Abstract
Numerous plant hormones interact during plant growth and development. Elucidating the role of these various hormones on particular tissue types or developmental stages has been difficult with exogenous applications or constitutive expression studies. Therefore, we used tissue-specific promoters expressing CKX1 and gai, genes involved in oxidative cytokinin degradation and gibberellin (GA) signal transduction, respectively, to study the roles of cytokinin and GA in male organ development. Accumulation of CKX1 in reproductive tissues of transgenic maize (Zea mays) resulted in male-sterile plants. The male development of these plants was restored by applications of kinetin and thidiazuron. Similarly, expression of gai specifically in anthers and pollen of tobacco (Nicotiana tabacum) and Arabidopsis resulted in the abortion of these respective tissues. The gai-induced male-sterile phenotype exhibited by the transgenic plants was reversible by exogenous applications of kinetin. Our results provide molecular evidence of the involvement of cytokinin and GA in male development and support the hypothesis that the male development is controlled in concert by multiple hormones. These studies also suggest a potential method for generating maintainable male sterility in plants by using existing agrochemicals that would reduce the expense of seed production for existing hybrid crops and provide a method to produce hybrid varieties of traditionally non-hybrid crops.
Evidence of the involvement of cytokinins and GAs in male reproductive development of flowering plants has resulted from the studies of exogenous applications and endogenous measurements of cytokinins and GAs in various wild-type and male-sterile plants (for review, see Sawhney and Shukla, 1994). Typically, deficiencies in endogenous cytokinins and GAs result in the delay or elimination of anthesis, whereas exogenous applications shorten the time to anthesis. Anthers of several male-sterile mutants including the sl-2 (stamenless-2) mutant of tomato (Lycopersicon esculentum; Sawhney and Shukla, 1994) and a genic male-sterile line of rapeseed (Brassica napus; Shukla and Sawhney, 1993) have lower endogenous cytokinin levels. Cytokinins have also been shown to reverse cytoplasmic male sterility in barley (Hordeum vulgare; Ahokas, 1982). In the female plants of dioecious species such as hemp (Cannabis sativa) and spinach (Spinacia oleracea), and the gynoecious line of cucumber (Cucumis sativus), the formation of male flowers is promoted by exogenous applications of GAs (Mitchell and Wittwer, 1962; Pike and Peterson, 1969; Mohan Ram and Jaiswal, 1972; Chailakhyan and Khrianin, 1978a, 1978b). GAs also rescue fertility of male-sterile mutants of barley, cosmos (Cosmos bipinnatus), and tomato (Phatak et al., 1966; Kasembe, 1967; Rana and Jain, 1968; Sawhney and Greyson, 1973; Schmidt and Schmidt, 1981). Conversely, 2-chloroethyl-trimethyl ammonium chloride, a GA biosynthesis inhibitor, reduces the number of male flowers in cucumber (Mitchell and Wittwer, 1962). Moreover, when the endogenous GA levels were analyzed, male-sterile mutants of tomato (sl-2) and rice (Oryza sativa) were deficient in GAs (Sawhney, 1974, 1992; Nakajima et al., 1991).
The requirement of GAs in male development is also supported by the studies of GA biosynthesis mutants. GA-deficient mutants in Arabidopsis (ga1, ga2, ga3, ga4, and ga5), perhaps one of the best characterized groups of mutants, were identified by the GA-reversible dwarf phenotype (Koornneef and van der Veen, 1980). Unlike other less characterized cytokinin- or GA-restorable male-sterile mutants, these Arabidopsis mutants have been confirmed as GA-deficient mutants through biochemical analysis and cloning of the genes (for review, see Olszewski et al., 2002). In addition to the dwarf stature, the mutants have poorly developed stamens but produce normal female reproductive tissues capable of setting seeds by pollinating with wild-type pollen (Koornneef and van der Veen, 1980). Application of GA4+7 completely restores the morphology and fertility of the flowers. Similarly, tomato GA-deficient mutants (gib-1 and gib-2) are also male sterile and the fertility can be restored by applications of GAs (Nester and Zeevaart, 1988; Jacobsen and Olszewski, 1991). More recently, Izhaki et al. (2002) found that a GA-induced gene, GIP, can serve as a molecular marker for GA response in anthers. Expression of GIP is promoted by GA3 and inhibited by paclobutrazol. They concluded that GAs are involved in regulating post-meiotic anther development in petunia (Petunia hybrida).
Interestingly, in some instances GAs can promote female reproductive development or induce male sterility. For example, GA treatments stimulate the growth of female reproductive organs in tomato, castor bean (Ricinus communis), and maize (Zea mays), and induce male sterility in lettuce (Lactuca sativa) and pepper (Capsicum annuum; for review, see Sawhney and Shukla, 1994). GA-deficient dwarf (an1, d1, d2, d3, and d5) mutants of maize become andromonoecious (fully developed stamens on ears; Neuffer et al., 1997). An1 and D3 appear to be orthlogs to the GA genes in Arabidopsis based on phylogenetic comparisons (Bensen et al., 1995; Winkler and Helentjaris, 1995), yet these genes have opposite effects on sex determination.
Despite active and continuous interests in investigating the involvement of cytokinins and GAs in male reproductive development, the evidence has not been conclusive. Cytokinins and GAs, like all other hormones, influence many processes in plant growth and development. Studying male development by exogenous applications of these plant hormones in wild-type or mutant plants is complicated by abnormal growth phenotypes resulted from pleiotropic effects, as well as differences in uptake, translocation, metabolism, and responsiveness of the hormone in various tissues or developmental stages. Therefore, the role of plant hormones in reproductive development should be investigated through studies with tissue-specific hormone manipulations.
In this study, we transformed maize with CKX1 driven by anther and pollen promoters, Arabidopsis with gai (GA insensitive) driven by anther and pollen promoters, and tobacco (Nicotiana tabacum) with gai driven by an anther promoter. In many plants, oxidative cytokinin degradation appears to be the major pathway for cytokinin inactivation (Armstrong, 1994). The purification of cytokinin oxidase (CKX), an enzyme involved in oxidative cytokinin degradation, has led to the cloning of CKX1 (Houba-Hérin et al., 1999; Morris et al., 1999). Identified by its GA insensitivity, gai is a semidominant gene that negatively regulates GA responses (Koornneef et al., 1985; Peng et al., 1997). The anther and pollen abortion phenotype displayed by the transgenic plants demonstrates direct involvement of cytokinins and GAs in male reproductive development. In addition, kinetin applications restore the normal growth and fertility of stamens in gai-induced male-sterile transgenic plants, which sheds new light on the interplay of hormones in male development.
RESULTS
CKX1-Transformed Maize Plants Display Male-Sterile Phenotype
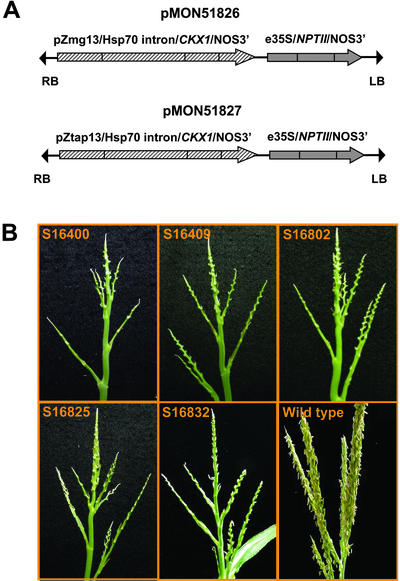
The complete maize CKX1 gene (Morris et al., 1999), encoding the transit peptide and mature protein was inserted into binary vectors to generate pMON51826 and 51827 (Fig. 1A). In pMON51826, CKX1 was under the control of a maize pollen-specific promoter, pZmg13 (Hanson et al., 1989), whereas a maize anther-specific promoter, pZtap (Brown and Fromm, 1999), was used in pMON51827. These constructs were introduced into maize by Agrobacterium tumefaciens-mediated transformation (Ishida et al., 1996). Thirty-three and 31 independent R0 transgenic plants were generated containing pMON51826 and pMON51827, respectively. Despite the difference in the promoters, 29 events each of pMON51826 and pMON51827 displayed a similar complete, sporophytic male-sterile phenotype. At the apex of the plants where the tassels ordinarily develop, the male-sterile transgenic plants produced rudimentary terminal structures that lacked recognizable male florets or spikelets (Fig. 1B). Some of these transgenic plants were also shorter and had narrower leaves than wild-type plants.
Figure 1.
R0 transgenic maize plants transformed with pMON51826 and pMON51827 displayed a male-sterile phenotype. A, T-DNA regions of pMON51826 and pMON51827. B, R0 transgenic plants of pMON51827 (S16400 and S16409) and pMON51826 (S16802, S16825, and S16832). The sizes of the sterile tassels on the transgenic plants were variable and noticeably smaller than wild-type tassels. All of the transgenic sterile tassels in the figure are enlarged approximately 2- to 3-fold.
Pollen from wild-type plants successfully fertilized several male-sterile transgenic plants to yield F1 seeds. About one-half of the independently transformed CKX1 events did not produce fertile ears when out-crossed. However, this female sterility was typical under our regeneration condition at the time when these transgenic plants were produced. The detection of a single copy of the transgene by DNA gel-blot analysis in five lines shown in Figure 1 was consistent with their F1 segregation ratio of 1:1 of the NPTII (neomycin phosphotransferase type II gene) based on an ELISA (data not shown). These results also suggest that the female gamete development is normal in these transgenic plants. When grown to maturity, all of the transgenic F1 plants that were positive by the NPTII ELISA inherited the male-sterile phenotype.
CKX1 Expression Is Detected in Male Tissues and Leaves of Transgenic Maize Plants
The most prominent phenotype of the transgenic plants was the male sterility. In addition, most of the transgenic plants generated from both vectors also exhibited other less pronounced phenotypic abnormalities such as height reduction, narrow leaves, and poor ear development. Cytokinins are involved in many processes of plant growth and development. The reduction of endogenous cytokinin levels during these processes may result in negative effects on plant growth. In Figure 2A, the CKX1 protein (CKX1) was undetectable in tissue of wild-type plants under our experimental procedures, which is consistent with previous studies (Bilyeu et al., 2001). In contrast, CKX1 was detected in the young leaves and tassels of transgenic plants, even though the expression of CKX1 in the transgenic plants is under the control of male-specific promoters. A lack of promoter specificity could explain the presence of CKX1 in tissues other than tassel and, in turn, cause the unexpected phenotype observed in transgenic plants. On the other hand, the identification of a putative transit peptide in its predicted protein sequence and the presence of glycosylation in the native CKX1 gene suggest that CKX1 is a secreted protein (Houba-Hérin et al., 1999; Morris et al., 1999). However, export of the transgenic CKX1 from the male tissues is unlikely because the tassel is not a photoassimilate source tissue during development.
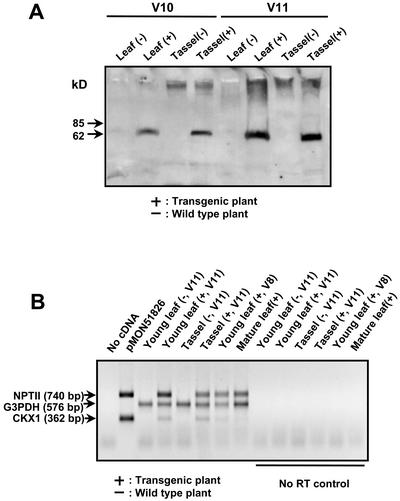
Figure 2.
Expression analysis of CKX1 in transgenic maize plants of line S16832. A, The 70-kD CKX1 protein band was detected in transgenic leaves (youngest) and tassels at two stages (V10 and V11) in the western blot. Sixty micrograms of leaf total protein and 20 μg of tassel total protein were loaded on the gel. B, RT-PCR was performed on the total RNA isolated from leaves and tassels of transgenic and wild-type plants. G3PDH was used as a positive cDNA control. CKX1 mRNA was detected in the tassels as well as the young leaves (V11 stage) of transgenic plants. CKX1 mRNA was also present in the young leaves at the V8 stage, but CKX1 transcripts were not detected in the mature leaves. The “no RT” controls consisted of a duplicated set of samples in which reverse transcriptase (RT) was not added in the cDNA synthesis step.
RNA analysis was performed on the F1 plants of line S16832 to determine if the presence of CKX1 in the leaves of transgenic plants with phenotype abnormalities was due to leaky expression of the male-specific promoters or secretion of the CKX1 from male-specific tissues. CKX1 transcripts in these transgenic plants were not detected by total RNA gel-blot analysis under these experimental conditions, so an RT-PCR was performed. As shown in Figure 2B, in addition to tassels, the CKX1 mRNA was detected in the young leaves of transgenic plants at the V11 stage (Ritchie et al., 1997). The CKX1 mRNA was also present in the young leaves at V8, a stage before tassel development in transgenic plants. However, unlike NPTII, which was driven by the enhanced cauliflower mosaic virus (CaMV) 35S (e35S) constitutive promoter, the CKX1 transcripts were not detected in the mature leaves. The detection of CKX1 expression in non-male tissues by RT-PCR was not due to contamination by genomic DNA, because the negative controls that lack RT were absent of any visible PCR products.
The promoter, pZmg13, in line S16832 was isolated from maize. The pollen specificity of pZmg13 was validated by driving the expression of a sensitive cytotoxic protein, barnase (Hartley, 1988). In these experiments, 50% of the pollen was aborted in transgenic maize plants with this pZmg13/barnase fusion (Williams, et al., 1997). One possible explanation of the leaky expression of pZmg13 in these male-sterile transgenic plants could be the close proximity of the CaMV 35S enhancers to CKX1 in the transformation constructs (Fig. 1A). Evidence for enhanced expression of endogenous promoters due to close proximity of CaMV 35S enhancers has been described (Weigel et al., 2000) and could account for the expression and phenotypes observed in nonreproductive tissues. This would also explain the phenotypic abnormalities in transgenic plants containing these vectors.
Exogenous Application of Kinetin and Thidiazuron (TDZ) Partially Restore the Male Development of Sterile Transgenic Maize Plants
Two previously registered agrochemicals were tested in fertility restoration experiments. Kinetin is a synthetic cytokinin that is not oxidized by CKX1 (Bilyeu et al., 2001), and TDZ is a known CKX inhibitor (Hare and Van Staden, 1994) and a cytokinin agonist (Yamada et al., 2001). The F1 seeds from the crosses between wild-type plants and several male-sterile R0 events were sown. To identify transgenic plants from these segregating populations, an NPTII ELISA was performed on leaf samples collected at the seedling stage of all plants. We had determined previously that these lines had a single transgene insert and that the NTPII ELISA positive phenotype cosegregated with the male-sterile phenotype. The NPTII ELISA was subsequently used to select male-sterile F1 transgenic plants for chemical treatments. Plants were either treated sequentially at three rates at the V4, V7, and V10 developmental stages or once at a higher rate at the V7 developmental stage (Table I). In several instances, the tassels of chemically treated transgenic plants were more developed than controls treated with surfactant only (Fig. 3A). These preliminary chemical application conditions were not sufficient to completely rescue the male-sterile phenotype but suggested the requirement of TDZ and kinetin for improving male development. The degree of floral development restoration varied and was limited to older tissues of the tassel. In these treated transgenic plants, the earlier flowers produced viable pollen (Fig. 3B, I), but the later flowers did not (Fig. 3B, II) even though filaments and anthers had developed. The observation of the aborted pollen in the later flowers supports the previous hypothesis of the requirement of cytokinins in pollen development. Also, on the same tassels that the restored flowers were observed, sectors that lacked floral development remained. These results suggest that continuous supplementation of exogenous chemicals are necessary to fully restore the fertility of these male-sterile transgenic plants.
Table I.
Tassel fertility rating of the preliminary fertility restoration study
| Line | TDZ and Kinetin Applications at V4 + V7 + V10
|
TDZ and Kinetin Applications at V7
|
|||
|---|---|---|---|---|---|
| 0, 0a | 0, 20 | 3, 20 | 0, 100 | 3, 100 | |
| 16802 | 0 | 0 | ++ | 0 | + |
| 16825 | 0 | 0 | ++ | 0 | + |
| 16832 | 0 | 0 | ++ | 0 | 0 |
| 16400 | 0 | 0 | 0 | 0 | + |
| 16409 | 0 | 0 | 0 | 0 | + |
Two to three transgenic F1 plants were used in each treatment group. Plants were either treated repeatedly in three different rates at three V4, V7, and V10 developmental stages or once in a higher rate at V7 developmental stage. Rates are indicated by milligram per plant of TDZ and kinetin, respectively, in the presence of 0.25% (v/v) Sylgard 309 surfactant. The fertility rating was based on the visual observation. 0, No or very little floral development; +, advance floral development observed in some areas of tassels; ++, increased areas of advanced floral development and anthersis observed in some flowers.
“#, #” indicate milligram per plant of TDZ and kinetin in each treatment.
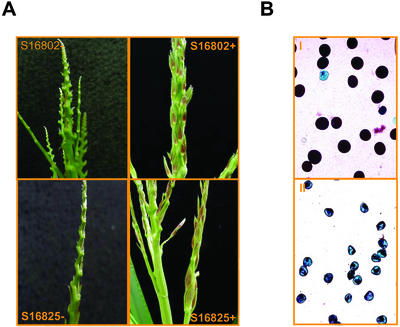
Figure 3.
Improved male organ development of F1 transgenic maize plants treated with kinetin and TDZ. A, F1 transgenic plants sprayed with a mixture of kinetin and TDZ (+) or surfactant alone (−). Advanced floral development was visible on chemically treated plants of lines S16802 and S16825 (+, Table I), whereas plants treated with surfactant only were devoid of floral development. B, Although florets appeared to develop on the transgenic plants after kinetin and TDZ applications, the pollen production was insufficient for pollination (++, Table I). Pollen viability stains were then performed using Alexander's solution (Alexander, 1969). The chemically restored florets produced viable (purple and symmetrical in shape) pollen (I) similar to wild type. Some of the transgenic plants failed to rescue completely resulting in nonviable (blue and asymmetrical in shape) pollen (II).
gai-Induced Male-Sterile Phenotype in Transgenic Tobacco Plants
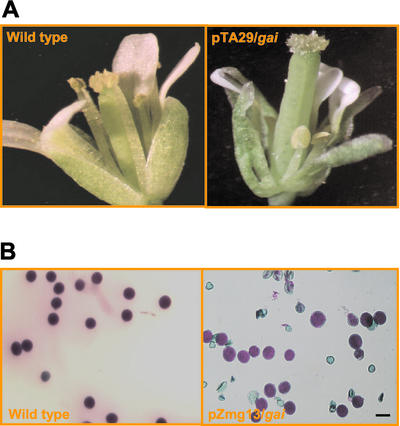
The involvement of GA in male development was investigated in transgenic tobacco plants. A binary vector, pMON42169 (Fig. 4A), containing the Arabidopsis gai (Peng et al., 1997) driven by a tobacco anther-specific promoter, pTA29 (Koltunow et al., 1990), was introduced into tobacco by A. tumefaciens-mediated transformation (Horsch et al., 1985). Among 22 independent kanamycin-resistant (kanr) R0 transgenic events generated, 18 plants displayed a male-sterile phenotype. Three of the male-sterile plants were also slightly shorter in stature. F1 plants generated from crosses with wild-type pollen inherited the male-sterile phenotype. Figure 4B shows the anthers of male-sterile transgenic and wild-type plants at stage 12 of tobacco anther development (Koltunow et al., 1990) with fully opened flowers. These male-sterile transgenic plants did not produce any visible pollen.
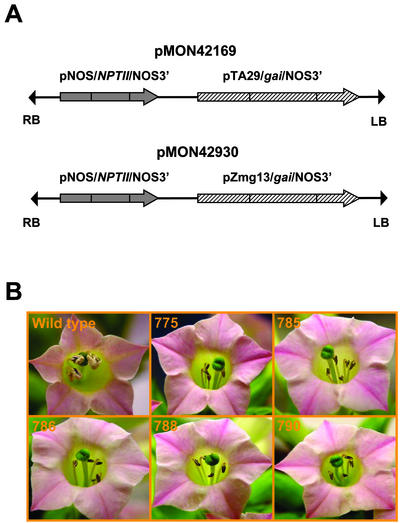
Figure 4.
Male-sterile phenotype of R0 transgenic tobacco plants. A, T-DNA regions of pMON42169 and 42930. B, Lines 775, 785, 786, 788, and 790 are R0 transgenic events containing pMON42169 among 17 that displayed the male-sterile phenotype.
gai Expression Is Detected in Anthers of Transgenic Tobacco Plants
Total RNA was isolated from anthers between stages −1 and +2 for RNA gel-blot analysis, because the TA29 promoter initiates transcription at stage −1 of tobacco anther development (Koltunow et al., 1990). All five of the transgenic plants showed strong expression of gai (Fig. 5). The absence of a detectable band on the gel blot of the wild-type plant indicated that the gai message was from the expression of the gai transgene.
Figure 5.
RNA gel-blot analysis of male-sterile transgenic tobacco plants. Total RNA isolated from anthers of wild-type and transgenic plants was hybridized with a gai probe. The bottom panel is the corresponding ethidium bromide-stained samples to visualize RNA quantity and quality.
Floral and Pistil Development Are Unaffected in Transgenic Tobacco Plants
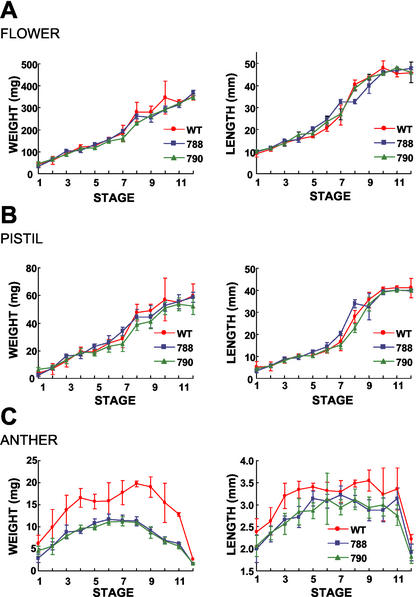
Unlike CKX1-transformed maize plants, most of the transgenic tobacco plants developed morphologically similar to wild-type plants except for the male-sterile phenotype. The production of F1 seeds of the transgenic plants by fertilization with wild-type pollen suggested that female reproduction was not affected. In Figure 6, we monitored the floral bud, anther, and pistil development of lines 788 and 790 and compared them with wild-type plants. Floral bud and pistil development of these transgenic plants were similar to wild-type plants based on weight and length measurements at various stages. In contrast, the anthers of these transgenic plants were noticeably smaller and lighter in weight as early as stage 1 of development. This was consistent with the TA29 promoter expression profile previously described. It appeared that altering GA responses by expressing gai in the anthers did not result in any other pleotropic effects commonly associated with hormonal changes.
Figure 6.
Floral development of wild-type and male-sterile transgenic tobacco plants. Changes in organ weight and length of wild-type and transgenic plants were measured at stages defined by Koltunow et al. (1990). Data points represent the mean of five measurements including sds. A, Flower; B, pistil; C, anther. Anther fresh weights represent the collective weight of all five anthers within the flower bud.
Pollen Sacs of Transgenic Tobacco Plants Collapse during Anther Development
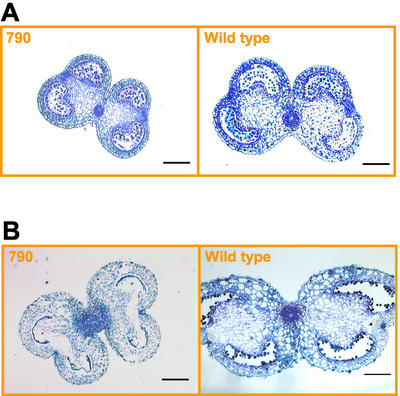
Anther sections were examined with a light microscope to investigate cellular development of male-sterile transgenic plants. Transgenic anthers (line 790) were smaller in size than wild type but otherwise morphologically indistinguishable at stage −1 (Fig. 7A). Subsequent stages of anther development in transgenic plants became developmentally abnormal. The pollen sacs of transgenic anthers were completely absent at stage 5, whereas the wild type had expanded and developed microspores (Fig. 7B). Despite the collapse of the pollen sacs and the absence of pollen grains at dehiscence, no other abnormalities in the transgenic anther tissues were observed.
Figure 7.
Cross sections of male-sterile transgenic (line 790) and wild-type tobacco anthers. Anthers were collected at stage −1, 7-mm bud length (A), and stage 5, 20-mm bud length (B). Scale bars = 200 μm.
These anther sections of pTA29/gai plants were similar to those observed in cell ablation studies by Koltunow et al. (1990). Using a chimeric pTA29/DTA (diphtheria toxin A chain) transgene, the authors showed that the destruction in the anthers of transgenic plants was limited to the tapetum and the pollen sac supported by the tapetum. They concluded that the TA29 gene is activated after tapetal cell differentiation and that the destruction of the tapetum has no effect on anther differentiation and/or function later in development. Together with our findings, it is clear that GAs are required for the autonomous function of the tapetum.
Exogenous Kinetin Applications Restore Fertility of Male-Sterile Transgenic Tobacco Plants
Several plant growth regulators were applied to the F1 male-sterile, transgenic tobacco plants including GA3, kinetin, and TDZ. The GA3-treated transgenic plants were spindly with elongated internodes, but remained male sterile. These results were expected because gai is GA insensitive and suggest that the male sterility exhibited by the transgenic plants was not due to the reduction of endogenous GA levels. Applications with kinetin, a synthetic cytokinin, restored the fertility to the transgenic plants and resulted in normal fertilization and seed development. However, applications of TDZ did not restore fertility in the male-sterile plants.
F1 plants from six male-sterile transgenic lines were selected based on resistance to kanamycin and grown to flowering to verify the inheritance of the male-sterile phenotype. Each plant was then treated with a solution containing 15 mg of kinetin every other day for 2 weeks. The plants continued to develop male-sterile flowers for 10 d after the first application of kinetin. The flowers that developed 10 d postapplication were fully fertile and produced seeds. The fertility restoration was observed in all but two lines tested. As shown in Figure 8, flowers from two of the restored transgenic lines (788 and 790) were visibly shedding pollen. Under the growth conditions of our greenhouse, the transition from stage −2 to 12 for a tobacco floral bud occurred in approximately 10 d. Only kinetin applications before stage −1 were effective in restoring fertility. This developmental stage coincides with the onset of expression of the TA29 promoter. The production of fertile flowers continued for 11 d postapplication, indicating that continuous kinetin treatments were not necessary for the restoration of fertility in the older floral buds of male-sterile plants. These results imply that the inhibition of anther development in the transgenic plants by pTA29/gai is limited to a precise period of anther development. Once treated with kinetin through the inhibitory stages, anthers resume normal development. Interestingly, kinetin applications did not restore the development of lines 770 and 785, but rather converted the anthers to petals (line 785 is shown in Fig. 8).
Figure 8.
Fertility restoration of F1 transgenic tobacco plants treated with kinetin. Transgenic plants from lines 788 and 790 sprayed with kinetin (indicated by “+”) displayed normal pollen dehiscence, whereas line 785 has anther to petal conversion.
The kinetin-restored flowers were morphologically normal and also had typical seed yields. When the seeds obtained from self-pollinated, kinetin-restored flowers were compared with the crossed seeds of sterile flowers and the self-pollinated seeds of wild-type flowers, no significant differences were observed in seed weight (Table II). Lines 788 and 790 both contain a single copy of the transgene based on DNA gel-blot analysis (data not shown) and were included in the experiments to simplify the segregation. Both lines had seedling ratios of kanr versus kans at 1:1 by cross-pollination and 3:1 by self-pollination. These ratios correlate with the expected Mendelian ratios for segregation of a single dominant gene.
Table II.
Seed yields of male-sterile transgenic plants resulted from crosses (C) with wild-type pollen and selfing (S) after fertility restoration by kinetin application
| Seed | Seed Weighta | (Kanr/Kans)b | χ2 |
|---|---|---|---|
| mg | |||
| 788 (C) | 122.6 ± 10.9 | 84 /81 | 0.05 (1:1) |
| 788 (S) | 113.0 ± 18.9 | 115 /41 | 0.14 (3:1) |
| 790 (C) | 132.2 ± 17.2 | 82 /86 | 0.10 (1:1) |
| 790 (S) | 110.2 ± 13.5 | 133 /38 | 0.81 (3:1) |
| Wild type (S) | 124.4 ± 15.7 | – | – |
Nos. represent the mean of five seed sets ± se.
Media-grown seedlings were assayed for the presence or absence of the selectable marker kanamycin. Kans, Kanamycin sensitive.
gai Causes Anther and Pollen Abortion in Arabidopsis
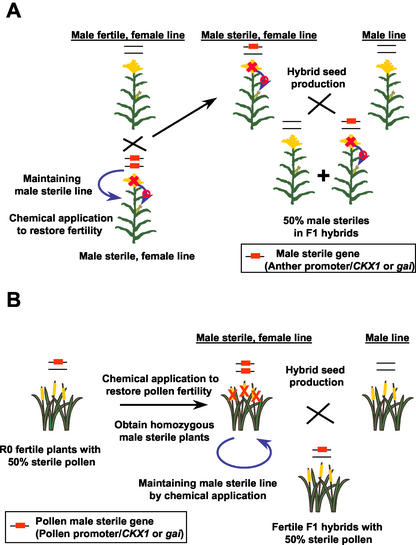
The pMON42169 binary vector used in tobacco transformation was also introduced into Arabidopsis to determine if the gai-induced male sterility could be applied to other species. Complete male sterility was observed in seven of the 17 transgenic Arabidopsis plants generated. Most of these male-sterile plants also displayed other phenotypes similar to GA-deficient mutants. For example, the transgenic male-sterile flower in Figure 9A had shorter filaments. These phenotypes could be the result of leaky expression of the TA29 promoter in Arabidopsis. However, the female reproductive tissues of these plants appeared phenotypically normal and set seed when fertilized with wild-type pollen. Currently, we are investigating fertility restoration of these plants with kinetin applications.
Figure 9.
Anther and pollen abortion in transgenic Arabidopsis plants caused by gai. Many transgenic Arabidopsis plants containing anther-expressed gai (pMON42169) were male sterile, whereas plants containing pollen-expressed gai (pMON42930) showed a pollen abortion phenotype. A, Wild-type and male-sterile flowers; B, 50% pollen abortion in transgenic plants. Pollen viability stains were performed using Alexander's solution (Alexander, 1969). Scale bars = 20 μm.
We also expressed gai under the control of the Zmg13 promoter (Hanson et al., 1989). Twenty-five of 32 transgenic Arabidopsis plants containing the pMON42930 construct (Fig. 4A) exhibited a partial pollen abortion phenotype. Seventeen plants had approximately 50% pollen abortion (Fig. 9B) that was most likely caused by the gametophytic expression of a single insertion of the transgene. As a comparison, wild-type plants grown in the same conditions have a natural pollen abortion rate of 1% to 2%. Although detailed molecular and genetic characterizations have yet to be completed, these preliminary results suggest that GAs are also involved in pollen development.
DISCUSSION
The isolation of reproductive tissue-specific promoters and genes involved in hormone pathways enabled us to investigate the roles of hormones in plant reproduction more directly and precisely. These techniques have led to a better understanding of hormone function and interactions. For example, in tobacco, ethylene was found to be required for ovule development (De Martinis and Mariani, 1999), whereas the increase of auxins altered male development (Spena et al., 1992). Similarly, we have demonstrated that the accumulation of CKX1 in maize tassels and the expression of gai in tobacco anthers caused male sterility in transgenic plants. It is clear now that many of these reproductive developmental processes implicate more than one hormone, and these processes could, in turn, provide model systems for studying the molecular interaction between hormones.
Molecular Approaches Suggest the Involvement of Cytokinins in Maize Male Reproductive Development
In maize, although the pistillate (ear) and staminate (tassel) organs are at different positions along the stem, early events in the development of both ear and tassel florets are bisexual and morphologically identical (Dellaporta and Calderon-Urrea, 1994). It has been suggested that cytokinins are essential for normal floral growth and development (Rastogi and Sawhney, 1989). The lack of floret structures in the tassels of the transgenic plants indicates that the initiation of male floral development is inhibited by a cytokinin deficiency. Thus, cytokinins may play a critical role in maize male floral development. Although the leakiness of the transgene and abnormal vegetative phenotype displayed by the transgenic plants have cautioned the interpretation of the male-sterile phenotype, the chemical restoration experiments suggest that the cytokinin deficiency caused by the transgene is responsible for the male-sterile phenotype. Kinetin and TDZ applications rescued the transgenic plants to various stages of the male development. The restoration of viable pollen was achieved in some florets but not in others, even when the floret development appeared normal. Also, on the same tassels that the restored florets were observed, sectors that lacked floral development remained. Failure to obtain complete rescue of fertility was most likely due to exhaustion of exogenously applied chemicals during male floret maturation and pollen development. These results imply that cytokinins are continuously required throughout male organ development both sporophytically and gametophytically.
Molecular Approaches Confirm the Involvement of GAs in Both Anther and Pollen Development
Studies of endogenous GA levels in male-sterile mutants and the effects of exogenous GA applications on mutant and wild-type plants support the involvement of GAs in male reproductive development. Nevertheless, similar experiments also suggest GA applications can induce male sterility and promote gynoecium development in some plant species. More recently, the cloning of ga mutants in Arabidopsis and d (dwarf) mutants in maize have confirmed that these two classes of mutants affect a similar array of genes involved in the GA biosynthetic and signal transduction pathways, yet have opposite effects on male sex expression. In this study, the male sterility caused by reducing the GA sensitivity specifically in male tissues of tobacco and Arabidopsis has verified the former examples. It is necessary to conduct the experiments in maize to determine if the role of GAs in male development is species dependent as previously suggested.
These results also suggest the potential of utilizing gai to study the involvement of GAs during anther development in a detailed spatial and temporal manner with molecular approaches. Depending on the promoters employed, gai could specifically abort the development of a particular cell type or developmental stage if such a process is GA dependent. For example, the pollen abortion phenotype caused by the pollen promoter-driven gai has confirmed the widely held speculation that gametophytic male development requires GAs. Earlier studies with GA-deficient mutants could not obtain these results because observations typically ended after the early stages of stamen development.
The GA-Insensitive Male Sterility Phenotype in Transgenic Tobacco Plants Is Reversed by Kinetin
The ability of kinetin to restore male fertility caused by GA insensitivity indicates the direct interaction of these two hormones in male reproductive development. Male-sterile transgenic plants respond to the treatment of kinetin, but not TDZ, which suggests that de novo biosynthesis of cytokinins in anthers is required for normal development. GA responses may stimulate the biosynthesis of cytokinins and, therefore, the male sterility in these GA-insensitive transgenic plants may be due to a lack of cytokinin production. Alternatively, cytokinin accumulation may enhance GA responses rather than be regulated by GA responses. In lettuce and celery (Apium graveolens), cytokinins were reported to increase GA activity in seed germination (Khan, 1971; Thomas and Van Staden, 1995). Therefore, exogenous applications of cytokinins could overcome gai-induced GA insensitivity in transgenic plants and restore male fertility. Interestingly, with the GA-reversible male-sterile tomato mutant, sl-2, reduction of indole-3-acetic acid by lowering growing temperatures restores anther fertility without GA applications (Singh and Sawhney, 1991). Perhaps GA activity in anther development is modulated by the relative amounts of cytokinins and auxins.
Floral Organ Identity in Male-Sterile Transgenic Flowers
Phytohormones not only affect sex expression in plants as mentioned previously, but also floral organ identity. In Arabidopsis, exogenous benzylaminopurine suppressed the normal function of floral meristem identity and generated abnormalities that resemble known phenotypes of floral organ identity mutants, such as clv1, ap1, ap2, and ap3 (Venglat and Sawhney, 1996). Under the growth conditions in our greenhouse, the application of kinetin converted most of the male-sterile stamens to petals in two transgenic lines (770 and 785), whereas the conversion occurred infrequently in other fertility-restored lines and wild-type controls. These organ conversions are also less consistent than fertility restoration, varying with environment and among individual progeny. The expression pattern of three tobacco homeotic floral organ identity genes representing a, b, and c functions (NAP1-1, NTDEF, and NAG1, respectively; GenBank accession nos. AF009126, X96428, and L23925) were analyzed by RT-PCR in several transgenic lines. RNA isolated between stages −1 and +2 of sepals, petals, stamens, and pistils showed no obvious differences in transcript levels of the three homeotic genes between wild-type and transgenic plants before and after kinetin applications (data not shown). However, a detailed spatial and temporal expression analysis has yet to be performed.
Chemically Reversible Male Sterility in Hybrid Production
Regulating male fertility by manipulating hormones in male reproductive tissues through genetic engineering is an attractive approach to hybrid seed production. The CKX1- or gai-induced male sterility potentially could be applied to create a reversible, dominant male sterility system in which the transgene could be maintained in the homozygous state in the foundation fields by exogenous hormone applications (Fig. 10A). In the production fields, hemizygous male-sterile plants from the cross of homozygous male-sterile and isogenic wild-type plants would be used as female pollen recipients to produce F1 hybrid seeds. Although 50% of the F1 hybrids sown by the grower will be male sterile, excess pollen from the interplanted fertile maize plants are sufficient for full yields. Alternatively, CKX1 or gai could be expressed in pollen to create gametophytic male sterility. Complete male sterility is achieved in transgenic plants that are homozygous for the transgene, and these can be maintained by chemical applications. When used in hybrid seed production, all F1 hybrids are hemizygous for the pollen sterility gene (Fig. 10B). These hemizygous plants would be fully fertile even though each would produce 50% fewer viable pollen.
Figure 10.
The potential use of CKX1- or gai-generated, reversible male sterility in hybrid seed production. A, When expressed in anthers, the CKX1 or gai is a dominant male sterility gene that can be maintained as a homozygous female parental line by chemical fertility restoration. In the hybrid seed production fields, hemizygous plants derived from a cross between homozygous transgenic and isogenic wild-type plants are used as females to produce F1 hybrids with 50% of them being fertile. B, When the CKX1 or gai is specifically expressed in pollen, only the transgenic plants that are homozygous for the transgene are male sterile. These male-sterile transgenic plants can be maintained by kinetin and TDZ applications. When used in production, hemizygous fertile F1 hybrids are produced.
The use of hybrid crops is one of the most important advancements in agriculture in recent years. In general, hybrids are more disease and insect resistant and have wider environmental adaptability, improved vegetative growth, and increased yield. Many methods have been utilized to produce hybrid crops including cytoplasmic male sterility, chemical hybridization, and mechanical emasculation. Numerous technical and economical disadvantages associated with these methods have prevented widespread use of hybrids in self-pollinated crops such as wheat (Triticum aestivum), rice, rapeseed, and cotton (Gossypium hirsutum). Reversible male sterility systems engineered through biotechnology could provide ideal solutions for producing and utilizing the full potential of hybrids in agriculture.
MATERIALS AND METHODS
Vector Construction
A 1.7-kb CKX1-containing DNA fragment was inserted into expression cassettes driven by pZmg13 and pZtap promoters isolated from maize (Zea mays) and terminated by the 3′-untranslated region of the nopaline synthase gene (NOS3′). These cassettes also included an intron from a heat shock protein (HSP70 intron) from maize to increase the expression of CKX1. The resulting CKX1 expression cassettes were cloned into binary vectors using an enhanced CaMV 35S promoter (e35S)-driving NPTII as the selectable marker to generate pMON51826 and 51827 (Fig. 1A). All other genetic elements used in the binary vector were identical to those described by Ye et al. (1999).
The Arabidopsis gai mutant (Koornneef et al., 1985) was obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). RNA was isolated from floral tissue, and the gai cDNA was amplified by RT-PCR (Life Technologies, Gaithersburg, MD). Primers were designed for RT-PCR based on the published gai sequence (Peng et al., 1997). The cDNA was confirmed by sequencing and subcloned into expression cassettes driven by pTA29 (Koltunow et al., 1990) and pZmg13 (Hanson et al., 1989), and terminated by NOS3′. The resulting gai expression cassettes were cloned into binary vectors using the nopaline synthase gene promoter (pNOS)-driving NPTII as the selectable marker to generate pMON42169 and 42930 (Fig. 4A).
Plant Material, Transformation, and Growth Condition
The binary vectors, pMON51826 and pMON51827, were electroporated into Agrobacterium tumefaciens ABI strain and introduced into maize embryos (LH198XHiII) by A. tumefaciens-mediated transformation (Ishida et al., 1996). H99 pollen was used to pollinate R0 male-sterile transgenic plants to produce F1 seeds.
The A. tumefaciens strain containing pMON42169 was transformed into leaf discs of tobacco Nicotiana tabacum cv Samsun by the cocultivation method (Horsch et al., 1985). R0 male-sterile transgenic plants were pollinated by wild-type pollen to produce F1 seeds. To identify the transgenic plants in the subsequent generations, seeds were surface sterilized and germinated on Murashige and Skoog medium (M0404, Sigma, St. Louis) containing 100 mg L−1 kanamycin. All plants were grown in greenhouses at 28°C/21°C (day/night) with a 16-h photoperiod (400 μmol m−2 s−1) and 50% relative humidity.
Arabidopsis plants ecotype Columbia were grown in a growth chamber at 24°C with a 16-h photoperiod (120 μmol m−2 s−1) and 70% relative humidity. The binary Ti plasmids pMON42169 and pMON42930 were introduced into Arabidopsis via A. tumefaciens-mediated vacuum infiltration (Bechtold et al., 1993). To select the transgenic plants, seeds collected from vacuum infiltrated plants were surface sterilized and germinated on Murashige and Skoog medium (M0404) containing 50 mg L−1 kanamycin (Sigma).
Transgenic Maize F1 Segregation Analysis
The antibiotic resistance and the NPTII ELISA from leaf protein extracts determined the presence of NPTII in the maize F1 plants. An antibiotic solution containing 1 g L−1 kanamycin, 1 g L−1 paromomycin (Sigma), and 0.6% (v/v) Silwet L77 surfactant (Loveland, Greenley, CO), was applied to plants at V2 or V3 stages. Three days after application, the kanamycin-susceptible plants displayed leaf chlorosis symptoms. The NPTII Pathoscreen Kit was purchased from Agdia (Elkhart, IN) and used for NPTII ELISAs following the manufacturer's protocol.
CKX1 Western Blot
Protein samples were prepared in an extraction buffer containing 1× phosphate-buffered saline, 1 protein inhibitor cocktail tablet (20 mL; Boehringer Mannheim/Roche, Basel), and 0.05% (v/v) Tween 20, and quantified by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Twenty or 60 μg of total protein was separated on a 10% to 20% (w/v) Ready Gel and blotted to a 0.2-μm polyvinylidene difluoride membrane following the manufacturer's protocol (Bio-Rad). The blot was probed with rabbit anti-CKX1 polyclonal antibody (Bilyeu et al., 2001) at a dilution of 1:10,000 (v/v) and then incubated with horseradish peroxidase-labeled anti-rabbit antibody at a 1:5,000 (v/v) dilution. The immunocomplexes were visualized by enhanced chemiluminescence according to the instructions of the manufacturer (Amersham Pharmacia, Piscataway, NJ).
CKX1 RT-PCR
Total RNA was isolated at various stages from leaves and tassels of maize plants by using TRIzol Reagent (Life Technologies) followed by first strand cDNA synthesis (SuperScript Preamplication System, Life Technologies) with 2 μg of RNA in a 20-μL reaction. One microliter of the resulting cDNA mix was used in a 30-μL PCR solution containing 1× reaction buffer, 15 mm MgCl2, 0.2 mm dNTPs, 6% (v/v) dimethyl sulfoxide, 0.2 mm of each primer, and 0.5 units of Taq DNA Polymerase. The reaction was amplified for 30 cycles. Each cycle consisted of denaturation at 92°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, after an initial denaturation for 2 min. Immediately after the last cycle, the samples were incubated at 72°C for 5 min to complete extension. The gene encoding glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a positive control (Russell and Sachs, 1989). The sequences of the primers used to amplify NPTII, G3PDH, and CKX1 were 5′-CGCTTGGGTGGAGAGGCTATTC-3′ and 5′-GAAGGCGATAGAAGGCGATGCG-3′, 5′-CCCCATGTTCGTTGTTG-3′ and 5′-TATCCCCACTCGTTGTCGTACC-3′, and 5′-CGGCACGCTGTCCAACGC-3′ and 5′-GGCTCTGGTTCACGAACACC-3′, and generated DNA fragments of 741, 576, and 362 bp, respectively. All chemicals and enzymes were from Sigma unless otherwise noted.
Isolation of Tobacco Anther RNA and RNA-Blot Analysis
Total RNA was isolated from tobacco anthers at stages between −1 and +2 by TRIzol Reagent from Life Technologies following manufacturer's protocols. Total RNA samples (10 μg) were electrophoresed on a 1.2% (w/v) agarose/formaldehyde gel and transferred to a positively charged nylon membrane (Boehringer Mannheim/Roche). The probe used in the hybridization was prepared by PCR with primers designed to the gai-coding region. The blot was analyzed by the same DIG system as used in the DNA gel-blot analysis.
Light Microscopy of Tobacco Anthers
Tobacco floral buds at relevant stages were fixed overnight at 4°C in a solution containing 4% (w/v) paraformaldehyde and 0.5% (v/v) glutaraldehyde in 100 mm phosphate buffer (pH 7.0) by vacuum infiltration. The following day, the samples were rinsed with 1× phosphate-buffered saline (Boehringer Mannheim/Roche) containing 0.05% (v/v) Tween 20, and then the tissue samples were placed in 30% (v/v) ethanol for 30 min. Further processing was conducted on a Tissue-Tek VIP automated processor (Sakura Finetek, Torrance, CA) through a series of graded ethanol wash steps: 50%, 75%, and 85% (v/v) ethanol at 37°C for an hour each; 95% and 100% (v/v) ethanol at 40°C and 45°C for 2 h each; and finally with paraffin at 58°C for 10 h with four solution changes. Sections (10 μm) were cut from embedded tissue using a microtome (Reichert Jung 2030 model, Leica Microsystems, Wetzlar, Germany) and mounted on Probeon Plus slides (Fisher Scientific, Pittsburgh), which were then dried overnight at 37°C.
For Toluidine Blue O stain, slides were deparaffinized through a series of xylene and ethanol washes (5 min each) and then rehydrated. Slides were stained for 5 min in 1% (w/v) Toluidine Blue O (Fisher Scientific) in 1% (w/v) Borax (Sigma) and then rinsed in water for 2 min and dehydrated quickly through a series of ethanol washes (95%, 95%, 100%, and 100% [w/v] for 1 min each). These were subsequently placed into two changes of xylene and mounted with Permount (Fisher Scientific). Slides were observed under a BH-2 microscope (Olympus, Melville, NY).
Chemical Applications
NPTII ELISA positive maize plants were identified as the male-sterile transgenic plants among F1-segregating populations and treated at V4, V7, and V10 stages. The application rates for each plant were 0, 20, and 100 mg of kinetin with or without 3 mg of TDZ in the presence of 0.25% (v/v) Sylgard 309 surfactant (Willbur-Ellis, Fresno, CA). Two to three plants were tested at each rate combination.
Kanr tobacco F1 seedlings were transferred to soil and grown to flowering. After male sterility was observed in the first few flowers, plants from each line were divided into four treatments. One group received 15 mg of kinetin (Sigma) per plant, whereas the other two groups received either 10 mg of GA3 (Sigma) or 10 mg of TDZ (Sigma) per plant. The chemicals were in an aqueous solution containing 0.25% (v/v) Sylgard 309 surfactant. The control group received the surfactant solution only. All applications were made through foliar spray every other day for 2 weeks.
ACKNOWLEDGMENTS
The authors wish to thank Monsanto Crop Transformation Team for plant transformation, St. Louis and Mystic Trait Development teams for greenhouse care, and Roy O. Morris (University of Missouri, Columbia) for providing CKX1 antibody.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.102.018598.
LITERATURE CITED
- Ahokas H. Cytoplasmic male sterility in barley: evidence for the involvement of cytokinins in fertility restoration. Proc Natl Acad Sci USA. 1982;79:7605–7608. doi: 10.1073/pnas.79.24.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Armstrong DJ. Cytokinin oxidase and the regulation of cytokinin degradation. In: Mok DWS, Mok MC, editors. Cytokinins. Chemistry, Action and Function. Boca Raton, FL: CRC Press; 1994. pp. 139–154. [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Paris Life Sci. 1993;316:1194–1199. [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. Cloning and characterization of the maize An1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilyeu KD, Cole JL, Laskey JG, Reikhof WR, Esparza TJ, Kramer MD, Morris RO. Molecular and biochemical characterization of a cytokinin oxidase from Zea mays. Plant Physiol. 2001;125:378–386. doi: 10.1104/pp.125.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Fromm ME, inventors. March 9, 1999. Glyphosate as a gametocide. PCT Patent Application No. WO99/46396.
- Chailakhyan MK, Khrianin VN. The influence of growth regulators absorbed by the root on the sex expression in hemp plants. Planta. 1978a;138:181. doi: 10.1007/BF00391176. −184. [DOI] [PubMed] [Google Scholar]
- Chailakhyan MK, Khrianin VN. Effect of growth regulators and role of roots in sex expression in spinach. Planta. 1978b;142:207. doi: 10.1007/BF00388214. −210. [DOI] [PubMed] [Google Scholar]
- Dellaporta DL, Calderon-Urrea A. The sex determination process in maize. Science. 1994;266:1501–1505. doi: 10.1126/science.7985019. [DOI] [PubMed] [Google Scholar]
- De Martinis D, Mariani C. Silencing gene expression of the ethylene-forming enzyme results in a reversible inhibition of ovule development in transgenic tobacco plants. Plant Cell. 1999;11:1061–1071. doi: 10.1105/tpc.11.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DD, Hamilton DA, Travis JL, Bashe DM, Mascarenhas JP. Characterization of a pollen-specific cDNA clone from Zea mays and its expression. Plant Cell. 1989;1:173–179. doi: 10.1105/tpc.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare PD, Van Staden J. Inhibitory effect of thidiazuron on the activity of cytokinin oxidase isolated from soybean callus. Plant Cell Physiol. 1994;35:1121–1125. [Google Scholar]
- Hartley RW. Barnase and barstar, expression of its cloned inhibitor permit expression of a cloned ribonuclease. J Mol Biol. 1988;202:913–915. doi: 10.1016/0022-2836(88)90568-2. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N, Pethe C, d'Alayer J, Laloue M. Cytokinin oxidase from Zea mays: purification, cDNA cloning and expression and expression in moss protoplasts. Plant J. 1999;17:615–626. doi: 10.1046/j.1365-313x.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol. 1996;14:745–750. doi: 10.1038/nbt0696-745. [DOI] [PubMed] [Google Scholar]
- Izhaki A, Borochov A, Zamski E, Weiss D. Gibberellin regulates post-microsporogenesis in petunia anthers. Physiol Plant. 2002;115:442–447. doi: 10.1034/j.1399-3054.2002.1150314.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Olszewski NE. Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiol. 1991;97:409–414. doi: 10.1104/pp.97.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasembe JNR. Phenotypic restoration of fertility in a male-sterile mutant by treatment with gibberellic acid. Nature. 1967;21 53 :668. [Google Scholar]
- Khan AA. Cytokinin: Permissive role in seed germination. Science. 1971;171:853–859. doi: 10.1126/science.171.3974.853. [DOI] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB. Different temporal and spatial gene expression pattern occur during anther development. Plant Cell. 1990;2:1201–1224. doi: 10.1105/tpc.2.12.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant. 1985;65:33–39. [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana L. Heynh Theor Appl Genet. 1980;58:25–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Mitchell WD, Wittwer SH. Chemical regulation of flower sex expression and vegetative growth in Cucumis sativus L. Science. 1962;136:880–881. doi: 10.1126/science.136.3519.880. [DOI] [PubMed] [Google Scholar]
- Mohan Ram HY, Jaiswal VS. Induction of male flowers on female plants of Cannabis sativa by gibberellins and its inhibition by abscissic acid. Planta 10. 1972;53:2543–2545. doi: 10.1007/BF00385397. [DOI] [PubMed] [Google Scholar]
- Morris RO, Bilyeu KD, Laskey JG, Cheikh NN. Isolation of a gene encoding a glycosylated cytokinin oxidase from maize. Biochem Biophys Res Commun. 1999;255:328–333. doi: 10.1006/bbrc.1999.0199. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yamaguchi I, Kizawa S, Murofushi N, Takahashi N. Semi-quantification of GA1 and GA4 in male sterile anthers of rice by radioimmunoassay. Plant Cell Physiol. 1991;32:511–513. [Google Scholar]
- Nester JE, Zeevaart JAD. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am J Bot. 1988;75:45–55. [Google Scholar]
- Neuffer MG, Coe E, Wessler SR. Mutants of Maize. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Olszewski N, Sun T-P, Gubler F. Gibberellin signaling: biosynthesis, catabolism, and response pathway. Plant Cell. 2002;14:S61–S80. doi: 10.1105/tpc.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, Harberd NP. The GAI gene defines a signal pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatak SC, Wittwer SH, Honma S, Bukovac MJ. Gibberellin-induced anther and pollen development in a stamenless tomato mutant. Nature. 1966;209:635–636. [Google Scholar]
- Pike LM, Peterson CE. Gibberellin A4/A7, for induction of staminate flowers on the gynoecious cucumber. Euphytica. 1969;18:106–109. [Google Scholar]
- Rana RS, Jain HK. Gibberellin-induced expression of male potential in a stamenless mutant of Cosmos. Naturwissenschaften. 1968;55:301–302. doi: 10.1007/BF00591722. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Sawhney VK. In vitro development of angiosperm floral buds and organs. Plant Cell Tissue Organ Cult. 1989;16:145–174. [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO. How a Corn Plant Develops: Special Report No 48. Ames: Iowa State University of Science and Technology Cooperative Extension Service; 1997. [Google Scholar]
- Russell DA, Sachs MM. Differential expression and sequence analysis of the maize glyceraldehyde-3-phosphate dehydrogenase gene family. Plant Cell. 1989;1:793–803. doi: 10.1105/tpc.1.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney VK. Morphogenesis of the stamenless-2 mutant of tomato: III. Relative levels if gibberellins in the normal and mutant plants. J Exp Bot. 1974;25:1004–1009. [Google Scholar]
- Sawhney VK. Floral mutants in tomato: development, physiology, and evolutionary implications. Can J Bot. 1992;70:701–707. [Google Scholar]
- Sawhney VK, Greyson RI. Morphogenesis of the stamenless-2 mutant in tomato: II. Modification of sex organs in the mutant and normal flowers. Can J Bot. 1973;51:2473–2479. [Google Scholar]
- Sawhney VK, Shukla A. Male sterility in flowering plants: Are plant growth substances involved? Am J Bot. 1994;81:1640–1647. [Google Scholar]
- Schmidt H, Schmidt V. Untersuchungen an pollensterilen, stamen-ähnlichen mutanten von Lycopersicon esculentum Mill.: II. Normalisierung von ms-15 und ms-33 mit gibberellinsäure (GA3) Biol Zentralbl. 1981;100:691–696. [Google Scholar]
- Shukla A, Sawhney VK. Metabolism of dihydrozeatin in floral buds of wild-type and a genic male sterile line of rapeseed. J Exp Bot. 1993;44:1497–1505. [Google Scholar]
- Singh S, Sawhney VK. Plant hormone and temperature in relation male sterile in “stamenless-2”mutant of tomato. Rep Tomato Genet Coop. 1991;41:53. [Google Scholar]
- Spena A, Estruch JJ, Prinsen E, Nacken W, Van Onckelen H, Sommer H. Anther-specific expression of the rolB gene of Agrobacterium rhizogenes increases IAA content in anthers and alter anther development and whole flower growth. Theor Appl Genet. 1992;84:520–527. doi: 10.1007/BF00224147. [DOI] [PubMed] [Google Scholar]
- Thomas TH, Van Staden JV. Dormancy break of celery (Apium graveolens L.) seeds by plants derived smoke extract. Plant Growth Regul. 1995;17:195–198. [Google Scholar]
- Venglat SP, Sawhney VK. Benzylaminopurine induces phenocopies of floral meristem and identity mutants in wild-type Arabidopsis plants. Planta. 1996;198:480–487. doi: 10.1007/BF00620066. [DOI] [PubMed] [Google Scholar]
- Williams ME, Leemans J, Michiels F. Male sterility through recombinant DNA technology. In: Shivanna KR, Sawhney VK, editors. Pollen Biotechnology for Crop Production and Improvement. Cambridge, UK: Cambridge University Press; 1997. pp. 237–257. [Google Scholar]
- Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM et al. Activation tagging in Arabidopsis. Plant Physiol. 2000;122:1003–1013. doi: 10.1104/pp.122.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler RG, Helentjaris T. The maize dwarf3 gene encodes a cytochrome P450-mediated early step in gibberellin biosynthesis. Plant Cell. 1995;7:1307–1317. doi: 10.1105/tpc.7.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Suzuki T, Terada K, Takei K, Ishikawa K, Miwa K, Yamashino T, Mizuno T. The Arabidopsis AHK4 histidine kinase is a cytokinin-binding receptor that transduces cytokinin signals across the membrane. Plant Cell Physiol. 2001;42:1017–1023. doi: 10.1093/pcp/pce127. [DOI] [PubMed] [Google Scholar]
- Ye GN, Stone D, Pang SZ, Creely W, Gonzalez K, Hinchee M. Arabidopsis ovule is the target for Agrobacterium in planta vacuum infiltration transformation. Plant J. 1999;19:249–257. doi: 10.1046/j.1365-313x.1999.00520.x. [DOI] [PubMed] [Google Scholar]