Abstract
The her-2 (neu, erbB-2) oncogene encodes a 185-kDa transmembrane receptor tyrosine kinase. HER2 overexpression occurs in numerous primary human tumors and contributes to 25–30% of breast and ovarian carcinomas. Synthesis of HER2 is controlled in part by an upstream open reading frame (uORF) present in the transcript. We used synthetic capped and polyadenylated mRNAs containing sequences derived from the 5′ region of the her-2 transcript fused to firefly luciferase (LUC) reporter to examine this ORF’s effect on translation in cell-free systems derived from reticulocytes, wheat germ and Neurospora crassa, and in RNA-transfected HeLa cells. The uORF reduced translation of the downstream cistron in all systems. [35S]Met-labeling of in vitro translation products obtained indicated that the uORF also affected downstream start-site selection. Primer extension inhibition (toeprint) assays of ribosomes loaded at initiation codons in reticulocyte lysates indicated that the uORF affected the interaction of ribosomes with the primary her-2 AUG codon.
INTRODUCTION
In eukaryotes, genes specifying products involved in growth control and development often contain short upstream open reading frames (uORFs) in their mRNA 5′ leaders. One of these genes, her-2 (neu, erbB-2), which encodes a 185-kDa transmembrane receptor tyrosine kinase that is a member of the epidermal growth factor receptor family, contains a single uORF in its mRNA [1]. The HER2 protein is overexpressed in a large number of cancers including approximately 30% of breast cancers, and HER2 overexpression plays an important role in promoting metastasis [2, 3]. In a fraction of cases, the HER2 receptor level varies without changes in mRNA levels, indicating that post-transcriptional mechanisms participate in control of its expression [4, 5]. Since the expression of many translation factors, such as eIF2α, is altered in different tumors [6, 7], it is possible that the her-2 uORF has a translational regulatory role during oncogenesis, as well as possible roles in growth and development.
Two distinct translational mechanisms were originally found to control HER2 protein synthesis [8]. The first is a cell-type-dependent mechanism that causes translation of her-2 mRNA to increase in some transformed cells in comparison with primary cells. The second is a cell-type-independent repression of downstream translation mediated by the uORF. Recently, the translational effect of the uORF was observed to be abrogated in some tumor cells [9]. The work described here is aimed at understanding the roles in translation of the her-2 uORF and the multiple in-frame initiation codons near the 5′ end of the her-2 coding region. Experiments with reporter constructs showed that the uORF was inhibitory to translation in vitro in mammalian, plant, and fungal systems and in vivo in mammalian cells. In vitro translation of her-2 occurred primarily from the first in-frame start codon when that start was the only one present. Primer extension inhibition (toeprint) mapping of ribosomes on the mRNA in vitro showed that ribosomes were clearly associated with the first her-2 start codon in the absence but not the presence of the uORF. In vitro studies raised the possibility that alternative start codons might be used when the uORF was present and/or absent, and that the uORF could change the initiation event at the first her-2 start codon so that it was not detected by toeprinting. Finally, additional features of the her-2 leader rendered the translation of reporter transcripts exquisitely sensitive to ionic conditions in the heterologous fungal cell-free translation system.
MATERIALS AND METHODS
Plasmids
Plasmid pEQ582 [1], containing the wild-type her-2 5′leader, uORF and coding region fused to β-galactosidase, was used as a template for PCR. Primer CCS3 (5′-CAAGAGATCTGCGCCCGGCCCCCACC-3′) and CCS4 (5′-CCACCTGGTGACCTGGTAGAGGTGGCG-3′) were used to amplify the region containing the her-2 5′ leader, uORF and the coding region containing the first three her-2 in-frame ATG codons. These primers also introduced a BglII restriction site upstream of the 5′ leader and a BstEII site downstream of her-2 ATG3. Plasmid pEQ581 [1], differing from pEQ582 by a mutation of the uORF ATG codon to AAG, was also used as a PCR template. Due to the GC-rich 5′-leader, betaine (Sigma-Aldrich) was used in the PCR reactions at a 1 M final concentration to improve amplification [10].
The PCR products from pEQ582 and pEQ581 templates were digested with BglII and BstEII, gel purified and ligated to pR301 [11] which was digested with the same restriction enzymes which cut between the T7 promoter and the firefly luciferase reporter coding region. This resulted in constructs pCS604 and pCS605 that produced synthetic mRNA containing the her-2 5′ leader followed by a HER2-LUC fusion reading frame. Additional constructs containing or lacking the uORF ATG and lacking her-2 ATG1 were made using mutagenic primers and megaprimer PCR to yield the set of plasmids pCS608–609 (Figs.1 and 2). Another matched set of constructs in the pR301 vector contained luciferase fused to the second codon of HER2 (pCS701–704, Figs. 1 and 2). These were obtained by PCR using primers CCS13 (5′-CTATAGATCTGCGCCCGGCCC-3′) as the forward primer (containing restriction site BglII), and CCS17 (5′-AGGCGGTGACCTCCATGGTGCT-3′), CCS18 (5′-AGGCGGTGACCTCCTTGGTGCT-3′) as the reverse primer (containing restriction site BstEII).
Fig. 1.
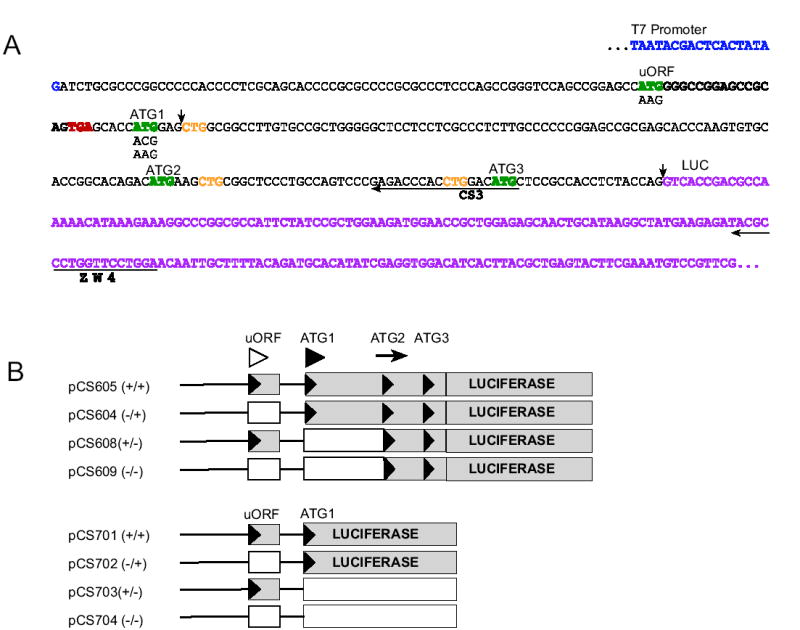
A. The 5′ leader regions, her-2 sequence, and constructs used in this study. The sequence shown begins with the T7 RNA polymerase-binding site (blue) and ends with the LUC coding region (purple). The upstream open reading frame ATG and the 3 in-frame ATGs (ATG1, ATG2, and ATG3) are in green. The in-frame CTGs are in yellow. The termination codon of the uORF region is in red and the mutations are shown directly below the sequence. The sequences whose reverse complements were synthesized and used as primers (CS3 and ZW4) for toeprint analyses are indicated by a horizontal arrow below the sequence. For the shorter constructs (pCS701–704), the luciferase reporter sequence was fused directly after the second codon after ATG1, the arrows in the sequence represent where the sequences were fused to make these shorter constructs. B. Constructs composed of the 97 nt her-2 5′ leader including the uORF and intercistronic region, and the N-terminal coding sequence that includes 3 in-frame ATGs. Wild-type (pCS605) and mutated sequences (lacking the uORF ATG codon and/or the first ATG codon, pCS604, 608, and 609) were fused in-frame to a firefly luciferase reporter. The symbols in parentheses represent the presence and/or absence of an ATG (e.g. The −/+ indicates the absence of the uORF ATG and the presence of the first her-2 ATG). These constructs were used to produce capped and polyadenylated synthetic RNAs. Shorter constructs composed of the 97 nt her-2 5′ leader including the uORF region and the first 2 codons of her-2 fused in-frame with firefly luciferase. The black triangles represent the ATG codons and the white boxes represent the coding regions that would not be translated if the ATG codons that were mutated were responsible for initiating translation. Additional symbols above the constructs correspond to the uORF ATG (open arrowhead), ATG1 (filled arrowhead) and ATG2 (arrow) respectively.
Fig. 2.
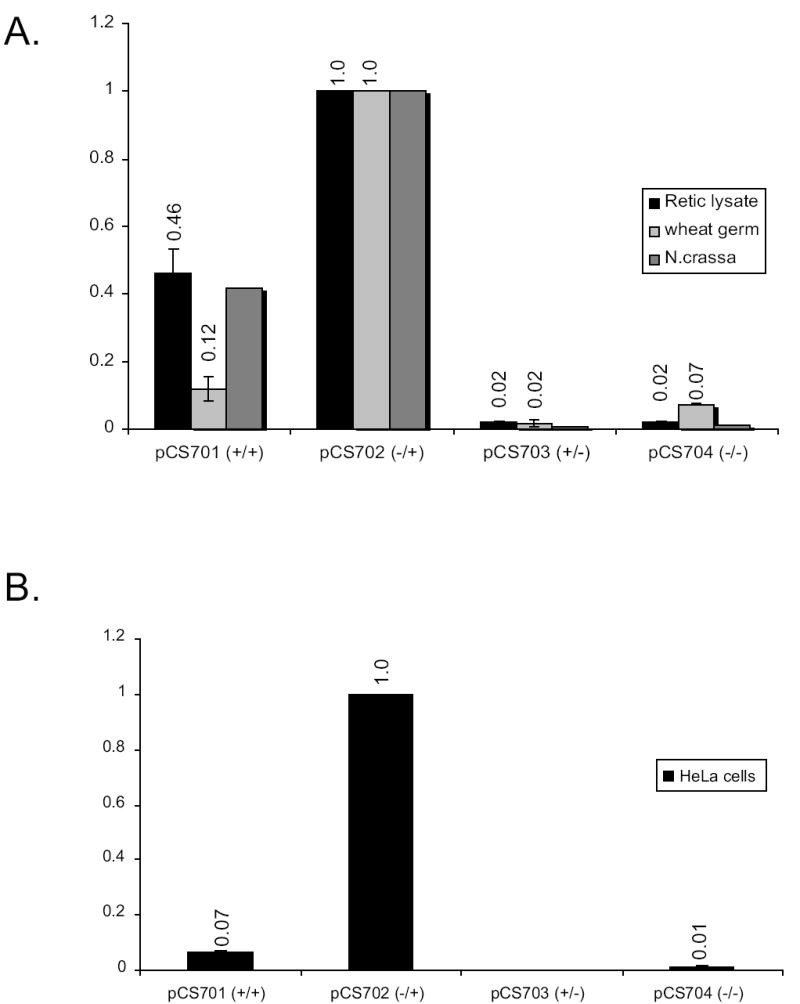
Luciferase enzyme activity assays. A. In-vitro translation reactions were performed for each extract and luciferase activity assays performed for rabbit reticulocyte lysates, wheat germ extracts, and N. crassa extracts. A dual luciferase (LUC) reporter assay system was used to measure firefly LUC and Renilla LUC (internal control) for constructs pCS701–704. All firefly LUC values were normalized to the internal control. The level of normalized firefly LUC expression from multiple independent experiments (two experiments using N. crassa, four experiments using wheat germ and reticulocyte lysates) with each construct were averaged and normalized to the expression from pCS702, and the standard deviations calculated for experiments using wheat germ and reticulocyte lysate (error bars). B. Luciferase enzyme activity obtained from RNA transfection into HeLa cells as describe in Experimental Procedures using RNA from constructs pCS701–704.
Preparation of Synthetic RNA
Plasmid templates were purified using the Wizard Plus Midi Prep kit (Promega). For in vitro studies, capped, polyadenylated RNA was synthesized with T7 RNA polymerase from EcoRI-linearized plasmid templates and yields of RNA were quantified as described [12]. For in vivo studies, capped and polyadenylated mRNAs were prepared as previously described [13, 14]; the yield of RNA transcripts were quantified by measuring UV-absorbance at 260nm and the quality of each RNA preparation assessed by SYBR gold (Molecular Probes; Eugene, OR) staining following fractionation on formaldehyde/1% agarose gels.
Cell-free translation and primer extension inhibition (toeprint) assays
Translation reaction conditions using nuclease-treated rabbit reticulocyte lysates and wheat germ extracts (Promega) were essentially those specified by the supplier, using a final reaction volume of 10 μl instead of 50 μl. Preparation of translation extracts from Neurospora crassa and the reaction conditions for in vitro translation were as described [11, 15, 16], except that to achieve maximum activity for her-2 containing reporters, K+ and Mg2+ final concentrations were 100 mM and 1.75 mM respectively. For firefly luciferase activity measurements, equal amounts (6 ng) of mRNA were used to program each of the three types of translation reactions (reticulocyte lysate, wheat germ extract or N. crassa extract); synthetic mRNA encoding Renilla (sea pansy) luciferase was added to all reactions to serve as an internal control [15]. All translation reaction mixtures were incubated at 25°C for luciferase assays; translation was halted by freezing reaction mixtures in liquid nitrogen after 30 min (N. crassa and wheat germ) or 45 min (reticulocyte lysates).
For [35S]Met labeling of polypeptides, synthetic RNA (60ng) was used to program reticulocyte lysates and wheat germ extracts. As a positive control, the reporter firefly luciferase, lacking any her-2 sequences, was placed downstream of the T7 RNA polymerase promoter (T7-LUC) [17]. Translation reactions were incubated for 45 min and 30 min respectively. [35S]Met was used at a final concentration of 0.5 μCi/μl. The reaction mixtures for both reticulocyte lysates and wheat germ extracts were mixed with SDS-containing loading buffer but were not heated prior to SDS-PAGE. Radiolabeled products were examined by SDS-PAGE and analyzed by phosphorimaging.
The toeprint assays were accomplished as described [17] using primers ZW4 (5′-TCCAGGAACCAGGGCGTA-3′) and CS3 (5′-CATGTCCAGGTGGGTCTC-3′). Cycloheximide (CYH) was added to a final concentration of 0.5 mg/ml as described [18]. The results shown are representative of at least three independent experiments. The radiolabeled products were examined using a Molecular Dynamics PhosphorImager and ImageQuant software.
Cell Culture and RNA transfections
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Sigma; St. Louis, MI) containing 10 % fetal bovine serum. Transient RNA transfections into HeLa cells were performed using DMRIE-C (Invitrogen; Carlsbad, CA), as specified by the manufacturer. Briefly, approximately 2 × 105 cells were seeded per well of a 6-well plate 18 hr prior to transfection. Cells were then transfected with 4 μg of capped and polyadenylated mRNAs from templates pCS701–704 and harvested ~ 16 hr post transfection. Transfections were done independently three times and transfection efficiencies were assessed by co-transfecting cells with Renilla luciferase RNA (1 μg/well); luciferase activities were measured using the Dual-Luciferase assay (Promega).
RESULTS
The her-2 uORF inhibits downstream translation in cell-free systems based on luciferase assays
To explore the role of the her-2 uORF in controlling translation, we used synthetic capped and polyadenylated mRNAs containing sequences derived from the 5′ region of the her-2 transcript, including the uORF and part of the HER2 coding region, fused to the firefly luciferase (LUC) reporter (Fig. 1A). For constructs pCS604–609, 51 codons specifying the amino terminus of HER2, which includes three in-frame Met codons, were fused to LUC; for short constructs pCS701–704, the first two codons of her-2 were fused to LUC (Fig.1B). Rabbit reticulocyte lysates, wheat germ extracts, and Neurospora extracts were programmed with mRNAs transcribed from these constructs.
The mRNAs specifying LUC fused to HER2 after the second codon (Fig. 1B) produced active enzyme when her-2 AUG1 was present (Fig. 2A). LUC activity in the pCS701 (+/+) and pCS702 (−/+) constructs was derived primarily from initiation at her-2 AUG1 because eliminating this codon (constructs pCS703 (+/−) and pCS704 (−/−)) substantially reduced LUC activity in each extract tested. When the wild-type construct containing the uORF and her-2 AUG1 (pCS701, +/+) was compared with that lacking the uORF (pCS702, −/+), the uORF yielded an approximate 2-fold inhibitory effect when a rabbit reticulocyte cell-free system was used. Under optimized salt conditions in Neurospora, the inhibitory effect of the uORF was comparable to that of reticulocyte lysates. An approximate 10-fold inhibitory effect was seen in the wheat germ system.
When N. crassa extracts were used to assay the translational function of the her-2 uORF with the constructs in which LUC was fused to HER2 after its second codon, the standard reaction conditions used previously [11, 15] did not support translation of the HER2-LUC reporters (Fig. 3). We found that, in N. crassa extracts, the synthesis of the fusion polypeptide from these transcripts was profoundly sensitive to K+ and Mg2+ concentrations. While the K+ and Mg2+ concentrations used previously (150 mM and 3.75 mM, respectively) did not support translation of these mRNAs, when they were adjusted to 100 mM and 1.75 mM, there was a two-order of magnitude increase in luciferase production from her-2-LUC transcripts containing HER2 AUG1. The reason why changing these ion concentrations in the fungal extract so dramatically affected the translation of mRNA containing the her-2 sequences is not known, but presumably, these ions influence the structure of the mRNA (which is 80% GC in its 5′-leader) in a way that affects translation or affects the activity of factors involved in translation. The effect of these changes in ion concentrations on transcripts lacking her-2 sequences was small (less than 2-fold) (data not shown).
Fig. 3.
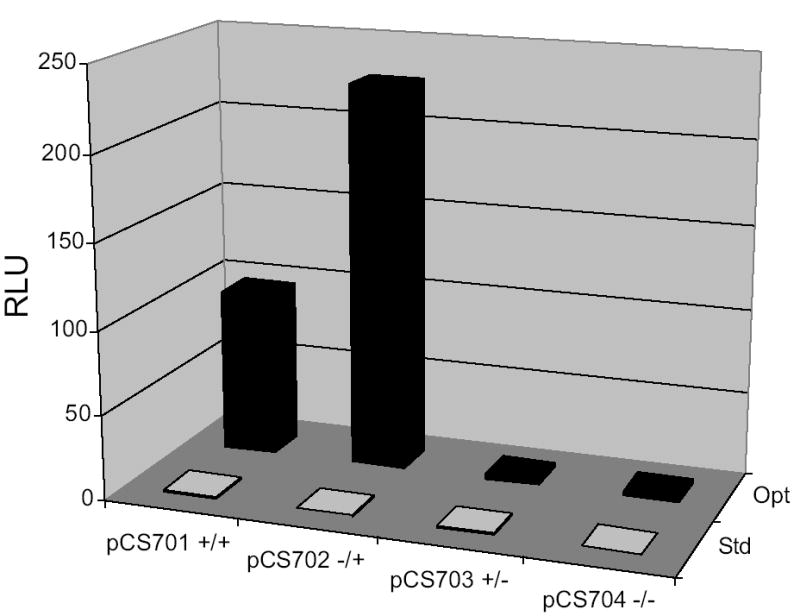
Luciferase activity assays show that translation of mRNAs from pCS701–704 containing the her-2 5′-UTR are highly sensitive to K+ and Mg2+ concentrations in N. crassa extracts. N. crassa in-vitro translation reactions were performed in parallel using 150 mM K+ and 3.75 mM Mg2+ salt concentrations as previously described [11, 15] (denoted Std, standard), or 100 mM K+ and 1.75 mM Mg2+, which yielded optimal synthesis of luciferase from mRNAs containing the her-2 5 leader (denoted Opt). After 30 min of incubation at 25ºC, LUC synthesis was determined by measuring enzymatic activity [expressed as RLU (relative light units)] using 5 μl aliquots from each reaction mixture. The N. crassa optimized salt concentrations showed less than a 2-fold effect on translation of the mRNAs lacking her-2 sequences (T7-LUC) but a 200-fold effect on transcripts containing the her-2 5′-UTR (data not shown). Using optimized salt concentrations, the construct lacking the uORF (pCS702) showed a ~2-fold increase in reporter expression compared to wild-type (pCS701).
To examine the effects of the uORF in vivo, transient RNA transfections into HeLa cells were performed using short pCS701–704 synthetic mRNAs and the luciferase they produced was measured (Fig. 2B). Luciferase synthesis in vivo was strongly dependent on her-2 AUG1. There was an approximately 15-fold inhibitory effect of uORF on expression of the reporter gene as determined by comparison of the enzyme activity synthesized from mRNAs containing or lacking the uORF. These results using transfected mRNAs are consistent with previous experiments using a different reporter system and plasmid transfections assays, which showed the uORF had a ~5-fold effect in both primary and transformed cells [8].
Luciferase activity measurements with the long pCS604–609 constructs that contained 51 residues of her-2 fused to LUC were complicated by the observation that constructs lacking her-2 AUG1 produced more enzyme activity per unit mRNA than constructs containing AUG1, instead of substantially reducing it (data not shown). The simplest explanation for this is that the 51-residue N-terminal extension reduced the specific activity of the reporter enzyme, and that shortening the N-terminus reduced the inhibitory effect. Thus, it is complex to assess enzyme activity data by direct comparison of constructs, since the uORF appears to affect downstream start site selection (see below), but the results were consistent with the uORF having an inhibitory effect when matched constructs (either containing or lacking AUG1) were compared (data not shown).
The her-2 uORF both quantitatively and qualitatively affects translation of the luciferase reporter based on [35S]Met labeling
To assess the effect of the uORF and the potential use of alternative start sites, [35S]Met labeling experiments were performed using equal amounts of mRNAs from both long and short constructs to program rabbit reticulocyte lysates (Fig. 4A). Reaction mixtures programmed with firefly LUC encoding mRNA lacking her-2 sequences were used as controls. In reticulocyte lysates, the long construct lacking the uORF (pCS604, −/+) produced a single major product whose migration was consistent with its predicted mass of 68-kDa (Fig. 4A, lane 2). In contrast, the long construct which contained the uORF (pCS605, +/+) produced two major products in reticulocyte lysates (Fig. 4A, lane 1), an upper band that could represent initiation at her-2 AUG1 (~68 kDa) and a lower band (~63 kDa) presumably resulting from initiation at an in-frame downstream start codon (see Fig. 1A). The absence of the lower band from lysates programmed with the pCS604 (−/+) mRNA argues against the lower band representing a degradation product or a C-terminally truncated product. Quantitation of radioactivity indicated that the larger product was reduced approximately 2-fold by the uORF (Fig. 4A, compare lanes 1 and 2). These data are consistent with the uORF having both quantitative and qualitative effects on downstream her-2 translation. In wheat germ extracts, the uORF strongly inhibited the production of [35S]Met labeled luciferase reporter whether expressed as either a long or short N-terminal fusion (Fig. 4B, lanes 1 and 6). Surprisingly, in reticulocyte lysates, regardless of the presence (pCS608, +/−) or absence (pCS609, −/−) of the uORF, translation of mRNA from the long constructs that lacked her-2 AUG1 generated polypeptide products of approximately the same size as those observed for the wild-type pCS605 (+/+) construct (Fig. 4A, compare lanes 3 and 4 with lane 1). Similar results were found in a wheat germ cell-free system where two major products (an upper band at ~68 kDa and a lower band at ~63 kD) were again observed after the translation of mRNA from the long construct pCS609 (−/−) (Fig. 4B, lane 4). While elucidating which codon, in the absence of AUG1, was used to initiate the synthesis of the ~68 kDa product has not yet been determined, it is evident that this product was obtained in translation extracts from mammals and plants (Fig 4A and 4B) from a template demonstrably lacking AUG1 (see sequencing reaction results for pCS609 in Fig. 5).
Fig. 4.
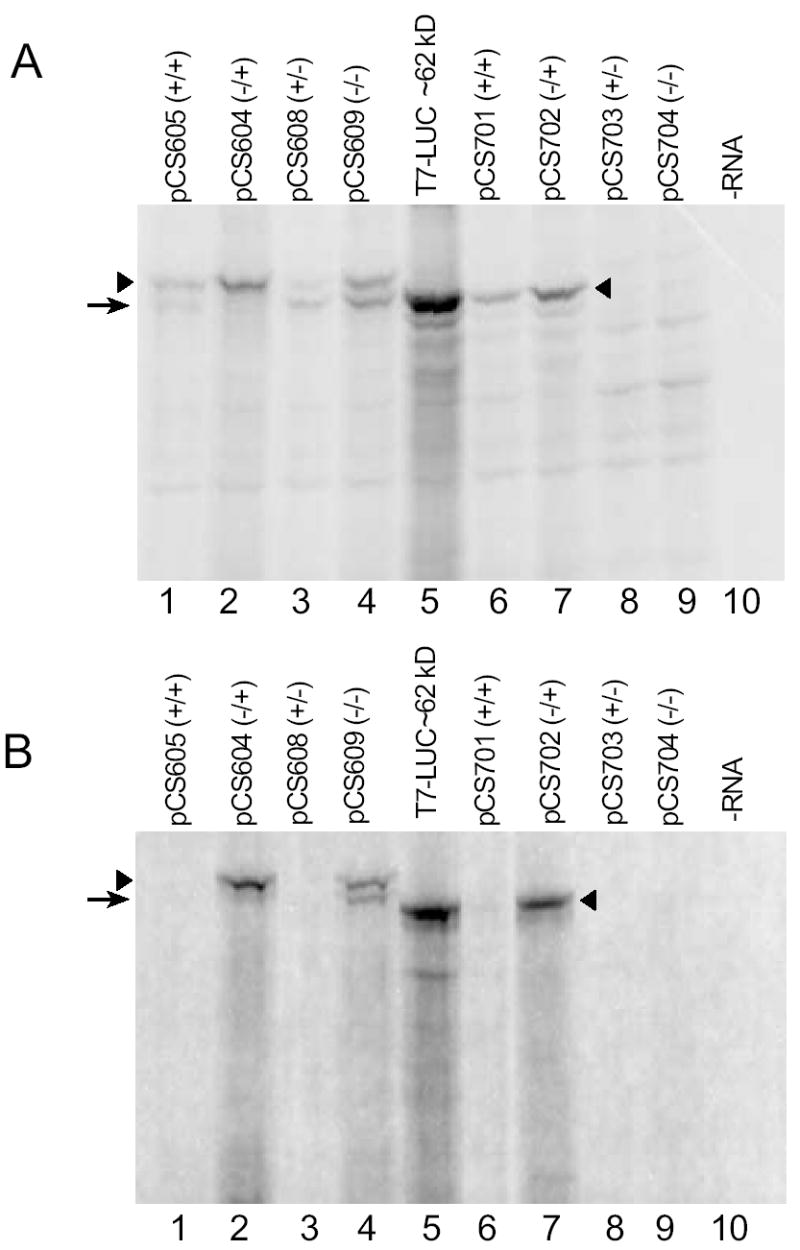
[35S]Met labeling of products obtained from each of the constructs shown in Fig.1B. A. Synthetic RNA (60 ng) were used to program reticulocyte lysates (10 μl). Reactions were incubated for 45 min at 25ºC. Radiolabeled products were examined by SDS-PAGE and phosphorimaging. Controls included a reaction mixture programmed with a firefly LUC encoding mRNA lacking her-2 sequences (T7-LUC) and a reaction mixture to which no RNA was added (-RNA). B. Synthetic RNA (60 ng) were used to program wheat germ extracts (10 μl). Reactions were incubated for 30 min at 25ºC. In each panel, filled arrowheads correspond to polypeptide products (where present) arising from (i) initiation at AUG1 (lanes 6-7); (ii) initiation at AUG1 or possibly at a nearby CUG codon (lanes 1–4). The arrow corresponds to polypeptide products (where present) arising from possible initiation at AUG2 (lanes 1–4).
Fig. 5.
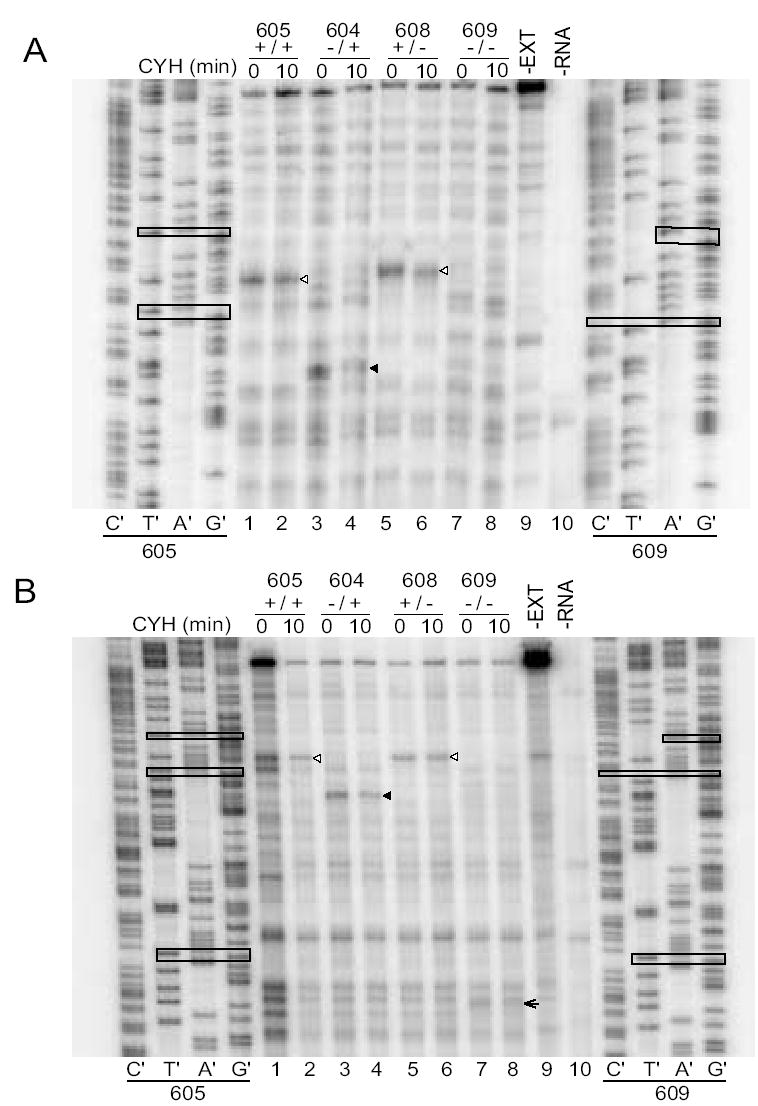
Primer extension inhibition (toeprint) assays examine initiation on transcripts containing or lacking the her-2 uORF AUG. A. Rabbit reticulocyte lysates (10 μl) were programmed with mRNA (60 ng) from long constructs pCS604–609. Cycloheximide (CYH) was added at time 0 (prior to incubation of translation reactions) or 10 min (when translation was underway). The reactions were incubated for a total period of 15 min and toeprinted with primer CS3. Toeprints obtained when CYH is added at time 0 should show where ribosomes first initiate translation; toeprints obtained when CYH was added after 10 min should reveal where primary initiation events and reinitiation events are occurring. Open arrowheads correspond to the uORF and filled arrowheads correspond to AUG1. B. Toeprint assay using a primer (ZW4) that is further downstream for visualization of the second her-2 AUG codon. When both the uORF AUG and HER2 AUG1 are mutated, ribosomes load on AUG2 (arrowhead). In these reactions, CYH was added at time 0 and after 10 min with a total reaction time of 15 min. These results indicate that most ribosomes initiate translation predominantly at the first AUG codon encountered in the transcript. Open arrowheads correspond to the uORF, filled arrowheads to HER-2 AUG1, and the arrow to HER-2 AUG2. Sequencing reactions of both 605 (+/+) and 609 (−/−) are shown on the left and right sides of the toeprint gel respectively. The sequencing reactions in panel A displayed some migration artifacts due to compression in the GC-rich regions. In the sequencing lanes, the uORF AUG, her-2 AUG1, and mutations that eliminate each, are boxed in both panels; her-2 AUG2 is boxed in panel B. The increased intensity of the specific signals in Lane 1 is due to an increase in the amount of cDNA sample loaded.
For the short constructs in which LUC was fused directly after the second HER2 codon, (pCS701–704), the presence of the uORF also inhibited protein synthesis from AUG1 2-fold in reticulocyte lysates (Fig. 4A, compare lanes 6 and 7). For the short constructs lacking AUG1, full-length fusion polypeptides were not produced (Fig. 4A, lanes 8 and 9), consistent with the loss of production of luciferase activity (Fig. 2); indicating AUG1 was crucial for initiation in these constructs.
Toeprinting analyses of her-2 uORF control
A primer extension inhibition (toeprint) assay was used to directly map the positions of the ribosomes at her-2 initiation codons. The purpose of these studies was to determine where ribosomes associated with the mRNA in the presence and absence of a functional uORF. All her-2 constructs examined by LUC assay, [35S]Met labeling, and RNA transfections were analyzed by toeprinting. Cycloheximide (CYH) was added to extracts prior to incubation of translation reactions (T0) or added after the translation reaction was underway for 10 min. Adding CYH at T0 should show where ribosomes first initiate translation, and adding it to extracts at T10 can reveal primary initiation events or reinitiation events [18] and other translation events such as ribosome stalling at uORF termination codons.
The results of toeprinting mRNAs produced from the long constructs are shown in Fig. 5A and 5B. The region downstream of AUG2 is not shown because there were no differences in signals among constructs. Regardless of when CYH was added, a toeprint corresponding to the uORF initiation codon was observed when the uORF was present (Fig. 5A, lanes 1, 2, 5 and 6; Fig. 5B, lanes 1, 2, 5 and 6) but not when it was absent (Fig. 5A, lanes 3, 4, 7 and 8; Fig. 5B, lanes 3, 4, 7 and 8). These results indicate that the uORF initiation codon is a site that recruits ribosomes for translation. In striking contrast, her-2 AUG1 showed a strong signal when the uORF was absent (Fig. 5A, lanes 3 and 4, Fig. 5B, lanes 3 and 4) but did not yield a strong toeprint signal when the uORF was present (Fig. 5A, lanes 1, 2, 5 and 6; Fig. 5B, lanes 1, 2, 5 and 6), indicating that the uORF altered translation from this site. The loss of this strong signal when both the uORF and AUG1 were absent (Fig. 5A, lanes 7 and 8; Fig. 5B lanes 7 and 8) verifies it corresponds to AUG1. There are some weak toeprint bands in lanes 7 and 8 that suggest the possibility that some ribosomes are recognizing the mutated AUG1 or alternatively could correspond to ribosomes recognizing the nearby CUG1. Possibly, some of the background bands in the region of gels in which toeprints corresponding to ribosomes at AUG1 are observed could arise from interactions between the RNA in this region and RNA-binding proteins. Importantly, when the start codons for both the uORF and AUG1 were mutated, a toeprint that corresponds to her-2 AUG2 (arrowhead) became stronger (Fig. 5B, compare the signal in lanes 7 and 8 to the other lanes). However, there was no evidence of initiation at AUG3 after analyzing the toeprint gels. The shorter constructs showed similar results to the longer constructs (Fig. 6) in that the majority of ribosomes associated with the first AUG encountered on the transcripts, but did not show significant fraction of ribosomes associated with AUG1 when the uORF was present.
Fig. 6.
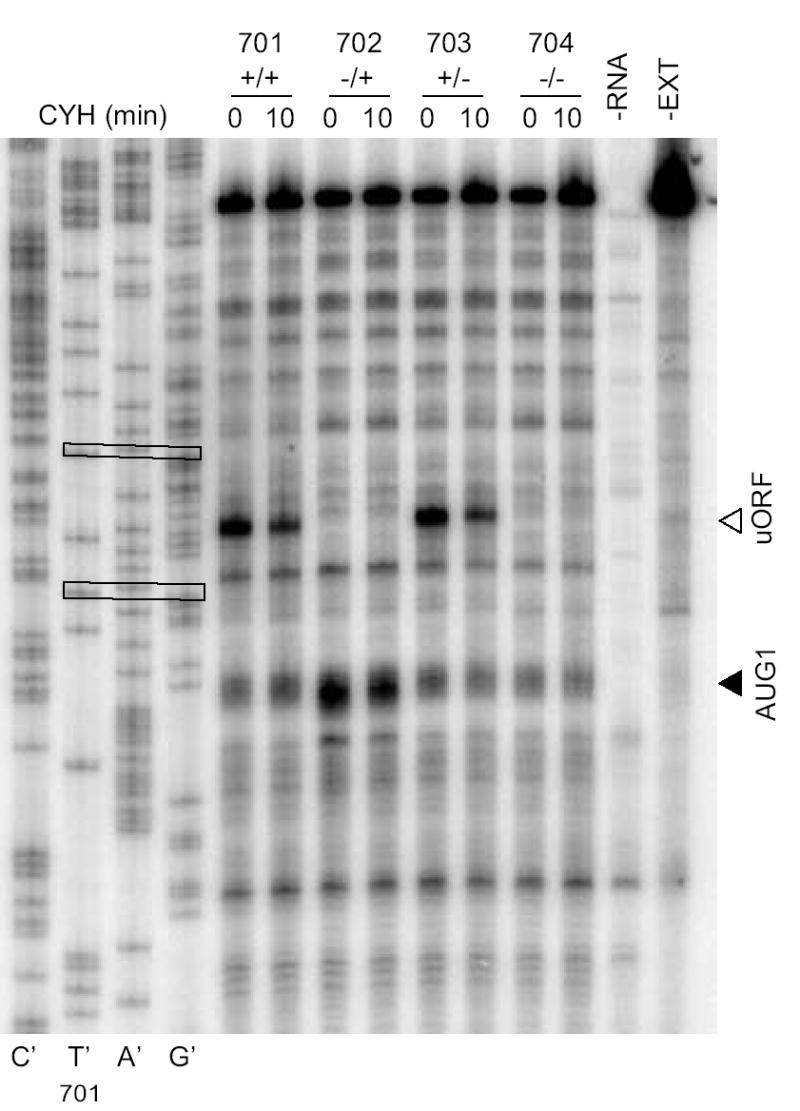
Toeprint assays to examine initiation on transcripts containing or lacking the her-2 uORF AUG and/or her-2 AUG1 using shorter constructs, pCS701–704. Rabbit reticulocyte lysates were programmed with mRNA and CYH was added at time 0 or after 10 min and reactions were incubated for a total period of 15 min. Ribosomes are preferentially associated with the uORF AUG when it is present. In the sequencing lanes, the uORF AUG and her-2 AUG1 are boxed.
DISCUSSION
HER2 is overexpressed in human cancers and its overexpression is associated with poor prognosis [19]. In breast cancer, overexpression of HER2 occurs in ~30% of cases and is often attributed to gene amplification [20]. The bulk of the transforming events mediated by overexpression of HER2 are the result of enhanced signaling through the PI3K/Akt pathway. Cells sensitive to the cytotoxic effects of herceptin (which interferes with HER2 activity) become resistant upon expression of a constitutively activated Akt [21, 22]. Additional translational mechanisms modulate HER2 protein expression [1]. The her-2 uORF represses translation of the downstream coding region; however, an element in the 3′ UTR of the transcript can reverse the inhibitory effect of the uORF in some cells [9]. The results described here show that synthesis of HER2 protein is quantitatively and qualitatively affected by its uORF. The uORF affects downstream translation in reticulocyte, wheat germ, and Neurospora cell-free systems as well as in transfected HeLa cells.
Both measurements of reporter enzyme activity (Fig. 2) and [35S]Met data (Fig. 4) support the idea that the uORF is inhibitory to translation. Furthermore, the presence of [35S]Met products with smaller sizes indicated that the uORF causes some ribosomes to initiate from alternative in-frame downstream start codons (Fig. 4). The synthesis of polypeptide products in long constructs lacking her-2 AUG1 that are similar to those observed in the wild-type long construct pCS605 (+/+) (Fig. 4A), regardless of whether the uORF is present or absent, is unclear. We speculate that other initiation events might occur in the absence of AUG1, such as initiation from a nearby in-frame CUG codon (see Fig. 1A). This CUG codon has a G at −3 and a G at +1, putting it in a reasonable “Kozak consensus” motif [23].
uORF-mediated initiation from a downstream start codon would result in N-terminally truncated HER2 polypeptides. A similar mechanism by which uORFs control downstream translation is seen in C/EBPα and C/EBPβ mRNAs containing a uORF [24]. In the case of her-2, this might generate a primary translation product that would lack its signal sequence and thus might not enter the ER as would be expected for full-length HER2. The presence of the uORF in her-2 may, therefore, serve as a cis-regulatory element that controls the site of translation initiation and possibly reinitiation to AUG2 resulting in a truncated product (~62 kDa) as seen in Fig. 4A, lanes 1 and 3. Such a truncated product might induce proliferation similar to the truncated products seen in C/EBPα and C/EBPβ [24]. The direct demonstration of the production of shorter polypeptides from the authentic her-2 coding region is consistent with the results of reporter studies suggesting alternative downstream reinitiation at a heterologous ATG codon is stimulated by this uORF [1]. Possibly, the uORF regulates reinitiation at different downstream start codons, as seen for Saccharomyces cerevisiae GCN4 [25] and mammalian ATF4 [26–28]. That is, the uORF might enable ribosomes to access a more remote downstream initiation codon by reducing initiation at a more proximal one.
The toeprint data confirm that the uORF has a very strong effect on how ribosomes access the first her-2 AUG codon, since no ribosome signal is detected there when the uORF is present. Since ribosomes are not observed there when the uORF is present, we cannot determine whether the ribosomes reach the her-2 AUG codon by leaky scanning or reinitiation. Why ribosomes are not observed at her-2 AUG1 (or AUG2, if the uORF were stimulating initiation from this site) when the uORF is present is not clear. The experiments using the short constructs (pCS701–704) measuring LUC activity (Fig. 3 and Fig. 4) and [35S]Met-incorporation (Fig. 5) establish that her-2 AUG1 is necessary for expression. Thus, the absence of a toeprint signal from this codon when the uORF is present (Fig. 7) cannot be due to its not having a role in translation. One possibility for the absence of signal is therefore that ribosomes that arrive at the her-2 AUG1 codon after translating the uORF bind that codon differently than when the uORF is absent and are lost during toeprinting compared to ones that arrive there when the uORF is absent.
N-terminally truncated HER2 receptors have been previously shown to have enhanced cell transformation activity compared with the full-length product [29–31]. These deletions within the extracellular domain (ECD) of HER2 have been shown to increase autokinase and transformation activities. Herceptin (trastuzumab), which binds to the ECD of HER2, was not effective against tumors expressing truncated products [21]. Additionally, it did not block receptor activation and downstream signal transduction pathways that are involved in disease progression [21]. The resistance to herceptin therapy may be due to increased levels of these N-terminally truncated proteins. The possibility that the uORF may promote ribosomal bypass of the first initiation codon to a downstream initiation codon signifies that further elucidation of how this uORF affects gene expression and protein synthesis will be valuable in the determination of therapeutic strategies.
Acknowledgments
This work was supported by grants to M.S.S. from the Medical Research Foundation of Oregon and the NIH (GM47498), to A.G. from the NIH (AI26672), to J. P. from the Canadian Institutes for Health Research (MOP-11354), and to C.S. and M.S.S. from the NIH (Research Supplement for Underrepresented Minorities). E.H.P. is supported by a K.M. Hunter/Canadian Institutes for Health Research doctoral research award.
References
- 1.Child SJ, Miller MK, Geballe AP. Translational control by an upstream open reading frame in the HER-2/neu transcript. J Biol Chem. 1999;274:24335–24341. doi: 10.1074/jbc.274.34.24335. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Buhring HJ, Sures I, Jallal B, Weiss FU, Busch FW, Ludwig WD, Handgretinger R, Waller HD, Ullrich A. The receptor tyrosine kinase p185HER2 is expressed on a subset of B-lymphoid blasts from patients with acute lymphoblastic leukemia and chronic myelogenous leukemia. Blood. 1995;86:1916–1923. [PubMed] [Google Scholar]
- 5.Oshima M, Weiss L, Dougall WC, Greene MI, Guroff G. Down-regulation of c-neu receptors by nerve growth factor in PC12 cells. J Neurochem. 1995;65:427–433. doi: 10.1046/j.1471-4159.1995.65010427.x. [DOI] [PubMed] [Google Scholar]
- 6.Clemens MJ, Bommer UA. Translational control: the cancer connection. Int J Biochem Cell Biol. 1999;31:1–23. doi: 10.1016/s1357-2725(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 7.Rosenwald IB, Wang S, Savas L, Woda B, Pullman J. Expression of translation initiation factor eIF-2alpha is increased in benign and malignant melanocytic and colonic epithelial neoplasms. Cancer. 2003;98:1080–1088. doi: 10.1002/cncr.11619. [DOI] [PubMed] [Google Scholar]
- 8.Child SJ, Miller MK, Geballe AP. Cell type-dependent and -independent control of HER-2/neu translation. Int J Biochem Cell Biol. 1999;31:201–213. doi: 10.1016/s1357-2725(98)00068-5. [DOI] [PubMed] [Google Scholar]
- 9.Mehta A, Trotta CR, Peltz SW. Derepression of the Her-2 uORF is mediated by a novel post-transcriptional control mechanism in cancer cells. Genes Dev. 2006;20:939–953. doi: 10.1101/gad.1388706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henke W, Herdel K, Jung K, Schnorr D, Loening SA. Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res. 1997;25:3957–3958. doi: 10.1093/nar/25.19.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang P, Wang Z, Sachs MS. Evolutionarily conserved features of the arginine attenuator peptide provide the necessary requirements for its function in translational regulation. J Biol Chem. 2000;275:26710–26719. doi: 10.1074/jbc.M003175200. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Sachs MS. Arginine-specific regulation mediated by the Neurospora crassa arg-2 upstream open reading frame in a homologous, cell-free in vitro translation system. J Biol Chem. 1997;272:255–261. doi: 10.1074/jbc.272.1.255. [DOI] [PubMed] [Google Scholar]
- 13.Park EH, Lee JM, Blais JD, Bell JC, Pelletier J. Internal translation initiation mediated by the angiogenic factor Tie2. J Biol Chem. 2005;280:20945–20953. doi: 10.1074/jbc.M412744200. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier J, Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985;40:515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Fang P, Sachs MS. The evolutionarily conserved eukaryotic arginine attenuator peptide regulates the movement of ribosomes that have translated it. Mol Cell Biol. 1998;18:7528–7536. doi: 10.1128/mcb.18.12.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Gaba A, Sachs MS. A highly conserved mechanism of regulated ribosome stalling mediated by fungal arginine attenuator peptides that appears independent of the charging status of arginyl-tRNAs. J Biol Chem. 1999;274:37565–37574. doi: 10.1074/jbc.274.53.37565. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Sachs MS. Ribosome stalling is responsible for arginine-specific translational attenuation in Neurospora crassa. Mol Cell Biol. 1997;17:4904–4913. doi: 10.1128/mcb.17.9.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaba A, Wang Z, Krishnamoorthy T, Hinnebusch AG, Sachs MS. Physical evidence for distinct mechanisms of translational control by upstream open reading frames. EMBO J. 2001;20:6453–6463. doi: 10.1093/emboj/20.22.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(Suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 20.Bofin AM, Ytterhus B, Martin C, O’Leary JJ, Hagmar BM. Detection and quantitation of HER-2 gene amplification and protein expression in breast carcinoma. Am J Clin Pathol. 2004;122:110–119. doi: 10.1309/8A2D-JFT0-7NE6-EWHE. [DOI] [PubMed] [Google Scholar]
- 21.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 22.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 23.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucl Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 25.Hinnebusch AG. Translational regulation of Gcn4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 26.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 27.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bargmann CI, Weinberg RA. Oncogenic activation of the neu-encoded receptor protein by point mutation and deletion. EMBO J. 1988;7:2043–2052. doi: 10.1002/j.1460-2075.1988.tb03044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 31.Segatto O, King CR, Pierce JH, Di Fiore PP, Aaronson SA. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988;8:5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]


