Abstract
Ethylene or its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) can stimulate hypocotyl elongation in light-grown Arabidopsis seedlings. A mutant, designated ACC-related long hypocotyl 1 (alh1), that displayed a long hypocotyl in the light in the absence of the hormone was characterized. Etiolated alh1 seedlings overproduced ethylene and had an exaggerated apical hook and a thicker hypocotyl, although no difference in hypocotyl length was observed when compared with wild type. Alh1 plants were less sensitive to ethylene, as reflected by reduction of ACC-mediated inhibition of hypocotyl growth in the dark and delay in flowering and leaf senescence. Alh1 also had an altered response to auxin, whereas auxin levels in whole alh1 seedlings remained unaffected. In contrast to wild type, alh1 seedlings showed a limited hypocotyl elongation when treated with indole-3-acetic acid. Alh1 roots had a faster response to gravity. Furthermore, the hypocotyl elongation of alh1 and of ACC-treated wild type was reverted by auxin transport inhibitors. In addition, auxin up-regulated genes were ectopically expressed in hypocotyls upon ACC treatment, suggesting that the ethylene response is mediated by auxins. Together, these data indicate that alh1 is altered in the cross talk between ethylene and auxins, probably at the level of auxin transport.
In the dark, ethylene-treated seedlings display a short root, an exaggerated apical hook concomitant with radial swelling and an inhibition of hypocotyl elongation (Knight and Crocker, 1913). By using exogenously applied ethylene or its precursor 1-aminocyclopropane-1-carboxylic acid (ACC), this so-called triple response was exploited for isolation of mutants in Arabidopsis (Bleecker et al., 1988; Guzmán and Ecker, 1990; Harpham et al., 1991; Van Der Straeten et al., 1993; Roman et al., 1995). Characterization of these ethylene-related mutants has led to the elucidation of a pathway for ethylene signaling (Stepanova and Ecker, 2000).
New screening assays could potentially uncover novel mutants with defects in the cross talk of the ethylene pathway with other hormones (Smalle and Van Der Straeten, 1997; Smalle et al., 1997). Ghassemian et al. (2000) identified alleles of ETHYLENE INSENSITIVE 2 while screening for mutants with increased sensitivity for abscisic acid. The ethylene-insensitive root (eir1-1) mutant turned out to have a defect in the auxin efflux carrier Atpin2 (Luschnig et al., 1998; Sieberer et al., 2000). We demonstrated that the effect of ethylene on hypocotyl elongation in the light is opposite to that in the dark (Smalle et al., 1997). As for ethylene, hypocotyl elongation in the light can be stimulated by auxins (Smalle et al., 1997; Gray et al., 1998). In the dark, auxins play a limited role in hypocotyl growth (Jensen et al., 1998). Earlier observations have linked auxin and ethylene pathways at later stages of development. Ethylene production is predominantly known to be enhanced by exogenous application of high concentrations of auxins (Yu and Yang, 1979; Woeste et al., 1999). In addition, a number of Arabidopsis mutants show cross-resistance to several hormones (Smalle and Van Der Straeten, 1997, and refs. therein). In contrast, processes in which ethylene controls auxins are relatively rare. However, ethylene has been shown to reduce auxin transport (Morgan and Gausman, 1966). In addition, ethylene can mediate differential growth in the apical hook region, most probably by controlling auxin levels. This interaction is defective in the hookless1 mutant (Lehman et al., 1996).
Here, we report on the isolation and physiological characterization of a new mutant that displays defects in ethylene and auxin response, further confirming a close interaction between both signaling pathways. Our data suggest that the ethylene-induced hypocotyl elongation in the light is mediated by auxin and probably stimulates auxin transport.
RESULTS
Isolation of a Novel Mutant alh1
ACC stimulates hypocotyl elongation in the light. The response is most pronounced on a low nutrient medium (LNM). This trait is a genuine ethylene effect, because Ag+ ions block the response (Smalle et al., 1997). In addition, the competitive inhibitor 1-methylcyclopropene (MCP) reversed the ACC stimulation of hypocotyl elongation (Table I). The elongation response was used to screen for constitutive response mutants in the absence of ACC. Thirty thousand and 40,000 seedlings treated with ethyl methanesulfonate and fast-neutron bombardment respectively, were analyzed, of which 80 candidate mutants were isolated. Thirty-two were confirmed by rescreening after self-fertilization. Knowing that ethylene-treated hypocotyls do not exceed twice their normal size, the number of candidates was narrowed down to five mutants, thus excluding most light-signaling mutants with long hypocotyl phenotype. On one-half-strength Murashige and Skoog (MS/2) medium, one of the mutants displayed epinastic cotyledons and leaf blades. These traits are typical for ethylene- or auxin-treated plants. The characteristics segregated in a semidominant fashion (mutant:intermediate:wild type, 21:54:25). The mutant was named ACC-related long hypocotyl 1 (alh1). However, in view of the phenotypes mentioned above and below, “alh1” might as well stand for auxin-related long hypocotyl 1.
Table I.
Effect of MCP on ACC-stimulated hypocotyl growth in wild-type Col-0
| Growth Medium | Air | 250 μL L−1 MCP |
|---|---|---|
| LNM (n ≥ 20) | 1.42 ± 0.23 | 1.54 ± 0.24 |
| LNM + 10 μm ACC (n ≥ 20) | 2.50 ± 0.47 | 1.62 ± 0.36 |
| Increase | 76% | 5% |
Values are mean hypocotyl length (in millimeters) ± sd; Measurements were done 8 d after germination.
On media containing either 200 μm CoCl2, an ethylene biosynthesis inhibitor, or 100 μm AgNO3, an ethylene action inhibitor, alh1 retained its long hypocotyl. Both alh1 and wild type showed a similar reduction of hypocotyl elongation of about 20%, implying that the hypocotyl phenotype in the light is probably not caused by ethylene overproduction.
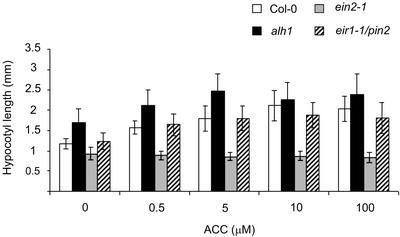
On LNM in the light, the length of alh1 hypocotyls exceeded that of wild type by at least 40% (Fig. 1). This observation suggests that part of the pathway controlling hypocotyl elongation in the light is constitutively active in alh1. The hypocotyl length on various concentrations of ACC indicated that alh1 is hypersensitive to ACC reaching the maximal response at a lower concentration than wild type (Fig. 1). On higher concentrations of ACC, alh1 hypocotyl length does not differ significantly from the wild type. This indicates that ACC-induced hypocotyl elongation and alh1-induced hypocotyl elongation are not additive (Fig. 1). Therefore alh1 most likely acts in the ACC/ethylene-regulated pathway. As opposed to the nonreacting ethylene-insensitive ein2-1, the ethylene-insensitive root 1 (eir1-1/pin2) mutant reacts in a wild type-like fashion (no significant difference at 100 μm ACC with P > 0.05). When grown on LNM supplemented with 50 μm ACC, pin1 mutants had an increase in hypocotyl elongation of only 68% (2.46 sd 0.5 mm treated versus 1.46 sd 0.26 mm untreated), whereas wild type had an increase of 82% (3.05 sd 0.52 mm treated versus 1.64 sd 0.27 mm untreated). Also pin3-3 mutant seedlings had a smaller increase in hypocotyl elongation upon ACC treatments. On 50 μm ACC, they showed only 32% increase in length (1.42 sd 0.25 mm treated versus 1.07 sd 0.12 mm untreated), whereas wild type had 63% (1.75 sd 0.30 mm treated versus 1.07 sd 0.12 mm untreated). This suggests a significant role for both PIN3 and PIN1 in the elongation process under given conditions.
Figure 1.
Effect of ACC on hypocotyl elongation in alh1 in the light. Seedlings of wild type (white bars), alh1 (black bars), ein2-1 (gray bars), and eir1-1/pin2 (striped bars) grown for 10 d on LNM medium supplemented with ACC in a range of concentrations. Data are mean ± sd (n > 20).
Map Position
The alh1 mutation was positioned on the genome by using microsatellite markers and AFLP markers (Bell and Ecker, 1994; Peters et al., 2001). As indicative traits for mutant selection, both the long hypocotyl and the rosette phenotype were scored. Alh1 was mapped in the vicinity of nga 692 on the bottom arm of chromosome 1 to a region overspanning the last 35 BACs (Table II). The ethylene mutants etr1, ein5, and ein7 all map to a different region on chromosome 1 (Roman et al., 1995).
Table II.
Map position of the alh1 mutation
| Marker | Recombinational Distance to alh1 |
|---|---|
| cM | |
| nga128 | 27.2 |
| nga280 | 27.2 |
| nga111 | 5.6 |
| AthATPase | 5.6 |
| nga692 | 5.6 |
| alh1 | 0.0 |
alh1 Displays Constitutive Auxin and Ethylene Responses
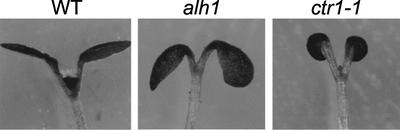
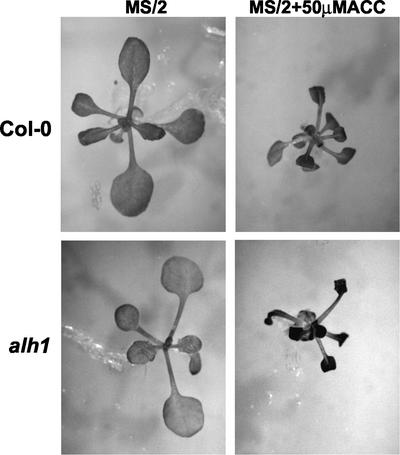
Besides longer hypocotyls in the light, alh1 seedlings and full-grown plants displayed additional traits characteristic of a constitutive auxin or ethylene response. Light-grown alh1 seedlings at the cotyledon stage are phenotypically intermediate between wild type and the ctr1-1 mutant with mildly epinastic cotyledons (Fig. 2) and thus resemble the auxin-overproducing sur1 seedlings (Boerjan et al., 1995). Leaf surface area was reduced throughout alh1 rosette development and the edges of leaf blades curled down. However, as petioles were longer, alh1 did not show the dwarfism characteristic for ctr1-1 (Kieber et al., 1993). As a consequence, the rosette diameter of full-grown alh1 plants was even larger than that of the wild type (Table III). In general, alh1 seedlings were smaller than the wild type during the early stages of development (first 3 weeks) and gradually became larger when reaching full expansion. Bolting and flowering were delayed (Table III). Alh1 inflorescences displayed an increased apical dominance as a result of a decrease and delay in secondary branching (Table III). Etiolated alh1 seedlings displayed a partial triple response, characterized by an exaggerated apical hook and a thicker, but not shorter, hypocotyl (Fig. 3).
Figure 2.
Morphology of alh1 at a stage of development relative to the wild type and the mutant ctr1-1. Plants germinated and grown in the light for 7 d on MS/2 medium at the cotyledon stage.
Table III.
Biometric analysis of alh1 relative to the wild type, ctr1-1, and etr1-3
| Assay | Wild Type | alh1 | ctr1-1 | etr1-3 |
|---|---|---|---|---|
| Rosette diameter (full-grown; cm) | 6.4 ± 1.4 | 7.4 ± 1.3 | 1.6 ± 0.3 | 6.5 ± 1.4 |
| Apical dominance (number of branches per inflorescence, n = 20) | 15.0 ± 5.0 | 9.0 ± 5.0 | N.D. | 13.0 ± 6.0 |
| Root length (mm) | 23.1 ± 2.0 | 18.3 ± 3.7 | 2.0 ± 0.5 | 23.6 ± 4.2 |
| Rosette leaf surface area (leaf 9, full-grown; cm2) | 1.83 ± 0.73 | 1.41 ± 0.58 | 0.05 ± 0.0 | 2.33 ± 0.69 |
| Petiole length (rosette leaf 9, full-grown; cm) | 1.2 ± 0.3 | 1.6 ± 0.3 | 0.4 ± 0.1 | 1.1 ± 0.3 |
| Leaf number at bolting | 18.0 ± 3.0 | 19.0 ± 4.0 | 23.0 ± 2.0 | 24.0 ± 3.0 |
| Bolting time (d after sowing) | 57.4 ± 3.6 | 69.5 ± 6.7 | 74.4 ± 5.1 | 60.7 ± 4.8 |
| Free auxin (pmol g−1 fresh wt) | 16.2 ± 1.2 | 16.0 ± 2.2 | N.D. | N.D. |
| Auxin conjugates (pmol g−1 fresh wt) | 5,325 ± 1,332 | 4,335 ± 660 | N.D. | N.D. |
| 3H after auxin accumulation in basal stem part (cpm) | 50.1 ± 11.9 | 50.6 ± 13 | N.D. | N.D. |
Values are means ± sd. N.D., Not determined.
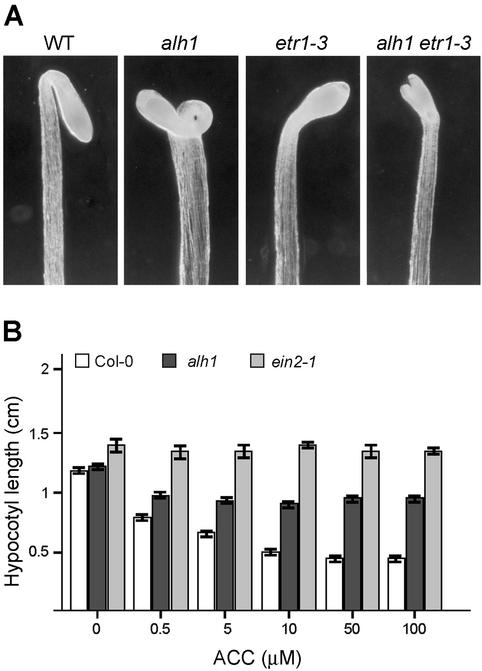
Figure 3.
Constitutive and insensitive responses to ACC of etiolated alh1 seedlings. A, Apical hook region of seedlings germinated and grown on MS/2 in the dark for 4 d. B, Hypocotyl length of seedlings germinated and grown in the dark for 7 d on MS/2 medium supplemented with the denoted concentrations of ACC. White bars, Wild type; black bars, alh1; gray bars, ein2-1.
alh1 Seedlings Overproduce Ethylene in Continuous Dark
To verify whether the partial triple response phenotype was caused by ethylene overproduction, we measured ethylene production in alh1 seedlings by using photo-acoustic detection. Although production levels in alh1 (0.63 sd 0.15 pL seedling−1 h−1) were far below those in an ethylene overproducer, eto2 (14.74 sd 1.53 pL seedling−1 h−1), alh1 produced 4-fold more ethylene than wild-type plants (0.15 sd 0.02 pL seedling−1 h−1) under these conditions. The ethylene production of alh1 seedlings under long-day conditions was not detectably different from wild type (data not shown). In addition, etiolated double mutant alh1 etr1-3 seedlings resembled the ethylene-insensitive etr1-3 mutant (Fig. 3A), supporting the fact that the alh1 constitutive response in the dark is due to increased ethylene biosynthesis levels.
alh1 Shows Characteristics of Ethylene Insensitivity
Treatment with ACC inhibited alh1 hypocotyl elongation in the dark to a lesser extent than in the wild type. Hypocotyl elongation at 100 μm ACC in the dark was only approximately 20% inhibited for alh1 against 65% in wild-type plants (Fig. 3B). At 50 μm ACC, alh1 hypocotyls were approximately twice as long as those of the wild type. Treatment of etiolated alh1 and wild-type seedlings with 10 μL L−1 ethylene gave a similar result (data not shown). Therefore, the reduction in ACC sensitivity is probably not caused by an altered ACC uptake or metabolism.
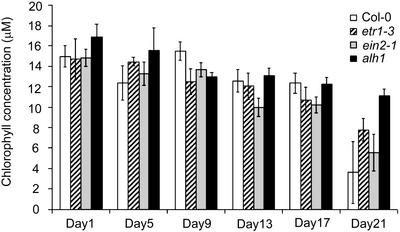
Decreased ethylene sensitivity is frequently accompanied by a delay in leaf senescence (Grbíc and Bleecker, 1995; Oh et al., 1997). Other reports mention a clear capability for auxins to influence this process (Grossmann and Retzlaff, 1997; Noh and Amasino, 1999). Low concentrations of auxins can delay leaf senescence, whereas high concentrations can stimulate it due to concomitant higher ethylene production levels. Total chlorophyll content can be used as a marker for senescence of tissues (Thomson and Plat-Aloia, 1987). At the end of the expansion phase of rosette leaves 7 and 8, chlorophyll levels were higher in alh1 when compared with both wild type and the ethylene-insensitive mutants etr1-3 and ein2-1 (Fig. 4). Twenty-one days after the end of leaf expansion, chlorophyll degradation was most pronounced in the wild type, less pronounced in ein2-1 and etr1-3, and the lowest in alh1 (Fig. 4). These results suggest that the delay in senescence cannot be due to mere ethylene insensitivity.
Figure 4.
Chlorophyll levels during rosette development of wild-type and ethylene mutants alh1, etr1-3, and ein2-1. Leaf discs of full-grown rosette leaves 7 and 8 were harvested immediately after the leaf expansion phase (d 1) and subsequently 4, 8, 12, 16, and 20 d later. Total chlorophyll levels were determined for the wild type (Col), alh1, etr1-3, and ein2-1. Error bars represent se.
With respect to ethylene-induced inhibition of leaf expansion in rosettes, alh1 appeared as sensitive as wild type, because when treated with ACC, the size of the alh1 leaf blades was similar to that of wild type (Fig. 5). On the molecular level, ethylene-treated alh1 rosettes showed a wild type-like induction of the ethylene-inducible ACC oxidase gene (At-ACO2) transcription (data not shown).
Figure 5.
ACC sensitivity in light-grown alh1 plants. Wild-type Col-0 and mutant plants were germinated and grown for 3 weeks in the light on MS/2 medium in the absence or presence of 50 μm ACC.
alh1 Roots React More Quickly to Gravistimulation
Seedling root elongation of wild type and alh1 did not differ in response to ACC (range from 0.05–50 μm ACC). Even the ein2-1 and etr1-3 mutants had a reduced root length of approximately 10% to 50% at the lowest and the highest ACC concentration tested, respectively (data not shown).
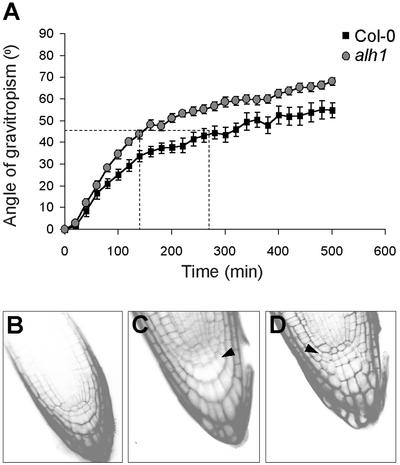
Several auxin mutants typically show defects in their response to gravitropic stimuli (Muday, 2001). We tested whether alh1 responds differently than wild type upon changes in direction of gravity. Therefore seedlings grown on vertical plates were rotated over 90°, and the angle of gravitropism was followed by time lapse imaging. Alh1 roots reacted more quickly than wild type, but no difference in growth rate was observed. Both wild type and alh1 gained 4 mm sd 1 mm of root length after 8 h. However, whereas alh1 roots had reached an angle of 45° after 150 min, wild-type roots needed 280 min to reach the same angle (Fig. 6A).
Figure 6.
a, Kinetic analysis of root gravitropism in alh1 and wild type. Seedlings were germinated and grown for 6 d in the light on MS/2 medium and turned for 90° at time 0. Each point represents the mean of at least 15 measurements. The error bars indicate se. b through d, Organization of columella cells in propidium iodide stained root tips from wild-type (b) and alh1 (c and d) plants. Arrows indicate extra cells.
Blancaflor et al. (1998) have shown the importance of columella cells in gravitropic growth. In addition, auxin distribution in these cells may be of crucial importance to the process (Swarup et al., 2001; Friml et al., 2002). Inspection of the root tips of alh1 plants revealed an abnormal organization of the columella. In contrast to the very stable cell order in Columbia (Col-0) wild type (Fig. 6B), alh1 mutants showed striking phenotypic variations. Of 36 plants, 72% showed a complete disorganization of the columella cells (Fig. 6D), 16% had an additional columella column (Fig. 6C), and 11% had wild-type phenotype with the characteristic four rows and four columns of columella cells (Fig. 6B; Dolan et al., 1993). In Col-0 plants, only 10% of 40 plants had an abnormality, deviating from the pattern in Figure 6B.
The Long Hypocotyl Phenotype in alh1 Is Related to Enhanced Auxin Signaling
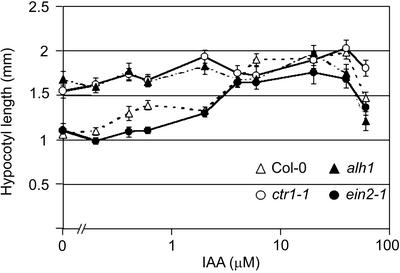
Hypocotyl elongation can be stimulated by auxin (Romano et al., 1995), whereas on LNM and in the light, auxin also mediates ethylene effects (Smalle et al., 1997). A dose-response relation for hypocotyl length after treatment with different auxin concentrations was established (Fig. 7). Whereas in the Col-0 wild type the hypocotyl length clearly increased between 6 and 40 μm indole-3-acetic acid (IAA), ctr1-1 and alh1 showed only a small difference in hypocotyl length. The ethylene-insensitive mutants etr1-3 and ein2-1 had an elongation comparable with that of wild type (Fig. 7; etr1-3 data not shown). For all lines except ctr1-1, 60 μm IAA was supra-optimal.
Figure 7.
Effect of IAA on hypocotyl elongation in Col-0, ctr1-1, alh1, and ein2-1. Black triangles, alh1; white triangles, wild-type Col-0; black circles, ein2-1; white circles, ctr1-1. Seedlings were grown on LNM supplemented with the indicated concentration of IAA for 10 d in a long-day photoperiod. Error bars represent se.
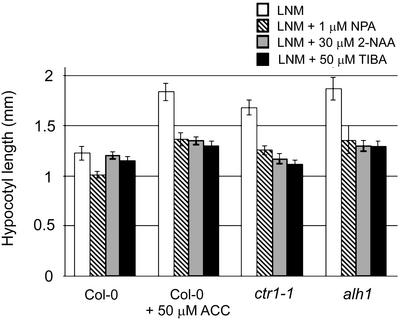
As a consequence, a direct involvement of auxins in the phenotype of alh1 was tested. The content of free and conjugated auxins in rosettes and transport in stems were not significantly different from wild type (Table III). In addition, alh1 hypocotyl elongation was studied on medium containing α-naphthylphthalamic acid (NPA), a potent auxin transport inhibitor (Morgan, 1964). Under these conditions, ctr1-1 and alh1 on LNM and Col-0 wild type on LNM supplemented with 50 μm ACC had a reduced hypocotyl length (Fig. 8). NPA did not completely abolish the increase in hypocotyl elongation in ACC-treated wild type, suggesting that a factor different from auxin transport is also involved in ACC-mediated hypocotyl elongation. The NPA effect was confirmed with another auxin transport inhibitor, TIBA. The anti-auxin 2-NAA, a competitive inhibitor, reduced the increase in hypocotyl elongation caused by ACC (Fig. 8), confirming the observations with the auxin transport inhibitors.
Figure 8.
Relation of the long hypocotyl and higher auxin transport in alh1. Seedlings were grown on LNM supplemented with the indicated concentration of NPA, tri-iodo-benzoic acid (TIBA), or 2-naphthaleneacetic acid (2-NAA) for 10 d in a long-day photoperiod. White bars, LNM; striped bars, LNM + 1 μm NPA; gray bars, LNM + 30 μm 2-NAA; black bars, LNM + 50 μm TIBA. Error bars represent se.
Furthermore, the auxin-insensitive mutants axr1-3 and axr2 showed limited, if any, ACC-induced hypocotyl elongation. In contrast, another auxin-insensitive mutant, aux1-7, displayed a strongly stimulated hypocotyl elongation upon ACC treatment (Table IV), implying that the AUX1 gene product is not required for the observed response to ACC.
Table IV.
Effects of ACC on hypocotyl growth in auxin-related mutants
| Growth Medium | Wild Type | aux1-7 | axr1-3 | axr2 |
|---|---|---|---|---|
| LNM (n = 10) | 1.14 ± 0.19 | 1.34 ± 0.13 | 1.16 ± 0.11 | 1.24 ± 0.14 |
| LNM + 50 μm ACC (n = 10) | 2.20 ± 0.45 | 2.44 ± 0.43 | 1.20 ± 0.14 | 1.34 ± 0.17 |
| Increase | 93% | 83% | 3% | 8% |
Values are mean hypocotyl lengths (in millimeters) ± sd; Measurements were done 10 d after germination.
Our data suggest that the ethylene effect on hypocotyl elongation is mainly mediated through auxins. Supportive evidence for this hypothesis results from kinetic analysis of developing seedlings. The major difference in growth rate between ACC-treated Col-0 hypocotyls and untreated seedlings occurred between the 3rd and the 4th d after germination (Fig. 9A). This growth phase coincided with an increase in β-glucuronidase (GUS) activity in the hypocotyl of ACC-treated plants, carrying an auxin-inducible promoter, linked to the UIDA gene (Fig. 9B). At 3.5 d of age, the staining in hypocotyls of non-treated plants was limited to the hypocotyl-root junction. After treatment with ACC, strong GUS activity was observed all over the hypocotyl. The latter observation might indicate a role of the auxin-inducible SAUR AC1 gene in elongation processes, as was suggested earlier (Gil et al., 1994). A similar effect, although less pronounced, was observed in DR5-GUS hypocotyls (Fig. 9B). Roots of DR5 seedlings seemed to be stained more intensely. However, we do not know whether that is due to an increase in UIDA activity or the compaction of the root resulting from ACC treatment (Fig. 9B). Note the general retardation in growth caused by the lack of nutrients in LNM (Smalle et al., 1997).
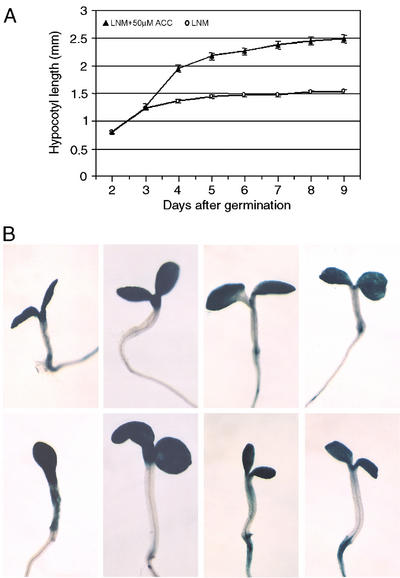
Figure 9.
Ectopic expression of SAUR AC1-GUS and DR5-GUS reporter genes in developing ACC-treated seedlings. A, Kinetic study of the effect of ACC on hypocotyl elongation in Col-0 seedlings. Black triangles, Col-0 seedlings grown on LNM + 50 μm ACC; white circles, Col-0 seedlings on LNM. B, Seedlings grown on LNM (top row) and on LNM + 50 μm ACC (bottom row). From left to right: 3.5-d-old SAUR AC1-GUS, 8-d-old SAUR AC1-GUS, 3.5-d-old DR5-GUS, and 8-d-old DR5-GUS.
DISCUSSION
Alh1 Illustrates the Complexity of Signaling Networks in Plants
The contrasting phenotypes of alh1 suggest that this mutation affects several signaling networks. The study of alh1 indicates that interactions between ethylene and auxin signaling are mediated by different mechanisms under different growth conditions, at different developmental stages, and in different tissues. Moreover, it is remarkable that ethylene can have opposite effects on hypocotyl growth, depending on whether plants were grown in light or in the dark (Smalle et al., 1997). Alh1 shows that the triple response in ethylene-treated etiolated seedlings and the ethylene-induced promotion of hypocotyl elongation under light can be uncoupled to some extent. This uncoupling of ethylene responses has also been shown with C-EIN2 transgenics. The carboxy terminus of EIN2 is sufficient to cause constitutive responses in ein2-5 mutant transgenics grown in the light, but it cannot induce triple response in the dark (Alonso et al., 1999). This suggests the existence of two partially separated ethylene-signaling networks depending on the light conditions. Alh1, which has a constitutive ethylene response in the light and decreased sensitivity in the dark, might be an additional key component in the separation of hormone responses in different developmental stages and conditions.
Plant hormones, like auxins, through their own signaling network, may have an effect on the ethylene-signaling routes that use common components. For instance, MAP kinase cascades and two-component systems have been shown to be implicated in a myriad of processes (Innes, 2001; Morris, 2001; Hwang et al., 2002). In addition, protein degradation seems a likely nod for several networks, like jasmonic acid, light, auxin, and cytokinin signaling (Hellmann and Estelle, 2002; Smalle et al., 2002; Xu et al., 2002). This posttranslational control of regulatory factors may allow fine-tuning the balance between different hormones. Ethylene responses could be yet another signaling route controlled by protein degradation.
Alh1 Shows an Altered Ethylene-Auxin Interaction Controlling Hypocotyl Growth
Auxin has been reported to stimulate ethylene production (Yu and Yang, 1979; Rodrigues-Pousada et al., 1999; Woeste et al., 1999). Nevertheless, hypocotyl length can be increased in light-grown ethylene-insensitive mutants by exogenous auxins, suggesting that auxins act downstream of ethylene in the elongation process or indicating the existence of an ethylene-independent pathway that controls hypocotyl growth (Fig. 7; Romano et al., 1995). In addition, ethylene and auxin have been reported to act independently in the inhibition of root and hypocotyl elongation in light-grown Arabidopsis plants on a rich medium (Fujita and Syono, 1996; Collett et al., 2000). However, seedlings grown for 3 d on LNM and treated with the ethylene precursor ACC show ectopic and higher expression of the auxin-inducible SAUR AC1 gene in the hypocotyl (Fig. 9B). SAUR AC1 has been postulated to be important for cell elongation (Gil et al., 1994; Gil and Green, 1997). At this stage, non-treated seedlings have fully expanded cotyledons, whereas ACC-treated seedlings have not (Fig. 9B). Thus, ACC could extend the elongation period of the hypocotyl at the expense of cotyledon expansion (Smalle et al., 1997). In addition, the site of enhanced expression of the auxin-inducible SAUR AC1 gene corresponds with the central region of the hypocotyl (Fig. 9B). In this part of light-grown hypocotyls, large increases in cell length have been reported to occur in the time window from d 3 to 5 after germination (Gendreau et al., 1997). This observation was confirmed for seedlings grown in the presence of ACC on LNM (Fig. 9A).
Furthermore, the axr1-3 and axr2 mutations, which confer a strong inhibition of auxin-induced SAUR AC1 expression, block the ACC-induced hypocotyl elongation response (Gil et al., 1994; Timpte et al., 1995). In contrast, a defect in the AUX1 gene had no effect on ACC-stimulated hypocotyl elongation and resulted in only a very mild reduction of auxin-induced SAUR AC1 mRNA accumulation (Table IV; Gil et al., 1994). Thus, ACC could stimulate hypocotyl elongation by intensifying or prolonging auxin signaling in a pathway that involves the AXR1 and AXR2 gene products, but not AUX1. AXR1 is involved in modifying the SCF-TIR (Skp-Cdc53-F-Box-Transport Inhibitor Response) complex, which uses AXR2 as a substrate as well as other AUX/IAA proteins (Dharmasiri and Estelle, 2002). It is conceivable that ethylene also has an effect on protein degradation. Earlier findings have already confirmed that cytokinins and jasmonic acid could exert their activity through protein degradation complexes (Smalle et al., 2002; Xu et al., 2002).
The influx carrier AUX1 and the efflux carrier EIR1/PIN2 are part of the auxin transport system. They are considered root specific (del Pozo et al., 1998; Luschnig et al., 1998; Nagpal et al., 2000; Swarup et al., 2001). Other auxin transport proteins, such as PIN1, PIN3, and other PIN family members, are probably involved in the response in hypocotyls. PIN3 is necessary for differential growth in root and hypocotyl, whereas PIN1 has a role in auxin transport in stems (Okada et al., 1991; Friml et al., 2002a, 2002b). Like pin1 mutants, pin3 mutants had a smaller, but significant, ACC-stimulated increase in hypocotyl elongation compared with the wild type. This suggests that both auxin efflux carriers are necessary for the full effect. The fact that these mutations did not cause a total absence of ACC-stimulated hypocotyl elongation may be due to redundancy of auxin efflux carriers. In that case, ethylene could have a general effect on several auxin efflux carriers. Moreover, the ethylene-induced elongation response in the hypocotyl might rely on the same mechanisms that are involved in differential growth. Double mutants between pin mutants could help clarifying these observations.
The effect on auxin transport varies depending on the species, the developmental stage, and the environmental conditions (Abeles et al., 1992). Although in many cases, ethylene inhibits auxin transport, stimulation of the process also has been observed (Morgan and Gausman, 1966; Goldsmith, 1977). As in Arabidopsis roots, ethylene might stimulate auxin transport in hypocotyls through PIN-like auxin transport proteins (Friml et al., 2002a, 2002b). Together, the data suggest that auxin acts after ethylene, positively controlling hypocotyl elongation.
The alh1 mutation might affect the ethylene-auxin crosstalk, regulating auxin transport in hypocotyls. This is supported by a much reduced IAA-promoted growth in alh1 and ctr1-1 (Fig. 7). The response controlled by ethylene in cross talk with auxins is probably near its maximum in both mutants. This is not due to an intrinsic higher auxin content in alh1 seedlings, because auxin measurements in seedlings indicated no differences between alh1 and wild type. Also, the phenotype of the alh1 mutant in the dark argues against a general auxin overproduction as in sur1/alf1/rty/hls3 (Boerjan et al., 1995; Celenza et al., 1995; King et al., 1995; Lehman et al., 1996). Whereas the light-grown seedlings of alh1 and sur1 mutants resemble each other, etiolated sur1 seedlings have the opposite phenotype of alh1 seedlings, displaying no apical hook and a short hypocotyl. Although the alh1 seedling phenotype was largely reverted by auxin transport inhibitors, we did not find any difference from wild type in an auxin accumulation assay. Therefore, we propose that alh1 is mutated in a component influencing the downstream part of the auxin-signaling pathway. Whether ALH1 is a positive or negative regulator cannot be revealed at this point, because the alh1 mutation, being semidominant, can be caused by either a gain or loss of function. In addition, stimulatory effects of auxin-mediated gibberellin signals in alh1 cannot be fully excluded at this point, because auxin transport inhibitors can diminish the rate of biosynthesis of gibberellins (Ross, 1998). However alh1 showed the same relative elongation of the hypocotyl upon gibberellin treatment as the wild type (data not shown). Therefore it is unlikely that alh1 is a gibberellin-signaling mutant.
Finally, it should be mentioned that ACC-mediated hypocotyl elongation on LNM is probably not solely due to auxin cross talk. The response could not be inhibited completely by auxin transport blockers, indicating the existence of an auxin-independent pathway as well.
Differential Growth in alh1
Auxin is known to play a role in gravitropism, which is caused by a more pronounced cell expansion on the upper side of the root (Maher and Martindale, 1980; Rashotte et al., 2000). It recently became clear that auxin transport, through proteins as AUX1 and PIN-family members, is a pivotal element in the gravitropic response and that columella cells in the root cap are essential for a full response (Blancaflor et al., 1998; Swarup et al., 2001). Alh1 has extra cells in the columella region. This could enhance the gravity perception of the plant root and thus cause the faster gravitropic response.
Differential growth also occurs upon the formation of an apical hook in dark grown seedlings. This phenomenon is thought to be dependent on unequal auxin distribution in the hypocotyl (Lehman et al., 1996). When wild-type seedlings are treated with ethylene in the dark, the curvature of the hook is exaggerated. In alh1 seedlings grown in air in the dark, we detected a partial triple response that was restricted to an exaggeration of apical hook formation and a thicker hypocotyl (Fig. 3). Etiolated alh1 seedlings also overproduce ethylene. In many aspects, including apical hook formation, hypocotyl elongation in the light and root gravitropism, alh1 has the opposite phenotype of the pin3 mutants (Friml et al., 2002b). The pin3 mutation is a recessive and thus loss of function mutation. Enhancement of the activity of a PIN3-like auxin transporter with tissue-specific functions could conversely cause a alh1 like phenotype. However, the PIN3 gene does not map to the region determined for the ALH1 gene.
MATERIALS AND METHODS
Plant Material
Seeds mutagenized by ethyl methanesulfonate and fast neutron were purchased from Lehle Seeds (Tucson, AZ). The Col-0 and Landsberg erecta wild types of Arabidopsis and the ethylene mutants aux1-7, axr1-3, axr2, etr1-3, ctr1-1, and ein2-1 all in Col-0 background were obtained from the Arabidopsis Biological Resource Center (Columbus, OH). The alh1 mutant was backcrossed to the Col-0 wild type. The pin1 mutant is in the Enkheim background, whereas the pin3-3 mutant has a Columbia background.
Media and Treatments
Seeds were sown and plants were grown under sterile conditions as described (Smalle et al., 1997). ACC, 2-NAA, TIBA, and IAA were obtained from Sigma-Aldrich (St. Louis); AgNO3 was from Merck (Darmstadt, Germany); CoCl2 was from UCB Pharma (Brussels), and NPA was from Greyhound (Merseyside, UK). All hormone and inhibitor solutions were added to the medium after filter sterilization. MCP was supplied by the Department of Organic Chemistry (Ghent University, Ghent, Belgium). For MCP gassing, seedlings were grown in 30 μmol m−2 s−1 photosynthetic photon flux density. Treatment with MCP was performed for 20 h d−1. Flushing of the growth chamber occurred during the subjective morning for 4 h with four refreshments per hour.
Segregation Patterns
The increase in hypocotyl length, the delay in senescence, and the presence of an exaggerated apical hook in the dark are traits that cosegregated in a population of 104 F2 plants of a alh1 backcross with Col-0 in a semidominant fashion (mutant:intermediate:wild type, 21:54:25); confirmation of the characteristics was obtained from F3 populations.
Mapping of the alh1 locus was performed with simple sequence length polymorphism markers (Bell and Ecker, 1994). alh1 was crossed to Ler. The F2 population was scored for mutant and wild-type plants. The phenotypes were confirmed in F3 to distinguish between the wild-type, homozygous, or heterozygous alh1. Per F2 individual, DNA was prepared from a single leaf or from a small population in the next generation with a single-step protocol (Thomson and Henry, 1995) or the DNeasy mini kit (Qiagen, Hilden, Germany), respectively. A total of 58 F2 individuals were scored. Map distances were determined by computational analysis using Joinmap (Stam, 1993) with the Kosambi and Haldane algorithms.
Isolation of a Double Mutant and Epistatic Analysis
The phenotype of the alh1 etr1-3 mutant could be observed in the F1 because both mutations display a degree of dominance. The double mutant was isolated by screening the F2 for strong ACC-insensitive seedlings (elongating roots on 10 μm ACC) with a rosette morphology of untreated alh1 seedlings. F3 populations were analyzed to allow identification of double homozygous lines.
Biometrics
Hypocotyl measurements were performed on seedlings grown for 10 d in 16 h of light/8 h of darkness. All seedlings were grown on horizontal plates, except for the kinetic study of hypocotyl growth, for which the seedlings were grown on vertical plates. Hypocotyl length (of light- and dark-grown seedlings) was measured using a Stemi SV11 binocular (Zeiss, Jena, Ger-many). Rosette diameter was measured on 5-week-old plants using a ruler with 1-mm precision. Petiole length and leaf blade surface area were measured from rosette leaf 9 of 5-week-old plants (at this developmental stage of the rosette, growth of leaf 9 had ceased). Branching was measured from plants with senescing apical meristem. Petiole length, surface area, and silique length were measured by pressing and taping the respective seedlings or plant organs onto 3MM paper (Whatman, Clifton, NJ), scanning the image, and computing distance or surface with the ScionImage software (Scion Corp., Frederick, MD).
For measurements of gravitropism, plants were grown for 6 d on vertical plates containing MS/2 medium in a 16-h-light/8-h-dark photoperiod. Plates were rotated over 90°. The roots were photographed every 15 min, and the angle of gravitropic curvature was measured using the ScionImage software. Root tip staining was done in a 10 μg mL−1 propidium iodide (Sigma-Aldrich) solution for 2 min, before visualization on a confocal laser scanning microscope.
Chlorophyll Levels
To determine chlorophyll concentrations, a set of 30 plants was used for each line. At each harvesting point, five plants were randomly chosen. Immediately after leaves stopped growing (end of elongation, i.e. d 1) 0.5-cm2 discs were harvested from the center of the widest part of the leaf blade of leaves 7 and 8, starting at 7 weeks of age. The leaf discs were frozen in liquid nitrogen. The same procedure was repeated 4, 8, 12, 16, and 20 d later. Determination of chlorophyll concentration was performed according to Graan and Ort (1984). Chlorophyll content was expressed in micromolar.
GUS Staining
The lines containing the auxin-inducible reporter constructs SAUR AC1-GUS and DR5-GUS lines were kind gifts from Pamela J. Green (Michigan State University, East Lansing) and Thomas J. Guilfoyle (University of Missouri, Columbia; Gil and Green, 1997; Ulmasov et al., 1997). For each treatment, 10 to 15 seedlings were harvested after 8 h of light at d 3. The second samples were taken at d 8, 2 d before the emergence of the first leaves. Seedlings were submerged in 90% (v/v) acetone for 30 min and washed with 1 m phosphate buffer for 15 min. The seedlings were subsequently incubated for 18 h in 0.1 m phosphate solution containing 0.5 mm Fe(CN)2, 0.5 mm Fe(CN)3, and 2 mm 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (ImmunoSource, Antwerp, Belgium). Destaining was done in 70% (v/v) ethanol for at least 8 h.
Hormone Measurements
For ethylene measurements, 150 seeds were sterilized and sown on LNM agar (Smalle et al., 1997) in 10-mL vials. The seeds were kept at 4°C for 48 h for stratification, then exposed to light for 4 h to stimulate germination, and incubated in the dark for 4 d. The vials were subsequently capped, and ethylene emanation was measured every 2.6 h during 24 h using photo-acoustic detection (Bijnen et al., 1996).
IAA was prepared from 3-week-old rosettes. Samples were ground in liquid nitrogen, transferred into 80% (v/v) MeOH, and extracted overnight at −20°C. [13C6]IAA (100 pmol, Cambridge Isotope Laboratories Inc., Andover, MA) was added for isotope dilution purposes. After centrifugation (20,000 rpm, 15′, 4°C), IAA was purified by a combined solid phase extraction procedure and methylated before analyses (Prinsen et al., 2000). Quantification was done by microLC-(ES+) tandem mass spectrometry in single reactant monitoring mode (Prinsen et al., 1998). The chromatograms obtained were processed by means of Masslynx software (Micromass, Manchester, UK). Concentrations were expressed in picomoles per gram fresh weight. IAA conjugates were purified and analyzed as described for IAA after alkaline hydrolysis (Prinsen et al., 2000).
For the auxin accumulation assay, the lower 2 cm of the bolting stem of 4-week-old plants was cut and put upside down in an Eppendorf tube containing 20 μL of an auxin solution. The latter had an overall concentration of 1.45 μm including 2.4 nCi of 3-(5(n)-3H)(IAA) (Amersham). After 18 h, 5 mm from the basal side (that was not in the liquid), was cut off and extracted in ethanol. These samples were measured using a scintillation counter (1409, PerkinElmer Wallac, Gaithersburg, MD).
ACKNOWLEDGMENTS
We thank Mira Haegman and Els Fostier (Ghent University, Ghent, Belgium) for excellent technical support; Jan Goeman and Johan Van Der Eycken (Ghent University) for supplying 1-MCP; Pamela J. Green (Michigan State University, East Lansing) and Thomas J. Guilfoyle (University of Missouri, Columbia) for the SAUR-GUS and DR5 transgenic lines, respectively.
Footnotes
This work was supported by the Fund for Scientific Research (Flanders; grant nos. G.0281.98 and G.0345.02) and by the European Union (grant for Access to Research Infrastructures Action of the Improving Human Potential Program). L.C. is a postdoctoral research assistant of the Fund for Scientific Research (Flanders). A.D.P. is a Research Assistant of the Fund for Scientific Research (Flanders).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010850.
LITERATURE CITED
- Abeles FB, Morgan PW, Salveit ME., Jr . Ethylene in Plant Biology. Ed 2. New York: Academic Press; 1992. [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bijnen FGC, Reuss J, Harren FJM. Geometrical optimization of a longitudinal resonant photoacoustic cell for sensitive and fast trace gas detection. Rev Sci Instrum. 1996;67:2914–2923. [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Collett CE, Harberd NP, Leyser O. Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol. 2000;124:553–561. doi: 10.1104/pp.124.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Timpte C, Tan S, Callis J, Estelle M. The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science. 1998;280:1760–1763. doi: 10.1126/science.280.5370.1760. [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Estelle M. The role of regulated protein degradation in auxin response. Plant Mol Biol. 2002;49:401–409. [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organization of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Friml J, Benkova E, Bililou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jurgens G et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002a;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002b;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Fujita H, Syono K. Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol. 1996;37:1094–1101. doi: 10.1093/oxfordjournals.pcp.a029059. [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Green PJ. Regulatory activity exerted by the SAUR-AC1 promoter region in transgenic plants. Plant Mol Biol. 1997;34:803–808. doi: 10.1023/a:1005875300606. [DOI] [PubMed] [Google Scholar]
- Gil P, Liu Y, Orbovic V, Verkamp E, Poff KL, Green PJ. Characterization of the auxin-inducible SAUR-AC1 gene for use as a molecular genetic tool in Arabidopsis. Plant Physiol. 1994;104:777–784. doi: 10.1104/pp.104.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith MHM. The polar transport of auxin. Annu Rev Plant Physiol. 1977;28:439–478. [Google Scholar]
- Graan T, Ort DR. Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem. 1984;259:14003–14010. [PubMed] [Google Scholar]
- Gray WM, Östin A, Sandberg G, Romano CP, Estelle M. High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7197–7202. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbíc V, Bleecker AB. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 1995;8:595–602. [Google Scholar]
- Grossmann K, Retzlaff G. Bioregulatory effects of the fungicidal strobilurin kresoxim-methyl in wheat (Triticum aestivum) Pestic Sci. 1997;50:11–20. [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpham NVJ, Berry AW, Knee EM, Roveda-Hoyos G, Raskin I, Sanders IO, Smith AR, Wood CK, Hall MA. The effect of ethylene on the growth and development of wild type and mutant Arabidopsis thaliana (L.) Heynh. Ann Bot. 1991;68:55–62. [Google Scholar]
- Hellmann H, Estelle M. Plant development: regulation by protein degradation. Science. 2002;297:793–797. doi: 10.1126/science.1072831. [DOI] [PubMed] [Google Scholar]
- Hwang D, Chen HC, Sheen J. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 2002;129:500–515. doi: 10.1104/pp.005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes RW. Mapping out the roles of MAP kinases in plant defense. Trends Plant Sci. 2001;6:392–394. doi: 10.1016/s1360-1385(01)02058-1. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:455–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight LI, Crocker W. Toxicity of smoke. Bot Gaz. 1913;55:337–371. [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EP, Martindale SJB. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem Genet. 1980;18:1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Morgan DG. Influence of α-naphthylphthalamic acid on the movement of indolyl-3-acetic acid in plants. Nature. 1964;201:476–477. doi: 10.1038/201476a0. [DOI] [PubMed] [Google Scholar]
- Morgan PW, Gausman HW. Effects of ethylene on auxin transport. Plant Physiol. 1966;41:45–52. doi: 10.1104/pp.41.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PC. MAP kinase signal transduction pathways in plants. New Phytol. 2001;151:67–89. doi: 10.1046/j.1469-8137.2001.00167.x. [DOI] [PubMed] [Google Scholar]
- Muday GK. Auxins and tropisms. J Plant Growth Regul. 2001;20:226–243. doi: 10.1007/s003440010027. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 2000;123:563–573. doi: 10.1104/pp.123.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol. 1999;41:181–194. doi: 10.1023/a:1006342412688. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SA, Park J-H, Lee GI, Paek KH, Park SK, Nam HG. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997;12:527–535. doi: 10.1046/j.1365-313x.1997.00527.x. [DOI] [PubMed] [Google Scholar]
- Peters JL, Constandt H, Neyt P, Cnops G, Zethof J, Zabeau M, Gerats T. A physical amplified fragment-length polymorphism map of Arabidopsis. Plant Physiol. 2001;127:1579–1589. [PMC free article] [PubMed] [Google Scholar]
- Prinsen E, Van Dongen W, Esmans E, Van Onckelen H. Micro and capillary liquid chromatography-tandem mass spectrometry: a new dimension in phytohormone research. J Chromatogr A. 1998;826:25–37. [Google Scholar]
- Prinsen E, Van Laer S, Öden S, Van Onckelen H. Auxin analysis. In: Tucker GA, Roberts JA, editors. Methods in Molecular Biology. 141: Plant Hormone Protocols. Totowa, NJ: Humana Press; 2000. pp. 49–65. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;122:481–490. doi: 10.1104/pp.122.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Pousada R, Van Caeneghem W, Chauvaux N, Van Onckelen H, Van Montagu M, Van Der Straeten D. Hormonal cross-talk regulates the Arabidopsis thaliana 1-aminocyclopropane-1-carboxylate synthase gene 1 in a developmental and tissue-dependent manner. Physiol Plant. 1999;105:312–320. [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano CP, Robson PRH, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- Ross JJ. Effects of auxin transport inhibitors on gibberellins in pea. J Plant Growth Regul. 1998;17:141–146. [Google Scholar]
- Sieberer T, Seifert GJ, Hauser MT, Grisafi P, Fink GR, Luschnig C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr Biol. 2000;10:1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Kurepa J, Yang PZ, Babiychuk E, Kushnir S, Durski A, Vierstra RD. Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. Plant Cell. 2002;14:17–32. doi: 10.1105/tpc.010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Van Der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. [Google Scholar]
- Stam P. Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J. 1993;3:739–744. [Google Scholar]
- Stepanova AN, Ecker JR. Ethylene signaling: from mutants to molecules. Curr Opin Plant Biol. 2000;3:353–360. doi: 10.1016/s1369-5266(00)00096-0. [DOI] [PubMed] [Google Scholar]
- Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D, Henry R. Single-step protocol for preparation of plant tissue for analysis by PCR. BioTechniques. 1995;19:394–400. [PubMed] [Google Scholar]
- Thomson WW, Plat-Aloia KA. Ultrastructure and senescence in plants. In: Thomson WW, Nothnagel EA, Huffaker RC, editors. Plant Senescence: Its Biochemistry and Physiology. Rockville, MD: American Society of Plant Physiologists; 1987. pp. 71–80. [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M. The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J. 1995;8:561–569. doi: 10.1046/j.1365-313x.1995.8040561.x. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Straeten D, Djudzman A, Van Caeneghem W, Smalle J, Van Montagu M. Genetic and physiological analysis of a new locus in Arabidopsis that confers resistance to 1-aminocyclopropane-1-carboxylic acid and ethylene and specifically affects the ethylene signal transduction pathway. Plant Physiol. 1993;102:401–408. doi: 10.1104/pp.102.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeste KE, Vogel JP, Kieber JJ. Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol Plant. 1999;105:478–484. [Google Scholar]
- Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Wen P, Huang D, Xie D. The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell. 2002;14:1919–1935. doi: 10.1105/tpc.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-B, Yang SF. Auxin-induced ethylene production and its inhibition by aminoethoxyvinylglycine and cobalt ion. Plant Physiol. 1979;64:1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]