Abstract
Sterols are important not only for structural components of eukaryotic cell membranes but also for biosynthetic precursors of steroid hormones. In plants, the diverse functions of sterol-derived brassinosteroids (BRs) in growth and development have been investigated rigorously, yet little is known about the regulatory roles of other phytosterols. Recent analysis of Arabidopsis fackel (fk) mutants and cloning of the FK gene that encodes a sterol C-14 reductase have indicated that sterols play a crucial role in plant cell division, embryogenesis, and development. Nevertheless, the molecular mechanism underlying the regulatory role of sterols in plant development has not been revealed. In this report, we demonstrate that both sterols and BR are active regulators of plant development and gene expression. Similar to BR, both typical (sitosterol and stigmasterol) and atypical (8, 14-diene sterols accumulated in fk mutants) sterols affect the expression of genes involved in cell expansion and cell division. The regulatory function of sterols in plant development is further supported by a phenocopy of the fk mutant using a sterol C-14 reductase inhibitor, fenpropimorph. Although fenpropimorph impairs cell expansion and affects gene expression in a dose-dependent manner, neither effect can be corrected by applying exogenous BR. These results provide strong evidence that sterols are essential for normal plant growth and development and that there is likely a BR-independent sterol response pathway in plants. On the basis of the expression of endogenous FK and a reporter gene FK::β-glucuronidase, we have found that FK is up-regulated by several growth-promoting hormones including brassinolide and auxin, implicating a possible hormone crosstalk between sterol and other hormone-signaling pathways.
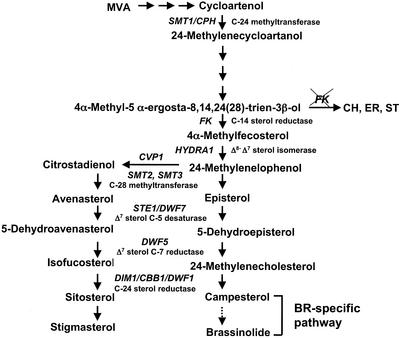
Sterols are part of the vast family of isoprenoids, a group of structurally related secondary metabolites. These compounds are essential for both animals and plants because they are components of membranes and as such affect cellular functions. The best known sterol is cholesterol, whose signaling functions in cell division, cell growth, cell death, and various developmental processes have been extensively studied in animals (Edwards and Ericsson, 1999). Whereas plants also produce dozens of different sterols, including cholesterol, only brassinosteroids (BR) derived from campesterol have been shown to act as hormone signals. BR hormones play critical roles in regulating cell expansion, morphogenesis, apical dominance, leaf and chloroplast senescence, and gene expression. Cellular defects in BR biosynthesis or response often result in a characteristic dwarf syndrome due to the defect of cell expansion (Altmann, 1998; Clouse and Sasse, 1998). The most common of the plant sterols are sitosterol, stigmasterol, and campesterol; they are produced by a bifurcated sterol biosynthetic pathway involving a common precursor (see Fig. 1; Noguchi et al., 2000). To date, only BRs have been demonstrated to have regulatory roles on postembryonic growth. Other sterols have been considered mainly as membrane structural components (Hartmann, 1998). However, recent studies of the fackel (fk) mutant in Arabidopsis have raised the possibility that other sterols besides BR may also be important for both embryonic and postembryonic development (Clouse, 2000; Jang et al., 2000; Schrick et al., 2000). The FK gene encodes a sterol C-14 reductase, catalyzing a reaction that is upstream of the branch point in the biosynthetic pathway that leads to BRs on one branch and sitosterol and stigmasterol on the other. The levels of both BR and sterols were reduced in fk mutants. The mutants also accumulate the substrate of sterol C-14 reductase plus several atypical sterols not detectable in the wild-type plants (Jang et al., 2000; Schrick et al., 2000). A specific role for FK-dependent sterols in cell division and cell expansion has been postulated on the basis of the unique embryonic and postembryonic phenotypes of fk mutants and of the unique expression pattern of FK in embryos and meristems. It is most critical that unlike other BR-deficient mutants, fk mutants could not be rescued by exogenous application of BRs. Although these studies strongly suggest that FK-mediated sterol biosynthesis is required for normal plant growth and development, the underlying molecular mechanisms are unknown.
Figure 1.
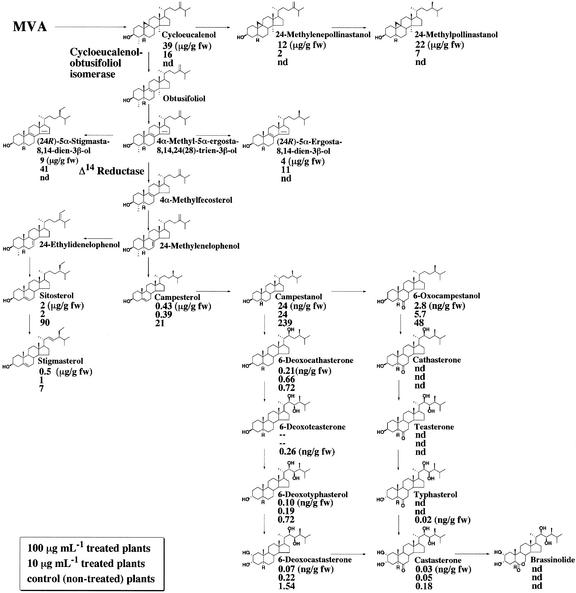
Sterol biosynthesis in Arabidopsis. A simplified pathway illustrates the synthesis of C5-sterols including campesterol, sitosterol, and stigmasterol in Arabidopsis. Three atypical sterols (5α-cholesta-8,14-dien-3β-ol [CH], (24R)-5α-ergosta-8,14-dien-3β-ol [ER], and (24R)-5α-stigmasta-8,14-dien-3β-ol [ST]) accumulated in fk mutants are shown.
In contrast to the limited understanding of sterol function in plant development, numerous studies have indicated that sterols are essential for embryonic and adult development in animals (Edwards and Ericsson, 1999). A well-known example is the involvement of cholesterol in the Hedgehog (Hh) signal transduction pathway that controls patterning. A critical step in Hh signaling is when secreted Hh proteins from signaling cells are modified by cholesterol before their release, allowing subsequent interaction of the Hh proteins with other membrane-bound proteins in the target cells to initiate signal transduction (Incardona and Eaton, 2000). Hh regulates cell growth and proliferation by promoting transcription of cyclin D and cyclin E (Duman-Scheel et al., 2002). In plants, previous genetic studies have revealed that BRs are important for cell elongation, however BRs were not implicated in controlling patterning. The dwarf phenotype associated with BR-deficient mutants is attributed to reduced cell size rather than reduced cell number, even though brassinolide (BL), the most active BR, is able to activate CycD3 (Hu et al., 2000) and to promote cell division (Clouse and Sasse, 1998; Hu et al., 2000).
There are an increasing number of genetic studies showing that non-BR sterols are important for plant pattern formation. For instance, two embryonic patterning mutants hydra1 and hydra2 were recently found blocked in sterol biosynthesis (Souter et al., 2002). HYDRA1 encodes Δ8-Δ7 sterol isomerase, catalyzing a reaction downstream of FK, and hydra2 turns out to be allelic to fk. Also, the smt1 mutants, which are blocked in sterol C-24 methyltransferase activity several steps upstream of FK, exhibited defects in embryonic patterning, although they were less severe than the hydra mutants (Diener et al., 2000). Furthermore, a homeodomain, Leu-zipper, and sterol/lipid-binding domain-containing protein PHABULOSA (PHB) has been found to be involved in determining shoot radial patterning. Abnormal expression of PHB can be found in the mutants as early as in the globular embryo stage. It is postulated that the activity of PHB is dependent on the ligand (sterol) binding and that the ligand synthesis or stability is positively regulated by active PHB via a feedback loop (McConnell et al., 2001). This further supports that sterol biosynthesis is part of the regulatory circuits that control plant development. Together these studies implicate a specific role for sterols in plant development that might be independent of BR (Clouse, 2002).
Although the aforementioned studies have suggested that sterols are critical for plant growth and development, it is not known which sterols regulated by FK participate in the control of various cellular activities. Furthermore, there is no knowledge yet of how sterol synthesis is regulated at the molecular level. In this report, we provide direct evidence to support the idea that sterols are effective regulators of plant development and gene expression. We also show that fk mutants can be phenocopied by treating wild-type Arabidopsis with fenpropimorph, an inhibitor of sterol C-14 reductase. Finally, we demonstrate that FK expression is subject to a complicated regulation by BR, sterols, and other hormones.
RESULTS
Sterols Affect Expression of Genes Important for Cell Growth
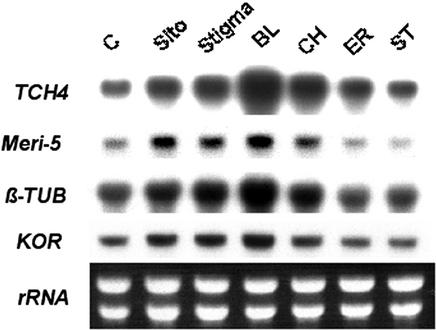
We previously showed that three atypical 8,14-diene sterols (CH, ER, and ST), collectively referred to as “fk sterols,” accumulated in fk mutants (Fig. 1). These fk sterols were made through alternative biochemical reactions that ended in a stable product. We also found that exogenous BR could not correct the defect of hypocotyl elongation in fk mutants (Jang et al., 2000). From these, it seems possible that the fk sterols might be the biologically active substances responsible for some aspects of growth and developmental defects observed in the fk mutants. This possibility can be tested through exogenous treatment with fk sterols obtained by chemical synthesis (Seto et al., 2000). To explore whether the effects of these sterols on developmental processes were mediated through the control of gene expression, we determined whether various sterols changed the expression of TCH4, Meri-5, β-TUBULIN, and KORRIGAN (KOR), a set of marker genes involved in cell expansion or cell division (Goda et al., 2002; Müssig et al., 2002; Yin et al., 2002). TCH4 and Meri-5 belong to the xyloglucan endotransglycosylase gene family (XET) that is involved in cell wall loosening, a mechanism required for plant cell expansion (Campbell and Braam, 1999). Tubulins are subunits of microtubules that are important for cytoskeleton, cell growth, and mitosis. KOR encodes a membrane-bond endo-β-1,4-glucanase that is critical for wall assembly, cell expansion, and cytokinesis (Zou et al., 2000). KOR also acts as a cellulase whose activity is required for cellulose synthesis (Lane et al., 2001; Sato et al., 2001; Peng et al., 2002). Our results show that the expression of all four genes was enhanced by BL (Fig. 2). Interestingly, both the common sterols sitosterol and stigmasterol and the fk sterol CH exhibited effects similar to BL, but similar concentrations of fk sterols ER or ST were ineffective in affecting the expression of these genes (Fig. 2). It is clear from these results that exposure to the specific sterols caused distinct and potentially important changes in the expression of various genes involved in plant cell division and growth. Both BL and the other sterols act as potent regulators of gene expression at the micromolar range.
Figure 2.
Both BL and sterols are activators of gene expression. Seedlings were grown in Murashige and Skoog liquid medium in continuous white light (90 μE m−2 s−1) at 25°C on a shaker. Two-DAG seedlings were treated with BL (10−6 m) or sterols (10−6 m) for 4 h. RNA blots were hybridized to probes specific to TCH4, Meri-5, β-TUBLIN (TUB), and KOR as described in “Materials and Methods.” Five micrograms of RNA was used for each sample. Ethidium bromide-stained rRNA bands were used as loading controls.
Distinct Gene Expression Pattern in fk and Other Sterol-Deficient Mutants
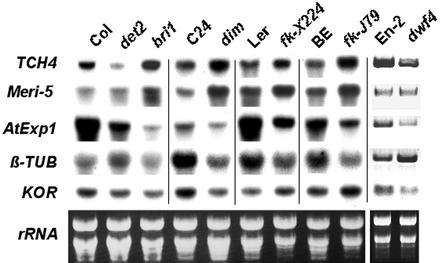
Whereas BR-deficient mutants det2 and dwf4 are blocked solely in BR-specific biosynthesis (Fig. 1), dim1, fk-J79, and fk-X224 are blocked in the sterol-specific pathway (Fig. 1), which results in a deficiency of both BR and other sterols. Intriguingly, whereas det2, dwf4, and dim1 can be complemented, neither fk-J79 nor fk-X224 can be rescued by exogenous BL. The availability of different BR and sterol mutants provides a unique opportunity for the investigation of plant sterol response at the molecular level. Consistent with the observation that the XET genes (TCH4 and Meri-5) were BL-inducible (Fig. 2), their expression was lower in BR-deficient mutants det2 and dwf4 than that in their respective wild types (Fig. 3). TCH4 and Meri-5 were unexpectedly expressed at a higher level in sterol-deficient mutants including dim1, fk-J79, and fk-X224, implicating factors other than BR affecting XET expression (Xu et al., 1995). Neither TCH4 nor Meri-5 was up-regulated dramatically in BR-signaling mutant bri1, although BL concentration in bri1 was 50- to 100-fold higher than that in the wild type (Noguchi et al., 1999). This result supports previous finding that BRI1 as a bona fide BR receptor (Wang et al., 2001). AtExp1 belongs to the expansin gene family that controls diverse cellular functions including cell wall extension (Cosgrove, 2000). AtExp1 was repressed in all BR-deficient mutants regardless of whether they were blocked in the BR-specific or the sterol-specific pathway. In contrast, the expression of β-TUBULIN was reduced in sterol-deficient mutants (dim1, fk-J79, and fk-X224) but was slightly activated in BR-deficient mutants (det2 and dwf4) when compared with their respective wild types. The expression of both AtExp1 and β-TUBULIN was reduced in bri1, indicating that neither AtExp1 nor β-TUBULIN is directly controlled by BR signaling. The expression of KOR varied in different mutant background, i.e. it was down-regulated in det2, dwf4, and dim1 but was up-regulated in fk mutants (Fig. 3). Whether the high expression of KOR is related to the uncontrolled cell division in fk mutants awaits further investigation. In summary, our results have demonstrated that sterol- and BR-deficient mutants are distinct in their gene expression profiles and the difference is likely due to direct or indirect effect of the their distinct sterol composition.
Figure 3.
Marker gene expression in fk and other BR-deficient mutants. Gene expression was determined in mutants blocked in BR-specific pathway (det2 and dwf4), in sterol-specific pathway (dim1, fk-X224 and fk-J79), in BR perception (bri1), and in their respective wild-type plants (Col for det2 and bri1, C24 for dim1, Landsberg erecta for fk-X224, BE for fk-J79, and En-2 for dwf4). RNA gel-blot analyses were performed using 8-DAG seedlings grown on Murashige and Skoog plates under a photoperiod of 16 h of light (90 μE m−2 s−1) and 8 h of dark at 25°C. Five micrograms of RNA was used for each sample. Ethidium bromide-stained rRNA bands were used as loading controls. Expression data for En-2 and dwf4 were obtained by reverse transcription-PCR.
The Expression of FK Is Affected by Sterols, BR, and Other Hormones
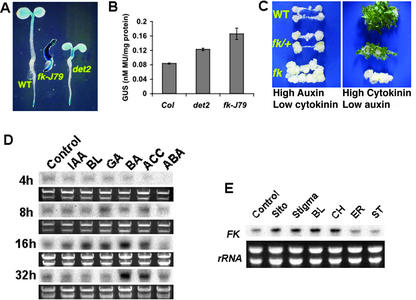
Because FK-dependent sterols are effective regulators of gene expression and FK expression is associated with regions with active cell growth (Jang et al., 2000), it is important to determine whether FK expression is affected by growth hormones. It has been reported that the expression of sterol biosynthetic genes in yeast is regulated by sterol intermediates (Smith et al., 1996) and that some BR biosynthetic genes in Arabidopsis are feedback-suppressed by BL (Mathur et al., 1998; Noguchi et al., 1999). To understand the effect of BR on FK, we first determine the expression of a promoter β-glucuronidase (GUS) fusion reporter gene FK::GUS (Jang et al., 2000) in 4-d after germination (DAG) wild-type (Benscheim [BE]), fk-J79, and det2 mutants. Interestingly, FK::GUS was expressed at a higher level in fk-J79 plants than in det2 or wild type in the absence of any exogenous hormones (Fig. 4A). FK::GUS expression in fk-J79 plants was notable throughout the whole seedlings instead of being restricted to the shoot and root tips as in the wild type. This abnormal expression pattern could be due to the loss of a putative feedback repression induced by sterols and/or BR. Quantitative GUS analysis revealed that FK::GUS was expressed at a higher level in det2 and fk-J79 than that in wild type, suggesting that FK might be feedback repressed by endogenous BR. Because FK::GUS expression was even higher in fk-J79 than that in det2, it is likely that factors other than BR could be responsible (Fig. 4B). One possibility is that fk mutant might have an altered hormone response that can activate FK, because Souter et al., (2002) have shown that hydra2 (allelic to fk) is hypersensitive to auxin. To test this hypothesis, a sensitive tissue culture assay using root explants (described in “Materials and Methods”) was carried out. Results showed that fk-J79 mutant was hypersensitive to auxin as indicated by exaggerated callus formation on Murashige and Skoog plates containing a high ratio of auxin to cytokinin (Fig. 4C). By contrast, fk was insensitive to high ratio of cytokinin to auxin because no shoots were able to regenerate from fk calli. The auxin hypersensitivity could be potentially important for patterning because auxin is known to be involved in patterning (Hardtke and Berleth, 1998) and some of the “auxin effects” may actually be achieved by the regulation of sterol synthesis and response. The auxin hypersensitivity found in fk suggests that sterols can affect auxin action and response possibly through modification of cell membranes or via crosstalk at the signaling level.
Figure 4.
Regulation of FK expression. A, The expression of reporter gene FK::GUS in wild-type, fk-J79, and det2 plants. Shown are 4-DAG seedlings stained with 5-bromo-4-chloro-3-indolyl-β-glucuronic acid for 24 h. B, Quantitative GUS analysis. Fifteen seedlings were pooled for each sample, and cell extract was used in an enzymatic assay using 4-methylumbelliferyl β-d-glucuronide (see “Materials and Methods”). The error bars represent ses of the means derived from three replicates. C, fk mutant is hypersensitive to auxin and is insensitive to cytokinin as reflected by exaggerated callus production and diminished shoot regeneration, respectively. For callus induction, root explants were sliced into 1-cm long sections, and five sections of each genotype were placed onto a CIM. CIM consisted of 1× Murashige and Skoog salts, B5 vitamins, 0.5 mg L−1 2,4-dichlorophenoxyacetic acid, 0.05 mg L−1 benzylaminopurine, 0.05 mg L−1 kinetin, and 0.7% (w/v) phytagar (Invitrogen). Callus induction was conducted for 10 d under 50 μE m−2 s−1 white light at 25°C. For shoot induction, callus was transferred to SIM and grown for 2 to 4 weeks under the same condition. SIM consisted of 1× Murashige and Skoog salts, 5 mg L−1 isopentenyl adenine, 0.15 mg L−1 IAA, and 0.7% (w/v) phytagar (Invitrogen). D, FK expression was enhanced by IAA, BL, gibberellin (GA), and cytokinin (BA). RNA gel-blot analysis was performed using 3-DAG wild-type seedlings grown in Murashige and Skoog liquid medium in continuous white light (90 μE m−2 s−1) at 25°C on a shaker. RNA samples were extracted from seedlings treated with hormone (10−6 m) for 4, 8, 16, or 32 h. Five micrograms of RNA was loaded in each lane. Ethidium bromide-stained rRNA bands were used as loading controls. E, Sterols enhance the expression of FK. RNA gel-blot analyses were performed using 2-DAG seedlings grown in Murashige and Skoog liquid medium in continuous white light (90 μE m−2 s−1) at 25°C on a shaker. Seedlings were treated with BL (10−6 m) or sterols (10−6 m sitosterol, stigmasterol, CH, ER, or ST) for 4 h. Five micrograms of RNA was loaded in each lane. Ethidium bromide-stained rRNA bands were used as loading controls.
To obtain more direct evidence to support the hypothesis that auxin and other hormones may affect FK expression, 3-DAG seedlings were used for RNA gel-blot analyses in a time-course experiment (Fig. 4D). Consistent with the results of quantitative GUS analysis (Fig. 4B), IAA caused approximately 2-fold induction of FK, which was obvious 16 h after the treatment. It is remarkable that several other hormones including BL could also induce FK expression. The effect of GA was similar to BL except that the induction occurred earlier with GA (8 h) than that with BL (16 h). Cytokinin (BA) and ethylene had the strongest effect on FK induction, and this effect persisted until 32 h. Abscisic acid did not affect FK expression under the conditions tested (Fig. 4D). In summary, many growth-promoting hormones can affect FK expression, consistent with the notion that FK expression is required for active cell growth. Another possibility for an elevated FK::GUS expression in fk-J79 mutant might be the accumulation of uncommon 8,14-diene sterols. To test this, 2-DAG wild-type seedlings were used in an RNA gel-blot analysis. Surprisingly, FK was activated by normal sterols (sitosterol and stigmasterol), BL, as well as one of the uncommon 8,14-diene sterols CH 4 h after the treatment (Fig. 4E). FK was induced more quickly by BL in this experiment (4 h) than in previous experiment (16 h, Fig. 4D) because FK is differentially expressed in different developmental stages (Jang et al., 2000; J.X. He and J.-C. Jang, unpublished data). It seems that 2-DAG seedlings used in this experiment responded more quickly to BL (Fig. 4E) than 3-DAG seedlings used in previous experiment (Fig. 4D). In conclusion, elevated expression of FK::GUS in fk mutant is likely due to a combinatory effect of altered sterol composition and/or response as well as altered responses to other hormones such as auxin.
The fk Mutant Can Be Phenocopied by Using Fenpropimorph
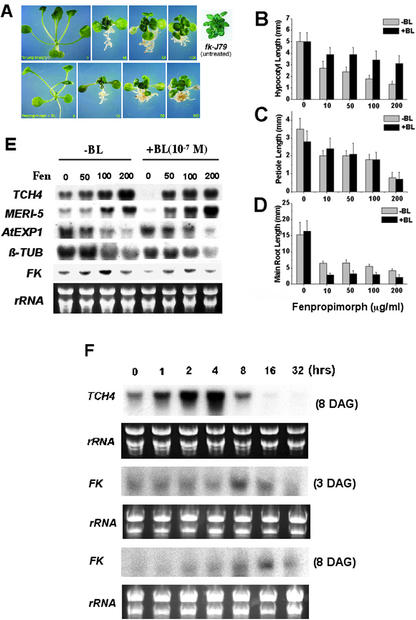
We have previously shown that fk-J79 mutant has reduced sterol C-14 reductase activity. Thus, it is hypothesized that the wild-type plants treated with specific inhibitors of the enzyme should display phenotypes similar to fk mutants. It has been shown that the yeast sterol C-14 reductase (ERG24) is the target of some anti-fungal agents such as morpholine or 15-azasterol (Lorenz, 1992). In tobacco (Nicotiana tabacum), sterol C-14 reductase is inhibited by fenpropimorph (I50 = 0.8 μm; Taton et al., 1989; Schaller et al., 1992). In this study, we found that fenpropimorph-treated wild-type Arabidopsis plants exhibited a dwarf stature with stunted shoots and roots, which resembled fk-J79 mutant (Fig. 5A, top panel). Furthermore, exogenous BL (10−7 m) failed to rescue the phenotype caused by fenpropimorph (Fig. 5A, bottom panel), suggesting that fenpropimorph blocked a reaction upstream of the bifurcation of BR- and sterol-specific biosynthetic pathway (Fig. 1) and that BL could not substitute sterols for their specific functions.
Figure 5.
Phenocopy of fk mutants using fenpropimorph. A, Wild-type plants were grown on Murashige and Skoog plates supplemented with 0, 10, 50, or 100 μg mL−1 of fenpropimorph for 3 weeks under a photoperiod of 16 h light (90 μE m−2 s−1) and 8 h dark at 25°C. B to D, The effects of fenpropimorph on Arabidopsis seedling development. The effects of fenpropimorph on the elongation of hypocotyl (B), petiole (C), and main root (D) were determined using wild-type (BE) plants grown on Murashige and Skoog plates under a photoperiod of 16-h-light (90 μE m−2 s−1) and 8-h-dark cycle at 25°C for 8 d. The error bars represent ses of the means derived from the measurements of 15 plants. E, Fenpropimorph (Fen) affects the expression of genes involved in cell division and expansion. RNA was extracted from wild-type (BE) plants grown on 1× Murashige and Skoog plates under a photoperiod of 16 h of light (90 μE m−2 s−1) and 8 h of dark at 25°C for 8 d. Five micrograms of RNA was loaded in each lane. Ethidium bromide-stained rRNA bands were used as loading controls. F, Transient activation of TCH4 and FK by BL. Wild-type (Col) seedlings of 3- or 8-DAG were grown in Murashige and Skoog liquid medium in continuous white light (90 μE m−2 s−1) at 25°C on a shaker. Samples were treated with BL (10−7 m) for 0, 1, 2, 4, 8, 16, or 32 h. Five micrograms of RNA was loaded in each lane. Ethidium bromide-stained rRNA bands were used as loading controls.
Quantitative analyses indicated that reduced length of the hypocotyl and petiole was positively correlated with fenpropimorph concentration (Fig. 5, B and C). More than 60% reduction of hypocotyl and petiole length was observed when 200 μg mL−1 fenpropimorph was used. Although the application of BL could promote hypocotyl elongation, a 20% to 30% reduction was still observed in the presence of both BL and fenpropimorph. Interestingly, BL was not effective at all in rescuing the reduction of petiole caused by fenpropimorph. Fenpropimorph was found to be as potent as BL in the inhibition of main root growth. An additive and saturated response was observed in the presence of both BL and fenpropimorph (Fig. 5D). It is intriguing that lateral root formation was promoted by low concentration (50 ppm) but was inhibited by high concentration (200 ppm) of fenpropimorph (data not shown). For the inhibition of lateral root elongation, 10 ppm of fenpropimorph was most effective, and higher concentrations appeared to be less effective (data not shown). Together these results clearly indicate that fenpropimorph exerts multiple effects on plant growth and development, and some of these effects are BR independent.
Fenpropimorph Causes Changes in Gene Expression Similar to That Observed in fk Mutants
To determine the impact of fenpropimorph on gene expression, we examined the marker genes whose expression pattern was altered in fk mutants (Fig. 3). The expression of TCH4 and Meri-5 was induced by fenpropimorph in the wild type, and the induction was correlated with the increase of fenpropimorph concentration (Fig. 5G). In contrast, fenpropimorph caused a concentration-dependent repression of AtExp1 and β-TUBULIN. The pattern of gene expression caused by fenpropimorph in the wild type was consistent with the pattern observed in two different fk mutants (Fig. 3). BL did not override the induction caused by fenpropimorph although BL alone nearly abolished the expression of TCH4 and Meri-5. Likewise, BL did not alter the effects of fenpropimorph on the repression of AtExp1 or β-TUBULIN. The results here suggest that the effect of exogenous BL cannot substitute that of sterols whose production is blocked by fenpropimorph (described below). Whereas BL could activate TCH4 and Meri-5 in a 4-h treatment in previous experiment (Fig. 2), it repressed their expression after a longer treatment in this experiment. To verify this discrepancy, we performed a time-course experiment by treating wild-type (Columbia [Col]) seedlings (8-DAG) with BL (10−7 m) for 0, 1, 2, 4, 8, 16, or 32 h. Consistent with previous reports (Xu et al., 1995; Iliev et al., 2002), TCH4 gene was induced by BL within 1 h and peaked in 4 h but dropped back to the basal level in 8 h. TCH4 expression was completely diminished by 16 h, indicating that the effect of BL was transient and a negative effect was triggered in longer term (Fig. 5F). This transient gene induction followed by a repression by BL treatment was also found for FK gene in both 3-DAG and 8-DAG seedlings (Fig. 5F). Overall, the pattern of gene expression in fenpropimorph-treated wild-type plants showed striking similarity to fk mutants (Fig. 3). These results provide strong evidence that fenpropimorph is a potent inhibitor of Arabidopsis sterol C-14 reductase.
Fenpropimorph Causes Changes in Sterol Composition
To examine the effects of fenpropimorph on sterol synthesis, the endogenous levels of BR and sterols in control and fenpropimorph-treated plants (10 and 100 μg mL−1) were determined. The results are summarized in Figure 6. A significant, dose-dependent reduction of endogenous BR was found in fenpropimorph-treated plants. The levels of 6-deoxocastasterone and 6-deoxotyphasterol, quantitatively the major BRs in Arabidopsis, were reduced to 5% to 26% of the control levels (Fig. 6). In addition to BRs, sterol levels were also affected by fenpropimorph. In plants treated with 10 ppm fenpropimorph, the Δ8,14 sterols (fk sterols) accumulated to a high level similar to that in the fk-J79 mutant (Jang et al., 2000). In addition, significant accumulations of cycloeucalenol, 24-methylenepollinastanol, and 24-methylpollinastanol were observed. In contrast, the levels of typical sterols such as campesterol, sitosterol, and stigmasterol were greatly reduced by exposure to 10 ppm fenpropimorph. In plants treated with 100 ppm fenpropimorph, drastic accumulations of cycloeucalenol, 24-methylenepollinastanol, and 24-methylpollinastanol were observed. In addition, a moderate accumulation of Δ8,14 sterols was found. The dramatic accumulation of 9β,19-cyclopropyl sterols and Δ8,14 sterols indicate that both cyclo-eucalenol-obtusifoliol isomerase and Δ14 sterol reductase are inhibited by fenpropimorph.
Figure 6.
Fenpropimorph inhibits sterol C-14 reductase in wild-type plants and causes sterol composition change including the accumulation of 8,14-diene sterols. Values shown are the content of each chemical (nano- or micrograms per gram fresh weight) for plants treated with 100 μg mL−1 fenpropimorph (top), with 10 μg mL−1 fenpropimorph (middle), or mock treated (bottom).
DISCUSSION
Sterols Affect Plant Development
A regulatory role of sterols in plant development was first proposed in the molecular genetic studies of fk mutants (Jang et al., 2000; Schrick et al., 2000). The current report focuses on the effects of sterols on gene expression and provides additional evidence to support that non-BR sterols have unique roles in plant growth and development. Results of our biochemical analysis indicate that fenpropimorph can block the activity of sterol C-14 reductase in wild-type plants and alter sterol composition. Fenpro-pimorph-treated wild-type plants display phenotypes and sterol profile similar to fk mutants. These developmental phenotypes remarkably cannot be rescued by exogenous BL, indicating that the role of sterols is distinct from BR. Recent cloning and mutant characterization of HYDRA1 (encoding Δ8-Δ7 sterol isomerase) and HYDRA2 (FK) has further strengthened this idea (Souter et al., 2002). Both hydra mutants contained altered sterol composition and displayed defects of embryonic patterning; neither phenotype has been observed in typical BR-deficient mutants. In addition, Arabidopsis plants with cosuppression of SMT2;1 resulted in a drastic reduction of sitosterol but an elevated level of campesterol (Schaeffer et al., 2001). These plants displayed pleiotropic phenotypes including reduced shoot growth, increased shoot branching, distorted flower morphology, and low fertility. All of these developmental defects can also be found in fk mutants (Topping et al., 1997; Jang et al., 2000; Schrick et al., 2000). Like fk mutants, neither hydra nor SMT2;1 cosuppression plants could be rescued by exogenous BL. cotyledon vascular pattern1 (cvp1) mutants were more recently found to have defects in STEROL METHYLTRANSFERASE2 (SMT2), a reaction one step downstream of HYDRA1 (Fig. 1). Although gross morphology of the cvp1 embryos was normal, the cvp1 plants displayed defects in vascular cell polarization and axialization in cotyledons during embryogenesis. The cvp1 mutants had increased campesterol and reduced sitosterol, and it was predicted that cvp1 contains higher levels of BR than that of wild type (Carland et al., 2002). However, neither exogenous sterols nor brassinazole, an inhibitor of BR synthesis, could rescue cvp1 mutant phenotype. The results of these recent studies strongly suggest that besides BR, sterols appear to have critical and independent signaling roles in plant development.
In our study, we have postulated that the unique phenotype of fk is caused by either the reduction of sterols and BRs or/and the accumulation of the substrate of FK or three atypical 8,14-diene sterols (fk sterols). Because neither BR nor sterols could rescue fk mutants (Jang et al., 2000), the three fk sterols might play a role in causing the mutant phenotype. The three fk sterols CH, ER, and ST accumulated at 1.6, 15, and 145 μm, respectively, in fk-J79 mutant (Jang et al., 2000). High concentrations of fk sterols may have toxic effects for cell growth and embryogenesis. To find out why fk mutants have embryonic defects, it will be imperative to determine the molecular and cellular effects of fk sterols at a broader range of concentrations during specific stages of embryo development. On the other hand, we cannot rule out the possibility that a reduction of downstream sterols is responsible for the embryonic phenotype of fk mutants. For example, although cvp1, dwf7/ste1, dwf5, and dwf1/dim are blocked in sterol biosynthesis downstream of 24-methylenelophenol (Fig. 1), they do not show obvious defects in embryonic patterning. In contrast, smt1/cph (Schrick et al., 2002), fk/hydra2, and hydra1, blocked in the pathway upstream of 24-methylenelophenol, display abnormal embryonic patterning. These results suggest that 24-methylenelophenol may be critical for normal embryo development (Clouse, 2002).
Sterols Affect Gene Expression in Arabidopsis
Although BR is known to control plant development and gene expression, little is known about how sterols affect gene expression in plants. Thirty BR-inducible genes were recently identified using DNA microarray analysis (Yin et al., 2002). Of the 30 genes, seven encoded cell wall-modifying enzymes including XETs, β-1,4 glucanases, polygalacturonase, pectin methylesterase, and expansin. Goda et al. (2002) have found that BR either up- or down-regulate a large number of genes in Arabidopsis. Genes encoding XET (TCH4, XTR6, BRU8, and BRU9), expansin (AtExp8 and BRU1), extensin, and arabinogalactan are among the ones induced by BR. TCH4 and AtExp5 were also found to be up-regulated by BR in an independent DNA microarray analysis using GeneChips (Müssig et al., 2002). Results of our current study indicate that BL, sitosterol, stigmasterol, and the atypical fk sterol CH have a similar effect in activating TCH4, Meri-5, β-tubulin, and KOR. Although one would expect that expression of these marker genes would be predictable in various BR- or sterol-deficient mutants, it was not entirely possible due to the multifaceted roles sterols have in many molecular and cellular mechanisms. Nevertheless, the expression profile of the marker genes was nearly identical between fk-J79 and fk-X224 mutants and was very similar in the dim1 mutant. In addition, the gene expression profile of sterol-deficient mutants (fk and dim1) is different from the profile of BR-deficient mutants (det2 and dwf4), indicating that endogenous sterols and BR have differential effects on regulating gene expression in Arabidopsis. Because TCH4 is highly induced by IAA or BR (Xu et al., 1995), the unusual high level of TCH4 and Meri-5 expression in fk mutants might be due to auxin hypersensitivity. This is strongly supported by the results of our tissue culture assays in which fk exhibits exaggerated callus proliferation stimulated by auxin (Fig. 4C). Furthermore, a recent study has shown that hydra2 (allelic to fk) has elevated auxin response and that the mutant phenotypes can be rescued partially by inhibition of auxin and ethylene signaling (Souter et al., 2002). Together, these results indicate that sterols are potentially important regulators. Altered sterol composition not only affects sterol-specific functions but also interferes with other hormone-signaling processes that require proper membrane properties to achieve normal functions. This is evidenced by numerous studies in animals demonstrating a crucial role of lipid rafts on plasma membrane where proteins with signaling function aggregate (Simons and Toomre, 2000).
Regulation of FK Expression
Genes encoding sterol biosynthesis enzymes are feedback-regulated by the end product ergosterol in yeast (Smith et al., 1996). This appears to be evolutionarily conserved in Arabidopsis because both DWF4 and CDP are down-regulated by BL. The CPD gene, encoding a sterol hydroxylase in the BR-specific biosynthetic pathway, requires the function of the BR receptor BRI1 for exogenous BL-induced feedback repression (Li et al., 2001). The expression of DWF4 is also feedback-regulated by the abundance and sensing capacity of BR. As a result, DWF4 was expressed at higher levels in dwf1 (dim1), cpd, and bri1 (BR-insensitive) mutants than in the wild type (Noguchi et al., 2000). Feedback repression of DWF4 and CDP by BR has been confirmed in recent DNA microarray analyses (Goda et al., 2002; Müssig et al., 2002).
Whereas genes involved in the BR-specific pathway (CPD and DWF4) are feedback-regulated by BL, FK is activated by BL. It has recently been found that the transcription of DWF7/STE1, DIM1/DWF1, SMT2, and SMT3 are not affected by BL (Carland et al., 2002; Goda et al., 2002). On the basis of these results, it seems that genes involved in upstream sterol-specific pathway (Fig. 1) are not feedback-regulated by BL. Consistent with this notion, our RNA gel-blot analyses show that FK is down-regulated in bri1 and dim1 and seems to be unchanged in dwf4 (data not shown). In addition, FK::GUS was expressed higher in fk than that in det2 or wild type (Fig. 4, A and B). BR-induced feedback repression cannot fully explain these seemingly contradictory events, because FK transcription is induced by BL (Fig. 4, D and E), and both det2 and fk-J79 are BR deficient. One possible explanation is that unlike genes involved in BR-specific pathway, intermediate sterols may have a role in regulating the transcription of FK (Fig. 4E). Differences of FK expression in the various mutants could be the result of accumulation of biosynthetic intermediates, each compound having a different effect on FK expression. On the other hand, we cannot rule out the possibility that results from using exogenously applied BL might not reflect a physiological response controlled by endogenous BL. Another possible explanation is that the accumulation of uncommon 8,14-diene sterols is responsible for regulatory changes in fk mutant, because we have found that CH can activate FK expression (Fig. 4E). However, FK is also activated by BL, sitosterol, and stigmasterol and yet fk mutant is deficient in all three compounds. One observation that surprised us is that the expression of FK::GUS is enhanced rather than reduced in fk mutant.
Besides BR and sterols, other compounds are also capable of regulating FK. We have remarkably found that FK is up-regulated not only by BL and sterols but also by different growth hormones including IAA, cytokinin, GA, and ethylene. Similar hormone regulation has been reported for other sterol biosynthetic genes. Both SMT2 and SMT3 are induced by a number of plant hormones including auxin, cytokinin, and ethylene; and all three hormones could promote cell division or cell elongation (Carland et al., 2002). The 2-fold induction of FK::GUS in fk mutant (Fig. 4B) is coincidentally similar to a 2-fold increase of FK expression in the presence of IAA, implicating the up-regulation of FK::GUS might simply due to IAA hypersensitivity of the fk mutant (Fig. 4C). Together these results suggest that multiple sterols act as regulatory molecules and that sterol response pathways may work in concert with other hormone-signaling pathways in the control of various cellular activities and development.
Fenpropimorph as a Tool for Chemical Genetic Studies
Sterol biosynthesis inhibitors are effective tools in probing the biosynthesis and regulatory functions of sterols across different kingdoms from yeast to humans (Parks et al., 1999; Moebius et al., 2000). In plants, both fenpropimorph and 15-azasterol are specific inhibitors of sterol C-14 reductase, and they affect the growth of tobacco calli (Schaller et al., 1992), Arabidopsis (Schrick et al., 2002), and barley (Hordeum vulgare) seedlings (Mercer et al., 1989). Bramble cells treated with 15-azasterol accumulate abnormal Δ8,14-diene sterols at the expense of Δ5-sterols (sitosterol, campesterol, and isofucosterol; Schmitt et al., 1980). In the present study, we have found that fk-J79 mutant can be phenocopied by treating the wild-type plants with fenpropimorph. Fenpropimorph inhibited the growth of hypocotyl, petiole and roots in a dose-dependent manner. Biochemical analyses revealed that sterol composition between fenpropimorph-treated wild-type plants and fk mutants is similar (Fig. 6). Fenpropimorph-treated wild-type plants most notably resembled fk mutants in the pattern of gene expression (Fig. 5E). All of these data strongly suggest that fenpropimorph can block the function of sterol C-14 reductase in Arabidopsis. Thus, it should be possible to use fenpropimorph to determine the biochemical consequences of blocking sterol C-14 reductase enzymes in different plants, tissues, and developmental processes and to perform chemical genetic studies to identify genes involved in sterol metabolism and signaling.
MATERIALS AND METHODS
Plant Materials and Chemicals
The Arabidopsis used in this study includes wild-type ecotypes Col, BE, En-2, and Landsberg erecta; BR-deficient mutants det2, dim1, dwf4, fk-X224, and fk-J79; BR-insensitive mutant, bri1; and wild-type plants homozygous for the transgene FK::GUS. The seeds were either generated in authors' lab or obtained from the Arabidopsis Biological Research Center (Ohio State University, Columbus). The fk sterols CH, ER, and ST were chemically synthesized in the authors' lab. Campesterol, sitosterol, and ethylene precursor 1-aminocyclopropane-1-carboxylic acid were purchased from Sigma-Aldrich (St. Louis). BL was purchased from CIDtech Research Inc. (Ontario, Canada) and fenpropimorph was a product of Riedel-deHaën (Sigma-Aldrich). IAA, gibberellic acid, cytokinin, and abscisic acid were purchased from Invitrogen (Carlsbad, CA).
Plant Growth and Tissue Culture Assay
All of the plants used in this study were grown either in liquid or on Murashige and Skoog plates containing 1× Murashige and Skoog salts (Invitrogen), 2% (w/v) Suc, Gamborg's B5 vitamins, and 0.8% (w/v) phytagar (Invitrogen). Seeds were surface-sterilized for 10 min in 50% (v/v) commercial bleach, rinsed three times with sterilized distilled water, and treated at 4°C for 3 d. Seeds were plated and treated at 4°C for additional 3 d before incubating at 25°C in the white light (90 μE m−2 s−1) for synchronized germination. For tissue culture assays, seeds were germinated and grown for 5 d on plates containing 1× Murashige and Skoog medium at room temperature in the dark. Seedlings were then transferred to a growth chamber (50 μE m−2 s−1 white light) and grown for 10 d at 25°C. For callus induction, root explants were collected and sliced into 1-cm-long sections, and five sections of each genotype were placed onto a callus induction medium (CIM). CIM consisted of 1× Murashige and Skoog salts, B5 vitamins, 0.5 mg L−1 2,4-dichlorophenoxyacetic acid, 0.05 mg L−1 benzylaminopurine, 0.05 mg L−1 kinetin, and 0.7% (w/v) phytagar. Callus induction was conducted for 10 d under 50 μE m−2 s−1 white light at 25°C. For shoot induction, callus was transferred to shoot induction medium (SIM) and grown for 2 to 4 weeks under the same condition. SIM consisted of 1× Murashige and Skoog salts, 5 mg L−1 isopentenyl adenine, 0.15 mg L−1 IAA, and 0.7% (w/v) phytagar.
The Effect of Fenpropimorph on Seedling Growth
For phenotypic analysis, seedlings (wild-type and fk-J79 mutants) were germinated and grown for 3 weeks on Murashige and Skoog plates containing different concentrations of fenpropimorph under a photoperiod of 16 h of light (90 μE m−2 s−1) and 8 h of dark at 25°C. Results were recorded using a dissecting microscope (SZH10, Olympus, Tokyo) with a CCD camera. For quantitative assay on organ growth, plants were germinated and grown for 8 d on Murashige and Skoog plates supplemented with fenpropimorph under the photoperiod of 16 h of light (90 μE m−2 s−1) and 8 h dark at 25°C.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The GC-MS analysis was carried out under the following conditions: a mass spectrometer (Automass JMS-AM150, JEOL, Tokyo) connected with a gas chromatograph (5890A-II, Hewlett-Packard, Wilmington, DE); electron ionization, 70 eV; source temperature, 230°C; column DB-5 (15 m × 0.25 mm, 0.25-μm film thickness; J&W Scientific, Folsom, CA); injection temperature, 280°C; column temperature program, 80°C for 1 min, then raised to 320°C at a rate of 30°C min−1, and held at this temperature for 5 min; interface temperature, 280°C; carrier gas He, flow rate 1 mL min−1; splitless injection. Fractions corresponding to BL, castasterone, and 6-deoxocastasterone were treated with pyridine containing methaneboronic acid (2 mg min−1), and fractions corresponding to teasterone, typhasterol, 6-deoxoteasterone, and 6-deoxotyphasterol were treated with pyridine containing methaneboronic acid (2 mg min−1) at 80°C for 30 min and then with N-methyl-N-trimethylsilyltrifluoroacetamide at 80°C for 30 min. Fractions corresponding to 6-deoxocathasterone, cathasterone, and sterols were treated with N-methyl-N-trimethylsilyltrifluoroacetamide at 80°C for 30 min.
Analysis of Endogenous BRs and Sterols
Wild-type seedlings (Ecotype BE) were germinated and grown for 3 weeks on Murashige and Skoog plates containing different concentrations of fenpropimorph under a photoperiod of 16 h of light (90 μE m−2 s−1) and 8 h of dark at 25°C. For BR and sterol analysis, seedlings were harvested and lyophilized in liquid N2. The lyophilized plant material (50 g fresh weight equivalent) was extracted with 500 mL of MeOH-CHCl3 (4:1) twice, and BL, castasterone, typhasterol, teasterone, cathasterone, 6-deoxocastasterone, 6-deoxotyphasterol, and [2H6]6-deoxoteasterone (50 ng each) were added to the extract as internal standards (Fujioka et al., 1997). After evaporation of the solvent in vacuo, the extract was partitioned between CHCl3 and water three times. The CHCl3-soluble fraction was subjected to silica gel chromatography (Sep-Pak Vac Silica, 10 g, Waters, Milford, MA). The column was subsequently eluted with 100 mL of CHCl3, 2% (v/v) MeOH in CHCl3, and 7% (v/v) MeOH in CHCl3. The 2% (v/v) MeOH and 7% (v/v) MeOH fractions were purified by Sephadex LH-20 column chromatography (column volume of 200 mL). The column was eluted with MeOH-CHCl3 (4:1). The effluents of elution volume to total column volume 0.6 to 0.8 were collected as the BR-containing fractions. After purification with an ODS cartridge (Sep-Pak Plus C18, Waters) with 20 mL of MeOH, eluates were subjected to ODS-HPLC (Senshu Pak Pegasil ODS, 10 × 30 mm + Senshu Pak Pegasil ODS, 20 × 250 mm; Senshu Scientific, Tokyo) at a flow rate of 8 mL min−1. Ninety percent (v/v) acetonitrile was used for the eluate derived from the 2% (v/v) MeOH fraction, and 70% (v/v) acetonitrile was used as a solvent for the eluate derived from the 7% (v/v) MeOH fraction. HPLC purification from the 7% (v/v) MeOH fraction yielded a BL fraction (retention time [Rt] from 8–10 min), a castasterone fraction (Rt from 10–14 min), a teasterone fraction (Rt from 17–20 min), a typhasterol fraction (Rt from 26–32 min), and a 6-deoxocastasterone fraction (Rt from 38–44 min). HPLC purification from the 2% (v/v) MeOH fraction yielded a cathasterone fraction (Rt from 20–24 min), a 6-deoxoteasterone fraction (Rt from 32–36 min), and a 6-deoxotyphasterol fraction (Rt from 48–56 min). Each fraction was analyzed by GC-MS after derivatization. The endogenous levels of BR, except of BL and cathasterone, were calculated from the peak area ratios of molecular ions of the internal standard and the endogenous BR. In the case of BL and cathasterone, fragment ions were used for the calculation. Ions of internal standards and endogenous BR were as follows: BL, m/z 338 and 332; castasterone, m/z 518 and 512; typhasterol and teasterone, m/z 550 and 544; 6-deoxocastasterone, m/z 504 and 498; 6-deoxotyphasterol and 6-deoxote-asterone, m/z 536 and 530; and cathasterone, m/z 193 and 187.
For the analysis of 6-deoxocathasterone and sterols, lyophilized plants (1 g fresh weight equivalent) were extracted with MeOH-CHCl3 (4:1). [2H6]campesterol (20 μg), [2H6]campestanol (500 ng), [2H6]6-oxoca-mpestanol (50 ng), and [2H6]6-deoxocathasterone (5 ng) were added to extract as internal standards. The extract was partitioned three times between CHCl3 (20 mL) and water (40 mL). The CHCl3-soluble fraction was purified with a silica gel cartridge (Sep-Pak Vac Silica, 2 g, Waters) using 40 mL of CHCl3. The eluent was subjected to ODS-HPLC (Senshu Pak ODS 1151-d, 4.6 × 150 mm, Senshu Scientific) at a flow rate of 1 mL min−1 with 100% (v/v) MeOH. Fractions were collected every 0.5 min (Rt, 2.5–18 min). Each fraction was analyzed by GC-MS after derivatization to the trimethylsilyl ether. The endogenous levels of campesterol, campestanol, and 6-oxocampestanol were calculated from the peak area ratios of molecular ions of the internal standard and the endogenous sterol. Molecular ions of the internal standard and the endogenous sterol were as follows: campesterol, m/z 478 and 472; campestanol, m/z 480 and 474; and 6-oxoca-mpestanol, m/z 494 and 488. The endogenous levels of 6-deoxocathasterone were calculated from the peak area ratios of m/z 193 for the internal standard and m/z 187 for the endogenous sterol. The endogenous levels of other sterols were roughly calculated from the areas of the total ion currents.
Gene Expression Analysis
Unless specified, seedlings were grown in 1× liquid Murashige and Skoog medium in continuous white light (90 μE m−2 s−1) on a platform shaker (140 rpm) for 2, 3, or 8 d and then treated with sterols or other hormones (10−6 m) for an indicated period of time. Two-DAG plants were used for testing the effects of BL and sterols, 3-DAG plants were used to test the effects of hormones on FK expression, and 8-DAG plants were used to analyze the marker gene expression in different mutants and fenpropimorph-treated wild-type plants. RNA extraction and RNA gel-blot analyses were performed as described (Jang et al., 2000). Probes used in the RNA gel-blot analyses were derived from either expressed sequence tag (Arabidopsis Biological Resource Center) or PCR using a cDNA library in pFL61 as template. The accession numbers for the probes are: ATU30476 (AtExp1), M20405 (β-tubulin), AA585915 (KOR), AF051338 (TCH4), and AA042665 (Meri-5). Some RNA samples were extracted by using the RNeasy Plant Mini Kit (Qiagen USA, Valencia, CA). Reverse transcription-PCR was carried out using the One Step RNA PCR Kit (AMV, Takara Shuzo, Ltd., Kyoto).
Histochemical GUS Enzyme Assay
Plants were grown for 4 DAG in Murashige and Skoog liquid medium on a platform shaker (140 rpm) at 25°C under a 16-h-light (90 μE m−2 s−1) and 8-h-dark regime. The reporter gene FK::GUS was integrated into det2 and fk-J79 by crossing with homozygous FK::GUS wild-type plants. Mutant and wild-type plants homozygous for FK::GUS were used for GUS activity assay according to Restrepo et al. (1990). Seedlings were incubated in a solution containing 1.2 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, 0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, and 10 mm EDTA for 24 h before chlorophyll was cleared by ethanol. Samples were documented using a dissecting microscope (SZH10, Olympus) with a CCD camera. Quantitative GUS analysis was performed as described (Hung et al., 1998). Fifteen 4-DAG seedlings were pooled and homogenized in 200 μL of cold extraction buffer containing 50 mm sodium phosphate (pH 7.0), 1 mm EDTA, 0.1% (w/v) sarkosyl, 0.1% (w/v) Triton X-100, and 10 mm dithiothreitol. The homogenized samples were centrifuged for 2 min, and 160 μL of supernatant was used in an enzymatic reaction with 40 μL of 5 mm 4-methylumbelliferyl β-d-glucuronide (in methanol) at 37°C for 30 min. The reaction was terminated by adding 200 μL of 0.2 m Na2CO3. A fluorescence spectrophotometer was used to determine the relative activities.
ACKNOWLEDGMENTS
We thank the Arabidopsis Biological Resource Center for seed stocks and expressed sequence tag clones and Dietz Bauer, John Price, and Eric Stockinger for critical reading of the manuscript.
Footnotes
This work was supported by the U.S. Department of Agriculture (grant no. 2002–35304–12500 to J.-C.J.). Salaries and research support was provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This is manuscript no. HCS 01–17.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.014605.
LITERATURE CITED
- Altmann T. A tale of dwarfs and drugs: brassinosteroids to the rescue. Trends Genet. 1998;14:490–495. doi: 10.1016/s0168-9525(98)01598-4. [DOI] [PubMed] [Google Scholar]
- Campbell P, Braam J. Xyloglucan endotransglycosylase: diversity of genes, enzymes and potential wall-modifying functions. Trends Plant Sci. 1999;4:361–366. doi: 10.1016/s1360-1385(99)01468-5. [DOI] [PubMed] [Google Scholar]
- Carland FM, Fujioka S, Takatsuto S, Yoshida S, Nelson T. The identification of CVP1 reveals a role for sterols in vascular patterning. Plant Cell. 2002;14:2045–2058. doi: 10.1105/tpc.003939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD. Plant development: a role for sterols in embryogenesis. Curr Biol. 2000;10:R601–R604. doi: 10.1016/s0960-9822(00)00639-4. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Arabidopsis mutants reveal multiple roles for sterols in plant development. Plant Cell. 2002;14:1995–2000. doi: 10.1105/tpc.140930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM. BRASSINOSTEROIDS: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Diener AC, Li H, Whoriskey WJ, Nes D, Fink GR. STEROL METHYLTRANSFERASE 1controls the levels of cholesterol in plants. Plant Cell. 2000;12:853–870. doi: 10.1105/tpc.12.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- Edwards PA, Ericsson J. Sterols and isoprenoids: signal molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J et al. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2002;130:1319–1334. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M-A. Plant sterols and the membrane environment. Trends Plant Sci. 1998;3:170–175. [Google Scholar]
- Hu Y, Bao F, Li J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000;24:693–701. doi: 10.1046/j.1365-313x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Hung CY, Lin Y, Zhang M, Pollock S, Marks MD, Schieffelbein J. A common position-dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis. Plant Physiol. 1998;117:73–84. doi: 10.1104/pp.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev EA, Xu W, Polisensky DH, Oh MH, Torisky RS, Clouse SD, Braam J. Transcriptional and posttranscriptional regulation of Arabidopsis TCH4 expression by diverse stimuli: roles of cis regions and brassinosteroids. Plant Physiol. 2002;130:770–783. doi: 10.1104/pp.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Eaton S. Cholesterol in signal transduction. Curr Opin Cell Biol. 2000;12:193–203. doi: 10.1016/s0955-0674(99)00076-9. [DOI] [PubMed] [Google Scholar]
- Jang J-C, Fujioka S, Tasaka M, Seto H, Takatsuto S, Ishii A, Aida M, Yoshida S, Sheen J. A critical role of sterols in embryonic patterning and meristem programming revealed by the fackel mutants of Arabidopsis thaliana. Genes Dev. 2000;14:1485–1497. [PMC free article] [PubMed] [Google Scholar]
- Lane DR, Wiedemeier A, Peng L, Höfte H, Vernhettes S, Desprez T, Hocart CH, Birch RJ, Baskin TI, Burn JE et al. Temperature-sensitive alleles of RSW2 link the KORRIGANendo-1,4-β-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 2001;126:278–288. doi: 10.1104/pp.126.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RT, Parks LW. Cloning, sequencing, and disruption of the gene encoding sterol C-14 reductase in Saccharomyces cerevisiae. DNA Cell Biol. 1992;11:685–692. doi: 10.1089/dna.1992.11.685. [DOI] [PubMed] [Google Scholar]
- Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton K. Role of PHABULOSA and PHAVOLUTAin determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- Mercer EI, Khalil IQ, Wang ZX. Effect of some sterol-biosynthesis-inhibiting functions on the biosynthesis of polyisoprenoid compounds in barley seedlings. Steroids. 1989;53:393–412. doi: 10.1016/0039-128x(89)90021-4. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Fitzky BU, Glossmann H. Genetic defects in postsqualene cholesterol biosynthesis. Trends Endocrinol Metab. 2000;11:106–114. doi: 10.1016/s1043-2760(00)00235-6. [DOI] [PubMed] [Google Scholar]
- Müssig C, Fischer S, Altmann T. Brassinosteroid-regulated gene expression. Plant Physiol. 2002;129:1241–1251. doi: 10.1104/pp.011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA. Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol. 2000;124:201–209. doi: 10.1104/pp.124.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Yoshida S, Yuan H, Feldmann KA, Tax FE. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999;121:743–752. doi: 10.1104/pp.121.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LW, Crowley JH, Leak FW, Smith SJ, Tomeo ME. Use of sterol mutants as probes for sterol functions in the yeast, Saccharomyces cerevisiae. Crit Rev Biochem Mol Biol. 1999;34:399–404. doi: 10.1080/10409239991209381. [DOI] [PubMed] [Google Scholar]
- Peng L, Kawagoe Y, Hogan P, Delmer D. Sitosterol-β-glucoside as primer for cellulose synthesis in plants. Science. 2002;295:147–150. doi: 10.1126/science.1064281. [DOI] [PubMed] [Google Scholar]
- Restrepo MA, Freed DD, Carrington JC. Nuclear transport of plant potyviral protein. Plant Cell. 1990;2:987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A, Bronner R, Benveniste P, Schaller H. The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by STEROL METHYLTRANSFERASE 2;1. Plant J. 2001;25:605–615. doi: 10.1046/j.1365-313x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Schaller H, Maillot-Vernier P, Belliard G, Benveniste P. Increased sterol biosynthesis in tobacco calli resistant to a triazole herbicide which inhibits demethylation of 14α-methyl sterols. Planta. 1992;187:315–321. doi: 10.1007/BF00195654. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Scheid F, Benveniste P. Accumulation of Δ8,14-sterols in suspension cultures of bramble cells cultured with an azasterol antimycotic agent (A25822B) Phytochemistry. 1980;19:525–530. [Google Scholar]
- Schrick K, Mayer U, Horrichs A, Kuhnt C, Bellini C, Dangl J, Schmidt J, Jürgens G. FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsisembryogenesis. Genes Dev. 2000;14:1471–1484. [PMC free article] [PubMed] [Google Scholar]
- Schrick K, Mayer U, Martin G, Bellini C, Kuhnt C, Schmidt J, Jürgens G. Interactions between sterol biosynthesis genes in embryonic development of Arabidopsis. Plant J. 2002;31:61–73. doi: 10.1046/j.1365-313x.2002.01333.x. [DOI] [PubMed] [Google Scholar]
- Sato S, Kato T, Kakegawa K, Ishii T, Liu Y-G, Awano T, Takabe K, Nishiyama Y, Kuga S, Sato S et al. Role of the putative membrane-bond endo-1,4-β-glucanase KORRIGAN in cell elongation and cellulose synthesis in Arabidopsis thaliana. Plant Cell Physiol. 2001;42:251–263. doi: 10.1093/pcp/pce045. [DOI] [PubMed] [Google Scholar]
- Seto H, Fujioka S, Koshino H, Takatsuto S, Yoshida S. Stereo and chemical course of acid-catalyzed double bond migration of cholesta-5,7-dien-3β-ol to 5∂-cholesta-8,14-dien-3β-ol. J Chem Soc Perkin Trans. 2000;1:1697–1703. [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Crowley JH, Parks LW. Transcriptional regulation by ergosterol in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5427–5432. doi: 10.1128/mcb.16.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K. hydramutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell. 2002;14:1–15. doi: 10.1105/tpc.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taton M, Benveniste P, Rahier A. Microsomal Δ8,14-sterol Δ14-reductase in higher plants: characterization and inhibition by analogues of a presumptive carbocationic intermediate of the reduction reaction. Eur J Biochem. 1989;185:605–614. doi: 10.1111/j.1432-1033.1989.tb15156.x. [DOI] [PubMed] [Google Scholar]
- Topping JF, May VJ, Muskett PR, Lindsey K. Mutation in the HYDRA1 gene of Arabidopsis perturb cell shape and disrupt embryonic and seedling morphogenesis. Development. 1997;124:4415–4424. doi: 10.1242/dev.124.21.4415. [DOI] [PubMed] [Google Scholar]
- Wang Z-Y, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/s0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Zou J, Niu Q-W, Nishizawa N, Wu Y, Kost B, Chua N-H. KORRIGAN, an Arabidopsis endo-1,4-b-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell. 2000;12:1137–1152. doi: 10.1105/tpc.12.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]