Abstract
A number of observations have implicated auxin in the formation of vascular tissues in plant organs. These include vascular strand formation in response to local auxin application, the effects of impaired auxin transport on vascular patterns and suggestive phenotypes of Arabidopsis auxin response mutants. In this study, we have used molecular markers to visualize auxin response patterns in developing Arabidopsis leaves as well as Arabidopsis mutants and transgenic plants to trace pathways of auxin signal transduction controlling the expression of early procambial genes. We show that in young Arabidopsis leaf primordia, molecular auxin response patterns presage sites of procambial differentiation. This is the case not only in normal development but also upon experimental manipulation of auxin transport suggesting that local auxin signals are instrumental in patterning Arabidopsis leaf vasculature. We further found that the activity of the Arabidopsis gene MONOPTEROS, which is required for proper vascular differentiation, is also essential in a spectrum of auxin responses, which include the regulation of rapidly auxin-inducible AUX/IAA genes, and discovered the tissue-specific vascular expression profile of the class I homeodomain-leucine zipper gene, AtHB20. Interestingly, MONOPTEROS activity is a limiting factor in the expression of AtHB8 and AtHB20, two genes encoding transcriptional regulators expressed early in procambial development. Our observations connect general auxin signaling with early controls of vascular differentiation and suggest molecular mechanisms for auxin signaling in patterned cell differentiation.
The vascular system of higher plants constitutes a continuous cellular network essential for solute transport and mechanic stability in plants (Esau, 1965; Nelson and Dengler, 1997). The network consists of interconnected vascular strands, each composed of two types of conducting tissue, phloem and xylem. Photoassimilates and numerous other substances are transported through sieve tubes in the phloem, whereas water and dissolved minerals are passed through the vessels of the xylem. Vascular tissues differentiate from procambial cells, which can be identified in young organ primordia (see “Materials and Methods” for definitions).
Vascular tissue patterning needs to be precisely regulated, because the conducting functions of vascular tissues require not only proper integration into the context of nonvascular tissues, but also perfect cell alignment and tissue continuity within vascular strands. Amazingly, the tight control mechanisms that mediate precise cell patterning within vascular strands still allow for highly flexible arrangements of vascular strands within the organs of many plants. Conspicuous regularities in vascular regeneration patterns and the possibility to induce the formation of vascular strands by local auxin application have implicated auxin in the formation of vascular strands (for review, see Sachs, 1981, 1991). In normal development, auxin is synthesized in shoot apical tissues and is actively transported toward the base of the plant (Sachs, 1981; for review, see Aloni, 1995; Lomax et al., 1995). A vascular inducing signal from young leaf primordia can be replaced by auxin application (Jacobs, 1952), suggesting that endogenous auxin promotes vascular development basal to its site of synthesis.
The cellular basis for the apical-basal transport of auxin has been studied extensively, and the activity of specific auxin influx and efflux proteins has been experimentally established (for review, see Lomax et al., 1995). The polarity of auxin transport is generally attributed to the restriction of auxin efflux carriers to the basal side of each cell (Rubery and Sheldrake, 1974; Raven, 1975). Recent cell biological studies further emphasize the role of auxin efflux carriers in controlling auxin transport, and genetic studies have identified genes with defined functions in either auxin influx or efflux (for review, see Bennett et al., 1998; Palme and Galweiler, 1999; Muday and DeLong, 2001). Auxin transport can experimentally be inhibited through a number of chemically heterogeneous compounds, among them 1-N-naphthylphtalamic acid (NPA), 2,3,5-triiodobenzoic acid (TIBA) and 2-chloro-9-hydroxyfluorene-9-carboxylic acid (HFCA), which inhibit polar auxin transport by interfering with auxin efflux (for a summary, see Lomax et al., 1995). Inhibition of auxin transport in young organs has been used to demonstrate the auxin-transport dependence of normal vascular strand patterning during Arabidopsis organogenesis, suggesting a role of auxin signals in normal organ development (Mattsson et al., 1999; Sieburth, 1999).
Potential vascular patterning genes have been identified by mutant phenotypes (Carland et al., 1999; Zhong and Ye, 1999; Deyholos et al., 2000; Koizumi et al., 2000). Although many of the identified gene products remain to be determined, available evidence suggests sterols (Szekeres et al., 1996; Yamamoto et al., 1997, 2001), small peptides (Casson et al., 2002), cytokinin (Mähönen et al., 2000; Inoue et al., 2001), and auxin in promoting vascular differentiation. Mutations in three genes, MONOPTEROS (MP)/AUXIN RESPONSE FACTOR 5 (AFR5), AUXIN RESISTANT 6 (AXR6), and BODENLOS (BDL)/IAA12, are associated with incomplete vascular systems and defects in the formation of the embryo axis and of the embryonic root (Berleth and Jürgens, 1993; Przemeck et al., 1996; Hamann et al., 1999; Hobbie et al., 2000). Interestingly, MP and IAA12/BDL encode members of two families of nuclear proteins, auxin response factors (ARFs) and AUX/IAA coregulators, which are believed to be involved in auxin-dependent gene regulation (for review, see Hagen and Guilfoyle, 2002; Leyser, 2002; Liscum and Reed, 2002). Recessive mutations in MP are presumed to eliminate completely the function of ARF5, whereas the dominant bdl mutation was localized in the IAA12 gene and is believed to stabilize the IAA12 protein (Hamann et al., 2002). ARF proteins bind to DNA and seem to act as homo- or heterodimers, whereas AUX/IAA proteins may regulate ARF function by interfering with ARF dimerization. Through this mechanism, dominant mutations in BDL could interfere with the function of MP or other redundantly acting ARF proteins (Hamann et al., 2002).
Many AUX/IAA genes are themselves rapidly induced by auxin and are widely used as reporters of local auxin responses (Abel and Theologis, 1996; Luschnig et al., 1998). Synthetic auxin response elements (AuxREs) are derived from conserved control elements in the promoters of rapidly auxin-inducible genes (for a summary, see Guilfoyle et al., 1998) and have been used to monitor the intensity of auxin responses in organ development (Sabatini et al., 1999; Friml et al., 2002). Although it would be desirable to directly measure the distribution of auxin in developmental processes, the spatial and temporal resolution of direct measurements is still technically limited. Moreover, if IAA is compartmentalized within cells, biochemical assays cannot distinguish between pools of biologically perceived and inaccessible auxin. Therefore, auxin response reporter gene expression remains another necessary tool in the study of developmental functions of auxin because it can reveal correlated patterns of auxin response and cell differentiation, even in highly dynamic developmental processes.
In this study, we have determined auxin response patterns in Arabidopsis rosette leaf primordia under normal and experimentally manipulated conditions. These patterns presaged the domains of procambial differentiation, suggesting a role of auxin in vascular differentiation during normal leaf development. The dependence of at least two transcriptional regulators expressed early in procambial development on the dosage of MP gene activity suggest mechanisms through which auxin can promote vascular differentiation and vascular tissue continuity.
RESULTS
Vascular Differentiation Occurs at Sites of Maximum Auxin Response
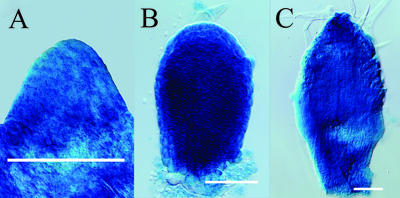
Auxin has been implicated in vascular differentiation under experimental conditions, but the distribution of auxin in naturally developing organs is unknown. To visualize the distribution of perceived auxin in early leaf primordia, we assessed the ability of a number of synthetic auxin response promoters to confer equal responsiveness to exogenously applied auxin in cells of the leaf primordium. Leaf primordia of various stages carrying synthetic AuxRE reporter gene constructs were exposed to the synthetic, poorly transported (McCready, 1963; McCready and Jacobs, 1963) auxin 2,4-dichlorophenoxy acetic acid (2,4-D; for details, see “Materials and Methods”). We found that a composite promoter comprising seven tandem repeats of the AuxRE TGTCTC motif and a 35S minimal promoter fused to a β-glucuronidase (GUS)-encoding reporter gene (referred to as DR5 in the following; Ulmasov et al., 1997b) conferred nearly homogenous GUS gene expression levels to all cells in the primordia (Fig. 1, A–C). We therefore chose DR5-driven GUS gene expression (DR5 expression in the following) as a suitable reporter for the distribution of perceived auxin in early leaf primordia.
Figure 1.
DR5::GUS reporter gene expression in leaf primordia exposed to 2,4-D. First rosette leaf primordia were incubated in culture medium containing 1 μm 2,4-D for 5 h, followed by histochemical detection of GUS activity. Age in DAG: A, 2; B, 3; C, 4. Scale bars = 50 μm in A and B, 100 μm in C. DIC optics.
The ontogeny of the Arabidopsis leaf vascular system has previously been described (Kinsman and Pyke, 1998; Donelly et al., 1999; Mattsson et al., 1999; Kang and Dengler, 2002). The first two vegetative leaf primordia emerge as small bulges at the flanks of the vegetative shoot meristem. Each primordium then enlarges to become a nearly cylindrical protrusion, slightly flattened on the adaxial side (Donelly et al., 1999). The formation of the lamina is associated with increased cell proliferation at the flanks of this promordium, which, in the first rosette leaf of Arabidopsis, occurs when the primordium has a length of approximately 200 μm. Further intense cell proliferation primarily in the basal part of the leaf increases the size of the lamina and determines the final shape of the leaf. The formation of primary and secondary veins is tightly associated with the major growth directions of the primordium (Kinsman and Pyke, 1998; Donelly et al., 1999; Mattsson et al., 1999; Kang and Dengler, 2002). Procambial cells in the position of the midvein become visible in early bulge-shaped primordia, and the midvein is extended acropetally as the still cylindrical early primordia increase in length. Just as the midvein is formed nearly concomitantly with the elongating central primordium, the formation of the lamina is immediately associated with the appearance of lobes of secondary procambial strands. Tertiary and quarternary veins are subsequently formed within and outside of the secondary vein lobes as the lamina further expands.
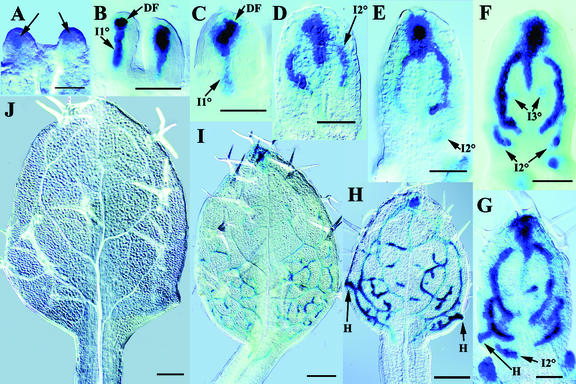
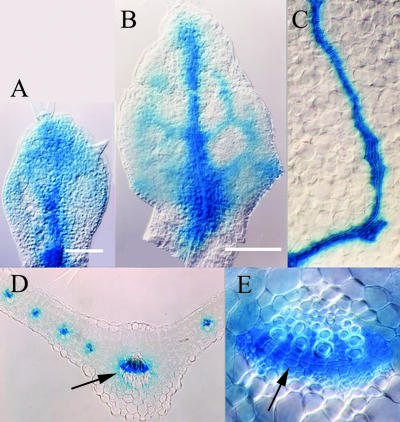
Throughout leaf development, DR5 expression was restricted to subepidermal cell layers. At very early stages of primordium development, a subset of the most distal subepidermal cells expressed DR5 (Fig. 2A). Interestingly, the position of this distal focus (DF) of DR5 expression was variable and could be either central or shifted to one side (Fig. 2C). However, irrespective of the precise position of this DF, there was always a zone of weaker DR5 expression between the DF and the base of the primordium, which we refer to as incipient primary vein (I1°; Fig. 2, B and C). DF expression was observed throughout leaf development at least up to 5 DAG, but its intensity varied in that DR5::GUS expression was usually but not always stronger in the DF than in incipient veins. Although the primordium rapidly increased in length, DR5 expression remained restricted to DF and I1° (Fig. 2, B and C). As the leaf lamina was initiated (Fig. 2D), DR5 expression in the midvein area decreased. With the appearance of markers of terminal differentiation, such as mature vessels, DR5 expression in the corresponding midvein segments had completely disappeared (Fig. 2H).
Figure 2.
Expression pattern of DR5::GUS in developing leaf primordia. First rosette leaf primordia, stages given in days after germination (DAG). A, Lateral view of 2-DAG primordia, with limited growth. Arrows point at DR5::GUS expression. B, Lateral view of 2-DAG primordia, somewhat older than in A. DF of expression in subepidermal cells and faint expression at the site of incipient primary vein (I1°). Note the absence of expression in the central apical dome of the shoot meristem. C, Abaxial view of 2-DAG primordium, with DF and I1°. D, Primordium at 3 DAG. Note diminished DR5::GUS expression in the basal part of I1°, whereas expression is visible in incipient secondary veins (I2°). E, Primordium at 3 DAG. Arrow indicates appearance of additional I2° in the basal region. F, Primordium at 4 DAG. Expression in basal I2°s and in incipient tertiary veins (I3°). G, Primordium at 4 DAG. Another I2° is appearing in a basal position. Expression is also seen at site of future hydathode (H). H, Primordium at 5 DAG. The vascular pattern, as visualized by DR5 expression and overt differentiation of vessels, comprises veins of all classes. DR5 expression is especially high in I3°s and in lateral positions where hydathodes will appear. I, Primordium at 6 DAG. The levels of DR5 expression are subsiding. Residual GUS activity primarily in veins in the basal part of the leaf after doubled assay time. J, Primordium at 7 DAG. Differentiated vessels, DR5 expression is no longer detectable. Scale bars = 50 μm in A through G, 200 μm in H through J. DIC optics.
At the onset of lamina formation, the width of the primordium increased rapidly (Fig. 2, D–J). Expression at DF continued to be strong through early stages of lamina expansion, whereas I1° expression subsided and two additional DR5 expression zones parallel to the leaf margin became apparent, indicating the first pair of I2°s (Fig. 2, D and E). DR5 expression was initially heterogenous in intensity within continuous expression domains but became more homogenous as individual veins matured (compare I2° expression domains in Fig. 2, D–F), marked by the formation of narrow procambial cells (Fig. 3). As previously reported, secondary veins form continuous lobes and emerge in a basipetal sequence (Kinsman and Pyke, 1998; Mattsson et al., 1999). DR5 expression preceded this emergence for each secondary vein lobe and each lobe was composed of a long stretch of DR5-expressing cells parallel to the leaf margin and of a number of cells connecting the basal end of the lobe with the midvein. A similar temporal pattern of DR5 expression and vein formation was observed for each of the subsequently emerging pairs of secondary vein lobes (Fig. 2, F and G). These lobes became first recognizable as heterogeneous DR5 expression domains parallel to the margin that subsequently became basally connected to the midvein and then differentiated into continuous lobes of elongated cell files. Although additional lobes of DR5 expression emerged in the basal part of the primordium, DR5 expression disappeared in more mature veins in the distal part of the leaf (Fig. 2, E–J). DR5 expression was generally absent or very weak in vein segments with mature vessel elements, suggesting that DR5 expression is restricted to specific stages of vein differentiation.
Figure 3.
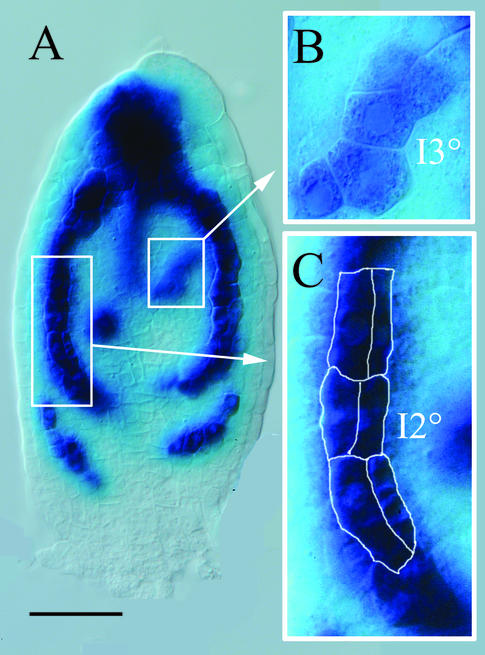
DR5::GUS expression in pre-procambial and procambial cells. Four-DAG first rosette leaf primordium. A, Extremely low DR5 expression in elongated procambial cells of I1° in the basal part and somewhat stronger expression in younger apical sections of I1°. Frames indicate magnified areas in B and C. B, Low, heterogenous DR5 expression cells of I3°. Note that these cells are still isodiametric in shape and cannot anatomically be distinguished from surrounding cells. C, Strong, homogenous DR5 expression in cells of I2°s, some of which have divided to form double rows of narrow procambial cells (outlined). Scale bar = 50 μm. DIC optics.
Tertiary and quarternary venation forms a more irregular pattern mainly within the intercostal areas between secondary vein lobes and the midvein. As for primary and secondary veins, tertiary vein DR5 expression domains marked sites of subsequent procambial differentiation (Fig. 2,G–J). Taken together, the same sequence of events was observed for all vein classes: DR5 expression preceded oriented cell divisions (Fig. 3, A–C), which produce continuous files of narrow procambial cells and disappeared at later differentiation stages. Thus, the DR5 marker visualizes an auxin response prepattern that precedes the appearance of anatomically recognizable procambial strands.
Proper Positioning of Auxin Perception Maxima Requires Polar Auxin Transport
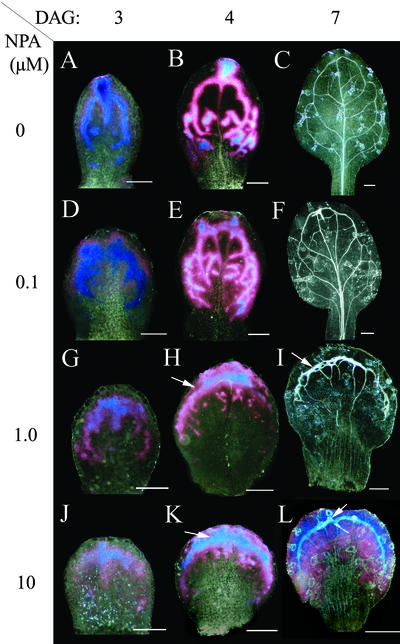
The distinct domains of high DR5 expression could be due to: (a) local accumulation of auxin that is derived from remote sources through polar auxin transport, (b) locally enhanced auxin synthesis or response within the incipient veins, or (c) a patterned signal unrelated to auxin, spuriously activating the DR5 marker. Only in scenario (a) would one expect that DR5 patterns are responsive to gradual changes in auxin transport, and we therefore assessed the position of DR5 expression domains in auxin transport-inhibited primordia. In the presence of 0.1 μm of auxin transport inhibitor NPA, the early DR5 expression domains close to the primordium margin were more pronounced than in leaf primordia grown under normal growth conditions (Fig. 4, A and D; red staining reflects lower levels of GUS activity than blue staining). DR5 expression became nearly restricted to the primordium margin at 1 μm NPA (Fig. 4, G and H), whereas even stronger inhibition of auxin transport resulted in delayed decrease of DR5 expression and delayed vascular differentiation at the margin (Fig. 4, J–L). We obtained similar results with the auxin transport inhibitors HFCA and TIBA (data not shown), suggesting that these shifts can be attributed to altered auxin transport properties of the leaf primordia rather than to drug-specific effects. Interestingly, broadening of the DR5 expression domain along the leaf margin was associated with the multiplication of midveins, suggesting that a sharp distal expression focus (DF) in normal development promotes the formation of a single midvein. Two separate DFs were occasionally observed, which may result in leaves with two distinct midveins, which are observed at low frequency in auxin transport-inhibited plants and in pinformed 1 mutants (Okada et al., 1991; Mattsson et al., 1999).
Figure 4.
Pattern of DR5::GUS expression in primordia exposed to auxin transport inhibitors. Ages are 3 DAG in left column (A, D, G, and J), 4 DAG in middle column (B, E, H, and K), and 7 DAG in right column (C, F, I, and L). Levels of NPA are 0 μm (A–C), 0.1 μm (D–F), 1.0 μm (G–I), and 10 μm (J–L). Note that with increasing concentration of NPA, the expression is gradually confined to tips and margins of primordia (arrows in H and K) coinciding with the site of final vascularization (arrows in I and L). Scale bars = 50 μm in A, D, G, and J; 100 μm in B, E, H, and K; and 200 μm in C, F, I, and L. Dark-field illumination, low concentration of GUS product as red and higher levels as blue staining.
Overall, the shift in DR5 expression pattern parallels the previously reported shift in vascular patterning of leaf primordia grown in the presence of auxin transport inhibitors (Fig. 4, C, F, I, and L; Mattsson et al., 1999; Sieburth, 1999). We conclude that DR5 expression domains mark incipient procambial patterns also under conditions of reduced auxin transport, suggesting functional involvement of auxin in vascular patterning.
Mutations in the MP Gene Result in Severe Auxin Insensitivity
The MP gene encodes an ARF transcription factor (Ulmasov et al., 1997a; Hardtke and Berleth, 1998), but early growth defects of mp mutants have precluded assessment of auxin sensitivity of mp mutants in traditional auxin response tests. To relate auxin responses in mp mutants to those in other auxin sensitivity mutants and in the Arabidopsis wild type, we subjected mp, the well-characterized auxin resistant1 (axr1) mutant (Lincoln et al., 1990), and the Columbia-0 (Col-0) background line to two auxin response tests at the seedling stage.
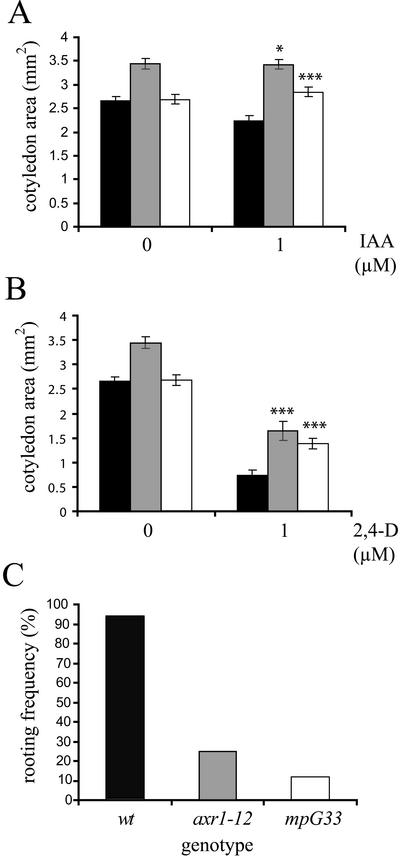
After germination, Arabidopsis wild-type cotyledons expand considerably, and this expansion is significantly reduced in the presence of micromolar concentrations of auxin. As shown in Figure 5A, neither mp nor axr1 mutants showed any response to 1 μm IAA, whereas the cotyledon area in the wild type dropped by 16%. Similar results were obtained upon exposure of germinating seedlings to 2,4-D (Fig. 5B). Wild-type and mp cotyledons had similar surface areas in the absence of 2,4-D, whereas mp cotyledons were approximately twice as large as wild-type cotyledons at 1 μm 2,4-D. All assays confirmed severe auxin insensitivity of the strong axr1 mutant allele axr1-12.
Figure 5.
Auxin responses in monopteros mutants. Genotypes: wild type (black), auxin-resistant 1-12 (gray), and monopteros G33 (white, mpG12 in A and B, mpG33 in C). A and B, Quantification of cotyledon area of seedlings grown for 10 d in the presence of 0 and 1 μm IAA (A) and in the presence of 0 and 1 μm 2,4-D (B). Size bars indicate se. Significance of genotype-dependent differences relative to wt type values as determined by Student t test analysis is indicated by asterisks (*, 0.01 ≤ P < 0.05; **, 0.001 ≤ P < 0.01; and ***, P < 0.001). Sample sizes of 10 to 50 cotyledons. C, Adventitious root formation in cotyledons exposed to 0.3 mg L−1 indole-butyric acid (IBA). Sample sizes of 222 to 475 cotyledons.
Auxin requirement for root initiation is well established, and adventitious root formation is efficiently induced by IBA. We noticed that excised wild-type cotyledons readily form roots when exposed to 0.3 mg L−1 IBA (Fig. 5C). No roots were formed on auxin-free medium, suggesting that endogenous auxin levels are insufficient and that root formation of excised cotyledons could be used to measure responsiveness of mutants to external auxin. Cotyledons of 5-d-old seedlings were cut off at the petiole and incubated in liquid medium supplemented with 0.3 mg L−1 IBA. The percentages of root producing cotyledons were determined for the three genotypes after 10 d of culture. Although 94% (373 of 395) of the wild-type cotyledons had formed roots at the basal end, only 25% (55 of 222) of the axr1 mutant cotyledons and only 11% (54 of 475) of the mp mutant had formed roots.
In conclusion, we consistently observed auxin insensitivity of mp mutants in two assays, and auxin perception defects were found to be similar to or even more severe than in axr1 mutants.
Expression of AtHB20 in Leaf Vascular Development
Several members of the homeodomain-leucine zipper (HD-ZIP) class of transcription factors are expressed in vascular tissues and may have functions in vascular tissue patterning (for summary, see Ye, 2002). Assessing expression patterns of HD-ZIP genes, we discovered the conspicuous expression pattern of AtHB20 (Hanson, 2000), a class I HD-ZIP gene, with high homology to the AtHB3/HAT7 gene (Mattsson et al., 1992; Schena and Davis, 1992). The GUS reporter gene was fused in frame to a fragment consisting of 1,978 bp upstream of the transcription initiation site and 549 bp of the 5′-transcribed region (AtHB20::GUS; see “Materials and Methods”) to study its expression in leaf primordia. As shown in Figure 6, AtHB20::GUS expression in 3-DAG first rosette leaf primordia was diffuse around the emerging veins in the apical part of the leaf and more restricted to positions close to the midvein in the basal part of the leaf (Fig. 6A). At later stages, expression was associated with the formation of new veins, but at this stage it was not exclusively restricted to procambial cells (Fig. 6B). In leaves close to maturity, expression was strong and restricted to veins (Fig. 6C). Cross sections of nearly mature leaves revealed expression in the fascicular cambium (Fig. 6, D and E).
Figure 6.
AtHB20::GUS expression in leaves. A, First rosette leaf primordium at 3 DAG: diffuse expression with elevated levels along I1° and at the tip of the primordium. B, 4 DAG: strong expression along the differentiating midvein and weak expression along I2°s. C, Highly localized and strong expression at late stage of secondary vein differentiation. D, Cross section of a 7-DAG leaf primordium: Expression is confined to cells in vascular bundles. E, Higher magnification of midvein in D (arrow) shows strong expression in fascicular cambium (arrow in E).
MP Dependent Regulation of Auxin-Responsive Genes
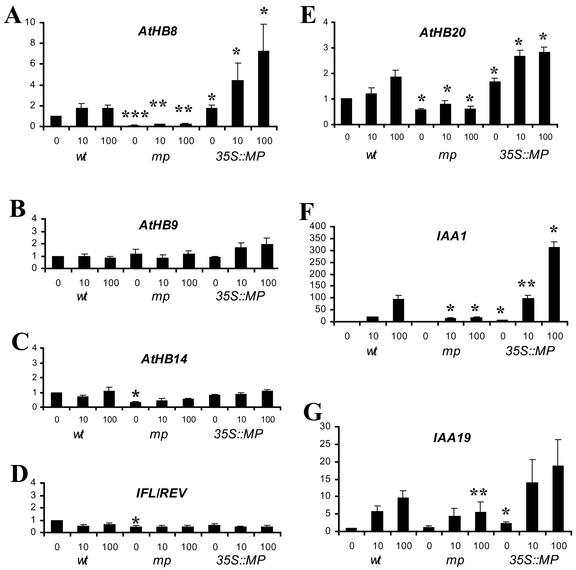
If recognizable procambial differentiation occurs at sites of auxin response maxima, some early regulators of procambial development might be auxin inducible. One gene expressed in procambial cells, AtHB8, encoding a class III HD-ZIP transcription factor, has been shown to be auxin inducible in Arabidopsis (Baima et al., 1995). We assessed the auxin inducibility of other class III HD-ZIP genes and the class I HD-ZIP AtHB20 gene, most of which are expressed in the vasculature, but also in other tissues (McConnell et al., 2001; see “Discussion”). Finally, we subjected two presumed primary auxin response genes, IAA1 and IAA19, to the same assay (Abel et al., 1995; Kim et al., 1997). We chose to measure transcript abundance on northern blots after only 30 min of auxin exposure, to restrict the analysis to primary gene regulatory responses to auxin (see “Materials and Methods”). We used an IAA concentration that is commonly used to visualize auxin-induced AUX/IAA gene expression (10 μm) and a 10-fold higher concentration to visualize possible plateau levels of auxin-induced gene expression.
As shown in Figure 7, expression of AtHB8 and AtHB20 and of IAA1 and 19 responded rapidly to the application of IAA, whereas the expression of AtHB9/PHAVOLUTA (PHV), ATHB14/PHABULOSA (PHB), and INTERFASCICULAR FIBERLESS1/REVOLUTA (IFL1/REV) remained essentially unchanged. We next determined whether the observed expression profiles were dependent upon MP gene activity. Because of the abnormal development and tissue composition of mp mutants, we focused our analysis on auxin responses in plants overexpressing the MP gene. To this end, we determined transcript levels of all seven genes in transgenic plants expressing a functional MP gene under the control of the 35S-cauliflower mosaic virus (35S::MP) at various auxin concentrations. At the seedling stage, 35S::MP plants are morphologically indistinguishable from wild type, which includes normal patterns of vascular tissues in all organs (data not shown). In 35S::MP plants, transcript abundance of AtHB8, AtHB20, IAA1, and IAA19 was increased, whereas no significant change was observed in the expression of AtHB9/PHV, AtHB14/PHB, and IFL1/REV. The response of AtHB8 and AtHB20 to increased MP gene activity was less dramatic than that of IAA1 and IAA19, but turned out to be significant in standard t tests. In contrast, the rapid auxin inducibility of AtHB20, although apparent in several independent experiments, is not sufficiently pronounced in the background of its variable expression to be confirmed as statistically significant. In conclusion, MP gene activity can be limiting in the auxin-dependent regulation of gene expression. The expression levels of primary auxin response genes of the AUX/IAA family are enhanced in 35S::MP plants and a qualitatively similar effect on the expression level was observed for two HD-ZIP genes expressed in procambial cells (see “Discussion”).
Figure 7.
Expression of HD-ZIP genes and AUX/IAA genes. Transcript abundance of the HD-ZIP genes AtHB8 (A), AtHB9 (B), AtHB14 (C), IFL1/REV (D), and AtHB20 (E) and of the AUX/IAA genes IAA1 (F) and IAA19 (G) were determined by northern-blot hybridization to total RNA from wild type (wt), mp mutant, and 35S::MP plants exposed for 30 min to 0, 10, and 100 μm IAA (indicated on x axes, genotypes below). Transcript levels (y axes) for each gene were calculated as multiples of the wild-type level at 0 μm IAA. Columns represent the mean ± se from 3 to 5 (A–D), and the results of two experiments (F and G). Significance of genotype-dependent differences between mp and 35S::MP values relative to wt type values under identical conditions as determined by Student's t test analysis is indicated by asterisks (*, 0.01 ≤ P < 0.05; **, 0.001 ≤ P < 0.01; ***, P < 0.001). Note that the absolute induction levels of IAA1 and IAA19 appear extremely high because of extremely low expression at 0 μm IAA.
In mp mutant seedlings, the amplitudes of auxin induction of IAA1 and IAA19 were reduced, indicating that MP is necessary for their proper auxin-inducible expression in wild-type plants, but also that this regulation seems to occur under partially redundant control. For AtHB8 and AtHB20, possible auxin inducibility changes cannot be expected to be sufficiently pronounced to be detectable in the background of the variable tissue composition of mp mutants. Overall AtHB8 expression levels were dramatically reduced in mp mutants, which could reflect the reduced amount of vascular tissue in the mutant.
DISCUSSION
The mechanisms controlling the differentiation of continuous vascular strands are largely unknown. Genetic screens in Arabidopsis and other species have identified a number of loci with potential functions in vascular differentiation (for review, see Nelson and Dengler, 1997; Dengler and Kang, 2001; Ye, 2002), some of which suggest a role of auxin in vascular development (for a summary, see Berleth et al., 2000). This role is further supported by the promoting influence of auxin on vascular differentiation (for a summary, see Lyndon, 1990; Aloni, 1995, and refs. therein) and by the responses of vascular patterns to altered auxin transport during organ development (Mattsson et al., 1999; Sieburth, 1999). In this study, we have explored the spatial relationship between the distribution of perceived auxin and vascular differentiation zones as well as pathways of auxin signaling in vascular differentiation during normal organogenesis. We find that the AuxRE DR5 marks zones of vascular differentiation before the procambium can be anatomically recognized and that the ARF MP is required for diverse auxin responses in Arabidopsis plants. We further show that the auxin signal transduction functions of MP include controlling the expression of auxin-inducible genes and that the expression of two transcriptional regulators expressed early in vascular development, AtHB8 and AtHB20, is positively correlated with the amount of MP gene activity.
Auxin Distribution and Vascular Patterning
Because of its nearly unbiased response to externally applied auxin, which we have also observed in mp mutant leaf primordia (data not shown), we have chosen the DR5 auxin-response reporter gene system to visualize the distribution of perceived auxin in the developing leaf primordium. We found that local DR5 expression, usually in the form of narrow reporter gene expression domains, preceded the earliest stages of anatomically detectable procambial differentiation. Initially, cells within these expression domains appeared not to be anatomically distinct from neighboring cells but they divided and differentiated to form files of narrow, aligned procambial cells in primordia of slightly later stages. Correlated with the formation of early procambial cell files, DR5 expression levels became more homogenous, a feature that could reflect changes in auxin conductivity of differentiating vascular cells. Finally, strong reduction or cessation of DR5 expression was correlated with the appearance of terminal differentiation markers, such as secondary wall formation in tracheary elements.
If the congruence of DR5 expression and vascular differentiation patterns is more than coincidental, it should also be observed under altered experimental conditions. Auxin transport inhibition has been shown to change patterns of vascular differentiation (Mattsson et al., 1999; Sieburth, 1999). Our observation of correspondingly altered DR5 expression patterns in leaf primordia suggests three important conclusions. First, DR5 expression domains are more likely to reflect accumulation sites of transported auxin rather than local changes in auxin production or perception or unrelated regulatory changes. Second, the persistent congruence of DR5 expression domains and vascular differentiation zones in a variety of auxin transport-inhibited conditions indicates an instrumental role of local auxin distribution in the positioning of vascular strands. This interpretation is consistent with the extremely early localized expression of the DR5 reporter and with the experimental induction of vascular strand formation by local auxin application (for a summary, see Sachs, 1981). Third, inhibition of auxin transport results in a shift of DR5 expression toward the leaf margin supporting the notion that major auxin sources are at the margins of young leaf primordia (Mattsson et al., 1999). Polar responses (increased differentiation in apical associated with reduced differentiation in basal positions) to auxin transport inhibition have been observed in other plant organs (Reed et al., 1998; Bhalerao et al., 2002) and most likely reflect the apical accumulation of auxin under these conditions. A possible role of auxin deduced from the spatial and temporal distribution of DR5 expression could be the positioning of vascular strands at sites of local auxin accumulation and the promotion of procambial continuity along a vascular strand.
It should be noted that, although the correlation of DR5 expression and procambial differentiation zones is far too close to be considered coincidental, it is not without exceptions, which may help to identify additional controls. First, correlation of DR5 expression and procambial differentiation may be restricted to leaf organs, where the procambial pattern is generated de novo from cells that would otherwise become mesophyll cells. In the growth of other organs, all tissues including the procambium are extended apically, while essentially maintaining a given radial pattern, and here, auxin may not accumulate to levels detectable by strong DR5 expression. In the root, for example, DR5 expression has been shown to be strongest in columella initials, whereas expression in the stele is faint (Sabatini et al., 1999). Second, isolated patches of DR5 expression, such as the DF or the hydathodes, do not differentiate to vascular tissue as early and as strongly as one might expect. The most direct conclusion from this observation is that auxin signals and signals from preexisting vasculature may have to act together to promote vascular differentiation. Given the abundantly documented requirement for factors other than auxin in vascular differentiation (for a summary, see Aloni, 1995; Ye, 2002), this finding would simply reflect the fact that auxin is necessary, but not sufficient to promote procambial development, but it would also indicate that other factors are not ubiquitous, but associated with preexisting vasculature. Finally, it should be emphasized that our findings do not imply that auxin-dependent vascular patterning is necessarily a self-organizing process. Although it is possible that in certain plant species, vascular strand positions are defined predominantly through self-organizing feedback interactions restricting high auxin levels to certain areas (Sachs, 1981), preferential routes of auxin transport or local modulations of auxin responsiveness could be rigidly specified in other plant species. Changing the auxin-transport properties within young leaf primordia may reveal to what degree self-organizing mechanisms are constrained by other cues. Although some venation patterns may turn out to be highly flexible (Mattsson et al., 1999; Sieburth, 1999), others could be entirely invariant. Irrespective of whether auxin has an influence on the venation pattern in a given species, as a non-cell autonomous signal preferably transported along cell files, it could integrate vascular differentiation and promote vein continuity.
Vascular Defects in Auxin-Insensitive Mutants
Involvement of auxin in vascular differentiation would suggest defective vascular systems at least in a subset of auxin-insensitive mutants. Although initial genetic screens for auxin sensitivity mutants focused on morphologically and anatomically intact adult plants, mutants with characteristic vascular defects, associated seedling morphology distortions, and auxin response defects have recently been reported (for a summary, see Berleth et al., 2000). For two mutants in this category, axr6 and bdl, insensitivity to external auxin application has been demonstrated (Hamann et al., 1999; Hobbie et al., 2000), whereas a third locus, MP, has been implicated in auxin-related developmental functions only indirectly, through defective cell axis formation in embryonic and postembryonic development (Przemeck et al., 1996) and by the identity of its gene product as a member of the ARF family of transcription factors (Ulmasov et al., 1997a; Hardtke and Berleth, 1998). In this study, we show that MP is involved in various types of auxin responses and that these include the regulation of auxin-inducible Arabidopsis genes. Together with auxin response assays in axr6 and bdl mutants (Hamann et al., 1999; Hobbie et al., 2000), these findings suggest that all three genes, AXR6, IAA12/BDL, and MP, are involved in auxin signal transduction beyond vascular development, consistent with the suggested molecular interaction of BDL/IAA12 and MP in auxin signaling (Hamann et al., 2002).
Vascular defects in mp mutant leaf organs are not random, and published data suggest that similar characteristics are observed in axr6 and bdl mutants (Hamann et al., 1999; Hobbie et al., 2000). The mp mutant allelic series (Berleth and Jürgens, 1993; Przemeck et al., 1996) suggests that higher order veins are particularly sensitive to reduced auxin signal transduction, secondary veins are intermediate, and the midvein is least affected by reduced auxin signal transduction. This graded auxin sensitivity of veins of different hierarchical orders could reflect distal auxin sources. The midvein elongating from the base of the leaf would be exposed to converging auxin from all parts of the primordium, even if this source becomes more dispersed upon lamina formation. By contrast, later formed veins would be exposed to less converging signal over a shorter period of time and would therefore depend more strongly on proper auxin signal transduction. Interestingly, a distal auxin source is also suggested by the DR5 expression and vascular response patterns in auxin transport-inhibited leaves. Here, the basal part of the leaf seems to become artificially insulated from distal signals and vascular differentiation is restricted to the leaf margin.
Genetic Hierarchy Controlling Vascular Differentiation
The class III HD-ZIP gene AtHB8 is expressed in procambial tissues and has also been functionally implicated in vascular tissue formation (Baima et al., 1995, 2001). We discovered a new tissue-specific expression profile of the class I HD-ZIP gene AtHB20, which in some ways resembled the expression of AtHB8. We found AtHB20::GUS to be expressed in very early leaf primordia at sites around emerging procambial strands. It will be interesting to determine whether AtHB20 expression can be functionally implicated in vascular development. Rapid auxin-induced increase of AtHB20 transcript levels remains below significance threshold levels because of high variability, but it could be that both AtHB8 and AtHB20 simply display slower auxin responses than AUX/IAA genes. In fact, it has been reported that AtHB8 transcript levels increase further upon prolonged auxin exposure (Baima et al., 1995). For IAA1, IAA19, and AtHB8, enhanced transcript levels are clearly observed already after 30 min of auxin exposure, suggesting that their auxin inducibility is due to modifications in a pre-existing signal transduction machinery. We explored the possibility that the expression levels of some of our test genes depend on MP gene activity. Because the highly abnormal development of mp mutants precludes stringent conclusions from gene expression profiles, our assessment of a role of MP in the regulation of both genes largely relies on their expression in 35S::MP plants. Seedlings overexpressing MP are phenotypically indistinguishable from wild-type seedlings but selectively overexpress the four auxin-inducible genes AtHB8, AtHB20, IAA1, and IAA19. These findings suggest that MP is a limiting component in the regulation of a number of auxin-inducible genes and may therefore be involved in their natural regulation. The activity of ARF transcription factors is believed to be constrained by interaction with AUX/IAA proteins (for review, see Hagen and Guilfoyle, 2002; Liscum and Reed, 2002; Leyser, 2002). In this regulatory context, it is plausible that the overexpression of an ARF product may overcome negative regulation by AUX/IAA proteins and lead to enhanced expression of auxin-inducible genes.
We further explored the transcript profiles of other class III HD-ZIP genes in response to auxin and various levels of MP gene activity. AtHB9, AtHB14, and IFL1/REV did not display an expression profile similar to AtHB8, although they are expressed in vascular tissues and may have functions in vascular development. The IFL1/REV gene is required for the formation of interfascicular fibers and secondary xylem formation but has also been demonstrated to have functions in the organization of shoot apical meristems (Talbert et al., 1995; Otsuga et al., 2001; Zhong and Ye, 2001). Therefore, despite its involvement in vascular tissue organization in the inflorescence stem, it seems likely that IFL1/REV is regulated in an entirely independent context. This is probably also true for AtHB9/PHV and AtHB14/PHB, which have been implicated in adaxial-abaxial pattern formation by their dominant mutant phenotypes. AtHB14/PHB is expressed primarily in adaxial domains of lateral organs, whereas the expression profile of AtHB9/PHV has not yet been determined (McConnell et al., 2001). These genes seem to be regulated in an unrelated context, although they may both be expressed in vascular tissues and may impinge on vascular bundle organization through their functions in adaxial-abaxial patterning.
In summary, the expression of AtHB8 and of two typical primary auxin response genes turned out to be auxin inducible and regulated by MP gene activity in a suitable genetic background, and the expression of AtHB20 shows strict dependence on MP gene activity and likely dependence on auxin signals. By overexpression phenotype, AtHB8 has been implicated in the organization of vascular tissues (Baima et al., 2001). A basic scenario is therefore that the expression of major regulators of vascular differentiation is controlled by auxin and thereby confined to a pattern that can be visualized in close approximation by DR5 expression. This basic concept is obviously modified by numerous regulatory inputs. The expression of AtHB8, for example, may well be influenced by adaxial-abaxial patterning cues (Kang and Dengler, 2002), and the final pattern is likely to be influenced by predisposing external as well as possible feed-back controls. The identification of early transcriptional regulators of procambial development and their control through factors implicated in procambial differentiation may help to define the patterning input of those factors in molecular detail.
MATERIALS AND METHODS
Definitions
Because the term “provascular” in the literature refers not only to procambial cells but also to the anatomically inconspicuous precursors of those cells, we have decided to exclusively use the term “procambial” in this paper. With this, we refer to anatomically recognizable progenitor cells of vascular tissues, which in Arabidopsis leaves are organized in files of narrow cells in young primordia and result from coordinated divisions and expansions of cells, to which we refer as “pre-procambial.” Because the first pair of rosette leaves appears simultaneously, we use the term “first rosette leaf primordium” to refer to one of those two primordia. We refer to DAG as days after exposure of imbibed seeds to light. We refer to the Col-0 line as “wild type” in all experiments. Nearly all genes in this study were identified independently in mutant and molecular searches. We have included all full names at first appearance in the text and then according to their first appearance in the literature. However, we made exceptions, where double names are common in the literature or are likely to be commonly used in the future.
Plant Material and Growth
Col-0 plants carrying single copies of the auxin-responsive promoter-GUS fusions 7xDR5, 2xD0, and AGH-3 (Ulmasov et al., 1997b; Murfett et al., 2001) were kindly provided by Jane Murfett and Tom Guilfoyle (University of Missouri, Columbia). Leaf primordia from each of these genotypes were subjected to 2,4-D treatment as described below, and the 7xDR5 was selected for further studies because of the even expression of the GUS reporter gene (Fig. 1). Col-0 plants carrying a full-length MP cDNA (Hardtke and Berleth, 1998), control sequenced and tested for functionality by normalization of mp mutant traits and fused to the cauliflower mosaic virus 35S promoter (Holthorf et al., 1995) in binary vector pGPTV (Becker et al., 1992; 35S::MP in the following), were kindly provided by Christian Hardtke (McGill University, Montreal). All experiments in mp mutant genetic background used the allele mpG12, except for the cotyledon rooting assay, where the mpG33 allele was used. Both alleles are molecularly characterized (Hardtke and Berleth, 1998). Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Seeds were surface-sterilized in 15% (v/v) commercial bleach, washed in distilled water, and stratified in growth medium at 5°C for 4 d. All plant material was germinated and grown in liquid medium (ready-to-use 0.5× Murashige and Skoog salt mixture, vitamins, 1.5% [w/v] Suc, buffered to pH 5.7; catalog no. 11118, Invitrogen, Carlsbad, CA) on a rotary shaker at 50 rpm and 25°C under continuous light (150 μE m−2 s−1).
Auxin Transport Inhibition
For exposure to NPA (Chem Service, West Chester, PA), TIBA (Sigma-Aldrich, St. Louis), and HFCA (Sigma-Aldrich), medium was adjusted to contain 0, 0.1, 1.0, and 10 μm inhibitor in an invariant volume of the inhibitor solvent, dimethyl sulfoxide (DMSO). Seeds were then stratified and grown in these media as described above and harvested at consecutive DAG.
Auxin Response Assays
For the cotyledon expansion assay, the growth medium was adjusted to contain 0 or 1.0 μm IAA (Sigma-Aldrich) or 2,4-D in an invariant volume of the inhibitor solvent DMSO. At 4 DAG of culture, seedlings of mp single-cotyledonous phenotype were selected from mp mutant lines and transferred to fresh auxin-containing medium. Wild-type seedlings were treated in the same manner. At 10 DAG, seedlings were fixed and washed as described below, and the area of cotyledons was measured from microscopy images using the Image-Pro-Plus software (Media Cybernetics, Inc., Silver Spring, MD). For the cotyledon-rooting assay, cotyledons were cut off at 5 DAG, transferred to new growth medium supplemented with 0.3 mg L−1 IBA (Sigma-Aldrich), and cultured under standard growth conditions (above). At 10 DAG, cotyledons were scored for the presence of adventitious roots.
Localization of GUS Activity
Epicotyls containing leaf primordia were dissected under water and transferred into GUS substrate solution, 50 mm sodium phosphate, pH 7, 5 mm K3/K4 FeCN, 0.1% (w/v) Triton X-100, and 2 mm 5-bromo-4-chloro-3-indolyl-beta-GlcUA (Duchefa Biochemie, Amsterdam, The Netherlands). After air evacuation, samples were incubated at 37°C for 3 to 4 h. Thereafter, samples were kept in water at 5°C overnight to remove excess substrate, fixed in a freshly made ethanol:acetic acid (6:1, v/v) solution overnight, washed twice in 96% (v/v) ethanol, and stored in 70% (v/v) ethanol. Dissected leaf primordia were mounted in clearing solution (chloral hydrate:glycerol:water, 9:1:3 [w/w/v]), analyzed on an AX-70 microscope (Olympus, Tokyo), and photographed using a digital camera (S1, Fuji Photo Film, Tokyo).
AtHB20::GUS Expression
The cloning of the AtHB20 gene has been described by Hanson (2000). The predicted HD-ZIP motifs are most closely related to AtHB3 (Mattsson et al., 1992) in the Arabidopsis genome (amino acid similarity, 93%). A 2,527-bp SalI-XhoI fragment (between positions 23,057 and 25,587 on BAC clone AC008261 at GenBank) was cloned into the SalI site in the pBI101.1 binary vector (BD Biosciences Clontech, Palo Alto, CA) to generate a translational fusion product with the GUS gene in the vector (AtHB20::GUS). On the basis of the sequence of a full-length cDNA clone (accession no. AY087631; Haas et al., 2002), the SalI-XhoI fragment contains 1,978 bp of upstream untranscribed sequence. Indistinguishable GUS expression patterns were observed in the progenies of four independent transformants.
Gene Expression Analysis
Aliquots of 50 to 100 Col-0, mp, or 35S::MP seeds were transferred to fresh medium at 3 DAG, incubated for 4 d, and transferred to the same medium adjusted to contain 0, 10, or 100 μm IAA in an invariant volume of the inhibitor solvent DMSO. After 30 min of incubation on a shaker, seedlings were dried by a quick squeeze between paper towels and then frozen in liquid nitrogen. RNA was purified as described by Chang et al. (1993) and quantified based on absorbance at 260 and 280 nm. Samples of total RNA (10 μg lane−1) were separated on MOPS-formaldehyde gel (Sambrook and Russell, 2001) and blotted onto Hybond-N (Amersham Biosciences AB, Uppsala) according to the manufacturer's instructions. Probes, [32P]dCTP-labeled by random-priming, were hybridized to filters in Church buffer as described by Sambrook and Russell (2001). Filters were washed twice for 10 min in 2× SSC and twice for 5 min in 0.2× SSC, all at 65°C. Hybridization signal was visualized and quantified in a phosphor imager (Personal FX, Bio-Rad, Hercules, CA). Intensity of signal was normalized against the signal intensity obtained with an Arabidopsis 18S rRNA probe.
Templates for probe labeling were obtained by PCR amplification using Taq-polymerase (Fermentas, Vilnius, Lithuania) according to the manufacturer's instructions and calculated annealing temperature of primers. PCR primers were: AtHB8f (CCGAAGGAAGTTACTCATCC), AtHB8r (TATGAGAATTACCTTCATCCC), AtHB9f (TCAATGCAACTTCCCACTTCC), AtHB9r (CCAACCTTTTCCTTCATCAAACA), AtHB14f (TGCTTCCTTTATCTTGCTCCT), AtHB14r (CCATCTTCCAAACATGTGCT), IFL/REVf (ATGAGATGTTCCCGGATGATG), IFL/REVr (GCAACAGCTTGTTCATAACTCACA), AtHB20–3′fCAAAGACCTATTCCCTTCATCG, AtHB20–3′r CCAATGCTCTACAAACCCAAA, AtHB20–5′f TCCAAATCTGCAAACAACAAA, AtHB20–5′r TGCCCCATCGTCTGATAGAT, IAA1f (AAGTCACCAATGGGCTTAACC), IAA1r (TCGGATCCTTTCATGATTCTG), IAA19f (GTGGTGACGCTGAGAAGGTTAA), IAA19r (GAACCAGCTCCTTGCTTCTTGT), 18SrRNAf (TTCCATTGCGTTTGAGAGGA), and 18SrRNAr (AGACTTGCCCTCCAATGGAT).
All northern hybridizations resulted in single bands. Probe specificity was ensured by BLAST search: similarity of hybridization probes with sequences in Arabidopsis databases, <21%; and for AtHB9, AtHB14, and IAA1 by control hybridization with closest match in the database, which resulted in a distinguishable signal.
ACKNOWLEDGMENTS
We thank Tom Guilfoyle and Jane Murfett for diverse auxin response marker lines; Christian Hardtke for the generation of 35S::MP constructs; and Johannes Hanson, Peter Engström, and Agneta Ottosson (Uppsala University, Sweden) for providing sequence information on the AtHB20 gene and help with Arabidopsis transformation. We also thank Enrico Scarpella and Naden Krogan (University of Toronto, Canada) for very helpful suggestions on the manuscript.
Footnotes
This work was supported by the Natural Science and Engineering Research Council (NSERC) of Canada (research grants to T.B. and microscope equipment grant to J.M.). W.C. was supported by an NSERC short-term student research fellowship and by an NSERC long-term postgraduate fellowship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.013623.
LITERATURE CITED
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol. 1995;25:533–549. doi: 10.1006/jmbi.1995.0454. [DOI] [PubMed] [Google Scholar]
- Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–11. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 531–546. [Google Scholar]
- Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, Morelli G. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- Baima S, Possenti M, Matteucci A, Wisman E, Altamura MM, Ruberti I, Morelli G. The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001;126:643–655. doi: 10.1104/pp.126.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1992;19:327–336. [Google Scholar]
- Bennett MJ, Marchant A, May ST, Swarup R. Going the distance with auxin: unravelling the molecular basis of auxin transport. Phil Trans R Soc Lond. 1998;353:1511–1515. doi: 10.1098/rstb.1998.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Jürgens G. The role of the MONOPTEROSgene in organizing the basal body region of the Arabidopsis embryo. Development. 1993;122:575–587. [Google Scholar]
- Berleth T, Mattsson J, Hardtke CS. Vascular continuity and auxin signals. Trends Plant Sci. 2000;5:387–393. doi: 10.1016/s1360-1385(00)01725-8. [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- Carland FM, Berg L, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B. Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell. 1999;11:2123–2137. doi: 10.1105/tpc.11.11.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson SA, Chilley PM, Topping JF, Evans IM, Souter MA, Lindsey K. The POLARISgene of Arabidopsis encodes a predicted peptide required for correct root growth and leaf vascular patterning. Plant Cell. 2002;14:1705–1721. doi: 10.1105/tpc.002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Dengler N, Kang J. Vascular patterning and leaf shape. Curr Opin Plant Biol. 2001;4:50–56. doi: 10.1016/s1369-5266(00)00135-7. [DOI] [PubMed] [Google Scholar]
- Deyholos M, Cordner G, Beebe D, Sieburth L. The SCARFACE gene is required for cotyledon and leaf vein patterning. Development. 2000;127:3205–3213. doi: 10.1242/dev.127.15.3205. [DOI] [PubMed] [Google Scholar]
- Donelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler N. Patterns of cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- Esau K. Vascular Differentiation in Plants. New York: Holt, Rinehart and Winston; 1965. [Google Scholar]
- Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Guilfoyle T, Hagen G, Ulmasov T, Murfett J. How does auxin turn on genes? Plant Physiol. 1998;118:341–347. doi: 10.1104/pp.118.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Volfovsky N, Town CD, Troukhan M, Alexandrov N, Feldmann KA, Flavell RB, White O, Salzberg SL. Full-length messenger RNA sequences greatly improve genome annotation. Genome Biol. 2002;3:29.1–29.12. doi: 10.1186/gb-2002-3-6-research0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–385. [PubMed] [Google Scholar]
- Hamann T, Benkova E, Baurle I, Kientz M, Jürgens G. The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev. 2002;16:1610–1615. doi: 10.1101/gad.229402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann T, Mayer U, Jürgens G. The auxin-insensitive bodenlosmutation affects primary root formation and apical-basal patterning in the Arabidopsis embryo. Development. 1999;126:1387–1395. doi: 10.1242/dev.126.7.1387. [DOI] [PubMed] [Google Scholar]
- Hanson J. Functional characterization of the pointed cotyledon subclass of HDZip genes in Arabidopsis thaliana. PhD thesis. Uppsala: Uppsala University; 2000. [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROSencodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M. The axr6mutants of Arabidopsis define a gene involved in auxin response and early development. Development. 2000;127:23–32. doi: 10.1242/dev.127.1.23. [DOI] [PubMed] [Google Scholar]
- Holthorf S, Apel K, Bohlmann H. Comparison of different constitutive and inducible promoters for the overexpression of transgenes in Arabidopsis thaliana. Plant Mol Biol. 1995;29:637–646. doi: 10.1007/BF00041155. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Jacobs WP. The role of auxin in differentiation of xylem around a wound. Am J Bot. 1952;39:301–309. [Google Scholar]
- Kang J, Dengler N. Cell cycling frequency and expression of the homeobox gene ATHB-8during leaf vein development in Arabidopsis. Planta. 2002;216:212–219. doi: 10.1007/s00425-002-0847-9. [DOI] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. Protein-protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci USA. 1997;94:11786–11791. doi: 10.1073/pnas.94.22.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. Bundle sheath cells and cell-specific plastid development in Arabidopsisleaves. Development. 1998;125:1815–1822. doi: 10.1242/dev.125.10.1815. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Sugiyama M, Fukuda H. A series of novel mutants of Arabidopsis thalianathat are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into question. Development. 2000;127:3197–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
- Leyser O. Molecular genetics of auxin signaling. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:377–398. doi: 10.1146/annurev.arplant.53.100301.135227. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton J, Estelle M. Growth and development of the axr1mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Reed JW. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol Biol. 2002;49:387–400. [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndon RF. Plant Development, the Cellular Basis. London: Unwin Hyman; 1990. [Google Scholar]
- Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 2000;14:2938–2943. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Söderman E, Svenson M, Borkird C, Engström P. A new homeobox-leucine zipper gene from Arabidopsis thaliana. Plant Mol Biol. 1992;18:1019–1022. doi: 10.1007/BF00019223. [DOI] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman J, Barton MK. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature. 2001;411:709–713. doi: 10.1038/35079635. [DOI] [PubMed] [Google Scholar]
- McCready CC. Movement of plant growth regulators in plants: I. Polar transport of 2,4-dichlorophenoxyacetic acid in segments of from the petioles of Phaseolus vulgaris. New Phytol. 1963;62:3–18. [Google Scholar]
- McCready CC, Jacobs WP. Movements of growth regulators in plants: II. Polar transport of radioactivity from indoleacetic acid (14C) and 2,4-dichlorophenoxyacetic acid (14C) in petioles of Phaseolus vulgaris. New Phytol. 1963;62:19–34. [Google Scholar]
- Muday GK, DeLong A. Polar auxin transport: controlling where and how much. Trends Plant Sci. 2001;6:535–542. doi: 10.1016/s1360-1385(01)02101-x. [DOI] [PubMed] [Google Scholar]
- Murfett J, Wang XJ, Hagen G, Guilfoyle TJ. Identification of Arabidopsis histone deacetylase HDA6 mutants that affect transgene expression. Plant Cell. 2001;13:1047–1061. doi: 10.1105/tpc.13.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T, Dengler N. Leaf vascular pattern formation. Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsisfloral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuga D, DeGuzman B, Prigge MJ, Drews GN, Clark SE. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001;25:223–236. doi: 10.1046/j.1365-313x.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- Palme K, Galweiler L. PIN-pointing the molecular basis of auxin transport. Curr Opin Plant Biol. 1999;2:375–381. doi: 10.1016/s1369-5266(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Raven JA. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 1975;74:163–172. [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubery PH, Sheldrake AR. Carrier-mediated auxin transport. Planta. 1974;188:101–121. doi: 10.1007/BF00388387. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:151–262. [Google Scholar]
- Sachs T. Cell polarity and tissue patterning in plants. Development Suppl. 1991;91:83–93. [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Ed 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schena M, Davis RW. HD-Zip proteins: members of an Arabidopsis homeodomain protein superfamily. Proc Natl Acad Sci USA. 1992;89:3894–3898. doi: 10.1073/pnas.89.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development. 1995;121:2723–2735. doi: 10.1242/dev.121.9.2723. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. ARF1, a transcription factor that binds to auxin response elements. Science. 1997a;276:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997b;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Demura T, Fukuda H. Brassinosteroids induce entry into the final stage of tracheary element differentiation in cultured zinnia cells. Plant Cell Physiol. 1997;38:980–983. doi: 10.1093/oxfordjournals.pcp.a029262. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Fujioka S, Demura T, Takatsuto S, Yoshida S, Fukuda H. Brassinosteroid levels increase drastically prior to morphogenesis of tracheary elements. Plant Physiol. 2001;125:556–563. doi: 10.1104/pp.125.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H. Vascular tissue differentiation and pattern formation in plants. Annu Rev Plant Physiol Plant Mol Biol. 2002;53:183–202. doi: 10.1146/annurev.arplant.53.100301.135245. [DOI] [PubMed] [Google Scholar]
- Zhong R, Ye Z-H. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye ZH. Alteration of auxin polar transport in the Arabidopsis ifl1mutants. Plant Physiol. 2001;126:549–563. doi: 10.1104/pp.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]