Abstract
Two cDNA clones, 3-ox and 2-ox, have been isolated from developing pumpkin (Cucurbita maxima) embryos that show significant amino acid homology to gibberellin (GA) 3-oxidases and 2-oxidases, respectively. Recombinant fusion protein of clone 3-ox converted GA12-aldehyde, GA12, GA15, GA24, GA25, and GA9 to GA14-aldehyde, GA14, GA37, GA36, GA13, and GA4, respectively. Recombinant 2-ox protein oxidized GA9, GA4, and GA1 to GA51, GA34, and GA8, respectively. Previously cloned GA 7-oxidase revealed additional 3β-hydroxylation activity of GA12. Transcripts of this gene were identified in endosperm and embryo of the developing seed by quantitative reverse transcriptase-polymerase chain reaction and localized in protoderm, root apical meristem, and quiescent center by in situ hybridization. mRNA of the previously cloned GA 20-oxidase from pumpkin seeds was localized in endosperm and in tissues of protoderm, ground meristem, and cotyledons of the embryo. However, transcripts of the recently cloned GA 20-oxidase from pumpkin seedlings were found all over the embryo, and in tissues of the inner seed coat at the micropylar end. Previously cloned GA 2β,3β-hydroxylase mRNA molecules were specifically identified in endosperm tissue. Finally, mRNA molecules of the 3-ox and 2-ox genes were found in the embryo only. 3-ox transcripts were localized in tissues of cotyledons, protoderm, and inner cell layers of the root apical meristem, and 2-ox transcripts were found in all tissues of the embryo except the root tips. These results indicate tissue-specific GA-biosynthetic pathways operating within the developing seed.
GA plant hormones regulate important processes in the life cycle of higher plants, including seed and seedling development (for review, see Pharis and King, 1985; Hooley, 1994; Hedden and Proebsting, 1999; Richards et al., 2001). For instance, by regulating plant height, GAs improve yields of important crops plants (Monna et al., 2002; Sasaki et al., 2002; Spielmeyer et al., 2002). However, the function of GAs in seed development is not entirely solved, even though there is growing evidence that GAs are important for early embryogenesis (Swain et al., 1997; Hays et al., 2002).
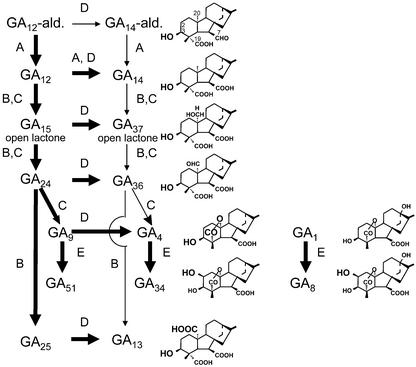
Recently, numerous genomic and cDNA clones encoding GA oxidases have been isolated from several plant species, some of which are expressed specifically in developing seeds (Hedden and Kamiya, 1997; Lange, 1998; Kang et al., 1999, 2002; Olszewski et al., 2002). Developing seeds, including those from pumpkin (Cucurbita maxima), proved to be very useful for unraveling GA biosynthetic pathways in cell-free systems, for isolation of involved enzymes, and for cloning the encoding genes (for review, see Graebe, 1987; Lange and Graebe, 1993; Hedden, 1999). However, catalytic properties of many of the GA oxidases from pumpkin seeds are unique, and, to date, have not been identified in other plant species. A principal pathway to the GA plant hormones can be drawn from GA12-aldehyde (Fig. 1). The first step, the oxidation at C-7 of GA12-aldehyde, results in GA12. This step is usually catalyzed by multifunctional microsomal cytochrome P450-monooxygenases (Helliwell et al., 2001). In developing pumpkin seeds, however, an additional GA 7-oxidase is active that belongs to the class of 2-oxoglutarate-dependent dioxygenases (Lange et al., 1994b; Lange, 1997). In this paper, it is shown that the recombinant pumpkin GA 7-oxidase also catalyzes the oxidation of GA12 to GA14 (Fig. 1), which initiates an “early” 3-oxidation pathway.
Figure 1.
Principal GA biosynthetic pathways in developing pumpkin seeds. In pumpkin embryos, reactions are catalyzed by GA 7-oxidase (A), seed-specific GA 20-oxidase (B), recently cloned GA 20-oxidase from pumpkin seedlings (C), GA 3-oxidase (D), and GA 2-oxidase (E). Structures and metabolic relations are discussed in the text. Bold arrows indicate major pathways.
The following three oxidation steps of the pathway are catalyzed by GA 20-oxidases that lead to the formation of C19-GAs (e.g. GA9; Fig. 1) and rarely to C20-GAs (e.g. GA25). Likewise, the recently cloned GA 20-oxidase from pumpkin seedlings catalyzes the formation of C19-GAs at high yields (T. Lange, A. Frisse, and M.J. Pimenta, unpublished data). However, GA 20-oxidase from developing pumpkin seeds produces mainly C20-GAs (Lange, 1994, 1998; Lange et al., 1994a). The latter GA 20-oxidase is specifically expressed in developing seeds and is, therefore, designated “seed-specific” GA 20-oxidase.
Hydroxylation of C19-GAs at the C-3β position leads to the formation of GA plant hormones (e.g. GA4; Fig. 1), which is catalyzed by GA 3-oxidases. However, further hydroxylation at the C-2β position inactivates hormonal function of GAs (e.g. GA34; Fig. 1). Unlike in most plants that have been investigated to date, in pumpkin endosperm a bifunctional GA 2β,3β-hydroxylase catalyzes both steps, 3-oxidation and 2-oxidation (not illustrated in Fig. 1; Lange et al., 1997). Moreover, this enzyme is unusual in hydroxylating C20-GAs more readily than C19-GAs.
In the present study, we report the isolation and molecular analysis of two genes from pumpkin embryos, coding for GA 3-oxidase and GA 2-oxidase. Expression patterns of both genes, together with the GA 7-oxidase, the two GA 20-oxidases, and the GA 2β,3β-hydroxylase gene, were studied in developing pumpkin seeds.
RESULTS
Isolation and Sequence Analysis of cDNA Clones
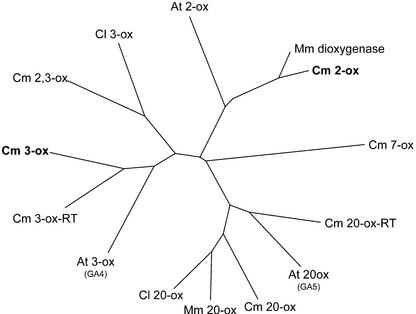
A cDNA plasmid library in pUC18, derived from pumpkin embryo poly(A+) RNA was screened for GA 3-oxidase and 2-oxidase cDNA molecules by a PCR-based cloning strategy (Israel, 1993) using degenerate primers that were designed according to homologous regions of published sequences of GA 3-oxidases and 2-oxidases, respectively. Two clones were isolated, designated 3-ox and 2-ox. DNA sequencing of inserts of clones 3-ox and 2-ox revealed open reading frames (ORFs) of 358 and 327 amino acids, respectively. Phylogenetically, 3-ox and 2-ox are as closely related to each other as they are to GA 7-oxidase and 20-oxidase (Fig. 2). They are approximately 25% identical at the amino acid level. Clone 3-ox from pumpkin embryo shows the highest phylogenetic homology to the GA 3-oxidase previously cloned from pumpkin seedlings (Fig. 2, Cm 3-ox-RT; T. Lange, A. Frisse, and M.J. Pimenta, unpublished data). Both share 63% identical amino acids. Phylogenetically, clone 3-ox groups with other GA 3-oxidases, and is closer related to the Arabidopsis GA 3-oxidase than to the GA 2β,3β-hydroxylase from pumpkin endosperm or to the GA 3-oxidase from watermelon (Citrullus lanatus; Chiang et al., 1995; Lange et al., 1997; Kang et al., 2002). The pumpkin 2-ox gene shows highest similarity to a dioxygenase of unknown function previously cloned from M. macrocarpus (MacMillan et al., 1997). Both share 84% identity, based upon their deduced amino acid sequences and, phylogenetically, both group with Arabidopsis GA 2-oxidase (Thomas et al., 1999; Fig. 2).
Figure 2.
Phylogenetic analysis of deduced amino acid structures of selected GA oxidases from diverse species. The tree was generated using ClustalW (version 1.8) and visualized using TREEVIEW. Shown are GA 7-oxidase from pumpkin (Cm 7-ox, accession no. U61386); GA 20-oxidases from Citrullus lanatus (Cl 20-ox, AF074710), Marah macrocarpus (Mm 20-ox, Y09112), pumpkin (Cm 20-ox, X73314; and Cm 20-ox-RT, AJ308480), and Arabidopsis (At 20-ox, X83379); GA 3-oxidases from C. lanatus (Cl 3-ox, AF074710), pumpkin (Cm 2, 3-ox, U63650; Cm 3-ox, AJ006453; and Cm 3-ox-RT, AJ302040), and Arabidopsis (At 3-ox, L37126); GA 2-oxidases from pumpkin (Cm 2-ox, AJ302041) and Arabidopsis (At 2-ox, AJ132437); and a dioxygenase of unknown function from M. macrocarpus (Mm dioxygenase, Y09113).
Substrate Specificity of Recombinant GA Oxidases
The catalytic properties of previously cloned GA 7-oxidase from pumpkin endosperm were reinvestigated (Table I; Lange, 1997). Recombinant GA 7-oxidase converted 14C-GA12 to two major (“W” and “X”) and two minor products (“Y” and “Z”; Lange, 1997). Full-scan mass spectra of methyl ester trimethylsilyl ether derivatives now reveal the identity of product W to be GA14 (Table I). The mass spectrum of the second major product, X, has similarity to 15-hydroxy GA12 (Gaskin and MacMillan, 1992). Mass spectra for Y and Z were contaminated with extraneous ions (Table I). No conversion of the 14C-labeled substrates GA14, GA25, GA13, GA9, and GA4 was obtained with recombinant GA 7-oxidase (data not shown).
Table I.
Metabolism of [14C]-GAs by cell lysates from Escherichia coli transformed with GA 7-oxidase cDNA (Lange, 1997), and with pUC18 clones 3-ox and 2-ox-ORF
| Clone | Substrate | Productsa
|
||||
|---|---|---|---|---|---|---|
| Compounds formed | % by HPLC | Kovats retention indices (KRI) | Characteristic ions at mass-to-charge ratio (% relative of base peak)b | |||
| 7-Oxidase | GA12cd | GA14 | 33 | 2,537 | 456 (0), 448 (0), 424 (16), 416 (12), 396 (15), 388 (9), 306 (32), 298 (30), 293 (45), 287 (47) | |
| X | 51 | 2,651 | 456 (3), 448 (1), 424 (35), 416 (26), 394 (43), 388 (30), 334 (14), 326 (6), 304 (18), 298 (19), 245 (45), 239 (31), 158 (100), 156 (63) | |||
| Y | 5 | 2,929e | ||||
| Z | 6 | |||||
| 3-ox | GA12aldcf | GA14ald | 19 | 2,574eg | ||
| GA12c | GA14 | 100 | 2,485g | 456 (1), 448 (3), 424 (27), 416 (25), 396 (11), 388 (9), 306 (36), 298 (40), 293 (47), 287 (32) | ||
| GA15c | GA37 | 100 | 2,739g | 440 (1), 432 (1), 350 (3), 342 (8), 318 (24), 310 (16), 292 (17), 284 (12), 243 (14), 237 (6) | ||
| GA24h | GA36 | 100 | 2,590g | 464 (0), 462 (0), 432 (19), 430 (3), 404 (14), 402 (0), 314 (45), 312 (12), 286 (100), 284 (39) | ||
| GA25h | GA13 | 100 | 2,586g | 494 (1), 492 (0), 479 (2), 477 (0), 402 (12), 400 (1), 312 (19), 310 (2), 284 (16), 282 (10) | ||
| GA9c | GA4 | 100 | 2,498g | 426 (4), 418 (2), 336 (14), 328 (5), 292 (72), 284 (36), 231 (96), 230 (100), 225 (43), 224 (42) | ||
| 2-ox-ORF | GA9h | GA51 | 68 | 2,564 | 420 (0), 418 (0), 388 (6), 386 (2), 330 (8), 328 (4), 286 (59), 284 (20), 270 (56), 268 (10), 227 (100), 225(49) | |
| GA4hi | GA34 | 100 | 2,669 | 508 (100), 506 (17), 290 (23), 288 (29), 263 (0), 261 (0), 231 (84), 229 (48), 225 (68), 223 (54) | ||
| GA1c | GA8 | 100 | 2,785 | 602 (50), 594 (100), 454 (18), 448 (18), 383 (8), 379 (18) | ||
Identification of [14C]-GA metabolic products by gas chromatography (GC)-mass spectrometry (MS) on the basis of mass spectra (Gaskin and MacMillan, 1992) and KRI of the methyl ester trimethylsilylether derivatives.
Based on ions above a mass-to-charge ratio of 50.
(1,7,12,18-14C4)-Labeled.
Incubation volumes 10 times the standard assay.
Spectrum was seriously contaminated with extraneous ions.
Incubation volumes 5 times the standard assay.
KRI obtained with PE-1HT capillary column (30 m, 0.32-mm i.d., 0.1-μm film, Perkin Elmer, Weiterstadt, Germany).
(17-14C)-Labeled.
Incubation volumes 1.6 times the standard assay.
The catalytic properties of recombinant fusion protein of clones 3-ox and 2-ox from pumpkin embryos were investigated by expression of the respective cDNA molecules in pUC18 and E. coli NM522 (Table I). Recombinant protein of clone 3-ox catalyzed oxidation at the C-3β position of 14C-GA12, -GA15, -GA24, -GA25, -GA9, and, less efficiently, -GA12-aldehyde to 14C-GA14, -GA37, -GA36, -GA13, -GA4, and -GA14-aldehyde, respectively (Table I). GA 2-oxidation activity was low in cell lysates prepared from the 2-ox full-length clone (data not shown), but it highly increased in cell lysates prepared from its predicted ORF (designated 2-ox-ORF) that was used, therefore, for further characterization (Table I). Recombinant protein of clone 2-ox-ORF oxidized 14C-labeled substrates GA4, GA1, and, less efficiently, GA9 to GA34, GA8, and GA51, respectively (Table I). No conversion of the 14C-labeled substrates GA12, GA24, GA25, and GA13 was obtained with recombinant protein of clone 2-ox-ORF (data not shown). No conversion of the 14C-labeled substrates GA12-aldehyde, GA12, GA15, GA24, GA25, GA13, GA9, GA4, GA14, and GA1 was obtained in standard incubation assays with cell lysates of E. coli harboring the pUC18 plasmid without the cDNA insert (data not shown).
Localization of Transcripts of GA Oxidases in Developing Pumpkin Seeds
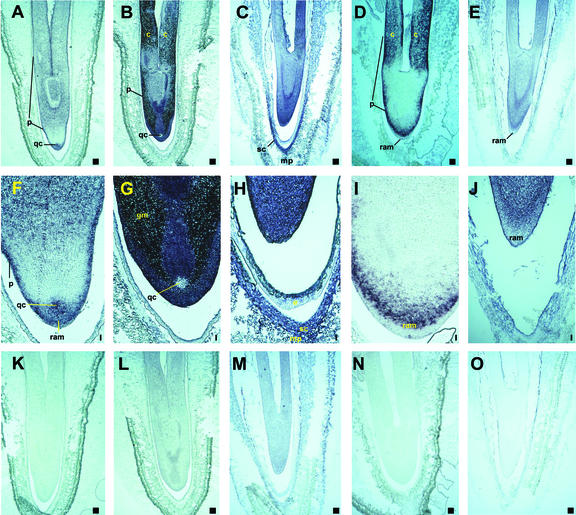
Expression pattern of five genes encoding GA oxidases were analyzed in developing pumpkin seeds by in situ hybridization (Fig. 3). GA 7-oxidase has been cloned previously from pumpkin endosperm (Lange, 1997). Seed-specific GA 20-oxidase gene has been cloned previously from pumpkin embryos (Lange et al., 1994a), the same tissue from which clone 3-ox (GA 3-oxidase) and clone 2-ox (GA 2-oxidase) have been isolated in this study. In addition, expression patterns of a recently cloned GA 20-oxidase gene from pumpkin seedlings (T. Lange, A. Frisse, and M.J. Pimenta, unpublished data) were analyzed in the developing seed. Distinct expression patterns of transcripts were detected in embryo tissues for these five GA oxidase genes. Detection of GA transcripts in pumpkin endosperm was difficult by in situ hybridization due to the fragile nature of the tissue. Transcripts of the GA 7-oxidase gene were mainly found in tissues of protoderm, the root apical meristem, and the quiescent center (Fig. 3, A, F, and K). Seed-specific GA 20-oxidase gene transcripts were identified mainly in the protoderm, ground meristem, and cotyledons, with weaker signals in the root apical meristem, and transcripts were not detected in the quiescent center (Fig. 3, B, G, and L). Transcripts of the recently cloned GA 20-oxidase gene from pumpkin seedlings were localized in all tissues of the embryo, and, in addition, in tissues of the inner seed coat at the micropylar end (Fig. 3, C, H, and M). Transcripts of the 3-ox gene are mainly present in protoderm, cotyledons, and inner cell layers of the root apical meristem (Fig. 3, D, I, and N). Transcripts of the 2-ox gene were found in all tissues of the embryo, except the root apical meristem (Fig. 3, E, J, and O).
Figure 3.
Localization of GA 7-oxidase (A, F, and K), seed-specific GA 20-oxidase (B, G, and L), recently cloned GA 20-oxidase from seedlings (C, H, and M), GA 3-oxidase (D, I, and N), and GA 2-oxidase (E, J, and O) mRNA in pumpkin seeds by in situ hybridization. All samples were sectioned longitudinally in a 90° angle with the plane of seeds at 50% (A, B, D, F, G, I, K, L, and N) or 36% (C, E, H, J, M, and O) mature index according to Graebe (1972). The sections were hybridized to either an antisense (A–J) or a sense (K–O) RNA probe of the entire respective cDNA (A, B, D, F, G, I, K, L, and N) or the predicted ORF (C, E, H, J, M, and O) that was labeled with digoxigenin. c, Cotyledon; gm, ground meristem; mp, micropylar end; p, protoderm; qc, quiescent center; ram, root apical meristem; sc, inner layer of the seed coat. Bar = 0.1 mm.
Transcript Levels of GA Oxidases in Developing Pumpkin Seeds
mRNA expression levels were determined for previously cloned genes encoding GA 7-oxidase, seed-specific GA 20-oxidase, and GA 2β,3β-hydroxylase (Lange et al., 1994a, 1997; Lange, 1997), as well as for the 3-ox (3-oxidase) gene in endosperm and embryos of developing pumpkin seeds by reverse transcriptase (RT)-PCR. Transcripts encoding GA 7-oxidase and seed-specific GA 20-oxidase genes were detected in endosperm and embryos (Table II). Transcripts of the GA 2β,3β-hydroxylase gene were specifically expressed in the endosperm, whereas transcripts encoding the 3-ox protein were detectable only in the embryo. Transcript levels of GA 20-oxidase and GA 2β,3β-hydroxylase genes were more than 15 times higher than those of GA 7-oxidase and GA 3-oxidase genes in the developing pumpkin seeds.
Table II.
Transcript levels by quantitative RT-PCR of GA 7-oxidase, 20-oxidase, 2β,3β-hydroxylase, and 3-ox in developing pumpkin seeds (40% maturity index)
| Tissue from | Transcript Levels
|
|||
|---|---|---|---|---|
| 7-Oxidase | 20-Oxidase | 2β,3β-Hydroxylase | 3-ox | |
| μg g−1 total RNA | ||||
| Endosperm | 20 | 1,100 | 330 | n.d.a |
| Embryo | 5.0 | 320 | n.d.a | 11 |
n.d., Not detectable.
DISCUSSION
We have isolated two cDNA clones, 3-ox and 2-ox, from developing pumpkin embryos that encode proteins homologous to GA 3-oxidase and GA 2-oxidase, respectively. Both cDNAs were cloned by a PCR-based cloning strategy adapted from Israel (1993). The recombinant protein of clone 3-ox has broad substrate specificity similar to the previously cloned GA 2β,3β-hydroxylase from pumpkin endosperm and to the previously cloned GA 3-oxidase from developing watermelon seeds (Lange et al., 1997; Kang et al., 2002). The recombinant enzyme catalyzes 3-oxidation of C20-GAs and C19-GAs, but, in contrast to the GA 2β,3β-hydroxylase, does not possess 2-oxidation of C20-GAs. Recombinant GA 3-oxidases from other plant species act principally on C19-GAs (Hedden, 1999). Phylogenetically, however, pumpkin clone 3-ox is closer related to GA 3-oxidases from pumpkin seedling and Arabidopsis than it is to pumpkin GA 2β,3β-hydroxylase and watermelon GA 3-oxidase (Fig. 2). The recombinant protein of clone 2-ox hydroxylates C19-GAs at C-2β position. Other recombinant GA 2-oxidases previously cloned from runner bean (Phaseolus coccineus), pea (Pisum sativum), and Arabidopsis further convert GA51 or GA34 to respective GA catabolites (Martin et al., 1999; Thomas et al., 1999). The 2-ox clone shares very high sequence identity with an unidentified dioxygenase from M. macrocarpus (MacMillan et al., 1997).
To date, pumpkin GA 7-oxidase is the only known 2-oxoglutarate-dependent enzyme that catalyzes the oxidation at C-7 of GA12-aldehyde to form GA12 (Fig. 1; Lange, 1998). In addition, the recombinant enzyme metabolizes GA12 to four other products, one of which has now been identified to be GA14, which initiates an “early” 3β-hydroxylation pathway (Graebe, 1987). However, 3-oxidation activity of GA 7-oxidase might take place in tissues only where GA 20-oxidase activities are low or not expressed, because both enzymes compete for the same substrate (see below).
Analysis of the transcript patterns of six genes encoding GA oxidases implies that GA-biosynthetic pathways are expressed in a tissue-specific manner in the developing pumpkin seed. In pumpkin, two GA 20-oxidases with different catalytic properties have been identified. The seed-specific GA 20-oxidase catalyzes the formation of C20-GAs (Lange et al., 1994a), and the recently identified 20-oxidase from seedlings produces mainly C19-GAs (T. Lange, A. Frisse, and M.J. Pimenta, unpublished data). In the seed coat next to the micropylar end of the developing seed, transcripts were identified that encode for the seedling GA 20-oxidase, which makes this tissue a potential site of C19-GA formation. Similar results were obtained with a watermelon GA 20-oxidase gene that is strongly expressed in the integument of the developing seed (Kang et al., 1999). Moreover, bioactive GAs were localized in the same tissue of Pharbitis nil seeds (Nakayama et al., 2002), suggesting a specific role for GAs in maternal tissues.
Our data show expression of GA 7-oxidase, seed-specific GA 20-oxidase, and GA 2β,3β-hydroxylase genes in endosperm tissues, as detected by quantitative RT-PCR. A second 20-oxidase gene recently cloned from pumpkin seedlings was also detected in endosperm tissues by RT-PCR (data not shown). Thus, the seedling 20-oxidase might account for the high yield of C19-GAs that was obtained by analysis of endogenous GAs and in metabolic studies (Blechschmidt et al., 1984; Lange et al., 1993a). Moreover, in cell-free systems from the endosperm, no 2-oxidation of C19-GAs was found, which makes this tissue a prime site of bioactive GA synthesis that potentially controls embryo development (Lange et al., 1993a, 1993b; Hays et al., 2002).
Transcripts of 2-ox gene were found in all parts of the embryo, except the root tip, which might indicate the tissues in which GA 2-oxidase helps to regulate the GA plant hormone pool. Other tissues, including cotyledons, protoderm, and inner cell layers of the root tip, show high transcript levels of seed-specific GA 20-oxidase and 3-ox gene, suggesting sites of C20-GA formation. However, the quiescent center contains high transcript levels of GA 7-oxidase gene, and low levels of seed-specific GA 20-oxidase gene, which indicates a site of bioactive GA-formation. Moreover, the root tip has high transcript levels of GA 7-oxidase and seedling 20-oxidase genes, but low transcript levels of seed-specific GA 20-oxidases, 3-ox, and 2-ox genes, suggesting a prime site of bioactive GA formation via the “early” 3-oxidation pathway. Imported GA precursors and bioactive GAs from the endosperm might add to the pool of bioactive GAs within the root tip, whereas in other tissues, imported GAs might get rapidly inactivated due to seed-specific GA 20-oxidase and/or 2-ox activities. Tissue-specific expression patterns as demonstrated in this study might account for high levels of endogenous C19-GAs as found in developing embryos (Blechschmidt et al., 1984; Lange et al., 1993b). However, in cell-free systems prepared from the embryo, tissue-specific expression patterns are disrupted that might explain the formation of C20-GAs in such enzyme preparations (Lange et al., 1993b). GAs are important, if not essential, for early embryogenesis (Swain et al., 1997). Using microspore derived embryos from Brassica napus, it has been demonstrated recently that GAs regulate embryo axis elongation (Hays et al., 2002). Moreover, GAs appear to be involved in controlling the abundance of several proteins associated with radicle protrusion during seed germination (Gallardo et al., 2002). Finally, GAs slow down cell doubling times of both quiescent center and root cap meristem, as shown in cultured root apices of tomato (Lycopersicon esculentum; Barlow, 1992), which might indicate some additional function of GAs also during embryogenesis.
MATERIALS AND METHODS
Construction of the pUC18 cDNA Plasmid Library
Plants of pumpkin (Cucurbita maxima L. cv Riesenmelone, gelb genetzt; van-Waverern, Göttingen, Germany) were grown in the Botanical Garden (Technical University, Braunschweig, Germany) in the summers of 1999 to 2001. Poly(A+) RNA (5 μg) from developing embryos of immature seeds with cotyledons of 40% the length of the seed lumen was used for the preparation of an oligo(dT)-primed cDNA library in pUC18 using commercial kits for cDNA synthesis and adaptor ligation (Amersham, Braunschweig, Germany). cDNA adaptor constructs were ligated into pUC18 plasmids and transformed into Escherichia coli NM522. A cDNA library of 1.7 × 106 independent cell-forming units (cfu) was obtained and amplified, 25% of which contained inserts >1,000 bp as shown by agarose gel electrophoresis of PCR products using pUC18-specific M13 primers.
PCR Screening of the pUC18 cDNA Library
For primary screening of GA 3-oxidase and GA 2-oxidase genes, the cDNA plasmid library in E. coli NM522 was subdivided into nine tubes, each containing approximately 100,000 cfu in 10 mL of l-broth, supplemented with ampicillin (50 μg mL−1), and grown for 16 h at 37°C with shaking. Plasmid DNA molecules isolated from each of the amplified cultures were used as template for a PCR-based screening procedure with degenerate primers (adapted from Israel, 1993): For GA 3-oxidase, forward primer 5′-ATG TGG (CT)(AC) N GA(AG) GGN TT(CT) AC-3′ and reverse primer 5′-GT(AG) TGN G(CG) NGC NA(AG) NCC CAT-3′ were used; and for GA 2-oxidase, forward primer 5′-GNN TNA A(CT) C A(CT) T A(CT) C CNC C-3′ and reverse primer 5′-GGN (GC) CN (GC) C(AG) AA(AG) TAN ATC AT-3′ were used (where N is a mixture of A, C, G, and T). The PCR reaction was initiated by heating to 94°C for 3 min, then subjected to 35 cycles of 94°C for 30 s, 55°C (for screening for GA 3-oxidase clones) or 50°C (for screening for GA 2-oxidase clones) for 30 s, and 72°C for 2 min. The reaction was completed by incubation at 72°C for 5 min. PCR products were analyzed by agarose gel electrophoresis in a 1% (w/v) agarose gel and visualized by ethidium bromide staining. Using the degenerate primer pairs for GA 3-oxidase, all nine tubes gave a PCR product of approximately 300-bp length, which is the expected size for the putative GA 3-oxidase. Bacteria from one tube were subdivided for secondary screening into 10 tubes, each containing approximately 10,000 cfu and re-amplified and rescreened as described above for the primary screen. After four more screening rounds, one tube containing 10 cfu was identified that gave PCR products of the expected size for putative GA 3-oxidase. Amplified bacteria of this tube were plated on l-broth agar, containing ampicillin (50 μg mL−1) at approximately 100 cfu per plate, and grown for 18 h at 37°C. Single colonies of 10 bacterial clones of this tube were rescreened as described above. One clone (designated 3-ox) was shown to give the approximately 300-bp PCR product. Screening for putative GA 2-oxidase clones was performed essentially as described above for GA 3-oxidase. Using degenerate primer pairs for GA 2-oxidase, one clone (designated 2-ox) gave a PCR-product of approximately 300-bp length, which is the expected size for a putative GA 2-oxidase. Both clones (3-ox and 2-ox) were used for DNA analysis and heterologous expression studies.
DNA Sequence Analysis
The pUC18 plasmid containing the cDNA insert of clone 3-ox was digested with AvaI and with HindIII, and fragments were subcloned in pBluescript SK− and pUC18, respectively. After restriction enzyme digestion, the remaining pUC18 vectors harboring the 5′ end of 3-ox were religated. Plasmid DNAs containing 3-ox and fragments thereof were sequenced on both strands by the dideoxynucleotide chain termination method from the M13 primers using the ABI Prism BigDye Termination Cycle Sequencing Kit (Perkin Elmer) and an ABI Prism 310 Genetic Analyzer (Perkin Elmer). The pUC18 plasmid containing the cDNA insert of clone 2-ox was custom sequenced on both strands (AGOWA, Berlin).
Heterologous Expression of Recombinant GA 3-Oxidase and GA 2-Oxidase
DNA sequence analysis revealed that the cDNA inserts of the two clones were not in frame to the lac promoter of pUC18. cDNAs of the clones 3-ox and 2-ox were excised by BamHI digest and subcloned using appropriate cloning sites of pUC18 and pBluescript SK− vector, respectively, and transformants with the cDNAs in sense and antisense orientation were selected. The predicted ORF of clone 2-ox was amplified by PCR (annealing temperature 55°C) using forward primer 5′-NGA ATT CAA TGA GAA GCT CCA CGT CCA TG-3 and reverse primer 5′-NGG ATC CGA TCA GAT GTT CGA ATC CTG TC-3′, cut by EcoRI and BamHI, and cloned into the appropriate cloning sites of pUC18 (and pBluescript SK− vector for preparation of riboprobes for in situ hybridization; see below). Protein induction and cell lysis were carried out as described by Lange (1997).
Preparation of 14C-Labeled Substrates
(1,7,12,18-14C4)-GA12-aldehyde (5.93 × 1012 Bq mol−1) and -GA12 (5.80 × 10l2 Bq mol−1) were prepared from R-2-14C-mevalonic acid (1.96 × 10l2 Bq mol−1; Amersham), and (14C)-GA15, (5.05 × 1012 Bq mol−1) and -GA1 (3.58 × 10l2 Bq mol−1) were prepared from l4C-GA12 by using a cell-free system from pumpkin endosperm (Lange and Graebe, 1993). (1,7,12,18-14C4)-GA9 (5.80 × 1012 Bq mol−1) was prepared by incubation of l4C-GA12 with recombinant GA 20-oxidase from Arabidopsis (Lange et al., 1997). [17-14C]GA25, and -GA9 (both 1.95 × 1012 Bq mol−1) were prepared from [17-14C]GA24 (1.95 × 1012 Bq mol−1, from Professor Lewis N. Mander, Australian National University, Canberra, Australia) by incubation with recombinant GA 20-oxidase from pumpkin embryo (Lange et al., 1994a) and from Arabidopsis (Lange et al., 1997), respectively. [17-14C]GA4 was prepared from [17-14C]GA9 by incubations with the recombinant 3-ox protein. All incubations with recombinant enzymes were performed by standard incubations as described below.
Standard Enzyme Assay and GC-MS Analysis of Incubation Products
E. coli cell lysates containing recombinant GA oxidases (350 μL) were incubated with 2-oxoglutarate and ascorbate (100 mm each, final concentrations), FeSO4 (0.5 mm), catalase (1 mg mL−1), and the 14C-labeled substrate [10 μL in 50% (v/v) methanol, 0.33 nmol for (1,7,12,18-14C4)-labeled Gas, and 1 nmol for (17-14C)-labeled GAs] in a total volume of 500 μL for 16 h at 37°C. Incubation products were extracted and analyzed by reverse-phase HPLC with on-line radio counting, using gradients of increasing methanol in acidic water, as described by Lange and Graebe (1993). More than 95% of the radioactivity originally added as substrate was recovered. Radioactive fractions were dried, derivatized, and analyzed by combined GC-MS (Xu et al., 2002). GA substrates and variations of the incubation and GC conditions are specified for particular experiments.
Quantitative RT-PCR
For quantification of GA 7-oxidase, 20-oxidase, and 2β,3β-hydroxylase transcripts, RT-PCR was performed as described by Lange et al. (1997). For quantification of GA 3-ox transcripts, three specific oligonucleotides were synthesized based on the cDNA sequence of the gene: forward primer, 5′-TCT CCA AGT ACT CCC CGA CTC CTA CCA GTG-3′; reverse primer, 5′-GGA TAA AGT CAG TCC AAG ATA TGG GGC GGT-3′; and RT primer, 5′-GTA GTA CAC GAA CAG TT-3′. The annealing temperature of PCR was 72°C. For preparation of the internal RNA standard, a pBluescript SK− plasmid containing clone 3-ox was digested with Eco147I that released a 121-bp fragment from the 3-oxidase cDNA. The vector containing the remaining cDNA was religated and used for standard RNA synthesis. The RT-PCR was performed as described elsewhere (Lange et al., 1997).
In Situ Hybridization
Tissues were fixed in 4% (w/v) p-formaldehyde and 0.25% (v/v) glutaraldehyde in 50 mm sodium phosphate buffer (pH 7.2). After dehydration by a graded series of ethanol, ethanol was replaced by Histoclear (Chandon, Frankfurt) and then gradually by liquid paraffin (Paraplast Plus, Sigma, Deisenhofen, Germany). Sections of 8-μm thickness were cut from the embedded samples with a microtome, mounted on microscopic slides previously coated with 2% (v/v) aminopropyltriethoxysilane in acetone, and fixed by drying overnight at 37°C. Paraffin was removed from the samples using Histoclear, which was then washed out with ethanol. Samples were hydrated by a graded series of ethanol and then treated with proteinase K (2 μg mL−1) at 37°C for 15 min. After acetylation, tissues were dehydrated by a graded series of ethanol and air dried. Samples were hybridized in a solution containing 50% (v/v) formamide, 0.3 m NaCl, 10 mm Tris-HCl (pH 7.5), 1 mm EDTA, 45 mm dithiothreitol, 10% (w/v) dextran sulfate, 1× Denhardt's solution, 0.17 mg mL−1 bovine liver tRNA, 0.5 mg mL−1 polyadenylic acid, and 100 to 300 ng mL−1 riboprobe at 50°C for 16 h. Sense and antisense riboprobes of full-length cDNAs encoding GA 7-oxidase (Lange, 1997), seed-specific GA 20-oxidase (Lange et al., 1994a), GA 2β-,3β-hydroxylase (Lange et al., 1997), and clone 3-ox, and of the predicted ORF encoding recently cloned GA 20-oxidase from pumpkin seedlings (Fig. 2, Cm 20-ox-RT; T. Lange, A. Frisse, and M.J. Pimenta, unpublished data), and clone 2-ox were synthesized using the DIG nucleic acid labeling kit according to the manufacturer's protocol with T7 and T3 RNA polymerases (Roche Molecular Biochemicals, Mannheim, Germany). Probes were hydrolyzed in 0.2 m bicarbonate at 60°C for 50 min. After hybridization and removal of unbound probes with RNase A (50 μg mL−1 at 37°C for 30 min), slides were washed two times in 0.2× SSC at 60°C for 20 min. Signals of hybridized probes were imaged using alkaline phosphatase-conjugated anti-DIG antibodies and nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche Molecular Biochemicals) as substrates.
ACKNOWLEDGMENTS
We thank Danele Hunecke and Anja Liebrandt for technical assistance.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. La880/4–1 and La880/4–2).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.015206.
LITERATURE CITED
- Barlow P. The meristem and quiescent centre in cultured root apices of the gib-1 mutant of tomato (Lycopersicon esculentum Mill) Ann Bot. 1992;69:533–543. [Google Scholar]
- Blechschmidt S, Castel U, Gaskin P, Hedden P, Graebe JE, MacMillan J. GC/MS analysis of the plant hormones in seeds of Cucurbita maxima. Phytochemistry. 1984;23:553–558. [Google Scholar]
- Chiang H, Hwang I, Goodman H. Isolation of the Arabidopsis GA4 locus. Plant Cell. 1995;7:195–201. doi: 10.1105/tpc.7.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SP, Puype M, Demol H, Vandekerckhove J, Job D. Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol. 2002;129:823–837. doi: 10.1104/pp.002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin P, MacMillan J. GC-MS of Gibberellins and Related Compounds. Bristol, UK: Cantock's Enterprise; 1992. [Google Scholar]
- Graebe JE. The biosynthesis of gibberellin precursors in a cell-free system from Cucurbita pepo L. In: Carr DJ, editor. Plant Growth Substances. Berlin: Springer; 1972. pp. 151–157. [Google Scholar]
- Graebe JE. Gibberellin biosynthesis and control. Annu Rev Plant Physiol. 1987;38:419–465. [Google Scholar]
- Hays DB, Yeung EC, Pharis RP. The role of gibberellins in embryo axis development. J Exp Bot. 2002;53:1747–1751. doi: 10.1093/jxb/erf017. [DOI] [PubMed] [Google Scholar]
- Hedden P. Regulation of gibberellin biosynthesis. In: Hooykaas PJJ, Hell MA, Libbenga KR, editors. Biochemistry and Molecular Biology of Plant Hormones. Amsterdam: Elsevier Press; 1999. pp. 161–188. [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Hedden P, Proebsting WM. Genetic analysis of gibberellin biosynthesis. Plant Physiol. 1999;119:365–370. doi: 10.1104/pp.119.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Chandler PM, Poole A, Dennis ES, Peacock WJ. The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci USA. 2001;98:2065–2070. doi: 10.1073/pnas.041588998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley R. Gibberellins: perception, transduction and responses. Plant Mol Biol. 1994;26:1529–1555. doi: 10.1007/BF00016489. [DOI] [PubMed] [Google Scholar]
- Israel D. A PCR-based method for high stringency screening of DNA libraries. Nucleic Acids Res. 1993;21:2627–2631. doi: 10.1093/nar/21.11.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Jun SH, Kim J, Kawaide H, Kamiya Y, An G. Cloning and molecular analyses of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon. Plant Physiol. 1999;121:373–382. doi: 10.1104/pp.121.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HG, Jun SH, Kim J, Kawaide H, Kamiya Y, An G. Cloning of gibberellin 3β-hydroxylase cDNA and analysis of endogenous gibberellins in the developing seeds in watermelon. Plant Cell Physiol. 2002;43:152–158. doi: 10.1093/pcp/pcf016. [DOI] [PubMed] [Google Scholar]
- Lange T. Purification and partial amino-acid-sequence of gibberellin 20-oxidase from Cucurbita maxima L. endosperm. Planta. 1994;195:108–115. doi: 10.1007/BF00206298. [DOI] [PubMed] [Google Scholar]
- Lange T. Cloning gibberellin dioxygenase genes from pumpkin endosperm by heterologous expression of enzyme activities in Escherichia coli. Proc Natl Acad Sci USA. 1997;94:6553–6558. doi: 10.1073/pnas.94.12.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T. Molecular biology of gibberellin synthesis. Planta. 1998;204:409–419. doi: 10.1007/s004250050274. [DOI] [PubMed] [Google Scholar]
- Lange T, Graebe JE. Enzymes of gibberellin synthesis. In: Lea PJ, editor. Methods in Plant Biochemistry. Vol. 9. London: Academic Press; 1993. pp. 403–430. [Google Scholar]
- Lange T, Hedden P, Graebe JE. Biosynthesis of 12α- and 13-hydroxylated gibberellins in a cell-free system from Cucurbita maxima endosperm and the identification of new endogenous gibberellins. Planta. 1993a;189:340–349. doi: 10.1007/BF00194430. [DOI] [PubMed] [Google Scholar]
- Lange T, Hedden P, Graebe JE. Gibberellin biosynthesis in cell-free extracts from developing Cucurbita maxima embryos and the identification of new endogenous gibberellins. Planta. 1993b;189:350–358. doi: 10.1007/BF00194431. [DOI] [PubMed] [Google Scholar]
- Lange T, Hedden P, Graebe JE. Expression cloning of a gibberellin 20-oxidase, a multifunctional enzyme involved in gibberellin biosynthesis. Proc Natl Acad Sci USA. 1994a;91:8552–8556. doi: 10.1073/pnas.91.18.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Schweimer A, Ward DA, Hedden P, Graebe JE. Separation and characterization of three 2-oxoglutarate-dependent dioxygenases from Cucurbita maxima L. endosperm involved in gibberellin biosynthesis. Planta. 1994b;195:98–107. [Google Scholar]
- Lange T, Robatzek S, Frisse A. Cloning and expression of a gibberellin 2β,3β-hydroxylase cDNA from pumpkin endosperm. Plant Cell. 1997;9:1459–1467. doi: 10.1105/tpc.9.8.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan J, Ward DA, Phillips AL, Sánchez-Beltrán MJ, Gaskin P, Lange T, Hedden P. Gibberellin biosynthesis from gibberellin A12-aldehyde in endosperm and embryos of Marah macrocarpus. Plant Physiol. 1997;113:1369–1377. doi: 10.1104/pp.113.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Proebsting WM, Hedden P. The SLENDER gene of pea encodes a gibberellin 2-oxidase. Plant Physiol. 1999;121:775–781. doi: 10.1104/pp.121.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y. Positional cloning of rice semidwarfing gene, sd-1: rice “green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 2002;9:11–17. doi: 10.1093/dnares/9.1.11. [DOI] [PubMed] [Google Scholar]
- Nakayama A, Park S, Zheng-Jun X, Nakajima M, Yamaguchi I. Immunohistochemistry of active gibberellins and gibberellin-inducible α-amylase in developing seeds of morning glory. Plant Physiol. 2002;129:1045–1053. doi: 10.1104/pp.010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N, Sun TP, Gubler F. Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell Suppl. 2002;14:S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharis R, King R. Gibberellins and reproductive development in seed plants. Annu Rev Plant Physiol. 1985;36:517–568. [Google Scholar]
- Richards DE, King KE, Ait-Ali T, Harberd NP. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:67–88. doi: 10.1146/annurev.arplant.52.1.67. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature. 2002;416:701–702. doi: 10.1038/416701a. [DOI] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA. 2002;99:9043–9048. doi: 10.1073/pnas.132266399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Reid JB, Kamiya Y. Gibberellins are required for embryo growth and seed development in pea. Plant J. 1997;12:1329–1338. [Google Scholar]
- Thomas SG, Phillips AL, Hedden P. Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc Natl Acad Sci USA. 1999;96:4698–4703. doi: 10.1073/pnas.96.8.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lange T, Altpeter F. Cloning and characterization of a cDNA encoding a multifunctional gibberellin 20-oxidase from perennial ryegrass (Lolium perenne L.) Plant Sci. 2002;163:147–155. [Google Scholar]