Abstract
Phytohormones as well as temporal and spatial regulation of the cell cycle play a key role in plant development. Here, we investigated the function and regulation of an alfalfa (Medicago sativa) A2-type cyclin in three distinct root developmental programs: in primary and secondary root development, nodule development, and nematode-elicited gall formation. Using transgenic plants carrying the Medsa;cycA2;2 promoter-β-glucuronidase gene fusion, in combination with other techniques, cycA2;2 expression was localized in meristems and proliferating cells in the lateral root and nodule primordia. Rapid induction of cycA2;2 by Nod factors demonstrated that this gene is implicated in cell cycle activation of differentiated cells developing to nodule primordia. Surprisingly, cycA2;2 was repressed in the endoreduplicating, division-arrested cells both during nodule development and formation of giant cells in nematode-induced galls, indicating that CycA2;2 was dispensable for S-phase in endoreduplication cycles. Overexpression of cycA2;2 in transgenic plants corresponded to wild type protein levels and had no apparent phenotype. In contrast, antisense expression of cycA2;2 halted regeneration of somatic embryos, suggesting a role for CycA2;2 in the formation or activity of apical meristems. Expression of cycA2;2 was up-regulated by auxins, as expected from the presence of auxin response elements in the promoter. Moreover, auxin also affected the spatial expression pattern of this cyclin by shifting the cycA2;2 expression from the phloem to the xylem poles.
Cyclins are the regulatory subunits of cyclin-dependent kinase complexes whose ordered, consecutive, and periodic activities drive the cell cycle. Cyclins are classified as G1 and mitotic cyclins. The latter group comprises the A- and B-type cyclins involved in the regulation of the cell cycle from the S to M phases. Cyclins are usually present for short periods in the cell cycle when they activate their CDK partners and determine the substrate specificity as well as the localization, maintenance and stability of these protein complexes. The activity of the cyclin-CDK complexes is tightly controlled during cell cycle and organ development.
In plants, where organogenesis takes place continuously, most cells maintain their ability to re-enter and to regulate the cell cycle, in response to a wide range of external signals. The phytohormones, mostly auxin and cytokinin endogenously exert a temporal and local control on the cell cycle (Stals and Inzé, 2001). They act at multiple levels affecting transcription of cell cycle genes or the activity of the cyclin-dependent kinases; moreover, their altered balance seems to be required at specific points of the cell cycle. It is largely unknown how these external and internal signals interact and how their actions are coordinated and integrated into developmental programs.
The plasticity of plant cell cycle may rely on the evolution of multiple cyclin forms. The sequence of the Arabidopsis genome indicates the existence of 30 cyclins classified as A, B, D, and H types (Stals and Inzé, 2001; Vandepoele et al., 2002), and similar complexity is expected for the other plant species. It is particularly intriguing why plants have evolved three subclasses of A-type cyclins (A1, A2, and A3), when in animal somatic cells, a single cyclin A protein is sufficient for cell cycle progression at the onset of S and M phases. The presence of multiple A-type cyclins suggests that they fulfill plant-specific roles. On the basis of the distinct expression patterns of the different plant A-type cyclin genes (Chaubet-Gigot, 2000), the combined action of these different A-type cyclins may be necessary for the global A-type cyclin-CDK complex activity, or alternatively, at least certain A-type cyclin-CDK complexes may provide functions under specific conditions.
From the A-type cyclin groups of alfalfa (Medicago sativa), only A2-type cyclins have been isolated; cycA2;1 from alfalfa subsp. varia A2 cell cultures (Meskiene et al., 1995) and cycA2;2 from root nodules induced by Sinorhizobium meliloti (Roudier et al., 2000). Their amino acid sequences exhibited 96% identity and shared relatively low similarity to the Arabidopsis A2-type cyclins (44%–50% identity). Medsa;CycA2;2 is one of the few plant cyclins with characterized cell cycle function (Roudier et al., 2000). Unlike other mitotic cyclins, neither the cyclin A2 mRNA nor the protein displays a marked oscillation during the cell cycle progression. The cyclin A2 protein interacts with the PSTAIRE type alfalfa cyclin-dependent kinase, Cdc2MsA, and the retinoblastoma protein. The CycA2;2-associated kinase activity is biphasic; a weaker activity peaks in the mid S phase, whereas the major one peaks during the G2/M transition, which suggests a dual function for CycA2;2 during DNA replication and preparation of mitosis. The protein is present in the nucleus from the late G1 phase to late prophase when it is abruptly degraded. This degradation is mediated by the destruction box motif present in all mitotic cyclins and recognized by the anaphase-promoting complex, an ubiquitin protein ligase that targets the protein to the 26S proteasome (Vodermaier, 2001).
Here, we studied the developmental role and hormonal regulation of the Medicago spp. cycA2;2 gene, including its function in the mitotic and endoreduplication cycles. Three root-derived organs, lateral roots, Rhizobium-induced root nodules, and galls formed by Meloidogyne incognita, an endoparasitic root knot nematode, were chosen for these studies, which share some common as well as distinct features in their developmental programs. Activation of the cell cycle in differentiated root cells is required for all of the three structures. In the case of lateral roots and nodules, cell proliferation leads to the de novo formation of persistent meristems, whereas in the case of galls, only limited cell division occurs transiently. Formation of the symbiotic nitrogen-fixing nodule cells and of the giant cells at the nematode feeding sites (NFSs) is accompanied by multiple rounds of endoreduplication cycles, duplication of the genome without cytokinesis, and extreme enlargement of the cells. Endoreduplication also occurs in Medicago spp. roots, however, the level of endoploidy is low, never exceeding a small percentage of 8C cells (Cebolla et al., 1999). Initiation of lateral roots is controlled by auxin, whereas nodule and gall formation are triggered by signals of the endosymbiotic or parasitic partners. In the case of nodules, the signals are the lipochitooligosaccharide Nod factors, produced by S. meliloti, the symbiotic partner of the different Medicago spp., which activate the cell cycle in the root inner cortex opposite to the protoxylem poles (Savouré et al., 1994; Yang et al., 1994). Cell proliferation leads to the formation of the nodule primordium, which then differentiates into three major nodule zones. The apical part (zone I) maintains the meristematic activity, whereas S. meliloti infection and morphogenesis of the symbiotic cells occur in the non-dividing submeristematic cell layers constituting nodule zone II. In this zone, cells undergo multiple rounds of endoreduplication cycles and gradual cell enlargement. Nitrogen fixation takes place in zone III where differentiation is irreversible for both the plant cells and the bacteria. In contrast to fixed sizes of zones I and II, zone III increases continuously due to constant production of nitrogen-fixing cells by persistent activity of the meristem. Distinct stages of nodule development are accompanied by induction of various sets of nodule-specific genes, called nodulins. Some of the early nodulin (enod) genes are also activated during lateral root development and nematode-induced gall formation (Favery et al., 2002). Moreover, coregulation of several enod genes by Nod factors and plant hormones indicates that Nod factors and plant hormones may act synergistically or via convergence of their signaling pathways during nodule development (Schultze and Kondorosi, 1998).
In this work, we show that Medsa;cycA2;2 plays role exclusively in the mitotic cycles, and in contrast to B-type cyclins studied previously (Cebolla et al., 1999) or other A-type cyclins (Joubes et al., 2000), it is not expressed during cell differentiation coupled to endoreduplication. We demonstrate the involvement of cycA2;2 in reactivation of cell cycle in differentiated cells and show that its expression is linked to meristems. In accordance with the presence of auxin response-like elements in its promoter, the Medsa;cycA2;2 gene is up-regulated by auxin. Moreover, auxin controls not only the expression level but also the spatial expression pattern of cycA2;2 opposite to the protoxylem poles that are likely required for the formation of lateral roots and nodules.
RESULTS
The Medsa;cycA2;2 Gene Structure
To study the regulation and involvement of the alfalfa cycA2;2 in organ development, we aimed at the isolation of its genomic clone. Southern-blot analysis of the alfalfa genomic DNA digested with EcoRI, which does not cut the cDNA, or with HindIII, which cuts the cDNA into two fragments (693 and 1,251 bp), resulted in multiple hybridizing fragments with the full-length Medsa;cycA2;2 cDNA probe (Roudier et al., 2000). This indicated that the coding region may be disrupted by several introns or that the allogamous tetraploid alfalfa may contain allelic variants of Medsa;cycA2;2. By screening an alfalfa genomic library with this probe under stringent conditions, seven strongly hybridizing phage clones were obtained. Their RFLP analysis indicated that they represent overlapping regions of the same gene. The clone with the longest 5′ region and the entire coding region was selected for further analysis. Sequencing of this 6,860-bp-long fragment (AY043242) revealed that the coding region was composed of 11 introns and 11 exons. The translation initiation site was localized at position 3,041, and the putative transcription start site, predicted on the basis of cDNA sequences, was at position 2,209: 21 bp downstream of a TATA box motif. The biggest intron (679 bp) was found in the 5′-untranslated region (UTR). Most introns started with GT and ended with AG, and most of the splicing sites were confirmed by computer analysis (Hebsgaard et al., 1996).
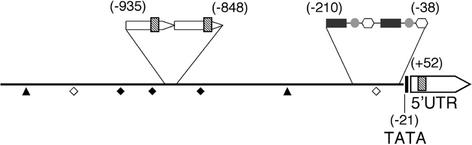
In the 5′-flanking region, several potential regulatory elements were present (Fig. 1). A sequence motif (GTCTC… AATAAG) highly similar to the auxin response elements (AuxRE, TGTCTC… AATAAG), identified previously in the soybean (Glycine max) GH3 promoter (Liu et al., 1994), was present at two positions (1,293–1,311 and 1,337–1,355) that were 916 to 898 and 872 to 854 bp upstream of the putative transcriptional initiation site, respectively. These two AuxRE elements were remarkably parts of 44-bp-long tandem direct repeats (1,274–1,317 and 1,318–1,355). A further AuxRE element (TGTCTC) was present in the first intron of the 5′-UTR at position 2,261. Putative binding sites for Myb and Myc transcription factors, implicated in cell proliferation, were also found. In addition, three distinct sequence motifs of 23, 18, and 11 bp were present as direct repeats in a 200-bp region (1,999–2,178) upstream of the TATA motif (Fig. 1).
Figure 1.
Putative regulatory elements in the 5 '-flanking region of the Medsa;cycA2;2 gene. Distances are given in base pairs from the beginning of the 5′-UTR. ♦ and ⋄, Myb-binding sites in the + or − strand, respectively; ▴, Myc-binding site in the + strand; ░⃞, Auxin response-like elements in the 44-bp tandem repeats and 5′-UTR; ▪, , and , 23-, 18-, and 11-bp direct repeats, respectively.
Expression of the cycA2;2 Gene Is Linked to Cell Proliferation
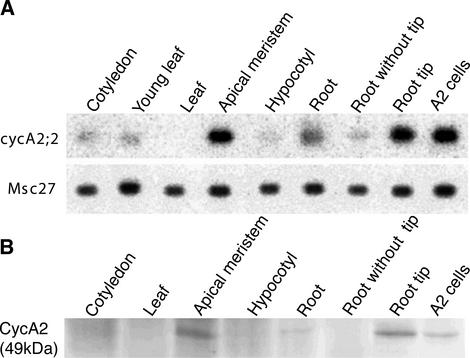
The expression pattern of cycA2;2 was first studied at transcriptional level by reverse transcriptase (RT)-PCR analysis in different M. sativa tissues/organs using primers specific for the cycA2;2 cDNA (Fig. 2A). In 10-d-old seedlings, the cycA2;2 gene showed its strongest expression in the meristematic tissues and in exponentially growing alfalfa A2 cell suspensions. Likewise, the highest level of the cyclin A2 protein, revealed by anti-CycA2 polyclonal antibodies (Roudier et al., 2000), was detected in the shoot and root meristems and in the actively proliferating A2 cell culture (Fig. 2B), whereas the protein was undetectable by western-blot analysis in tissues or organs containing mainly differentiated cells, like in leaves.
Figure 2.
Medsa;cycA2;2 expression in non-symbiotic tissues. A, RT-PCR analysis of Medsa;cycA2;2 and the constitutive Msc27 gene expression in different tissues of alfalfa and in exponentially growing A2 cells. B, Detection of the CycA2 protein by western-blot analysis. For A and B, the roots, hypocotyls, and cotyledons were harvested from 1-week-old seedlings, whereas leaves and apical meristems were from 10-d-old plants.
To visualize the spatial and temporal expression pattern of the Medsa;cycA2;2 gene during development, transgenic Medicago truncatula plants carrying a 2,310-bp-long cycA2;2 promoter fragment (including the 5′-UTR) fused to the β-glucuronidase (Gus) reporter gene (uidA from Escherichia coli) were generated. On the basis of Southern-blot analysis, 20 independent primary transformants were selected. Histochemical analysis of these primary cycA2;2pr-Gus transformants revealed identical Gus-staining pattern, however, with varying intensities. The control plants transformed with the pPR97 vector without the insert exhibited no Gus activity.
In the T1 progenies of independent lines expressing cycA2;2pr-Gus from moderate to high levels, a detailed analysis of Medsa;cycA2;2 promoter activity was carried out. Histochemical staining for Gus activity in different tissues and organs revealed, as it was expected, a direct link between cell proliferation and cyclin A2 expression, because blue staining was detected in apical shoot and root meristems (data not shown).
cycA2;2 Is Expressed in the Root Meristem and Involved in Lateral Root Initiation
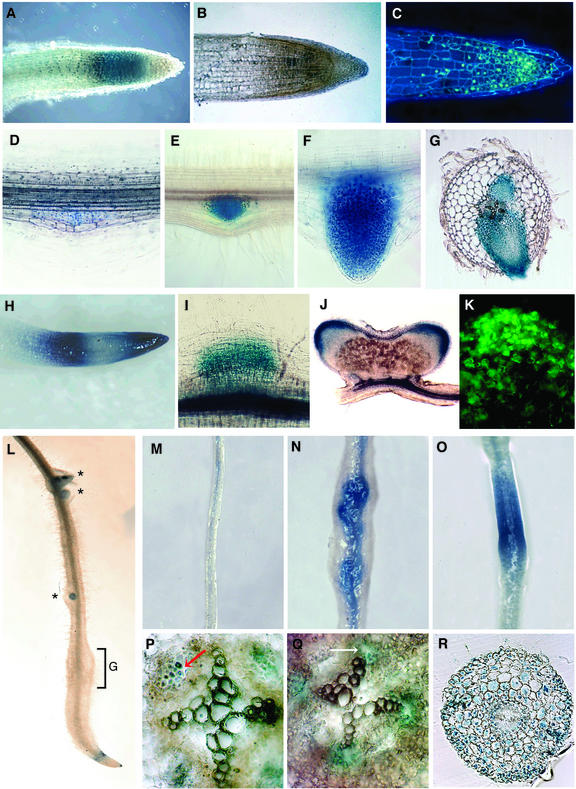
In transgenic plants, the expression pattern was followed from germination to primary and lateral root development. Gus activity was detected in the emerging radicle very early on after imbibition. In the root tip of 3-d-old seedlings, blue staining was visible in the meristematic zone and in the appearing root cap (data not shown). In the primary root of one-week-old plantlets, cycA2;2 expression was localized in the meristematic zone (Fig. 3A). The presence of cycA2;2 transcripts at the root tip was confirmed in longitudinal root sections both by in situ hybridization (Fig. 3B) and immunolocalization of the cyclin A2 protein (Fig. 3C).
Figure 3.
Expression of Medsa;cycA2;2 in the root and during lateral root, nodule, and gall development and its regulation by auxin. A, D through J, and L through R, Histochemical localization of Gus activity in transgenic M. truncatula carrying the cycA2;2pr-Gus fusion in primary roots (A), during different stages of lateral root development (D–H), in nodule primordium (I), in a 100-μm section of bilobed mature nodule (J), and in nematode-infected root (L) where G marks the gall and stars the lateral root primordia. G, A 70-μm transversal section showing Gus activity in developing lateral roots in front of protoxylem poles. B, Localization of the cycA2;2 mRNA in a 7-μm longitudinal root section by in situ hybridization (brown/magenta color). C and K, Immunolocalization of the CycA2 protein in the root and nodule meristems, respectively. M through R, cycA2;2-Gus expression in the nodulation-competent zone of transgenic M. truncatula without hormone treatment (M and P) and treatment for 3 d with 10 μm NAA (N), 1 μm NAA (Q), or 1 μm NPA (O and R). P through R, Eighty-micrometer transversal root sections originate from the application zone. Gus staining in the phloem (P) and in front of the xylem poles (Q) is indicated by arrows.
The Medsa;cycA2;2 promoter-driven Gus expression was induced during lateral root initiation, during all stages of primordium formation, both before (Fig. 3, D and E) and after (Fig. 3F) its emergence from the main root. The expression was strictly limited to cells of the lateral root primordium (Fig. 3G). During the initial growth and elongation of the emerging lateral roots, a zonation of the Gus staining was observed in the root tip where the Gus became transiently inactive in the middle region (Fig. 3H). This zonation was similar to the expression pattern of the Arabidopsis histone H4 gene (Atanassova et al., 1992) or Atcdc2a upon auxin treatment (Hemerly et al., 1993). The Gus activity may mark, in addition to the root apical meristem, dividing cells in the elongation zone. However, the exact reason for the three-zone expression pattern of these genes is still not clear. After further growth of the lateral roots, the Gus expression pattern became similar to that of the primary root.
Medsa;CycA2;2 Is Implicated in Nod Factor-Mediated Cell Cycle Activation and Cell Proliferation But Not in Symbiotic Cell Differentiation
Nodules, like lateral roots, develop in front of the protoxylem poles from de novo meristems. However, development of these organs differs in the triggering signals and the origin of the dividing cell type. Whereas auxin is a key regulator of lateral root development and cell division occurs in the pericycle, nodule development can be triggered by application of the symbiotic partner, S. meliloti or Nod factors provoking cell division in the inner cortex of Medicago spp. roots. Nodules develop in the emerging root hair zone located 5 to 8 mm above the root tip where cells have transient competence for nodule organogenesis.
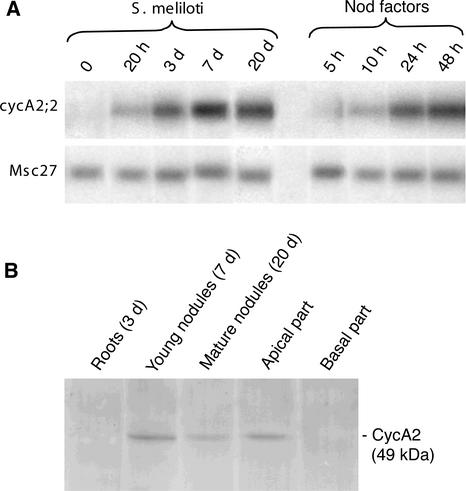
Previous studies (Savouré et al., 1994; Yang et al., 1994) indicated that Nod factors activate the cell cycle in G0-arrested cells; however, which cell cycle genes are the targets of the Nod factors and are required for re-entering the cell cycle was unknown. Because the expression of Medsa;cycA2;2 in the cell cycle was somewhat atypical for A-type cyclins starting already in the G1 phase (Roudier et al., 2000), we studied whether cycA2;2 was involved in the initiation of nodule development. The expression kinetic of cycA2;2 was analyzed by RT-PCR during early stages of nodogenesis (Fig. 4A). Alfalfa seedlings were either inoculated with S. meliloti strain Rm41 or purified Nod factors. Total RNA was isolated from the nodulation competent root zone before visible nodule development and later from developing nodules. Expression of Medsa;cycA2;2 was detectable at 20 h postinoculation with Rm41, which preceded the first division, whereas no signal was observed in the control root zone. Nod factor production in rhizobia starts only after their inoculation on the root surface, which may delay the actual induction of the cycA2;2 gene. Therefore, the roots were treated directly with purified Nod factors at 10−7 m concentration. In this case, expression of cycA2;2 started already 5 to 10 h after the application of Nod factors, and the transcript accumulation during the first 2 d was higher than upon inoculation with S. meliloti. In the Rm41-inoculated roots, the transcript level increased gradually and reached a maximum at 7 d postinoculation when nodule primordia appeared in these experiments. Expression of cycA2;2 was maintained in the growing nitrogen-fixing nodules (20 d postinoculation), however, due to the increasing mass of nodule zone III, the cycA2;2 transcripts, at the same concentration of total RNA samples, were represented at lower abundance.
Figure 4.
Expression of Medsa;cycA2;2 is induced during nodule initiation, and the presence of the CycA2 protein correlates with cell proliferation. A, RT-PCR analysis of cycA2;2 gene activation in the nodulation-competent root zone of alfalfa after S. meliloti or Nod factor application. B, Western-blot analysis of CycA2 protein during nodule development in alfalfa roots. Protein extracts were made from Rm41-inoculated root segments at the indicated time points and from the apical and basal parts of 3-week-old nitrogen-fixing nodules.
By western-blot analysis (Fig. 4B), the cyclin A2 protein was first detectable in the nodule primordia, usually 1 week after the inoculation with S. meliloti. After 3 weeks, the nodules contained the three characteristic central zones: the meristem (zone I), the differentiation or infection zone (zone II), and the nitrogen-fixing zone (zone III) as well as the typical peripheral tissue layers. After nodules dissection in apical (zones I and II) and basal (zone III) regions, western-blot analysis revealed that the CycA2 protein was present exclusively in the apical part, which contains actively proliferating cells and division-arrested cells undergoing multiple endoreduplication cycles along 10 to 15 cell layers.
In the cycA2;2pr-Gus transgenic plants, the first detectable Gus staining was observed 48 h after inoculation with strain Rm41 in the dividing inner cortical cells (data not shown); the Gus activity then increased concomitantly with the formation of the nodule primordium (Fig. 3I). In the mature differentiated nodules (Fig. 3J), the blue staining was restricted to the meristem and to the submeristematic cell layers but was absent in zone II where endoreduplication cycles occur. Location of cyclin A2 in the meristematic region was confirmed by in situ hybridization (not shown) as well as by immunolocalization of the cyclin A2 protein (Fig. 3K).
The expression pattern of cycA2;2 during nodule initiation indicated that it could be one of the cell cycle genes involved in the recruitment of differentiated cells for proliferation and development of secondary meristems upon developmental signals. Moreover, the exclusive presence of cycA2;2 transcripts in proliferating cells suggested that CycA2;2 may be necessary for the maintenance of mitotic activity, whereas it is dispensable or even undesirable for the endoreduplication cycles.
Expression of cycA2;2 Is Not Required for Giant Cell Development in Root Knot Nematode-Induced Galls
Nematodes induce redifferentiation of root cells to NFSs. Infection occurs usually in the vicinity of the root tip where second-stage infective juveniles penetrate the roots and migrate toward the vascular cylinder. Close to the xylem, the nematodes trigger the development of a few giant cells characterized by nuclear and cellular hypertrophy generated via endoreduplication cycles (Williamson and Hussey, 1996) and sequential mitosis without cytokinesis (Huang and Maggenti, 1969). Formation of giant cells and division of the neighboring root cells, results in the formation of root knots or galls. Involvement of Medsa;cycA2;2 in gall formation and in the development of NFSs was tested by infection of the cycA2;2pr-Gus transgenic plants with the root knot nematode M. incognita. Gus staining performed after various time periods of infection did not reveal, however, any Gus activity at any stage of NFS development, indicating that cycA2;2 was not implicated either in the redifferentiation of root cells or in the development of polyploid giant cells. Expression of cycA2;2pr-Gus in the lateral root primordia developing in a distance from the gall was unaffected by the nematode infection. On the other hand, as a result of nematode infection near the root tip, root growth was halted and progressively the cycA2;2pr-Gus expression was diminished in the root meristem (Fig. 3L) until further root growth started.
Overexpression of cycA2;2 in Transgenic Plants Does Not Result in Overproduction of the CycA2;2 Protein and Has No Effect on Plant Development
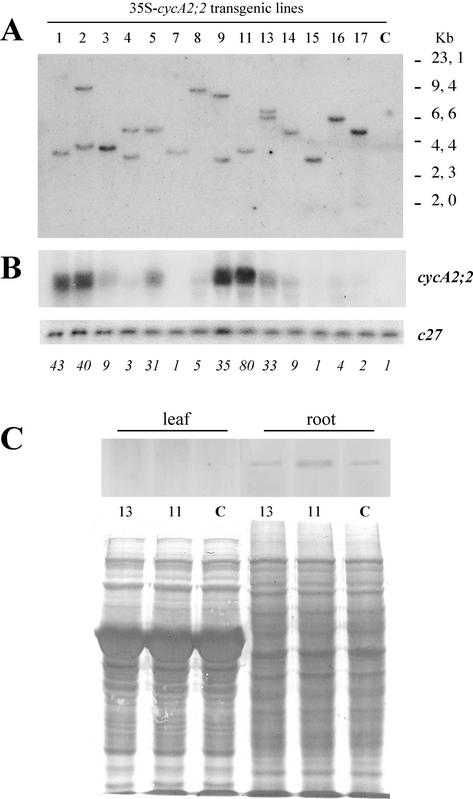
Overexpression of the Arabidopsis cyclins cycD3;1 and D2;1 (Riou-Khamlichi et al., 1999; Cockcroft et al., 2000) and ectopic expression of cyclin B1;1 (Doerner et al., 1996) have been shown to accelerate growth or to disturb meristem organization. As expression of the cycA2;2 gene was linked to cell proliferation and formation of meristems and was absent in the differentiating cells, we investigated how ectopic expression of the cycA2;2 gene from the 35S promoter will affect plant development. Fourteen independent transgenic lines, 10 with a single and four with two T-DNA insertions, were selected for further studies (Fig. 5A). Because the cycA2;2 gene has no detectable expression in wild type M. truncatula leaves, it was possible to test the expression level of the transgene directly in this organ. Northern analysis of the leaf RNA samples (Fig. 5B) revealed significant variations in the cycA2;2 transcript levels, from undetectable to high levels. For quantification of the loaded RNA samples, the blot was hybridized with the constitutive c27 cDNA probe. After normalization of the cycA2;2 hybridization signal with that of c27, one plant (line 11) displayed 80-fold, lines 1 and 2 about 40-fold, and lines 5, 9, and 13 a 30- to 35-fold increase in the cycA2;2 transcript levels. None of these plants exhibited abnormalities in their development or nodulation capacities (data not shown). Western-blot analysis of root and leaf protein extracts from control and transgenic plants with high- or medium-level expression revealed that none of these overexpressing plants contained proportionally increased CycA2;2 protein levels (Fig. 5C). In the leaves, the CycA2;2 protein was undetectable in all samples, whereas in the roots, the CycA2;2 protein was detectable, but the levels were similar to the control. Because the d-box pathway is active in plants and results in the degradation of the d-box containing cyclins by the anaphase-promoting complex and the 26S proteasome (Genschik et al., 1998), we tested how inhibition of the 26S proteasome affects the CycA2;2 protein levels. By western-blot analysis of control and transgenic seedlings treated for 36 h with the 26S proteasome inhibitor MG132 at 50 μm concentration, no increase in the amounts of CycA2;2 comparable with the cycA2;2 transcript levels in the transgenic plants was detected (data not shown). One has to note, however, that MG132, which is used generally in the in vitro assays or cell cultures, was toxic for the plants and inhibited further growth and development. Therefore, from this experiment, it is difficult to judge the involvement of the d-box pathway in the down-regulation of CycA2;2 in the transgenic plants. Thus, overexpression of the transgene in M. truncatula may be compensated by various mechanisms, including lower efficiency of translation and degradation of the CycA2;2 protein.
Figure 5.
Overexpression of cycA2;2 in transgenic M. truncatula plants does not correlate with increased level of the CycA2;2 protein. A, Southern-blot analysis of HindIII digested genomic DNAs hybridized with the hptII probe. B, Northern-blot analysis of cycA2;2 and c27 expression. Numbers below the blots show the relative signal intensities of cycA2;2 after normalization with the c27 signals. C, Western-blot analysis of the CycA2;2 protein with the stained protein membrane. C, Control.
Antisense Expression of cycA2;2 Aborts Regeneration of Somatic Embryos
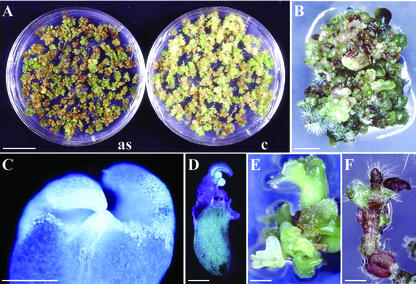
Because overexpression of cycA2;2 did not cause alterations in the amount of the CycA2;2 protein, we tested how antisense expression of cycA2;2 will affect plant development. M. truncatula was transformed with the full-length cDNA cloned in antisense orientation in pBinHyg as well as with pBinHyg without insert as control. Callus formation and development of somatic embryos were comparable in the control and antisense transformations, although somatic embryos obtained with the antisense construct were more clustered and displayed a more intense green color (Fig. 6A). However, differentiation of somatic embryos into plants was halted in the antisense transformants. In most cases, no regeneration occurred while production of embryos continued for 18 months in the absence of hormones (Fig. 6B). In these embryos, besides normal-looking shoot apical meristems (Fig. 6C), formation of secondary embryos was often observed at the site of apical meristem (Fig. 6D) and a single embryo resulted in the development of many others (Fig. 6E). In rare cases, a few leaves or leaf-like structures appeared, and hairs were produced at abnormal sites (Fig. 6F). These plantlets were violet-colored due to anthocyan production, were unable to develop roots, and died. Cultivation of the embryos with hormone supplements at varying combinations and concentrations did not improve their regeneration. The complete lack of root development and the failure of shoot development seem to indicate a requirement for cycA2;2 in the formation and activity of the apical meristems.
Figure 6.
Phenotype of the antisense cycA2;2 transformation in M. truncatula. A, Callus formation and development of somatic embryos transformed with the 35S-antisense cycA2;2 (as) or with the binary vector (c). B, Prolonged cultivation of the antisense somatic embryos. C, One hundred-micrometer-thick longitudinal section through the meristem of a somatic embryo. D, Development of secondary embryos on the primary one. E, Embryos deriving from a single one. F, Aborted development of a highly stressed plantlet. Bars = 20 mm in A; 1 mm in B, D, and E; 0.5 mm in C; and 2 mm in F.
Up-Regulation of the cycA2;2 Gene by Auxin
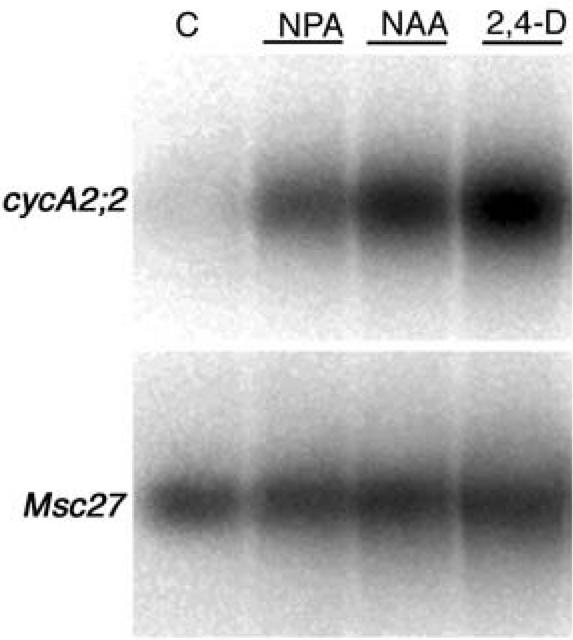
The presence of putative AuxREs in the cycA2;2 promoter and in the 5′-UTR suggested that the expression of this cyclin gene may be regulated by auxin. This was tested by RT-PCR in the emerging root hair zone of alfalfa plantlets (Fig. 7), where in the absence of phytohormones, only weak expression of cycA2;2 was detected. In contrast, a high increase in the cyclin transcript levels was observed after overnight treatment of the roots with 10 μm 1-naphthaleneacetic acid (NAA) or 2,4-dichlorophenoxyacetic acid (2,4-D) or with the auxin transport inhibitor N-1-naphtylphthalamic acid (NPA) at 1 μm. These results suggest that the AuxREs are functional and mediate up-regulation of the cycA2;2 gene in the presence of auxin.
Figure 7.
Up-regulation of the Medsa;cycA2;2 gene by auxins and a polar auxin-transport inhibitor. RT-PCR analysis showing the Medsa;cycA2;2 transcription levels after spot-inoculation of nitrogen starved alfalfa plantlets in the Nod factor sensitive root zone with 1 μm NPA, 10 μm NAA, 10 μm 2,4-D, or nitrogen-free medium for 14 h. Msc27 transcript levels served as control for the amount of RNAs.
To visualize both the cycA2;2 expression at cellular level and the morphological effects of hormones at the site of application, transgenic M. truncatula plants carrying the cycA2;2pr-GUS fusion were treated with NAA and NPA applied in agar blocks on the emerging root hair zone for 72 to 76 h, and then GUS staining was performed. As a control, transgenic cycA2;2pr-Gus roots were treated similarly but with hormone-free agar blocks which, consistently with the RT-PCR result, only occasionally exhibited a faint blue staining along the vascular tissues (Fig. 3M). Exogenous supply of auxins is known to trigger lateral root development. Application of agar blocks containing either 10 μm NAA (Fig. 3N) or 2,4-D (data not shown) induced the formation of lateral root primordia in all of the treated roots and simultaneously strong Gus staining in the primordia and in the vascular tissue (Fig. 3N). However, these experiments did not allow discrimination of whether cycA2;2 expression was activated directly by the auxin treatment, by the induction of lateral root development, or by both. Therefore, to uncouple the auxin effect from the developmental program, the transgenic roots were treated with the polar auxin transport inhibitor, NPA at low (1 μm) concentration, which did not trigger extensive cell division and formation of nodule-like structures. This treatment elicited homogenous Gus activity in the application zone with limited root swelling (Fig. 3O), demonstrating that local accumulation of auxins alone was sufficient for the activation of the cycA2;2 gene.
Auxin Controls the Spatial Expression of cycA2;2 in Medicago spp. Roots
To localize more precisely the cycA2;2 promoter activity in roots, especially in response to auxin, transgenic cycA2;2pr-Gus seedlings, pregrown for 2 d on hormone-free medium, were transferred on plates containing either 1 μm NAA or NPA or no hormones in the medium. The GUS staining was inspected after 3 d of incubation in a series of root cross sections. In the control roots, whenever the faint Gus staining was detectable, it was associated with phloem cells and was absent in front of the xylem poles (Fig. 3P). During the treatments, control roots grew (in average 14 mm), whereas treatment of roots with 1 μm NAA arrested root growth and triggered hyperplasia and root thickening but no lateral root development. In the NAA-treated roots, instead of the phloem-associated GUS staining, the GUS activity appeared in front of the xylem poles where both lateral roots and nodules initiate (Fig. 3Q). Treatment of the roots with NPA at 1 μm resulted in GUS staining in each cell type and cell layer from the pericycle to the epidermis (Fig. 3R). Because in the zone of application, the auxin transport inhibitor NPA provokes accumulation of auxins, this overall expression pattern is consistent with the auxin inducibility of cycA2;2.
DISCUSSION
Regulated changes in the cell cycle underlie many aspects of growth and differentiation where cyclins may be key regulatory elements by providing a spatial and temporal control of cyclin-dependent kinase activities. Most of our knowledge of cyclins came from studies on yeast and animals, however, in contrast to the overwhelming data obtained in cell cultures or isolated cells, surprisingly few studies have been devoted to the role of cyclins in plant organ development. From the A2-type cyclin group in plants, only the Arabidopsis Arath;cycA2;1 gene was studied in this respect (Burssens et al., 2000a).
Here, we present the organization, promoter structure, and expression pattern of a distantly related A2-type cyclin gene from alfalfa, Medsa;cycA2;2, during root developmental programs. This cyclin differs from Arath;cycA2;1 in cell cycle regulation (Burssens et al., 2000b; Roudier et al., 2000), in developmental expression pattern (this work; Burssens et al., 2000a) and in hormonal regulation (this work; Himanen et al., 2002), thereby it is unlikely that they would be orthologs. Here, we show that in contrast to Arath;cycA2;1 expressed in both dividing and non-dividing cells throughout plant development (Burssens et al., 2000a), expression of Medsa;cycA2;2 is restricted to proliferating cells designated to meristem formation during developmental programs. The lack of cycA2;2 transcripts in endoreduplicating cells further supports the involvement of cycA2;2 in the mitotic cycles, where its major role can be the preparation of cells for M-phase entry. Overexpression of the gene did not correlate with overproduction of the protein and had no effect on plant development, in contrast to the antisense expression that aborted plant regeneration. We show the presence of auxin response and auxin response-like elements in the Medsa;cycA2;2 promoter region that are likely responsible for direct activation of the gene by auxin. Moreover, this work provides the first indication that auxin, in addition to gene activation, controls the spatial expression by inducing Medsa;cycA2;2 in front of the xylem poles where lateral root and nodule initiation take place.
Organization of the Medsa;cycA2;2 Gene and Potential Regulatory Elements
The transcribed region of the Medsa;cycA2;2 gene spreads over 4.1 kb and is interrupted by 11 introns, one of them being in the 5′-UTR. This exon-intron organization is similar to that of the Arabidopsis cycA2 genes (Vandepoele et al., 2002). The promoter region of Medsa;cycA2;2, contains several potential regulatory elements. In a 44-bp-long tandem repeat and in the first intron of the 5′-UTR, putative auxin response-like elements were found. The TGTCTC and the related (G/T) GTCCCAT cis-acting AuxREs were identified by the analysis of the soybean GH3 (Liu et al., 1994) and the pea (Pisum sativum) PS-IAA4/5 promoters (Ballas et al., 1993), respectively. Composite AuxREs were found in the promoter of many auxin-responsive genes that in combination with constitutive or other coupling regulatory elements could potentially confer a wide range of tissue-specific and developmentally regulated expression patterns (Guilfoyle et al., 1998). It is uncertain, however, whether a single TGTCTC element would be active while two copies oriented as palindrome or as direct repeat conferred auxin responsiveness (Ulmasov et al., 1997). The Medsa;cycA2;2 promoter contains a slightly degenerated version of the TGTCTC sequence (GTCTC) as direct repeats as well as a proper TGTCTC element. Although this study demonstrated auxin responsiveness of Medsa;cycA2;2, the significance of these AuxREs as cis-acting elements remains to be assessed. By analyzing the 5′-upstream regions, we did not find AuxREs in the Arath;cycA2;1, Arath;cycA2;2, and Arath;cycA2;4 promoters, and only a single TGTCTC element was present in the promoter region of Arath;cycA2;3, which makes auxin regulation of the Arabidopsis cycA2 genes unlikely, at least by the same transcriptional regulator implicated in Medsa;cycA2;2 expression. However, recent work by Himanen et al. (2002) also showed responsiveness of Arath;cycA2;1 to auxin.
Three repeated sequences with possible regulatory function in the vicinity of the TATA box as well as two putative Myc- and five Myb-binding sites were present in the promoter region of Medsa;cycA2;2. In animal cells, the Myc and Myb transcription factors regulate cyclin A expression and likely mediate distinct control in cell cycle and differentiation program (Jansen-Durr et al., 1993; Rudolph et al., 1996; Bouchard et al., 1998; Muller et al., 1999). Auxin-responsive elements and Myc- and Myb-binding consensus sequences were also found in the promoters of the Arabidopsis cdk-A, Atcdc2a, and the tobacco (Nicotiana tabacum) mitotic cyclin Nicsy;cycB1;1 genes and were shown to influence significantly the expression level of these genes (Chung and Parish, 1995; Trehin et al., 1997). These results suggest that auxin and Myb/Myc transcription factors may be positive regulators of cyclin A2 expression and are part of a yet elusive general network that coordinates cyclin and cdk expression, and as such, cell division during plant development.
Auxin-Mediated Up-Regulation of cycA2;2 in Front of the Xylem Poles
Auxin responsiveness of the Medsa;cycA2;2 gene was demonstrated by RT-PCR experiments. Activation of cycA2;2 by exogenous application of auxins on Medicago spp. roots indicated that the AuxRE-like cis-elements are likely functional and induction of the cycA2;2 gene may rely on these AuxREs. The auxin effect on tissue- and cell type-specific expression pattern of cycA2;2 was studied in transgenic plants expressing the Gus reporter gene from the Medsa;cycA2;2 promoter containing all putative regulatory elements. Treatment of the transgenic plants with 10 μm NAA or 2,4-D resulted in the formation of multiple lateral root primordia and induction of the cycA2;2 gene in the newly induced primordia. Application of NPA at 1 μm concentration did not elicit any morphological response but activated cycA2;2 expression in most cell types, thus uncoupling cycA2;2 expression from morphogenesis and mitotic activity. The differences in the expression pattern and in the morphological responses elicited by auxins and the auxin transport inhibitor NPA may reflect, in the case of NPA, elevated auxin levels in all cell types. This could explain the overall activation of the cycA2;2 gene, which together with the lack or perturbation of the endogenous auxin gradient could interfere with lateral root development, which is dependent on the root basipetal auxin transport activities (Casimiro et al., 2001). In the case of Arath;cycA2;1, the promoter activity on NPA was limited to the vascular parenchyma (Himanen et al., 2002), which may indicate either indirect or different auxin responsiveness of Arath;cycA2;1.
Auxin responsiveness is considered to be auxin-mediated transcriptional up-regulation of a gene without impact on spatial regulation. In the case of Medsa;cycA2;2, auxin-treatment also affected the spatial expression pattern by suppressing the phloem-associated expression observed in the cycA2;2pr-Gus plants without hormone treatment and de novo-inducing cycA2;2 transcription in front of the xylem poles, where lateral roots initiate. This auxin-regulated spatial cyclin production may provide a novel insight and further complexity in hormonal regulation of the cell cycle in planta.
De Novo Induction of cycA2;2 in Differentiated Cells Reprogrammed for Nodule Development
While treatment of roots with auxin was sufficient for cycA2;2 transcription and triggering cell division in the pericycle and for development of lateral roots, it was insufficient for nodule development. Nod factors or S. meliloti in nitrogen-starved plants was able to induce cycA2;2 expression in the inner cortex long before the first cell division, which was likely achieved by combined action of Nod factors and phytohormones. It has been proposed that nitrogen starvation may cause an auxin burst and changes the endogenous hormone gradients in the root (Foucher and Kondorosi, 2000; Mathesius et al., 2000). Furthermore, recent studies have shown that Nod factors themselves inhibited specifically and transiently the auxin transport capacity in the nodulation competent zone (Boot et al., 1999; Mathesius et al., 1998). This inhibition of polarized auxin transport resulted in the transient accumulation of auxin in the inner cortex in front of protoxylem poles (Mathesius et al., 1998) that may represent a positional control for cortical cell division. Thus, Nod factors and nitrate limitation may modulate the auxin/cytokinin ratio in a cell-specific manner eliciting de novo expression of cycA2;2 in the inner cortex in front of the protoxylem poles. The early activation of cycA2;2 during lateral root and nodule organogenesis suggests that cycA2;2 is required for the reactivation of differentiated cells and can be a limiting factor for the activation of the PSTAIRE-type cyclin-dependent kinases.
CycA2 Is Involved in Postembryonic Meristem Formation and Activity
In plants, the major sites of cycA2;2 expression are the root and shoot apical meristems. In the aerial part, practically no expression was found in the differentiated tissues that were devoid of proliferating cells. In roots, in addition to the main root tip, strong Gus staining was detected during lateral root initiation and primordium formation; later, in the elongated lateral roots, the cycA2;2pr-Gus expression pattern was similar to that of the primary root. However, in the emerging lateral root, the central zone within the Gus positive region became transiently inactive. This was also reported for other auxin-responsive gene promoters such as Atcdc2a (Hemerly et al., 1993) that may reflect stage-, site-, and/or cell-specific regulation of the cell cycle during early root development. During gall formation, no cycA2;2pr-Gus expression was detected in the dividing cortical cells around the NFSs. This was somewhat unexpected because in these cells, as in the case of nodule development, the cell cycle was reactivated. Because gall development does not involve meristem formation, this may suggest that the cycA2;2 function is required for persisting mitotic cycles, leading primordium and secondary meristem formation. This hypothesis is further supported by the inability of the antisense cycA2;2 somatic embryos for shoot and root development.
CycA2;2 Is Not Required for Endoreduplication Cycles
Nodule differentiation is characterized by consecutive endoreduplication cycles in the zone II. Like in roots, the distance of the cells from the meristem along the longitudinal axis reflects their age. By aging, the cells enlarge proportionally with their nuclear DNA content, after each round of endocycles. This unique organization, the persistent local cell differentiation events and endocycles make the indeterminate nodule extremely valuable for studying cell cycle regulation during organ development and morphogenesis. In nodules, cycA2;2 expression was strong in the meristem and in the adjacent cell layers but was absent in zone II. In situ hybridization revealed expression of the S-phase-specific histone H3 gene both in the meristem and in the zone II, in a few randomly localized cells just undergoing endoreduplication (Cebolla et al., 1999). Despite our careful inspection of numerous nodule sections, no evidence was found for cycA2;2 expression in the zone II, neither with Gus staining nor with immunolocalization. This indicates that Medsa;CycA2;2 is not required for endocycles, not even for DNA synthesis, suggesting that in S-phase progression, the control of DNA replication may involve different A-type cyclins in the mitotic and endocycles. In line with that, an A3-type cyclin Lyces;CycA3;1 has been proposed to be involved in endoreduplication of the gel tissue in the tomato (Lycopersicon esculentum) fruit (Joubes et al., 2000). Similar to nodules, no cycA2;2 expression was detected in the galls during giant cell formation, providing a further independent confirmation that cycA2;2 is dispensable for the endoreduplication cycles. Moreover, no cycA2;2 expression was detected during trichome development or in etiolated hypocotyls, both involving repeated rounds of endoreduplication cycles (data not shown). The major activity of the CycA2;2-CdkA complex peaks at the G2-M transition, suggesting that its function is required for the preparation of cells to M phase entry. Because there is no M-phase in the endoreduplication cycle, this activity is likely needless or even should be avoided. The absence of cycA2;2 transcripts may even suggest that repression of the gene is needed for the exit from the mitotic cycle and for the transition either toward cell differentiation or endocycles.
MATERIALS AND METHODS
Isolation and Sequence Analysis of the Medsa;cycA2;2 Genomic Clones
An alfalfa (Medicago sativa subsp. sativa cv Nagyszénási) genomic library was constructed in the EMBL4 cosmid by cloning 15- to 20-kb MboI fragments obtained with partial digestion. The complexity of the library was 4 × 105 and the titer 1010. For the screening, filters were made in duplicates from eight plates, each containing 50,000 plaque-forming units. With the cycA2;2 cDNA probe, seven phage plaques hybridized strongly and several other weakly, which were not analyzed further. RFLP using a 290-bp probe to determine their relatedness. This probe was generated by PCR using as forward primer the 5′-GCTGGAGAGGTTTCAAGTCG-3′ and as reverse primer the 5′-ACATGAGGTTGAGCAGGCTT-3′ sequences in the presence of [α-32P]dCTP according to the Taq polymerase manufacturer instructions (Roche Diagnostics, Mannheim, Germany). The promoter region of the longest insert hybridizing with the probe was subcloned into pBluescript II KS vector (Stratagene, La Jolla, CA) and sequenced using Automatic Sequencer 373A (Applied Biosystems, Foster City, CA) with the dideoxy chain termination method (Big Dye Terminator, Applied Biosystems).
Construction of a Chimeric Medsa;cycA2;2 Promoter-Gus Gene, Plant Transformation, and Regeneration
cycA2;2pr-Gus was constructed by cloning the 2,310-bp Bsu36-XhoI promoter region in front of the Gus reporter gene (uidA from Escherichia coli) into the binary vector pPR97 (Szabados et al., 1995). The 35S sense and antisense cycA2;2 constructs were made in the binary vector pBinHyg (Gatz et al., 1992) by cloning the full-length cDNA in the two orientations. The resulting plasmids and the binary vectors without insert were electroporated into Agrobacterium tumefaciens strain EHA105 and then transformed into Medicago truncatula cv R108 using the transformation and regeneration procedure described by Trinh et al. (1998). Southern and northern blots were performed according to standard procedures (Sambrook et al., 1989).
Plant Material, Growth Conditions, and Treatments
Alfalfa subsp. sativa cv Sitel and M. truncatula T1 and T2 seeds were surface sterilized with 20% (v/v) Inov'chlore (Inov'chem, Tanneries, France) for 20 min with shaking, rinsed three times with sterile water, and germinated for 2 d on 0.7% (w/v) water agar plates. The seedlings were transferred in petri dishes containing nitrogen-free solid medium (solution I). The plates were then incubated in a growth chamber at 24°C under a 16-h photoperiod. Plant growth conditions, media are described in detail at http://www.isv.cnrs-gif.fr/embo2/manuels/pdf/module1.pdf. The alfalfa cell suspension was cultured as described previously (Roudier et al., 2000).
Expression of cycA2;2 in the Nod factor-sensitive zone was analyzed upon various treatments by RT-PCR. Four-day-old alfalfa plantlets grown on agar plates were spot-inoculated in the emerging root hair zone Sinorhizobium meliloti strain Rm41 at A600 = 0.4 or purified S. meliloti Nod factors at 10−7 m as described previously (Crespi et al., 1994) or treated with NPA (10−6 m), NAA (10−5 m), or 2,4-D (10−5 m) that was applied in the form of agar cubes of 0.5 cm. In the case of each treatment, 5- to 10-mm-long root segments, corresponding to the emerging root hair zone or the treated area, were excised from 40 plants and frozen in liquid nitrogen before RNA extraction.
To analyze the expression of the cycA2;2-Gus fusion in transgenic M. truncatula upon treatments with Rm41 or Nod factors, 3-d-old plantlets were placed in plastic growth pouches (Mega International, Minneapolis) as described by Journet et al. (1994) and inoculated 1 week later with Rm41 or purified Nod factors (10−7 m). For auxin treatments, NAA at 10−6 m or 10−5 m, 2,4-D at 10−5 m, and NPA at 10−6 m were added to warm 1% (w/v) agar (Mayoly Spindler, Chatou, France) and applied in the form of 0.5-cm agar cubes at the emerging root hair zone of plantlets grown on agar plates for 3 d. Gus activity in the treated roots or nodules was tested in at least 10 plantlets for each treatment.
For analyzing cycA2;2-Gus expression in galls, 3-week-old in vitro plants were inoculated with 2,000 disinfected Meloidogyne incognita second-stage juveniles as described previously (Sijmons et al., 1991). The inoculated plants were harvested from 3 to 21 d after inoculation, and NFSs were stained for histochemical localization of GUS.
Expression Analysis by RT-PCR
RT-PCR experiments were made with total RNAs extracted from different tissues or from treated root segments using the RNeasy Plant Mini Kit (Qiagen, Courtaboeuf, France). cDNA preparation, semiquantitative RT-PCR, gel electrophoresis, and DNA transfer blot were performed essentially according to Bauer et al. (1994). For PCR reactions, the Taq polymerase and buffer were from Roche Diagnostics. Primers used to amplify Medsa;cycA2;2 and the constitutively expressed Msc27 cDNAs were forward, 5′-AACCGCCATCCGCAAGAAGTA-3′; reverse, 5′-AAGCAAGAACCCCCCATAAGG-3′; and forward, 5′-GGAGGTTGAGGGAAAGTGG-3′; reverse, 5′-CACCAACAAAGAATTGAAGG-3′, respectively. Medsa;cycA2;2 and Msc27 probes were prepared by PCR using the same primers and in the presence of [α-32P]dCTP.
Western-Blot Analysis
For western-blot analysis, different organs exponentially growing A2 cell suspensions from young and mature nodules from S. meliloti-inoculated alfalfa plants were collected and ground in liquid nitrogen. Proteins extracted in Laemmli buffer (Laemmli, 1970) were separated on SDS-PAGE subjected to western-blot analysis according to standard procedures (Sambrook et al., 1989) Equal protein loading and transfer efficiency were verified by staining the membrane with red Ponceau. The rabbit anti-CycA2;2 polyclonal antibodies were used in 1:2,000 (v/v) dilution. The secondary alkaline phosphatase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) was used in 1:25,000 (v/v) and detected by a colorimetric reaction (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate, Sigma-Aldrich, St-Quentin Fallavier, France) according to the manufacturer's instructions.
In Situ Hybridization
Preparation of paraffin-embedded material and in situ hybridizations were performed as described by Crespi et al. (1994) and Coba de la Pena et al. (1997). The antisense and the control sense riboprobes generated from the Medsa;cycA2;2 cDNA were labeled with Dig-UTP-labeled probes. Hybridization with the sense probe developed at the same time as the antisense hybridizations revealed no significant signal.
Histochemical Localization of Gus, Microscopic Analysis, and Semithin Sectioning
Histochemical Gus staining was performed according to Pichon et al. (1992). The intact plants, organs, or sections were incubated in a 0.1 m sodium phosphate buffer, pH 7, containing 0.2 mm 5-bromo-4-chloro-3-indolyl-β-d-GlcUA (Biosynth AG, Staad, Switzerland), 5 mm EDTA, 0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, and 0.5% (v/v) Triton X-100, for a few hours or overnight to get intense and cell-specific labels but avoiding overstaining. A binocular microscope (M420, Wild, Heerbrugg, Switzerland) was used for whole-root observations. Before microscopic observation, the stained material was briefly cleared with sodium hypochloride as described by Pichon et al. (1992). For localization of Gus activity at cellular level, stained roots were post-fixed in 1% (w/v) paraformaldehyde in 100 mm potassium phosphate buffer (pH 7.0) and embedded in 7% (w/v) agarose. Microtome sections of 70 to 100 μm (Micro-cut H1200, Bio-Rad, Hercules, CA) were observed under bright- and dark-field as well as Nomarski optics using a Polycar microscope (Reichart-Jung, NuBlock, Germany).
Immunolocalization
For indirect immunofluorescence microscopy, the samples were fixed for 1 h at 4°C in 2% (w/v) paraformaldehyde in phosphate buffer (pH 7.2), embedded in LR-white resin (Sigma-Aldrich) at −10°C, polymerized under UV light, and cut. After blocking with 7% (w/v) milk, the samples were incubated overnight at 4°C with anti-CycA2;2 antibodies diluted at 1:400 and then with the secondary goat anti-rabbit FITC-conjugated antibody (Sigma-Aldrich) at a dilution of 1:60 for 1 h at room temperature. Preparations were examined using a Polyvar Reichert-Jung epifluorescence microscope equipped with standard fluorescence filters.
ACKNOWLEDGMENT
We are grateful to N. Mansion for her help in photographic work.
Footnotes
This work was supported by the Ministère de la Recherche et Technologie (to F.R.), by the Ministère des Affaires étrangères (to E.F.), and by the European Commission, European Cell Cycle Consortium Network Program (grant no. QLG2–99–00454 to G.H.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.011122.
LITERATURE CITED
- Atanassova R, Chaubet N, Gigot C. A 126 bp fragment of a plant histone gene promoter confers preferential expression in meristems of transgenic Arabidopsis. Plant J. 1992;2:291–300. doi: 10.1111/j.1365-313x.1992.00291.x. [DOI] [PubMed] [Google Scholar]
- Ballas N, Wong LM, Theologis A. Identification of the auxin-responsive element, AuxRE, in the primary indoleacetic acid-inducible gene, PS-IAA4/5, of pea (Pisum sativum) J Mol Biol. 1993;233:580–596. doi: 10.1006/jmbi.1993.1537. [DOI] [PubMed] [Google Scholar]
- Bauer P, Crespi MD, Szecsi J, Allison LA, Schultze M, Ratet P, Kondorosi E, Kondorosi A. Alfalfa Enod12 genes are differentially regulated during nodule development by Nod factors and Rhizobiuminvasion. Plant Physiol. 1994;105:585–592. doi: 10.1104/pp.105.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW. Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativassp. nigra roots. Mol Plant-Microbe Interact. 1999;12:839–844. [Google Scholar]
- Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol. 1998;8:202–206. doi: 10.1016/s0962-8924(98)01251-3. [DOI] [PubMed] [Google Scholar]
- Burssens S, de Almeida Engler J, Beeckman T, Richard C, Shaul O, Ferreira P, Van Montagu M, Inzé D. Developmental expression of the Arabidopsis thaliana cycA2;1gene. Planta. 2000a;211:623–631. doi: 10.1007/s004250000333. [DOI] [PubMed] [Google Scholar]
- Burssens S, Himanen K, van de Cotte B, Beeckman T, Van Montagu M, Inzé D, Verbruggen N. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta. 2000b;211:632–640. doi: 10.1007/s004250000334. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ et al. Auxin transport promotes Arabidopsislateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla A, Vinardell JM, Kiss E, Olah B, Roudier F, Kondorosi A, Kondorosi E. The mitotic inhibitor ccs52is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 1999;18:4476–4484. doi: 10.1093/emboj/18.16.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubet-Gigot N. Plant A-type cyclins. Plant Mol Biol. 2000;43:659–675. doi: 10.1023/a:1006303100592. [DOI] [PubMed] [Google Scholar]
- Chung S, Parish R. Studies on the promoter of the Arabidopsis thaliana cdc2agene. FEBS Lett. 1995;362:215–219. doi: 10.1016/0014-5793(95)00211-q. [DOI] [PubMed] [Google Scholar]
- Coba de la Pena T, Frugier F, McKhann HI, Bauer P, Brown S, Kondorosi A, Crespi M. A carbonic anhydrase gene is induced in the nodule primordium and its cell-specific expression is controlled by the presence of Rhizobiumduring development. Plant J. 1997;11:407–420. doi: 10.1046/j.1365-313x.1997.11030407.x. [DOI] [PubMed] [Google Scholar]
- Cockcroft CE, den Boer BG, Healy JM, Murray JA. Cyclin D control of growth rate in plants. Nature. 2000;405:575–579. doi: 10.1038/35014621. [DOI] [PubMed] [Google Scholar]
- Crespi MD, Jurkevitch E, Poiret M, d'Aubenton-Carafa Y, Petrovics G, Kondorosi E, Kondorosi A. enod40, a gene expressed during nodule organogenesis, codes for a non-translatable RNA involved in plant growth. EMBO J. 1994;13:5099–5112. doi: 10.1002/j.1460-2075.1994.tb06839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner P, Jorgensen JE, You R, Steppuhn J, Lamb C. Control of root growth and development by cyclin expression. Nature. 1996;380:520–523. doi: 10.1038/380520a0. [DOI] [PubMed] [Google Scholar]
- Gatz C, Frohberg C, Wendenburg R. Stringent repression and homogenous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 1992;2:397–404. doi: 10.1111/j.1365-313x.1992.00397.x. [DOI] [PubMed] [Google Scholar]
- Favery B, Complainville A, Vinardell JM, Lecompte P, Vaubert D, Mergaert P, Kondorosi A, Kondorosi E, Crespi M, Abad P. The endosymbiosis-induced genes enod40 and ccs52A are involved in endoparasitic-nematode interactions in Medicago truncatula. Mol Plant-Microbe Interact. 2002;15:1008–1013. doi: 10.1094/MPMI.2002.15.10.1008. [DOI] [PubMed] [Google Scholar]
- Foucher F, Kondorosi E. Cell cycle regulation in the course of nodule organogenesis. Plant Mol Biol. 2000;43:773–786. doi: 10.1023/a:1006405029600. [DOI] [PubMed] [Google Scholar]
- Genschik P, Criqui MC, Parmentier Y, Derevier A, Fleck J. Cell cycle-dependent proteolysis in plants: identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor MG132. Plant Cell. 1998;10:2063–2075. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle T, Hagen G, Ulmasov T, Murfett J. How does auxin turn on genes? Plant Physiol. 1998;118:341–347. doi: 10.1104/pp.118.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebsgaard SM, Korning PG, Tolstrup N, Englbrecht J, Rouze P, Brunak S. Splice site prediction in Arabidopsis thalianaDNA by combining local and global sequence information. Nucleic Acid Res. 1996;24:3439–3452. doi: 10.1093/nar/24.17.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inzé D. cdc2a expression in Arabidopsisis linked with competence for cell division. Plant Cell. 1993;5:1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Maggenti AR. Wall modifications in developing giant cells of Vicia faba and Cucumis sativus induced by root knot nematode, Meloidogyne javanica. Phytopathology. 1969;59:931–937. [PubMed] [Google Scholar]
- Jansen-Durr P, Meichle A, Pagano M, Finke K, Botz J, Wessbecher J, Draetta G, Eilers M. Differential modulation of cyclin gene expression by Myc. Proc Natl Acad Sci USA. 1993;90:3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubes J, Walsh D, Raymond P, Chevalier C. Molecular characterization of the expression of distinct classes of cyclins during the early development of tomato fruit. Planta. 2000;211:430–439. doi: 10.1007/s004250000306. [DOI] [PubMed] [Google Scholar]
- Journet EP, Pichon M, Dedieu A, de Billy F, Truchet G, Barker DG. Rhizobium meliloti Nod factors elicit cell-specific transcription of the ENOD12gene in transgenic alfalfa. Plant J. 1994;6:241–249. doi: 10.1046/j.1365-313x.1994.6020241.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu ZB, Ulmasov T, Shi X, Hagen G, Guilfoyle TJ. Soybean GH3promoter contains multiple auxin-inducible elements. Plant Cell. 1994;6:645–657. doi: 10.1105/tpc.6.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Charon C, Rolfe BG, Kondorosi A, Crespi M. Temporal and spatial order of events during the induction of cortical cell divisions in white clover by Rhizobium leguminosarum bv. trifoliiinoculation or localized cytokinin addition. Mol Plant-Microbe Interact. 2000;13:617–628. doi: 10.1094/MPMI.2000.13.6.617. [DOI] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe B. Auxin transport inhibition precedes nodule formation in white clover and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- Meskiene I, Bögre L, Dahl M, Pirck M, Thi Cam Ha D, Swoboda I, Heberle-Bors E, Ammerer G, Hirt H. cycMs3, a novel B-type alfalfa cyclin gene, is induced in the G0-to-G1 transition of the cell cycle. Plant Cell. 1995;7:759–771. doi: 10.1105/tpc.7.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Yang R, Idos G, Tidow N, Diederichs S, Koch OM, Verbeek W, Bender TP, Koeffler HP. c-Myb transactivates the human cyclin A1 promoter and induces cyclin A1 gene expression. Blood. 1999;94:4255–4262. [PubMed] [Google Scholar]
- Pichon M, Journet EP, Dedieu A, de Billy F, Truchet G, Barker DG. Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12in the differentiating root epidermis of transgenic alfalfa. Plant Cell. 1992;4:1199–1211. doi: 10.1105/tpc.4.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsiscell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Roudier F, Fedorova E, Györgyey J, Feher A, Brown S, Kondorosi A, Kondorosi E. Cell cycle function of a Medicago sativaA2-type cyclin interacting with a PSTAIRE-type cyclin-dependent kinase and a retinoblastoma protein. Plant J. 2000;23:73–83. doi: 10.1046/j.1365-313x.2000.00794.x. [DOI] [PubMed] [Google Scholar]
- Rudolph B, Rainer S, Zwicker J, Henglein B, Müller R, Ansorge A, Eilers M. Activation of cyclin-dependent kinases by Myc mediates induction of cyclin A, but not apoptosis. EMBO J. 1996;15:3065–3076. [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Savouré A, Magyar Z, Pierre M, Brown S, Schultze M, Dudits D, Kondorosi A, Kondorosi E. Activation of the cell cycle machinery and the isoflavonoid biosynthesis pathway by active Rhizobium meliloti Nod signal molecules in Medicagomicrocallus suspensions. EMBO J. 1994;13:1093–1102. doi: 10.1002/j.1460-2075.1994.tb06358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Kondorosi A. Regulation of symbiotic root nodule development. Annu Rev Genet. 1998;32:33–57. doi: 10.1146/annurev.genet.32.1.33. [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Grundler FMW, Von Mende N, Burows PR, Wyss U. Arabidopsis thalianaas a new model host for plant parasitic nematodes. Plant J. 1991;1:245–254. [Google Scholar]
- Stals H, Inzé D. When plant cells decide to divide. Trends Plant Sci. 2001;6:359–364. doi: 10.1016/s1360-1385(01)02016-7. [DOI] [PubMed] [Google Scholar]
- Szabados L, Charrier B, Kondorosi A, de Bruijn FJ, Ratet P. New plant promoter and enhancer testing vectors. Mol Breed. 1995;1:419–423. [Google Scholar]
- Trehin C, Ahn IO, Perennes C, Couteau F, Lalanne E, Bergounioux C. Cloning of upstream sequences responsible for cell cycle regulation of the Nicotiana sylvestris CycB1;1gene. Plant Mol Biol. 1997;35:667–672. doi: 10.1023/a:1005837931851. [DOI] [PubMed] [Google Scholar]
- Trinh TH, Ratet P, Kondorosi E, Durand P, Kamate P, Bauer P, Kondorosi A. Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp. falcatalines improved in somatic embryogenesis. Plant Cell Rep. 1998;17:345–355. doi: 10.1007/s002990050405. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Raes J, De Veylder L, Rouzé P, Rombauts S, Inzé D. Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell. 2002;14:903–916. doi: 10.1105/tpc.010445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodermaier HC. Cell cycle: waiters serving the destruction machinery. Curr Biol. 2001;11:R834–R837. doi: 10.1016/s0960-9822(01)00498-5. [DOI] [PubMed] [Google Scholar]
- Williamson VM, Hussey RS. Nematode pathogenesis and resistance in plants. Plant Cell. 1996;8:1735–1745. doi: 10.1105/tpc.8.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WC, de Blank C, Meskiene I, Hirt H, Bakker J, van Kammen A, Franssen H, Bisseling T. Rhizobiumnod factors reactivate the cell cycle during infection and nodule primordium formation, but the cycle is only completed in primordium formation. Plant Cell. 1994;6:1415–1426. doi: 10.1105/tpc.6.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]