanced striatal activation response to uncertainty in an understudied population—young adults who have used stimulants but are neither treatment-seeking nor stimulant-dependent—that is related to impulsivity. These users were all college students or college graduates, free of major medical and psychiatric problems, and limited in their stimulant exposure (21 lifetime uses on average). This may suggest that the observed differences from controls reflect pre-existing traits rather than chronic drug effects. Users showed a stronger uncertain – certain activation contrast than controls in part of the striatum (specifically the left caudate) during action selection. Previous investigations have suggested that the caudate is particularly important for the coding of stimulus salience (Aron, et al., 2004; Zink et al., 2003; Zink et al., 2004). Thus, one possible explanation for the difference is that these users find uncertain situations more salient when making decisions. Since salience contributes to action (Horvitz, 2000; Redgrave et al., 1999), finding uncertainty especially salient may create pressure to act in uncertain situations.
The striatum is associated with dopamine-mediated reinforcement processes (Robbins and Everitt, 1996), which raises the question as to whether the present findings suggest that stimulant users find uncertainty itself rewarding in the sense of having hedonic value. As self-reports on the subjective feelings associated with uncertainty were not obtained, this study cannot directly address that question. According to temporal difference models (see Schultz, 2001 for a review), however, dopamine can signal stimulus novelty and salience in the absence of reward, and does not simply signal reward, but rather a violation of reward expectancy. When expectancies are violated, new uncertainty is introduced. Likewise, novelty and high salience often reflect deviations from expected input. Thus, reward prediction errors and uncertainty more generally may share common currency in dopaminergic signals and striatal activation. This perspective is consistent with the incentive-salience hypothesis (Berridge and Robinson, 2003; Robinson and Berridge, 1993), which associates dopaminergic function with incentive motivation or “wanting”, as distinguished from pleasure or “liking.” According to the hypothesis, “liking” and “wanting” processes are related in that stimuli associated with reward (“liked”) normally become the focus of approach behavior (“wanted”), but they are functionally dissociable. Thus, while certain individuals may find uncertainty rewarding, greater salience in and of itself may be sufficient to explain increased striatal activity and increased propensity to act under uncertainty. Even in this case, “wanting” may be associated with subjective arousal (although Berridge and Robinson have suggested that both “liking” and “wanting” can be implicit), so future work in this area may benefit from comparing subjective reactions to uncertainty, as well as physiological measures of arousal such as heart rate and respiration, with performance and imaging measures.
In addition to the caudate, a number of other areas were activated in response to uncertainty more in stimulants users than controls, including the insula, thalamus, precuneus, medial frontal gyrus, and superior temporal gyrus. All but the last of these areas were also activated in healthy controls during performance of a two-choice prediction task rigged to maximize uncertainty, with 50% of responses given feedback as “correct” regardless of choices made (Paulus et al., 2001). The precuneus and thalamus were also activated by a similar two-choice guessing task (Elliot, 1999). Furthermore, the insula and precuneus were activated during selection of risky as compared with safe responses in the risky gains task (Paulus et al., 2003), where the safe options were inherently certain in their outcome while the risky ones were uncertain. In that study, subjects were not actually paid money based on performance but they did win and lose “cents” and had a running total up top as they performed the task, so that both uncertainty and some degree of reward salience were likely involved. Taken together, these previous findings suggest that, in addition to the caudate, the other areas activated more in users in response to uncertainty in the present study are associated with uncertain decision-making. It is also possible, however, that the neural substrates are different in users than never-users, so that the group contrast reflects not just stronger activation of the same areas but activation of different areas altogether. Future work with larger samples will allow for the within-group analyses necessary to test this alternative hypothesis.
The combination of an immature inhibitory control system and a drive for novel experiences has been proposed to increase the propensity of impulsive actions and risky behaviors in young adults (Chambers et al., 2003). Furthermore, individuals with substance use problems have been shown via questionnaires and laboratory tasks to be more impulsive than those without such problems (for a review, see Dawe and Loxton, 2004). We compared the stimulant user and control groups on measures of impulsivity and sensation-seeking and correlated scores with individuals’ activation in each of the clusters from the imaging analysis, with particular interest in the striatum. Users were more impulsive but not more sensation-seeking, and impulsivity (but not sensation-seeking) was significantly positively correlated with the uncertainty contrast activation for all subjects in a number of the areas of significant group difference, including one covering the thalamus and part of the left caudate. While the majority of that cluster is in the thalamus, a posthoc analysis confirmed the correlation for the caudate voxels in that cluster. A posthoc region of interest analysis, looking for BIS-correlated clusters solely within the striatum (volume threshold of 192 μl) revealed that while the left caudate voxels, separated from the thalamic ones, were too few to survive as an independent cluster, there was a right caudate cluster showing the same correlation. Impulsivity was also correlated with response entropy across subjects. These findings support and extend the association of impulsivity with substance use and suggest that personality factors preceding chronic drug exposure may play a role in individuals’ responses to uncertain situations. While users in this study did not have significantly higher sensation-seeking scores, this may be a power issue given the modest sample sizes. A previous study with similar groups and larger sample sizes found both higher impulsivity and sensation-seeking scores in young adult stimulant users than in controls (Leland and Paulus, 2005).
Much of drug use research assesses long-term or dependent users for evidence of the consequences of chronic use and addiction. In this study, we intentionally and specifically looked at young adults with limited exposure (21 times, on average) given our interest in factors that may predispose individuals to stimulant use. These individuals were all college students or college graduates, well-matched for years of education although not tested for executive function (which could relate to impulsivity differences; see Cheung et al., 2004). Since the groups were demographically similar and since the users had limited experience with stimulants, it is not surprising that the groups did not differ in reaction time or percent correct predictions. Users did demonstrate greater entropy in their selections than controls, but this was true for both the certain and uncertain conditions, with no significant interaction effect based on certainty. This is relevant to the fMRI results because it suggests that both groups experienced a similar difference in uncertainty between the certain and uncertain conditions, and that users had greater activation to this difference, rather than experiencing a larger uncertainty difference to begin with.
The user group had a wide range of lifetime stimulant uses (2–50), which we did not narrow with further exclusions due to the already small user sample (n=11). Also, complete data on use onset, recency, and patterns were unavailable. These factors are doubtless important for distinguishing between 1) effects that are more likely to predate drug use and those that may result from lasting effects of even subchronic use and 2) effects that may vary as a function of use patterns. Motivation for use (e.g., for self-medication, for recreation, or as a study aid) is also likely to account for a significant amount of the heterogeneity within young adult stimulant users.
Subjects were instructed not to use stimulants (or other drugs other than nicotine, caffeine, and alcohol) within 48 hours of the scan session. In order to encourage compliance, they were told that they may be asked to provide a urine sample for screening, but only self-reports were in fact obtained. Thus, it is possible that some individuals might have used drugs recently enough to confound group effects. A high level of agreement between self-report and random drug testing has been observed in individuals with drug related problems, however (Brown et al., 1992), and we believe that the individuals in our sample, with relatively low exposure and drug-related problems, were likely willing and able to abstain, especially given the prospect of being tested. Another possible confound is that the groups may have differed in recency of use of nicotine, caffeine, and/or alcohol, which were not prohibited and could have affected BOLD response. Since the group difference under consideration was itself a contrast (uncertain minus certain), however, such a substance-related influence would need to affect activation in these conditions differently to confound the results, since an influence common to both would be subtracted out in the contrast.
In summary, the observed relationship between uncertainty, impulsivity, and striatal activation in this study is consistent with the idea that some individuals are subject to more “action pressure” in the face of uncertainty. This could be one of the psychobiological bases for differences in risk-taking, given that one of the critical elements of a risky decision or risk situation, in addition to the potential for harm or reward, is uncertainty about outcomes. Such a trait may be beneficial in certain contexts, since increased risk-taking in uncertain situations can facilitate learning (Fiorillo et al., 2003), but it may also make individuals highly vulnerable to stimulants and other drugs that increase dopamine levels, which may play a significant role in mediating this behavior, far beyond normal physiological levels. Among young adults who have used stimulants, increased salience and action pressure associated with uncertain situations may be related to higher risk of future dependence, a question we are addressing in our current longitudinal study. Age-related and individual sensitivity to uncertainty, combined with repeated sensitization of the neural substrate of incentive motivation through stimulants, may partly explain why this category of substances carries such a high risk of compulsive, dependent use in members of this age group. If so, this research may constitute a first step toward an endophenotypic marker using functional neuroimaging and a specific behavioral probe to identify those at risk for stimulant misuse and related problems.
Acknowledgments
We would like to thank Alan Simmons for feedback on the manuscript. This research was supported by grants from NIDA (R01DA016663, R01DA018307) and by a VA Merit Grant.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Press; Washington, D.C: 1994. [Google Scholar]
- Aron AR, Shohamy D, Clark J, Myers C, Gluck MA, Poldrack RA. Human midbrain sensitivity to cognitive feedback and uncertainty during classification learning. J Neurophysiol. 2004;92:1144–1152. doi: 10.1152/jn.01209.2003. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Brown J, Kranzler HR, Delboca FK. Self-reports by alcohol and drug-abuse inpatients-factors affecting reliability and validity. Br J Addict. 1992;87:1013–1024. doi: 10.1111/j.1360-0443.1992.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the customary drinking and drug use record, CDDR. a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AM, Mitsis EM, Halperin JM. The relationship of behavioral inhibition to executive functions in young adults. J Clin Exp Neuropsychol. 2004;26:393–404. doi: 10.1080/13803390490510103. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev. 2004;28:343–351. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic-resonance-imaging (Fmri): - use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas ES, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh BC. Peril, chance, adventure: concepts of risk, alcohol use and risky behavior in young adults. Addiction. 1999;94:371–383. doi: 10.1046/j.1360-0443.1999.9433717.x. [DOI] [PubMed] [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clinical Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. The Mathematical theory of communication. University of Illinois Press; Urbana, Illinois: 1949. [Google Scholar]
- Slovic P. Perception of risk. Science. 1987;236:280–285. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Overview of findings from the 2003 national survey on drug use and health. Rockville, MD: 2004. (Office of Applied Studies, NSDUH Series H–25, DHHS Publication No. SMA 04–3964) [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; Stuttgart, Germany: 1988. [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence developmental periods of risk for dependence upon marijuana, cocaine, and alcohol developmental periods of risk for dependence upon marijuana, cocaine, and alcohol . Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23:8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America-cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]


